Abstract
Background
The aim of this study was to investigate the relationship between diabetes mellitus (DM) and left atrial (LA) remodelling in a group of patients with heart failure and reduced ejection fraction (HFrEF), and their combined impact on cardiac events (CE).
Methods
This study included 136 consecutive HFrEF patients (65 ± 11 years), 36 had DM, and 86 had increased LA stiffness (LASt). All patients underwent complete conventional and tissue Doppler echocardiographic measurements were made including LA volumes and function. LASt was calculated using the formula: LASt = E/e’ ratio / PALS.
Results
At 55 ± 37 months follow‐up, free survival from CE was 69% in patients without DM and 44.4% in those with DM (p < .0001). The CE free survival was lower in patients with increased LASt compared to normal LASt, (50 versus. 80%, p < .001), irrespective of the presence of DM (27 versus. 71%, p < .001).The best cut‐off value of LASt for predicting CE in the group as a whole was ≥ 0.82% [81% sensitivity, 72% specificity and AUC 0.82 (p < .001)]. LASt ≥ 0.82% also predicted CE in no DM patients [78% sensitivity, 71% specificity and AUC 0.80 (p < .001)] and was a stronger predictor in DM patients [85% sensitivity, 71% specificity and AUC = 0.847 (p < .001)].
Conclusion
High LA stiffness is associated with poor clinical outcome in patients with HFrEF. Diabetes has an additional incremental value in determining clinical outcome in those patients.
Keywords: diabetes mellitus, heart failure with reduced ejection fraction, Left atrial stiffness
Abbreviations
- 2D:
two dimensional
- DM:
diabetes mellitus
- EDD:
end‐diastolic dimension
- EF:
ejection fraction
- ESD:
end‐systolic dimension
- HF:
heart failure
- HFmrEF:
heart failure mid‐range ejection fraction
- HFrEF:
heart failure reduced ejection fraction
- LA:
left atrial/ left atrium
- LASt:
left atrial stiffness
- LAV max:
left atrial maximal volume
- LAVI max:
left atrial maximal indexed volume
- LV:
left ventricle/ ventricular
- PALS:
peak atrial longitudinal strain
- STE:
speckle tracking echocardiography
- TDI:
tissue Doppler imaging
1. INTRODUCTION
Heart failure (HF) is a known widespread epidemic and constitutes a major public health problem. (Bytyçi & Bajraktari, 2015) Diabetes mellitus (DM), a well‐established risk factor for coronary and myocardial disease, is closely related to HF through many mechanisms, (Nagoshi et al., 2011) for example impaired cardiac glucose metabolism which leads to left ventricular (LV) systolic and diastolic dysfunction. (Rosano et al., 2008) Patients with the two conditions combined are known to have higher risk of mortality and cardiac events (CE). (MacDonald et al., 2008) In patients with HF and reduced ejection fraction (HFrEF), studies have shown significant reduction of left atrial (LA) function as shown by deformation measurements, which implicate clinical outcome and prognosis. (Bytyçi et al., 2014; Rossi et al., 2018).
In HF, the commonly seen LA enlargement is associated with worse symptoms, frequent atrial fibrillation, reduced quality of life and poor prognosis. (Dini et al., 2002) In addition to structural changes, LA cavity remodelling and dysfunction also play an important clinical role in these patients. Impaired LA function results in raised pulmonary venous pressures (Bytyçi et al., 2019) and increased LA stiffness (LASt), a marker of myocardial fibrosis, which is an important substrate for arrhythmias. (Khurraml et al., 2016) However, the clinical value of those parameters in prognostic stratification of HFrEF patients and the additional role of DM has not yet been fully ascertained. The aim of this study was to investigate the relationship between DM and LA remodelling in HFrEF and their combined impact on CE.
2. METHODS
2.1. Study population
We studied 136 consecutive patients with clinical signs and symptoms of HF (New York Heart Association—NYHA class I‐III), and LV EF < 40%, according to the current ESC guidelines. (Ponikowski et al., 2016) Patients were divided into those with (n = 36) and without (n = 100) DM, as well as with (n = 68) or without (n = 68) increased LASt, based on a previous suggested cut‐off value of 0.99%, (Kurt et al., 2009) various combinations and permutations constituted subgroups. All patients were referred to the Cardiac, Thoracic and Vascular Department of the University of Pisa, Italy between January 2013 and December 2018, for thorough clinical assessment for potential diagnosis of HF, and all received detailed Doppler echocardiographic examination.
Exclusion criteria were as follows: atrial arrhythmia, history of congenital heart disease, pacemaker implantation, valvular surgery, cardiac transplantation, chronic obstructive pulmonary disease (COPD) or recent acute coronary syndrome, stroke, poor echo window and age < 18 years. The study was approved by the local institutional review board, and all patients gave written informed consent before enrolment in the study. The study was conducted in accordance with institutional policies, national legal requirements, the revised Helsinki Declaration and was approved by the local institutional review board (20,110,015,213).
2.2. Data collection
Detailed history and clinical assessment were obtained in all patients, in whom routine biochemical tests were also performed, including complete blood count, blood glucose, electrolytes and kidney function, as well as weight and height measurements.
2.3. Echocardiographic examination
All echocardiographic examinations were made by one experienced sonographer, using an iE33 X5‐matrix Ultrasound echocardiograph or an Epic Ultrasound echocardiograph (Philips, Andover, Massachusetts) equipped with a multi‐frequency transducer and harmonic imaging software as appropriate. All measurements were performed according to the recommendations of the European Association of Echocardiography / American Association of Echocardiography. (Lang et al., 2015).
2.4. LA structure and function measurements
LA size was measured at end‐ventricular systole when the LA was at its largest dimensions, in both the long‐axis view (anterior–posterior diameter) and the 4‐chamber view (longitudinal and transverse diameters). LA volumes were measured using the Simpson method from the apical 4‐ and 2‐chamber views, in line with the recommendations of the American Society of Echocardiography and European Society of Cardiology. (Lang et al., 2015) LA maximum volume (LAV max) was measured at end‐systole, just prior to mitral valve opening by tracing the LA inner border excluding the area under the mitral valve annulus, and LA minimum volume (LAV min) was measured at end‐diastole, directly after mitral valve closure. LA maximum volume index (LAVI max) was defined as LAV max divided by body surface area. Likewise, LAV min was measured and LAVI min calculated. (Galderisi et al., 2017).
2D speckle tracking software (Q‐LAB version 6.0, TMQ, Philips Medical Systems, Andover, MA) was used to trace the LA endocardial border in the apical 4‐chamber view, as recommended by the EACVI/ASE/Industry Task Force to standardize deformation imaging (Badano et al., 2018) while taking care to exclude the LA appendage and pulmonary veins from the LA cavity, and a composite LA longitudinal reservoir strain curve throughout the cardiac cycle was generated. Peak atrial longitudinal strain (PALS) was derived from the composite LA strain curve. LASt was calculated using the formula (Figure S1) (Kurt et al., 2009):
2.5. LV and RV structure and function measurements
LV volumes and EF were calculated from the apical 2‐ and 4‐chamber views using the modified Simpson's method. (Lang et al., 2015) Pulsed‐wave Doppler mitral velocity recordings were obtained from the apical four‐chamber view by positioning a 1–2 mm sample volume by the tips of the mitral valve leaflets, in diastole. Peak early and late LV diastolic velocities were measured as was E‐wave deceleration time (DT). RV long‐axis myocardial velocities were also studied using Doppler myocardial imaging technique and conventional protocols. LV E/e’ was calculated as the ratio between trans‐mitral peak E‐wave velocity and mean lateral and septal LV myocardial velocities. Linear internal measurements of the LV were made from the parasternal long‐axis view, carefully obtained perpendicular to the LV long axis, and measured at the level of the mitral valve leaflet tips. Mitral regurgitation severity was assessed by colour, continuous‐wave Doppler and other conventional quantitative parameters including the relative mitral regurgitation jet area into the LA. The flow velocity profile was graded: mild, moderate, or severe, according to the guidelines of the American Society of Echocardiography (Zoghbi et al., 2003) and European Society of Cardiology. (Lancellotti et al., 2013) Likewise, tricuspid regurgitation was assessed using colour Doppler and continuous‐wave Doppler techniques. Retrograde trans‐tricuspid peak pressure drop > 35 mmHg was taken as an evidence for pulmonary hypertension. (Rudski et al., 2010).
2.6. Follow‐up
Cardiovascular clinical events (CE) were prospectively reported during follow‐up. Information on patients' clinical outcome was obtained through clinical visits, personal communication with general physicians, and telephone interviews with patients and relatives, by trained research nurses. The primary study end point was cardiac events, combination of death and hospitalization for worsening HF, and secondary end points were cardiac death and hospitalization.
2.7. Statistical analysis
Data are summarized using frequencies (percentages) for categorical variables and mean ± standard deviation for continuous variables or median interquartile (IRQ) ranges, when appropriate continuous data were compared with two‐tailed Student t test and discrete data with chi‐square test. Analysis of variance and Bonferroni statistical tests were used to compare quantitative variables between more than two groups. Correlations were tested with Pearson coefficients. Patients’ survival curves were estimated using the Kaplan–Meier product limit, which were also compared between groups using the log‐rank test. A significant difference was defined as p value < 0.05 (2‐tailed). Statistical analysis was performed with SPSS Software Package version 22.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Clinical and echocardiographic data
One hundred thirty‐six patients were included in the study, with a mean follow‐up period of 55 ± 37 months (Figure S2). Patients’ mean age was 66 ± 11 years, 29% were females, NYHA class 1.92 ± 0.73, and 84.6% had raised BNP > 125pg/ml. 41% of patients were hypertensives, 26.5% were diabetics, and 33.1% had chronic renal failure. Mitral regurgitation was present in 28 patients (20.6%) (Table S1).
3.2. Clinical and echocardiographic data of patients with and without DM
The age, gender, LV end‐systolic dimension (LVEDD), LV end‐diastolic dimension (LVESD), LV EF and BNP level did not differ between patients with and without DM. Patients with DM had higher NYHA functional class (p = .02), worse right ventricle systolic longitudinal function‐RVs’ (p = .01), higher LV E/e’ ratio (p = .02) and LA stiffness (p = .002, Table S2) than those without DM.
3.3. Clinical and echocardiographic data of patients with and without increased LASt
Patients with increased LASt had larger LV dimensions (LVEDD p = .02 and LVESD p = .03, LVEDV p = .002 and LVESV p < .001) with lower EF (p = .001), higher E/e’ ratio (p < .001) and reduced RV e’ (p = .009) compared to those with normal LASt. Also, patients with increased LASt had larger LA volumes (LAV max and LAVI max p < .001 for both) and reduced LA strain (p < .001) (Supplement 4).
3.4. The impact of combined DM and LASt on clinical and echocardiographic data
Patients with neither DM nor increased LASt had smaller LVEDD (p = .03), larger LVESV (p = .03), lower E/e’ ratio (p < .001), smaller LA volumes (LAV max and LAVI max, p = .01 for both) and higher LA strain (p < .001) compared to the other groups. Patients with raised LASt, irrespective of DM had similar rise in E/e’ ratio (p < .001) and in addition, had larger LVESV (p = .03), larger LA volumes (LAV max and LAVI max p = .01 for both) and worse LA function (LA strain, p < .001) compared to the two groups with normal LASt (Table 1).
TABLE 1.
Comparison of clinical and echocardiographic data of HFrEF with and without DM/LASt
| Variable | Gr‐I | Gr‐II | Gr‐III | Gr‐IV | P |
|---|---|---|---|---|---|
| DM‐, LAST‐ | DM‐, LAST+ | DM+, LAST‐ | DM+, LAST+ | value | |
| (n = 54) | (n = 46) | (n = 14) | (n = 22) | ||
| Clinical data | |||||
| Age (years) | 64 ± 12 | 64 ± 12 | 66 ± 9.1 | 67 ± 7.2 | NS |
| Sex (female, n, %) | 15 (27.7) | 7 (15.2) | 5 (35) j | 2 (9.1) i,l | 0.02 |
| NYHA class | 1.81 ± 0.6 | 1.85 ± 0.7 | 2.0 ± 0.7 | 2.27 ± 0.7 | NS |
| AH (n, %) | 21 (38.9) | 20 (43.5) | 8 (57.4) | 7 (31.8) l | 0.03 |
| CRF (n, %) | 13 (24.1) | 20 (43.4) g | 4 (28.6) | 8 (36.3) | 0.01 |
| Haemoglobin (g/dl) | 13.4 ± 1.8 | 13.5 ± 1.8 | 13.6 ± 1.7 | 12.9 ± 1.9 | NS |
| Creatinine (mg/dl) | 105 ± 3.3 | 123 ± 3.4 | 103 ± 3.3 | 119 ± 3.4 | NS |
| BNP pg/ml | 403 ± 46 | 569 ± 53 | 397 ± 37 | 558 ± 48 | NS |
| BNP > 125 (n, %) | 43 (79.6) | 41 (89.1) | 12 (85.7) | 19 (86.3) | NS |
| Echocardiographic data | |||||
| LV EDD (cm) | 5.9 ± 0.7 | 6.4 ± 0.7 g | 6.2 ± 0.6 | 6.3 ± 0.7 | 0.03 |
| LV ESD (cm) | 4.7 ± 1.1 | 5.2 ± 0.8 | 4.9 ± 0.7 | 5.1 ± 0.8 | NS |
| EDV (ml) | 190 ± 47 | 220 ± 65 | 181 ± 35 | 214 ± 65 | NS |
| ESV (ml) | 129 ± 40 | 158 ± 57 g | 121 ± 21 j | 150 ± 51 | 0.03 |
| LV EF (%) | 32 ± 6.8 | 29 ± 7.2 | 34 ± 4.9j | 30 ± 5.1 | 0.04 |
| E/e' ratio | 11.5 ± 3.9 | 18.6 ± 7.2 a | 12.4 ± 4.8 d,f | 21.6 ± 10 c,e | <0.001 |
| E‐wave DT (msec) | 194 ± 71 | 172 ± 60 | 174 ± 64 | 137 ± 36j | 0.003 |
| TAPSE (cm) | 19 ± 3.5 | 17 ± 3.7 | 19 ± 2.5 | 18 ± 3.6 | NS |
| PAPS, mmHG | 35 ± 9.8 | 38 ± 11 | 37 ± 9.1 | 47 ± 12 c,k,l | 0.002 |
| Mitral regurgitation (n, %) | 6 (11.1) | 14 (30.4) g | 0 (0) h, j | 8 (36.3) i,l | 0.01 |
| LAV max (ml) | 68 ± 24 | 89 ± 35 g | 73 ± 24 j | 99 ± 45 i,l | 0.01 |
| LAVI max (ml/m2) | 36 ± 13 | 46 ± 17 g | 38 ± 12 j | 51 ± 22 i,l | 0.01 |
| LA strain, % | 23.8 ± 7.9 | 12.6 ± 5.3 a | 25.3 ± 9.1d | 10.2 ± 4.6 c,e,f | <0.001 |
| LASt (%) | 0.54 ± 0.2 | 1.73 ± 1.1a | 0.52 ± 0.16j | 2.8 ± 2.3 c,f,k | <0.001 |
a) p < .001; Gr. I versus II b) p < .001; Gr. I versus III c) p < .001; Gr. I versus IV, d) p < .001; Gr. II versus III e) p < .001; Gr. II versus IV f) p < .001; Gr. III versus. IV g) p < .05; Gr. I versus II h) p < .05; Gr. I versus III i) p < .05; Gr. I versus IV j) p < .05; Gr. II versus III k) p < .05; Gr. II versus IV l) p < .05; Gr. III versus. IV
Group I (DM‐, LASt‐): HFrEF without DM and without increased LASt; Group II (DM‐, LAST+): HFrEF without DM and with increased LASt; Group III (DM+, LASt‐):HFrEF with DM and without increased LASt; Group IV (DM+, LASt +): HFrEF with DM and with increased LASt; DM: diabetes mellitus; NYHA: New York Heart Association; AH: arterial hypertension; DM: diabetes mellitus; CRF: chronic renal failure; LV: left ventricle; EDD: end‐diastolic dimension; ESD: end‐systolic dimension; EDV: end‐diastolic volume; ESV: end‐systolic volume; TAPSE: tricuspid annular plane systolic excursion; LA: left atrium; LAV max/LAVI max: left atrial maximal volume/indexed.
3.5. Cardiac events
After a mean follow‐up of 55 ± 37 months, 26 (19.1%) patients died. Throughout the follow‐up, 51 (37.5%) experienced at least one CE and 25 (18.4%) hospitalization (Table 2). At follow‐up, the CE free survival was 69% in no DM patients and 44.4% in DM patients (X2 12.7; p < .0001, Figure 1a). The CE free survival was also lower in patients with increased LASt, irrespective of the presence of DM: 1) no DM with normal LASt (85%); 2) no DM with increased LASt (50%); 3) DM with normal LASt (71%); and 4) DM with increased LASt (27%) (X2 29.6; p < .0001, Figure 1b).
TABLE 2.
Rate of outcomes in HFrEF patients with and without DM
| End point | Overall | DM‐ | DM+ | LAST‐ | LAST+ |
|---|---|---|---|---|---|
| CE | 51 (37.5%) | 31 (31%) | 20 (55.5%) | 12 | 39 |
| Death | 26 (19.1%) | 15 (15%) | 11 (30.5%) | 6 | 20 |
| Hospitalization | 25 (18.4%) | 16 (16%) | 9 (25%) | 6 | 19 |
| Overall | DM‐, LAST‐ | DM‐, LAST+ | DM+, LAST‐ | DM+, LAST+ | |
|---|---|---|---|---|---|
| CE | 51 (37.5%) | 8 (14.8) | 23 (50%) | 4 (28.5) | 16 (72.7) |
| Death | 26 (19.1%) | 3 (5.55) | 12 (26.1) | 3 (21.4) | 8 (36.3) |
| Hospitalization | 25 (18.4%) | 5 (9.25) | 11 (23.9) | 1 (7.14) | 8 (36.3) |
DM‐: without diabetes; DM+: with diabetes; LASt‐: without increased LASt; LASt+: with increased LASt; DM: diabetes mellitus; LASt: left atrial stiffness; CE: cardiac events (death and hospitalization).
FIGURE 1.
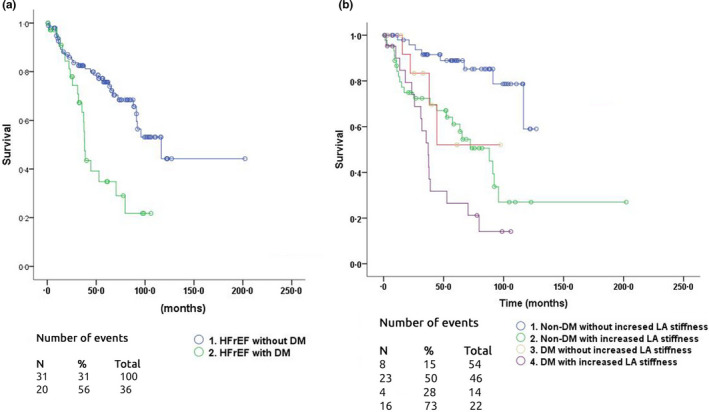
Survival free from all in patients CE in HFrEF patients: a) HFrEF patients without DM and HFrEF with DM (X (Nagoshi et al., 2011) 12.7; p < .0001); b) survival free from CE in patients CE in different groups of HFrEF
The best cut‐off LASt value for predicting CE in the group as a whole was ≥ 0.82% [81% sensitivity, 72% specificity and AUC 0.82 (p < .001)] (Figure 2a). LASt ≥ 0.82% also predicted CE in no DM patients [78% sensitivity, 71% specificity, AUC 0.80 (p < .001)], but in DM patients the same LASt cut‐off was a stronger predictor of CE [85% sensitivity, 71% specificity and AUC = 0.847 (p < .001)] (Figure 2b & 2c).
FIGURE 2.
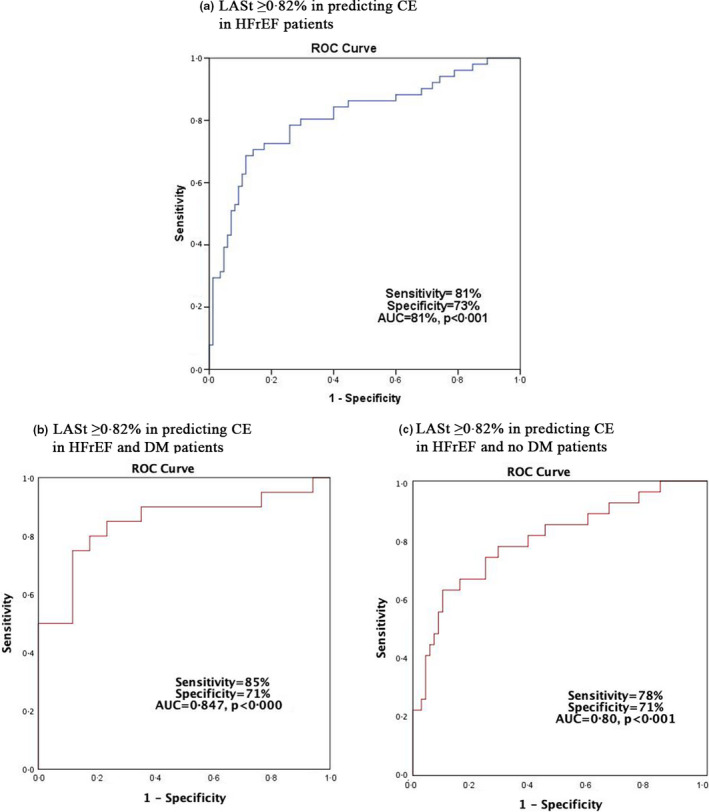
The best cut‐off LASt value for predicting CE
3.6. Impact of DM on CE in patients with increased LASt
Cardiac events were significantly higher in patients with increased LASt compared to those with normal LASt (59% versus.17.2%, p < .001). CE in patients with DM were more than in no DM (58.3% versus. 31.0%, p = .001). When DM was tested as a potential confounder to increased LASt, the CE were higher compared to increased LASt alone (73.9 versus. 30.7, p < .001). The prevalence of CE was also higher when DM was included in the model (51% versus. 38%, p < .001) (Table 3).
TABLE 3.
The impact of LASt and DM in cardiac events
| DM + versus DM‐ | LASt + versus. LASt‐ | DM+, LASt+ | |
|---|---|---|---|
| versus. DM‐LASt‐ | |||
| CE + versus. CE‐ (%) | 58.3 versus. 31.0, p = .001 | 59 versus.17.2, p < .001 | 73.9 versus. 30.7, p < .001 |
| RR | 1.88 (1.25–2.81p = 0.001) | 3.32 (1.35–8.16, p = .008) | 3.90 (2.40–6.64, p < .001 |
| PPV (%) | 58.3 (44.3–71.1) | 69.1 (58.7 – 77.9) | 74.0 (58.4 – 85.1) |
| NPV (%) | 69.5 (63.5–73.9) | 82.7 (75.1–88.3) | 77.8 (58.1–89.8) |
| Accuracy (%) | 66.2 (57.5 – 74.1) | 76.1 (59.7– 87.6) | 77.2 (69.2– 83.9) |
| Prevalence of CE (%) | 38.3 (30.1 – 46.9) | 38.2 (30.0 – 44.7) | 51.2 (35.1 – 67.1) |
DM: diabetes mellitus; LASt: left atrial stiffens; CE: cardiac events; (+): presence; (‐): absence: RR: relative risk; PPV: positive predictive value; NPV: negative predictive value.
3.7. Reproducibility
The reproducibility of LASt measurements in different territories and coefficient of variation (CV) was calculated in 10 random patients by two independent operators, blinded to each other's measurements. The CV was < 10% consistent with low variability and safe measurements, (Brunzendorf & Behrens, 2007) (Table S3).
4. DISCUSSION
4.1. Findings
The main findings in this study are summarized as follows: a) HFrEF patients without DM or increased LASt had smaller LV dimensions and volume, smaller LA dimensions and better LA function compared to other groups; b) patients with increased LASt have raised LV filling pressures and worse LA and RV cavity functions; and c) the CEs free survival is lower in patients with increased LA stiffness, particularly in those with DM.
4.2. Data interpretation
Our study shows that in patients with heart failure and reduced ejection fraction, LV diastolic function and LA function were less compromised in those with no DM or increased LASt compared to patients with DM and/or increased LASt. (Kristensen et al., 2018) Raised LASt is associated with LA cavity enlargement and compromised intrinsic myocardial properties. Such changes, in the absence of mitral valve disease, reflect significant diastolic LV disease and raised diastolic pressures. (Bytyçi et al., 2020 Apr 25; Khurraml et al., 2016; Melenovsky et al., 2015) Being chronic in nature and perpetual in deterioration, the LA response to increased cavity pressure is in the form of enlargement, remodelling and finally fibrosis. (Bytyçi et al., 2019) These changes result in worsening cavity function including contraction and stiffness, which eventually result in raised cavity pressure and pulmonary venous hypertension. (Bytyçi et al., 2019) Our findings confirm such patho‐mechanism in showing worse LA function and signs of raised cavity pressure in patients with raised LASt. The same nature of disturbances seems to be even worse in patients with addition DM. Diabetes is an important risk factor for coronary and peripheral vascular disease, (Rosenkranz et al., 2016) through many well‐established mechanisms. (Chiha et al., 2012) Thus, irrespective of the status of the coronary circulation in our patients, the results show that DM constitutes an additional risk for worsening LA function, probably through microcirculation disease. (Low Wang et al., 2016) Finally, our results show that raised LASt is the strongest predictor of survival in our patients’ sample. We have previously shown that LA cavity enlargement and myocardial fibrosis predict symptoms in patients with mitral and aortic valve disease. (Nicoll et al., 2017) The main symptoms in those patients were breathlessness and arrhythmias. The same principle applies in this study, although none of our patients had significant valve disease. A stiff left atrium cannot accommodate raised cavity pressure, particularly in systole irrespective of mitral regurgitation, which is transmitted to the pulmonary venous circulation and the right heart, with resulting right ventricular function disturbances, fluid retention and frequent hospitalization. Our result supports such pathophysiology in showing additional right ventricular systolic dysfunction in the group of patients with raised LASt, particularly those with DM. (Henein et al., 2015; Pinsky, 2016).
4.3. Limitations
The relatively small number of patients is an important limitation of this study, particularly with analysis and comparisons made between four subgroups. The exact duration of the raised LASt and DM was not known in our patients, variables which could influence their predictive accuracy of clinical events. The presence and nature of coronary artery disease were not available for all patients, who presented with clear clinical signs and symptoms of heart failure which we think took over the priority for management and prognostication.
4.4. Clinical implications
Our findings show that increased LASt is associated with lower CE free survival particularly in patients with DM. The LASt association with cavity enlargement and raised pressures, our results urge clinical pressure off‐loading treatment, for example with vasodilators, in patients with signs of raised LA pressure before further worsening dysfunction which may become intractable. Indeed, we and others have previously shown that patients with raised LA pressure do badly compared with those with normal LA pressure, particularly those who demonstrate persistently raised LA pressure at fast heart rate, which suggests poor diastolic reserve. (Nagueh et al., 1998) Finally, LA stiffness may serve as a good explanation of symptoms and prognosticator in patients with HF a reduced ejection fraction.
5. CONCLUSIONS
High LA stiffness is associated with poor clinical outcome in patients with heart failure and reduced ejection fraction. Diabetes has an additional incremental value in determining clinical outcome in those patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Supplementary Material
Bytyçi I, D’Agostino A, Bajraktari G, Lindqvist P, Dini FL, Henein MY. Left atrial stiffness predicts cardiac events in patients with heart failure and reduced ejection fraction: The impact of diabetes. Clin Physiol Funct Imaging. 2021;41:208–216. 10.1111/cpf.12688
REFERENCES
- Badano, L. P. , Kolias, T. J. , Muraru, D. , Abraham, T. P. , Aurigemma, G. , Edvardsen, T. , … Voigst, J‐U. (2018). Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European Heart Journal of Cardiovascular Imaging, 19(6), 591–600. [DOI] [PubMed] [Google Scholar]
- Brunzendorf, J. , & Behrens, R. (2007). How to type test the coefficient of variation of an indication. Radiation Protection Dosimetry, 123, 21–31. [DOI] [PubMed] [Google Scholar]
- Bytyçi, I. , & Bajraktari, G. (2015). Mortality in heart failure patients. Anadolu Kardiyol Derg, 15, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytyçi, I. , Bajraktari, G. , Fabiani, I. , Lindqvist, P. , Poniku, A. , Pugliese, N. R. , … Henein, M. Y. (2019). Left atrial compliance index predicts exercise capacity in patients with heart failure and preserved ejection fraction irrespective of right ventricular dysfunction. Echocardiography, 36, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Bytyçi, I. , Bajraktari, G. , Ibrahimi, P. , Berisha, G. , Rexhepaj, N. , & Henein, M. Y. (2014). Left atrial emptying fraction predicts limited exercise performance in heart failure patients. International Journal of Cardiology Heart Vessel, 4, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytyçi, I. , Bajraktari, G. , Lindqvist, P. , & Henein, M. Y. (2019). Compromised left atrial function and increased size predict raised cavity pressure: A systematic review and meta‐analysis. Clinical Physiology and Functional Imaging, 39, 297–307. [DOI] [PubMed] [Google Scholar]
- Bytyçi, I. , Dini, F. L. , Bajraktari, A. , Pugliese, N. R. , D'Agostino, A. , Bajraktari, G. … Henein, M. Y. (2020). Speckle tracking‐derived left atrial stiffness predicts clinical outcome in heart failure patients with reduced to mid‐range ejection fraction. Journal of Clinical Medicine, 9(5), 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiha, M. , Njeim, M. , & Chedrawy, E. G. (2012). Diabetes and coronary heart disease: A risk factor for the global epidemic. International Journal of Hypertension. 2012:697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini, F. L. , Cortigiani, L. , Baldini, U. , Boni, A. , Nuti, R. , Barsotti, L. , & Micheli, G. (2002). Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. American Journal of Cardiology, 89, 518–523. [DOI] [PubMed] [Google Scholar]
- Galderisi, M. , Cosyns, B. , Edvardsen, T. , Cardim, N. , Delgado, V. , Di Salvo, G. , … Habib, G. (2017). Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. European Heart Journal of Cardiovascular Imaging, 18, 1301–1310. [DOI] [PubMed] [Google Scholar]
- Henein, M. Y. , Holmgren, A. , & Lindqvist, P. (2015). Left atrial function in volume versus pressure overloaded left atrium. International Journal of Cardiovascular Imaging, 31, 959–965. [DOI] [PubMed] [Google Scholar]
- Khurraml, I. M. , Maqbool, F. , Berger, R. D. , Marine, J. E. , Spragg, D. D. , Ashikaga, H. , … Zimmerman, S. L. (2016). Association between left atrial stiffness index and atrial fibrillation recurrence in patients undergoing left atrial ablation. Circulation Arrhythmia Electrophysiology, 9:e003163. [DOI] [PubMed] [Google Scholar]
- Kristensen, S. L. , Rørth, R. , Jhund, P. S. , Shen, L. , Lee, M. M. Y. , Petrie, M. C. … BEST Investigators . (2018). Microvascular complications in diabetes patients with heart failure and reduced ejection fraction‐insights from the Beta‐blocker Evaluation of Survival Trial. European Journal of Heart Failure, 20, 1549–1556. [DOI] [PubMed] [Google Scholar]
- Kurt, M. , Wang, J. , Torre‐Amione, G. , & Nagueh, S. F. (2009). Left atrial function in diastolic heart failure. Circulation: Cardiovascular Imaging, 2, 10–15. [DOI] [PubMed] [Google Scholar]
- Kurt, M. , Wang, J. , Torre‐Amione, G. , & Nagueh, S. F. (2009). Left atrial function in diastolic heart failure. Circulation. Cardiovascular Imaging, 2, 10–15. [DOI] [PubMed] [Google Scholar]
- Lancellotti, P. , Tribouilloy, C. , Hagendorff, A. , Popescu, B. A. , Edvardsen, T. , Pierard, L. A. … Canna, G. L. (2013). Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. European Heart Journal of Cardiovascular Imaging, 14, 611–644. [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , Flachskampf, F. A. , … Voigt, J.‐U. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28(1–39), e14. [DOI] [PubMed] [Google Scholar]
- Low Wang, C. C. , Hess, C. N. , Hiatt, W. R. , & Goldfine, A. B. (2016). Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus ‐ Mechanisms, Management, and clinical considerations. Circulation, 133(24), 2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, M. R. , Petrie, M. C. , Varyani, F. , Östergren, J. , Michelson, E. L. , Young, J. B. , … McMurray, J. J. V. (2008). Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. European Heart Journal, 29, 1377–1385. [DOI] [PubMed] [Google Scholar]
- Melenovsky, V. , Hwang, S. J. , Redfield, M. M. , Zakeri, R. , Lin, G. , & Borlaug, B. A. (2015). Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation: Heart Failure, 8, 295–303. [DOI] [PubMed] [Google Scholar]
- Nagoshi, T. , Yoshimura, M. , Rosano, G. M. C. , Lopaschuk, G. D. , & Mochizuki, S. (2011). Optimization of cardiac metabolism in heart failure. Current Pharmaceutical Design, 17, 3846–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagueh, S. F. , Mikati, I. , Kopelen, H. A. , Middleton, K. J. , Quinones, M. A. , & Zoghbi, W. A. (1998). Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation, 98, 1644–1650. [DOI] [PubMed] [Google Scholar]
- Nicoll, R. , Zhao, Y. , Wiklund, U. , Diederichsen, A. , Mickley, H. , Ovrehus, K. … Cadematiri, F. (2017). Diabetes and male sex are key risk factor correlates of the extent of coronary artery calcification: A Euro‐CCAD study. Journal of Diabetes and Its Complications, 31, 1096–1102. [DOI] [PubMed] [Google Scholar]
- Pinsky, M. R. (2016). Erratum to: The right ventricle: Interaction with the pulmonary circulation. Critical Care, 20, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski, P. , Voors, A. A. , Anker, S. D. , Bueno, H. , Cleland, J. G. , Coats, A. J. , …ESC Scientific Document Group . (2016). 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure, 18, 891–975. [DOI] [PubMed] [Google Scholar]
- Rosano, G. M. , Fini, M. , Caminiti, G. , & Barbaro, G. (2008). Cardiac metabolism in myocardial ischemia. Current Pharmaceutical Design, 14, 2551–2562. [DOI] [PubMed] [Google Scholar]
- Rosenkranz, S. , Gibbs, S. R. , Wachter, R. , Marco, D. , Vonk‐Noordegraaf, A. , & Vachiéry, J. L. (2016). Left ventricular heart failure and pulmonary hypertension. European Heart Journal, 37(12), 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, A. , Dini, F. L. , Agricola, E. , Faggiano, P. , Benfari, G. , Temporelli, P. L. , … Ghio, S. (2018). Left atrial dilatation in systolic heart failure: A marker of poor prognosis, not just a buffer between the left ventricle and pulmonary circulation. Journal of Echocardiography, 16, 155–161. [DOI] [PubMed] [Google Scholar]
- Rudski, L. G. , Lai, W. W. , Afilalo, J. , Hua, L. , Handschumacher, M. D. , Chandrasekaran, K. … Schiller, N. (2010). Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography, 23(7), 685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- Zoghbi, W. A. , Enriquez‐Sarano, M. , Foster, E. , Grayburn, P. A. , Kraft, C. D. , Levine, R. A. … Weissman, N. J. (2003). American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. Journal of American Society of Echocardiography, 16, 777–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


