Abstract
Randomized controlled trial (RCT) populations often do not reflect those typically seen in clinical practice. This retrospective, observational cohort study analysed the real‐world data of people with type 2 diabetes (T2DM) prescribed basal insulin analogues from electronic medical records (EMRs) in the Explorys database, which includes data from 39 integrated healthcare systems in the United States, to determine how representative selected RCTs investigating insulin glargine 300 U/mL (Gla‐300) are of T2DM populations in a real‐world setting. Applying eligibility criteria derived from the EDITION 1, 2 and 3 (Gla‐300 vs. insulin glargine 100 U/mL [Gla‐100]) and BRIGHT (Gla‐300 vs. insulin degludec) RCTs, we observed that only 17% (33 345/191 218) of people captured in the real‐world database would have been eligible for such trials. Those who were ineligible tended to be older, had more comorbidities and a higher baseline hypoglycaemia rate than the eligible group. Using another large US EMR database (Optum Humedica) as corroboration, we found that 15% (36 285/235 697) would have been eligible to participate in the EDITION/BRIGHT RCTs. Furthermore, only 7% (1734/24 547) would have been eligible for the CONCLUDE (insulin degludec vs. Gla‐300) RCT. Our findings remind us of the value of real‐world data studies, complementing the results of RCTs, and providing additional insights into groups who would typically be excluded from RCTs.
Keywords: cohort study, database research, type 2 diabetes, basal insulin, randomized trial
1. INTRODUCTION
Randomized controlled trials (RCTs) are the gold standard setting in which to evaluate the efficacy and safety of therapies. 1 For example, in people with type 2 diabetes (T2DM), RCTs have been undertaken to compare the efficacy and safety of the basal insulin analogue insulin glargine 300 U/mL (Gla‐300) with insulin glargine 100 U/mL (Gla‐100) (the EDITION 1, 2 and 3 trials 2 , 3 , 4 ) and with insulin degludec (IDeg) (the BRIGHT and CONCLUDE trials 5 , 6 ). However, to create a relatively homogeneous population and to avoid factors that may confound treatment comparisons, RCTs often have strict eligibility criteria and consequently may not fully reflect all the populations treated in real‐world clinical practice. RCTs can also be prone to investigator selection bias, and to the selection of more motivated populations through the need for participants to provide informed consent and adhere to structured follow‐up. Such bias may affect population characteristics even if the effects on between‐group comparisons are mitigated by randomization.
Databases of electronic medical records (EMRs) often contain large amounts of data from a broad spectrum of people. Analysing data from EMRs allows the clinical effectiveness of a therapy to be assessed in a real‐world population, including those people who would have been excluded from most RCTs. The DELIVER series of studies, for example, has evaluated the effectiveness of Gla‐100, insulin detemir (IDet), Gla‐300 and IDeg using real‐world data drawn from a broad population with T2DM. 7 , 8
The aim of the present study was to determine the proportion and characteristics of people with T2DM treated with basal insulin analogues in the real world after applying eligibility criteria adapted from the EDITION 1, 2 and 3, BRIGHT and CONCLUDE trials.
2. METHODS
2.1. Study design and participants
In this retrospective, observational cohort study, EMRs were obtained for adults in the United States from the Explorys (IBM Watson Health, Armonk, New York) database. This database contains longitudinal medical data from 39 major integrated delivery networks across the United States, and includes 3 997 077 people with T2DM. Claims‐based data were not included in our analyses. Adults eligible for inclusion in this analysis were those with T2DM (presence of one or more International Classification of Diseases [ICD]‐ 9 or ‐10 diagnosis codes [ICD‐9: 250.x0; 250.x2; ICD‐10: E11] or one or more prescriptions for an antihyperglycaemic drug, plus exclusion of type 1 diabetes based on ICD‐9/‐10 codes) on March 1, 2015 (the index date, corresponding to the launch date of Gla‐300), who had received one or more prescriptions for a basal insulin analogue (Gla‐100, Gla‐300, IDet or IDeg), had been enrolled in the database, and had data available, for at least 12 months prior to the index date, and had one or more glycated haemoglobin (HbA1c) value and one or more blood pressure measurement available from the previous 12 months. The inclusion criteria used in our analysis were selected to enable the specific RCT exclusion criteria to be applied. Exclusion criteria were adapted from those of the EDITION 1, 2 and 3 and BRIGHT RCTs, 2 , 3 , 4 , 5 but did not match them exactly as exclusion criteria varied slightly among the trials. These adapted exclusion criteria, chosen to approximate all four trials, were HbA1c <53/>86 mmol/mol (<7 %/>10 %), uncontrolled severe hypertension (systolic blood pressure >180 mmHg and/or diastolic blood pressure >95 mmHg) and key comorbidities (myocardial infarction, heart failure, stroke, autonomic or diabetic neuropathy, diabetic foot ulcer, chronic or end‐stage renal disease, pyelonephritis, diabetic retinopathy or severe mental illness; Table S1) in the previous 12 months.
2.2. Outcomes and analysis
The proportion of people with T2DM in the Explorys database who would have been excluded from participating in RCTs based on eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT RCTs was estimated. The following characteristics for individuals eligible or ineligible for the RCTs based on these criteria (eligible and ineligible populations) were analysed: age, antihyperglycaemic medications, HbA1c, hypoglycaemic events, comorbidities and the Elixhauser comorbidity index 9 (an index that measures comorbidity based on ICD‐9/‐10 diagnosis codes and that includes prevalent comorbidities that are not encompassed by the well‐known Charlson index 10 ).
To ascertain whether similar results would be obtained with data from a different EMR database, a sensitivity analysis was undertaken. The same eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT RCTs were applied to EMRs in the Optum Humedica database, which included data for 14 940 692 people with T2DM. As with the Explorys analysis, claims data were not included in the Optum Humedica analysis. A further analysis of data from the Optum Humedica database was conducted using eligibility criteria adapted from another RCT, CONCLUDE (comparing Gla‐300 and IDeg in people with T2DM switching their basal insulin 6 ), results of which were not published at the time that the Explorys database analysis was conducted. CONCLUDE eligibility criteria were not pooled with those of EDITION 1, 2 and 3 and BRIGHT, as CONCLUDE purposefully included only those people who had at least one risk factor for hypoglycaemia. 6
As these analyses were exploratory and hypothesis‐generating, all analyses were descriptive and no statistical comparisons were made.
3. RESULTS
3.1. Study population
Of the 3 997 077 people with T2DM in the Explorys database, EMRs from 191 218 people who met the inclusion criteria for the present analysis (see Section 2.1) were evaluated (Figure 1). Based on the eligibility criteria adapted from EDITION 1, 2 and 3 and BRIGHT, only 17% (33 345/191 218) of these people would have been eligible to participate in the RCTs; 83% (157 873) met at least one exclusion criterion (Figure 1). More people aged >65 years were in the ineligible population (47%) than in the eligible population (40%; Table 1). While non‐basal insulins were more commonly prescribed in the ineligible population, prescriptions for other classes of antihyperglycaemic medications were similar in both populations (Table 1).
FIGURE 1.
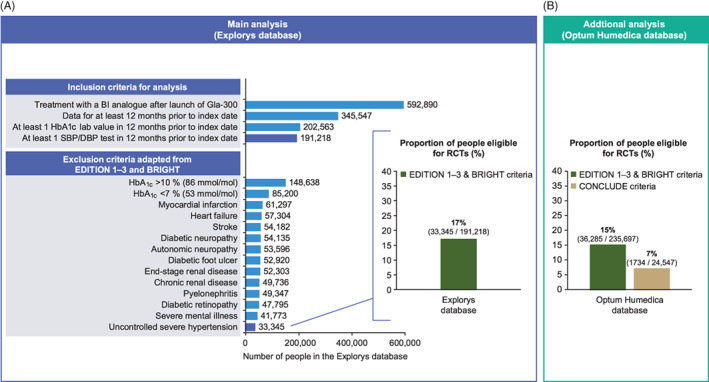
Schematic showing A, participant selection and percentage of people with type 2 diabetes (T2DM) in the Explorys database eligible to participate in randomized controlled trials (RCTs) using eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT trials, and B, the percentage of people with T2DM in the Optum Humedica database eligible to participate in RCTs using eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT trials and adapted from CONCLUDE. Main analysis: All exclusion criteria apply 12 months prior to the index date. Uncontrolled hypertension defined as systolic blood pressure >180 mmHg and/or diastolic blood pressure >95 mmHg at any point in the 12 months prior to the index date. Additional analysis: Eligibility criteria adapted from the CONCLUDE RCT are shown in Table S3. BI, basal insulin; DBP, diastolic blood pressure; Gla‐300, insulin glargine 300 U/mL; HbA1c, glycated haemoglobin; SBP, systolic blood pressure
TABLE 1.
Characteristics of people with type 2 diabetes from the Explorys database either eligible or ineligible for randomized controlled trials based on the eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT trials
| Characteristic | Ineligible population (n = 157 873) | Eligible population (n = 33 345) |
|---|---|---|
| Age group, n (%) | ||
| 18–34 years | 5689 (4) | 1714 (5) |
| 35–44 years | 10 387 (7) | 2423 (7) |
| 45–54 years | 25 008 (16) | 5676 (17) |
| 55–64 years | 41 527 (26) | 9730 (29) |
| ≥65 years | 74 590 (47) | 13 406 (40) |
| Hypoglycaemia 12 months prior to the index date | ||
| Incidence, n (%) | 33 333 (21) | 3143 (9) |
| Rate, events (per person‐year) | 86 698 (0.56) | 5554 (0.17) |
| HbA1c level, n (%) | ||
| <53 mmol/mol (<7%) | 50 645 (32) | 0 (0) |
| 53–<64 mmol/mol (7–<8%) | 36 205 (23) | 14 555 (44) |
| 64–<75 mmol/mol ( 8–<9%) | 24 579 (16) | 11 932 (36) |
| 75–86 mmol/mol (9–10%) | 16 674 (11) | 6858 (21) |
| >86 mmol/mol (>10%) | 29 770 (19) | 0 (0) |
| Antihyperglycaemic medications, n (%) | ||
| Basal insulin | 95 710 (61) | 20 530 (62) |
| Other insulin | 85 525 (54) | 14 377 (43) |
| Oral medications | 90 821 (58) | 20 319 (61) |
| Sulphonylureas | 43 536 (28) | 10 019 (30) |
| GLP‐1RAs | 10 833 (7) | 3107 (9) |
| Comorbidities in the 12 months prior to the index date, n (%) | ||
| Exclusion criteria | ||
| Autonomic neuropathy | 2583 (2) | 0 |
| Chronic renal disease | 28 737 (18) | 0 |
| Diabetic foot ulcer | 3995 (3) | 0 |
| Diabetic neuropathy | 361 (0) | 0 |
| Diabetic retinopathy | 8237 (5) | 0 |
| End‐stage renal disease | 6773 (4) | 0 |
| Heart failure | 30 750 (19) | 0 |
| Myocardial infarction | 54 922 (35) | 0 |
| Pyelonephritis | 2839 (2) | 0 |
| Severe mental illness | 32 480 (21) | 0 |
| Stroke | 18 684 (12) | 0 |
| Depression | 38 423 (24) | 2021 (6) |
| Hyperlipidaemia | 119 814 (76) | 23 233 (70) |
| Hypertension | 25 351 (16) | 889 (3) |
| Obesity | 35 201 (22) | 5154 (15) |
Abbreviations: GLP‐1RA, glucagon‐like peptide 1 receptor agonist; HbA1c, glycated haemoglobin.
3.2. Glycaemic control and hypoglycaemia
In the ineligible population based on eligibility criteria adapted from EDITION 1–3 and BRIGHT, 32% of people had good glycaemic control (HbA1c <53 mmol/mol [< 7 %]), while 19% had HbA1c levels >86 mmol/mol (>10 %; Table 1). The proportion of people who had one or more previous hypoglycaemic event and the annualized rate of these events were higher in the ineligible population than in the eligible population (Table 1).
3.3. Comorbidities
The mean (± SD) Elixhauser comorbidity index score was higher in the ineligible population (4.19 ± 2.81) than in the eligible population (2.16 ± 1.51), based on eligibility criteria adapted from EDITION 1, 2 and 3 and BRIGHT. A history of myocardial infarction was the most common comorbidity leading to exclusion (Table 1). Other nonexclusionary comorbidities were generally more common in the ineligible than the eligible populations (Table 1).
3.4. Further analyses using the Optum Humedica database
Of the 14 940 692 people with T2DM in the Optum Humedica database, 235 697 met the inclusion criteria for the sensitivity analysis using the same adapted EDITION 1, 2 and 3 and BRIGHT eligibility criteria. Of these, the percentage of people with T2DM eligible to participate in the RCTs based on these adapted criteria was 15% (36 285/235 697; Figure 1; Table S2), corroborating the results from the Explorys database. In a further analysis, using the Optum Humedica database and the eligibility criteria adapted from CONCLUDE, an RCT that specifically recruited people with at least one risk factor for hypoglycaemia, 6 the percentage of people with T2DM eligible to participate in this RCT was 7% (1734/24 547; Figure 1: Table S3). This estimate is based on a smaller subset of the Optum Humedica database than the EDITION 1, 2 and 3 and BRIGHT estimates, because the inclusion criteria required to enable the CONCLUDE exclusion criteria to be applied were more restrictive (for example, an estimated glomerular filtration rate (eGFR) value was required to enable the exclusion of those with eGFR 30–59 mL/min/1.73 m2; Table S3).
4. DISCUSSION
In the present analysis of data from the EMRs of people with T2DM in the Explorys database, the majority of people (83%) would have been excluded from the EDITION 1, 2 and 3 and BRIGHT RCTs, based on eligibility criteria adapted from the trials. Furthermore, of the 17% of people eligible to participate in these RCTs, it is likely that other, unmeasurable factors, specific to individual people (such as motivation to participate in trials) would see the potential pool of participants further reduced, so our figure may overestimate the representativeness of RCTs. These results appear robust, as the percentage of people eligible to participate based on eligibility criteria adapted from the EDITION 1, 2 and 3 and BRIGHT trials was similar (15%) when these criteria were applied to EMR data from a different US database, the Optum Humedica database. Our findings are not specific to the EDITION 1, 2 and 3 and BRIGHT RCTs, as an even smaller proportion of people in the Optum Humedica database were eligible for participation based on criteria adapted from CONCLUDE (7%), which recruited people with an increased risk of hypoglycaemia. 6
This lack of representativeness of clinical trial participants for the general T2DM population is not specific to studies of basal insulin analogues. Based on eligibility criteria adapted from five phase 3 studies of the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) liraglutide, 27% of people with T2DM (2483/9251) in northern Denmark would have been eligible for these RCTs, 11 with people primarily excluded owing to comorbidities or having HbA1c levels outside the criteria. 11 Eligibility for GLP‐1RA cardiovascular outcome trials has also been investigated, showing that only between 10% and 35% of people with T2DM would have been eligible to participate based on the eligibility criteria of six such trials. 12 One study reported that a higher proportion of people with T2DM (45%; 3156/7034) would have been eligible for phase 3 RCTs investigating glycaemic control with the sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor empagliflozin, 13 with people being excluded for reasons including use of glucose‐lowering drugs that were not permitted as per trial protocol or again having HbA1c levels outside the eligibility criteria. 13 However, another study using eligibility criteria from an empagliflozin cardiovascular outcome RCT reported that only 16% of people with T2DM in the United Kingdom had the same cardiovascular risk as those included in the RCT. 14 In a combined analysis of four cardiovascular outcome trials of SGLT2 inhibitors, the representativeness of the general T2DM population varied from 59% for DECLARE‐TIMI 58 to 16% for VERTIS‐CV, mainly reflecting the exclusion criteria of cardiovascular risk at baseline (eg, exclusion of people with cardiovascular risk factors but no established cardiovascular disease). 15 A second study examining cardiovascular outcome studies identified a similar trend for the generalizability of DECLARE‐TIMI (49.5%) and VERTIS‐CV (19.0%). 16 Together, these analyses of antihyperglycaemic therapies show that the proportion of people with T2DM eligible to participate in RCTs is generally low, but that the range is broad depending on which RCT criteria were applied. This is reflected in a study of the total population of people with T2DM in Scotland in 2008, which identified that between 4% and 51% of these people would be eligible for seven large RCTs that investigated various drug interventions. 17
From the present study, in general terms, the population ineligible based on criteria adapted from the EDITION 1, 2 and 3 and BRIGHT RCTs appears to be older, to have more comorbidities and to be at higher risk of hypoglycaemia based on higher rates and incidence of hypoglycaemia at baseline, than those eligible to participate. Therefore, it is noteworthy that this ineligible population represents people who may benefit most from reducing their risk of hypoglycaemia.
The strengths of the present study include the use of large, comprehensive, real‐world EMR databases, and particularly the consistency of results from analyses of the Explorys and Optum Humedica databases. However, this study was only designed to assess the proportion of people with T2DM who would be eligible for RCTs of Gla‐300 based on adapted criteria, and so is limited in that detailed analyses of the overlooked populations were not conducted. Related to this, it is worth noting that although the observed characteristics of people in the ineligible and eligible populations provide useful information (Table 1), the study design means that certain values may differ from expectations. For example, the prevalence of hypertension and obesity seems lower than that typically found in populations of people with T2DM, but as this information is from ICD codes it is possible that they are underestimates. Furthermore, the large difference between ineligible and eligible populations in the prevalence of depression (24% vs. 6%, respectively) may primarily reflect the inclusion of severe mental illness (ICD codes which include depressive disorders) as an exclusion criterion. This study is also limited specifically by the focus on trials of Gla‐300, and so cannot be fully generalized to all RCTs of basal insulin analogues. Another factor to consider is the pooling of eligibility criteria from the EDITION 1, 2 and 3 and BRIGHT trials, because although these trials were similarly designed there were some differences and the adapted criteria used in our analyses do not fully reflect each individual trial. For example, our adapted criteria used an upper HbA1c value of 10% (86 mmol/mol), whereas the EDITION 3 RCT included those with HbA1c between 7% (53 mmol/mol) and 11%, so this may have lowered our final estimates of eligibility. However, this can be balanced against the fact that our pooled adapted criteria did not include every eligibility criterion from each trial (for example, BRIGHT excluded participants with a body mass index of <25 kg/m2 or >40 kg/m2), which may increase our final estimates of eligibility. The requirement for laboratory data as a requirement for inclusion in this study reduced the total evaluable population and may have lowered our estimates of trial eligibility as shown in a previous study of cardiovascular outcome trials. 15 Of 592 890 people with T2DM in the Explorys database who received a basal insulin following the launch of Gla‐300, only 191 218 also had data for at least 12 months, and one or more HbA1c and one or more blood pressure measurement/s in the 12 months prior to the index date. In addition to reducing the population for our analyses, potentially excluding people who may have been eligible for the RCTs based on the adapted criteria used, the loss of the group of people with T2DM without laboratory measures may provide a selection bias in terms of selecting for a population who are more actively managed.
In conclusion, this analysis of data from EMRs demonstrates that because RCTs are designed to test the efficacy and safety of treatments in a very controlled setting, a large proportion of people with T2DM who may receive treatment with basal insulin analogues are not represented as they fail to meet the eligibility criteria for inclusion. RCTs that focus on specific subgroups of people with T2DM may better reflect other groups of people shown to be excluded in this EMR study (eg, older people in the EDITION SENIOR study 18 ); however, such focused RCTs are few in number and are still restrictive in their inclusion. Our finding reinforces the value of real‐world studies that assess the clinical effectiveness of basal insulin analogues, which can complement the results of RCTs. These results also highlight that such real‐world evidence may provide additional insights into diabetes management for those people who would typically be excluded from RCTs.
CONFLICT OF INTEREST
D.M. has served on Advisory boards for AstraZeneca, Ferrer, Merck, Novo Nordisk, Praxis Pharmaceutical and Sanofi, and on the Speakers' bureau for Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Menarini, Merck, Novartis, Novo Nordisk and Sanofi. J. Westerbacka and C.N. are employees of Sanofi. J. Wu was a Sanofi employee when the work was conducted but has since left the company. R.G. has provided consultancy work for Sanofi. B.E. has served on Advisory boards and received consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Mundipharma, Navamedic, Novo Nordisk and RLS Global, and has received research support from Sanofi.
AUTHOR CONTRIBUTIONS
J. Westerbacka, C.N. and J. Wu were involved in the concept and design of this analysis. R.G performed the descriptive analyses. All authors were involved in the interpretation of the data, writing and reviewing drafts of the manuscript and approved the final version for submission.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14264.
Supporting information
TABLE S1 International Classification of Diseases (ICD) codes for key comorbidities listed as exclusion criteria for the analysis of the EDITION 1–3 and BRIGHT trials.
Table S2 Number of people with T2DM included in the analysis of Optum Humedica eligible to participate in RCTs using eligibility criteria adapted from the EDITION 1–3 and BRIGHT trials.
Table S3 Number of people with T2DM in the analysis of Optum Humedica database eligible to participate in an RCT using eligibility criteria adapted from the CONCLUDE study.
ACKNOWLEDGMENTS
This study was funded by Sanofi. The authors received editorial/writing support in the preparation of this manuscript provided by Simon Rees, PhD, of Fishawack Communications Ltd, funded by Sanofi. The authors would like to thank Arjun Menon (previously of Accenture) for assistance with data analysis. These data were previously presented at the 79th American Diabetes Association Scientific Sessions, June 7–11, 2019, San Francisco, California, and the 55th Annual Meeting of the European Association for the Study of Diabetes, September 16–20, 2019, Barcelona, Spain.
Mauricio D, Westerbacka J, Nicholls C, Wu J, Gupta R, Eliasson B. How many people with type 2 diabetes fulfil the eligibility criteria for randomized, controlled trials of insulin glargine 300 U/mL in a real‐world setting? Diabetes Obes Metab. 2021;23:838–843. 10.1111/dom.14264
Funding information This study was funded by Sanofi. The authors received editorial/writing support in the preparation of this manuscript provided by Simon Rees, PhD, of Fishawack Communications Ltd, funded by Sanofi.
DATA AVAILABILITY STATEMENT
Requests concerning data access should be addressed to the corresponding author
REFERENCES
- 1. Schulz KF, Altman DG, Moher D, Group C . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726‐732. [DOI] [PubMed] [Google Scholar]
- 2. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755‐2762. [DOI] [PubMed] [Google Scholar]
- 3. Yki‐Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235‐3243. [DOI] [PubMed] [Google Scholar]
- 4. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin‐naive type 2 diabetes: the randomized head‐to‐head BRIGHT trial. Diabetes Care. 2018;41:2147‐2154. [DOI] [PubMed] [Google Scholar]
- 6. Philis‐Tsimikas A, Klonoff DC, Khunti K, et al. Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin‐treated patients with type 2 diabetes: the randomised, head‐to‐head CONCLUDE trial. Diabetologia. 2020;63:698‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey TS, Zhou FL, Gupta RA, et al. Glycaemic goal attainment and hypoglycaemia outcomes in type 2 diabetes patients initiating insulin glargine 300 units/mL or 100 units/mL: real‐world results from the DELIVER naive cohort study. Diabetes Obes Metab. 2019;21:1596‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sullivan SD, Bailey TS, Roussel R, et al. Clinical outcomes in real‐world patients with type 2 diabetes switching from first‐ to second‐generation basal insulin analogues: comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study. Diabetes Obes Metab. 2018;20:2148‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8‐27. [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 11. Knudsen JS, Thomsen RW, Pottegard A, Knop FK, Sorensen HT. Differences between randomized clinical trial patients and real‐world initiators of the glucagon‐like peptide 1 receptor agonist liraglutide. Diabetes Care. 2018;41:e133‐e135. [DOI] [PubMed] [Google Scholar]
- 12. Sciannameo V, Berchialla P, Orsi E, et al. Enrolment criteria for diabetes cardiovascular outcome trials do not inform on generalizability to clinical practice: the case of glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2020;22:817‐827. [DOI] [PubMed] [Google Scholar]
- 13. Munk NE, Knudsen JS, Pottegard A, Witte DR, Thomsen RW. Differences between randomized clinical trial participants and real‐world empagliflozin users and the changes in their glycated hemoglobin levels. JAMA Netw Open. 2020;3:e1920949. [DOI] [PubMed] [Google Scholar]
- 14. McGovern A, Feher M, Munro N, de Lusignan S. Sodium‐glucose co‐transporter 2 (SGLT2) inhibitor: comparing trial data and real‐world use. Diabetes Ther. 2017;8:365‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21:968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castellana M, Procino F, Sardone R, Trimboli P, Giannelli G. Generalizability of sodium‐glucose co‐transporter‐2 inhibitors cardiovascular outcome trials to the type 2 diabetes population: a systematic review and meta‐analysis. Cardiovasc Diabetol. 2020;19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saunders C, Byrne CD, Guthrie B, et al. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2013;30:300‐308. [DOI] [PubMed] [Google Scholar]
- 18. Ritzel R, Harris SB, Baron H, et al. A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: results from the SENIOR study. Diabetes Care. 2018;41:1672‐1680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 International Classification of Diseases (ICD) codes for key comorbidities listed as exclusion criteria for the analysis of the EDITION 1–3 and BRIGHT trials.
Table S2 Number of people with T2DM included in the analysis of Optum Humedica eligible to participate in RCTs using eligibility criteria adapted from the EDITION 1–3 and BRIGHT trials.
Table S3 Number of people with T2DM in the analysis of Optum Humedica database eligible to participate in an RCT using eligibility criteria adapted from the CONCLUDE study.
Data Availability Statement
Requests concerning data access should be addressed to the corresponding author


