Abstract
Background
Studies about the incidence and severity of coronavirus disease 2019 (COVID-19) in children are still significantly lower than those in adults. Moreover, data on the effect of COVID-19 in children with congenital heart disease (CHD) are limited. To the best of our knowledge, this study first reported mortality in a child with CHD who acquired COVID-19.
Case summary
A 16-month-old boy presented to the emergency department due to shortness of breath, fever, cough, and poor oral intake. He tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). He required mechanical ventilation for rapidly progressing respiratory failure. The patient had a large mid-muscular ventricular septal defect (VSD) that was closed percutaneously at the age of 13 months. Moreover, we followed his hospital sequelae from admission to death.
Discussion
This child had multiple risk factors, including malnutrition and persistent pulmonary hypertension (PH) after late closure of the VSD. The pre-existing PH could have been aggravated by the lung condition associated with COVID-19 and the respiratory failure triggered by SARS-CoV-2 infection. The patient presented with ventricular systolic dysfunction, elevated troponin serum levels and newly developed trifascicular block, which were indicative of myocardial injury. The elevated inflammatory markers and multi-organ dysfunction seem to corroborate multisystem inflammatory syndrome in children, which was described recently among paediatric patients with COVID-19.
Keywords: Coronavirus disease 2019, Congenital heart disease, Myocarditis, Multisystem inflammatory syndrome, Case report
Learning points
Persistent pulmonary hypertension after late ventricular septal defect closure and malnutrition could be risk factors for critical illness in children with severe acute respiratory syndrome coronavirus 2 infection.
Myocardial injury can be observed in paediatric patients with coronavirus disease 2019.
Introduction
The world has been experiencing the greatest healthcare challenge since December 2019 when coronavirus disease 2019 (COVID-19) was first detected in Wuhan, China.1 The first confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a child was reported in Shenzhen on 20 January 2020.2 Thereafter, several paediatric case reports and case series have been published.
The SARS-CoV-2 infection rate in children is significantly lower than that in adults. Data from China and the USA showed that only about 2% of all reported COVID-19 cases involved individuals aged below 18 years.3,4 Data on children with congenital heart disease (CHD) who acquire COVID-19 are limited. Moreover, comorbidities are associated with severe illness in children.5
Timeline
| Time | Events |
|---|---|
| 3 February 2020 | Percutaneous ventricular septal defect closure |
| 25 March 2020 | Hospital admission due to bronchiolitis |
| 29 March 2020 | Discharged home |
| 13 May 2020 | The patient had shortness of breath, fever, cough, vomiting, and poor oral intake |
| 15 May 2020 4:00 |
Presentation to the emergency department due to respiratory failure. The patient was suspected of coronavirus disease 2019. A swab test was performed to confirm the diagnosis. He had anaemia, elevated inflammatory markers, and high cardiac enzyme levels. Chest radiography revealed bilateral ground-glass opacities. |
| 15 May 2020 7:00 |
The patient required mechanical ventilation for rapidly progressing respiratory failure. He was transferred to the paediatric intensive care unit. Inotropic support was started. |
| 16 May 2020 | Reverse transcription polymerase chain reaction confirmed the diagnosis of coronavirus disease 2019. Treatment with hydroxychloroquine, azithromycin, and tocilizumab was initiated. Intravenous immunoglobulin was also administered. |
| 16–19 May 2020 | The patient presented with multi-organ dysfunction. High-frequency oscillatory ventilation and vasopressors were continuously administered. |
| 20 May 2020 | The patient experienced worsening shock, refractory hypoxaemia, and cardiac arrest, and eventually died. |
Case presentation
A 16-month-old Saudi Arabian boy presented to the emergency department due to shortness of breath. The patient experienced fever, poor oral intake, vomiting 1–2 times per day, and dry cough for 2 days. The patient had a large mid-muscular ventricular septal defect (VSD), with a previous history of percutaneous closure of VSD 3 months before the current presentation. The child had been in contact with his mother who tested positive for COVID-19. The mother’s manifestations started 8 days before the child developed symptoms. Upon presentation, the patient’s oxygen saturation was 70%. Hence, he required oxygen supplementation. COVID-19 test, other blood examinations, and chest radiography were performed. In the emergency room (ER), the condition of the child deteriorated. Therefore, he required mechanical ventilation for rapidly progressing respiratory failure. Premedication for endotracheal intubation was administered including atropine 0.02 mg/kg, ketamine 1 mg/kg, and rocuronium 1 mg/kg. During intubation, he developed bradycardia and asystole that required cardiopulmonary resuscitation (CPR) for 6 min and two doses of epinephrine (0.01 mg/kg/dose). Then, a successful return of spontaneous circulation was achieved, and the patient was transferred to the paediatric intensive care unit (PICU).
The following examination results were obtained. Assessment in the ER: weight, 7.1 kg; height, 71.5 cm (both below the 3rd percentile); temperature, 38.3°C; heart rate, 180 beats/min; respiratory rate, 70 cycles/min; blood pressure, 73/43 mmHg; and capillary refill time, >3 s. Chest examination: severe subcostal and intercostal recessions and bilateral fine crackles. Cardiac examination: grade I–II soft systolic murmur at the lower left sternal border. Abdominal examination: enlarged liver located 4 cm below the costal margin. Examination of the other systems: unremarkable results.
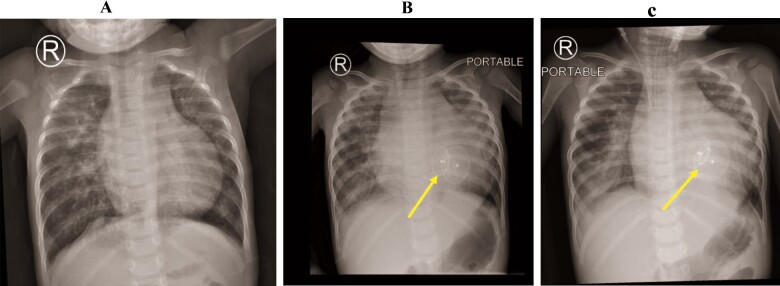
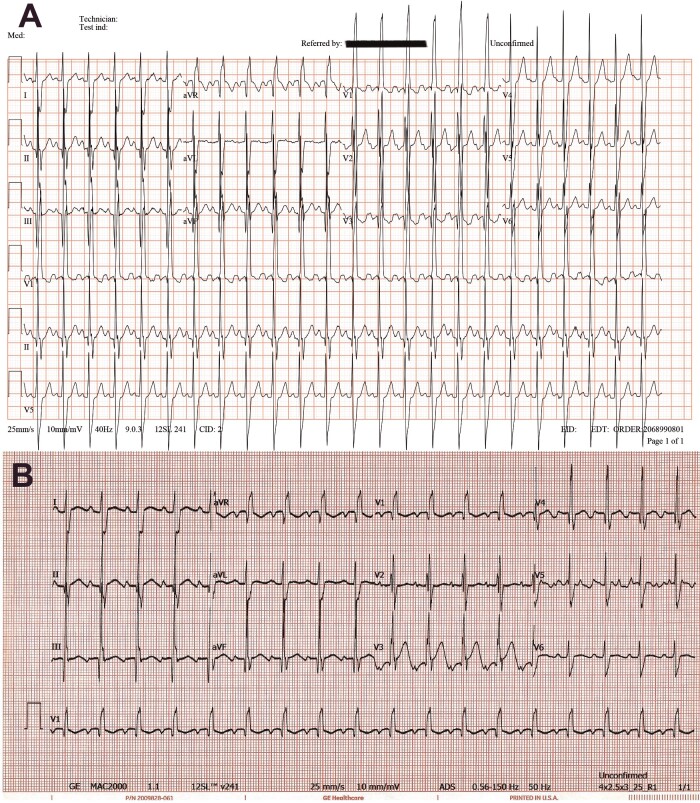
The patient tested positive for SARS-CoV-2 based on reverse transcription polymerase chain reaction. Initial laboratory testing revealed anaemia (haemoglobin 7.6 g/dL, normal range 9.6–15.6 g/dL), elevated D-dimer levels (40.56 mg/L, normal <0.5 mg/L), prolonged prothrombin time (23 sec, normal <12.5 s), increased aspartate transaminase enzyme levels (79 U/L, normal <37 U/L), lactic acidosis (7.7 mmol/L, normal <2 mmol/L), and elevated lactate dehydrogenase (LDH) levels (1284 U/L, normal <240 U/L) and high cardiac enzyme levels (troponin-I 2.07 µg/L, normal <0.04 µg/L). While in the PICU, the patient had thrombocytopenia (72–92 K/µL, normal >150 K/µL), lymphopenia (1.27–3.62 K/µL, normal range 4–10.5 K/µL), increased C-reactive protein (CRP) level (19 mg/L, normal <3 mg/L), and impaired liver and kidney functions (alanine transaminase 110–146 U/L, normal <78 U/L; urea 11.3–19.5 mmol/L, normal <6.4 mmol/L) (Table 1). The bacterial culture and respiratory and blood examination results were negative. Chest radiography revealed bilateral ground-glass opacities and cardiomegaly (Figure 1). Electrocardiogram upon admission showed sinus rhythm with trifascicular block and precordial T-wave inversion (Figure 2).
Table 1.
Serial laboratory results during hospital admission
| Normal reference range | Baseline levela | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|---|---|
| CBC | ||||||||
| WBC (K/µL) | 5.5–17.5 | 8.8 | 8.7 | 11.35 | 8.39 | 7.19 | 7.23 | 10.8 |
| Lymphocytes (K/µL) | 4–10.5 | 6.16 | 5.49 | 1.47 | 1.27 | 1.28 | 3.11 | 3.62 |
| Platelets (K/µL) | 150–450 | 347 | 160 | 88 | 92 | 83 | 75 | 72 |
| Haemoglobin (g/dL) | 9.6–15.6 | 9.9 | 7.6 | 8.6 | 8.9 | 8 | 8.3 | 8.5 |
| Coagulation profile | ||||||||
| Prothrombin time (s) | 9.4–12.5 | 13 | 23 | 23.2 | 20.8 | 13.9 | ||
| INR | 0.85–1.3 | 1.16 | 1.5 | 2.04 | 1.83 | 1.24 | ||
| APTT (s) | 25.1–36.5 | 34.4 | 27.1 | 27.6 | 25.4 | 27.5 | ||
| D-dimer (mg/L) | 0–0.5 | 40.56 | 28.27 | 4.15 | ||||
| Fibrinogen (mg/dL) | 200–393 | 118 | 385 | 297 | ||||
| CRP (mg/L) | 0–3 | <3 | <3 | 19 | ||||
| Ferritin (ng/ml) | 30–400 | 273 | 157 | |||||
| KFTs and electrolytes | ||||||||
| Na (mmol/L) | 136–145 | 140 | 140 | 148 | 149 | 153 | 154 | 155 |
| K (mmol/L) | 3.5–5.1 | 4.3 | 5 | 3.8 | 3.9 | 3.3 | 3 | 4 |
| Urea (mmol/L) | 2.5–6.4 | 5.8 | 4.3 | 11.3 | 19.5 | 13.6 | 14.2 | 19.5 |
| Creatinine (µmol/L) | 53–115 | 20 | 31 | 86 | 68.7 | 78 | 68 | 65.3 |
| Calcium (mmol/L) | 2.12–2.52 | 2.2 | 2.2 | 1.67 | 2.09 | 2.05 | 2.12 | 2.01 |
| Magnesium (mmol/L) | 0.70–1.0 | 0.82 | 0.81 | 0.66 | 1.07 | 0.86 | 0.76 | 1.02 |
| Liver function tests | ||||||||
| AST (U/L) | 15–37 | 47 | 79 | 367 | 510 | |||
| ALT (U/L) | 12–78 | 34 | 38 | 110 | 146 | |||
| GGT (U/L) | 5–85 | 12 | 13 | 33 | 22 | |||
| Bilirubin total (µmol/L) | 0–17 | 14 | 15 | 5.5 | 13.5 | |||
| Albumin (g/L) | 40.2–47.6 | 41 | 32.4 | 29.2 | 30.5 | 28.2 | ||
| Lactic acid, mmol/L | 0.4–2 | 7.7 | 1.3 | 0.9 | 1.1 | 1.4 | 1.7 | |
| Cardiac enzymes | ||||||||
| Troponin-I (µg/L) | 0.02–0.04 | 2.07 | ||||||
| Creatine kinase (IU/L) | 26–308 | 398 | ||||||
| Mass CK MB (µg/L) | 0–3.6 | 11.0 | ||||||
| LDH (U/L) | 100–240 | 1284 | ||||||
| Blood group | O positive |
ALT, alanine transaminase; APTT, activated partial thromboplastin time; AST, aspartate transaminase; CBC, complete blood count; CK, creatine kinase; CRP, C-reactive protein; GGT, γ-glutamyl transferase; INR, international normalized ratio; KFTs, kidney function tests; LDH, lactate dehydrogenase; WBC, white blood count.
The values obtained before cardiac catheterization.
Figure 1.
Chest radiography was conducted before ventricular septal defect closure (A), during the last admission in the emergency department (B), and immediately after mechanical ventilation (C). Results reveal bilateral ground-glass opacities and cardiomegaly. The ventricular septal defect device (yellow arrows).
Figure 2.
(A) Electrocardiogram was conducted after percutaneous ventricular septal defect closure. Results reveal sinus rhythm with incomplete right bundle branch block. (B) Electrocardiogram upon admission showing sinus rhythm with trifasicular block and precordial T-wave inversion.
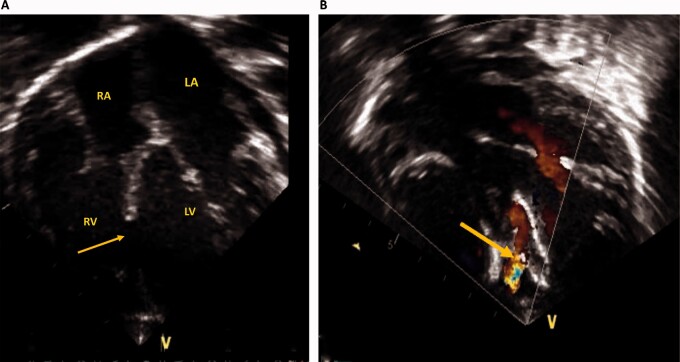
Echocardiography was performed after PICU admission. Results showed that the VSD device was in place, with a small residual leak across the device, mild tricuspid valve regurgitation, with an estimated systolic pulmonary pressure of 60 mmHg, moderate biventricular systolic dysfunction with a left ventricular ejection fraction of 40% (Video1), normal coronary arteries (Video2), and no pericardial effusion or vegetations. Two days after PICU admission, a follow-up echocardiography demonstrated unchanged ventricular systolic dysfunction and pulmonary hypertension (PH) (Figure 3).
Figure 3.
Echocardiography before and after ventricular septal defect device closure. (A) Apical four-chamber view showing a large mid-muscular ventricular septal defect (yellow arrow). (B) Apical four-chamber view with colour Doppler after device closure showing a small residual leak across the ventricular septal defect device (yellow arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
After a successful CPR, the patient was conscious with bilateral equal and reactive pupils. Fentanyl, epinephrine, and milrinone infusions were initiated in PICU. Then, norepinephrine infusion for vasodilatory shock was initiated. Six hours after PICU admission, oxygenation and ventilation were not maintained even with conventional mechanical ventilation. Thus, the patient was connected to high-frequency oscillatory ventilation (HFOV). Based on our protocol, he received the following medical therapy: hydroxychloroquine 6.5 mg/kg twice daily for Day 1; followed by 3.25 mg/kg twice daily for 5 days, azithromycin 10 mg/kg on Day 1; followed by 5 mg/kg once daily on Days 2–5, and tocilizumab 12 mg/kg repeated within 12 h. Intravenous immunoglobulin (total dose of 2 g/kg) was administered for suspected myocarditis.
Despite the provision of aggressive supportive care, the patient developed multiple organ dysfunction syndrome (MODS). HFOV and vasopressors were continuously administered for 5 days. Ultimately, he presented with worsening shock, refractory hypoxaemia, and cardiac arrest. The patient died on Day 6 of hospitalization.
At the age of 2 weeks, he developed shortness of breath and was diagnosed with a large mid-muscular VSD. He was referred for cardiac surgery but was never followed-up due to family living in a remote area. He returned to our institution at the age of 13 months. He was malnourished because of poor oral intake. Echocardiography was performed and revealed a large mid-muscular VSD (16 mm) with severe PH (70 mmHg).
Cardiac catheterization showed a baseline indexed pulmonary vascular resistance (PVRI) of 5.8 Wood units (WU)⋅m2, but it dropped to 2.6 WU⋅m2 on 100% oxygen. The baseline systolic pulmonary artery pressure was 71 mmHg; systemic blood pressure was 76/40 mmHg; QP/QS was 2.5 and the pulmonary vascular resistance/systemic vascular resistance (SVR) was 0.26. After a multidisciplinary consensus meeting and discussion with the family, percutaneous VSD closure was performed successfully without complications using a 19 mm Amplatzer septal occluder (St. Jude Medical, Plymouth, MN, USA).
One month before the current presentation, he had bronchiolitis that required hospitalization for 5 days. Echocardiography was repeated and revealed haemodynamically insignificant small residual VSD, PH (60 mmHg), and normal ventricular function (Video3). He was scheduled for a re-evaluation of PH after 6 weeks to allow complete lung recovery, but he presented earlier with COVID-19.
Discussion
There are only a few reported cases of COVID-19 in children with CHD worldwide. These cases included three patients from China6 and three patients from North America.5 Although these patients had a severe illness, they all survived. To the best of our knowledge, this study first reported mortality among children with CHD who acquired SARS-CoV-2 infection.
Consistent with previous reports,5 our patient had underlying CHD and malnutrition. These disorders likely contributed to the severity of the condition that required mechanical ventilation and resulted in MODS.
Our patient presented with fever, dyspnoea, and respiratory failure complicated by cardiac arrest and MODS. Chest radiography revealed bilateral lung infiltrations, which is consistent with previous reports.4,7 A chest computed tomography scan was not performed to limit the spread of infection. Similar to other studies,6 there were evident laboratory abnormalities throughout the disease course (Table 1), including elevated D-dimer, CRP, LDH, and cardiac enzyme levels. The lymphocytic and platelet counts were low as well.
The American Heart Association guidelines recommend the repair of CHD in patients with PH if the PVRI is <6 WU⋅m2 or the PVR/SVR is <0.3 at baseline.8 Accordingly, our patient underwent VSD device closure. The pre-existing PH could have been aggravated by the lung condition associated with COVID-19. Respiratory failure triggered by SARS-CoV-2 infection and MODS could have been correlated with COVID-19 and/or cardiac arrest.
SARS-CoV-2 infection can cause an exacerbated immune reaction that causes a surge in pro-inflammatory cytokines, which is known as a cytokine storm. The biomarkers of cytokine storm include D-dimer, CRP, LDH, troponin, and lymphopenia.9 All of these were observed in the current case.
The patient presented with ventricular dysfunction, elevated troponin serum levels, and newly developed trifascicular block, which were indicative of myocardial injury. The cause of myocardial damage might have been a multifactorial process, which includes viral myocarditis, MODS, and sequelae of cardiac arrest. The previous findings seem to corroborate multisystem inflammatory syndrome in children, which was described recently among paediatric patients with COVID-19.10
Conclusion
COVID-19 may cause life-threatening symptoms in children with underlying CHD.
Lead author biography

Jameel Al-Ata, FRCPC, is an associate professor of Pediatrics and Pediatric Cardiology at King Abdulaziz University, Jeddah, Saudi Arabia. After completing the American Board of Pediatrics, he performed a paediatric cardiology fellowship in Montreal Children’s Hospital (MCH), McGill University, Canada. He is an interventional paediatric cardiologist. He is also very involved in teaching students, residents, and fellows at King Abdulaziz University Hospital.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient’s parents in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
Contributor Information
Saud A Bahaidarah, Pediatric Cardiology Division, Department of Pediatrics, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia.
Ahmed M Dohain, Pediatric Cardiology Division, Department of Pediatrics, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia; Pediatric Cardiology Division, Department of Pediatrics, Cairo University, Kasr Al Ainy Faculty of Medicine, Al-Manial 11956, Cairo, Egypt.
Gaser Abdelmohsen, Pediatric Cardiology Division, Department of Pediatrics, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia; Pediatric Cardiology Division, Department of Pediatrics, Cairo University, Kasr Al Ainy Faculty of Medicine, Al-Manial 11956, Cairo, Egypt.
Abeer A Alnajjar, Pediatric Infectious Diseases Division, Department of Pediatrics, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia.
Jameel Al-Ata, Pediatric Cardiology Division, Department of Pediatrics, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 4. CDC COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA. et al. ; for the International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr 2020;174:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A. et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020;179:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK. et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015;132:2037–2099. [DOI] [PubMed] [Google Scholar]
- 9. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. et al. COVID-19 and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 10. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF. et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.