Abstract
Background and Aims
GS‐9688 (selgantolimod) is an oral selective small molecule agonist of toll‐like receptor 8 in clinical development for the treatment of chronic hepatitis B. In this study, we evaluated the antiviral efficacy of GS‐9688 in woodchucks chronically infected with woodchuck hepatitis virus (WHV), a hepadnavirus closely related to hepatitis B virus.
Approach and Results
WHV‐infected woodchucks received eight weekly oral doses of vehicle, 1 mg/kg GS‐9688, or 3 mg/kg GS‐9688. Vehicle and 1 mg/kg GS‐9688 had no antiviral effect, whereas 3 mg/kg GS‐9688 induced a >5 log10 reduction in serum viral load and reduced WHV surface antigen (WHsAg) levels to below the limit of detection in half of the treated woodchucks. In these animals, the antiviral response was maintained until the end of the study (>5 months after the end of treatment). GS‐9688 treatment reduced intrahepatic WHV RNA and DNA levels by >95% in animals in which the antiviral response was sustained after treatment cessation, and these woodchucks also developed detectable anti‐WHsAg antibodies. The antiviral efficacy of weekly oral dosing with 3 mg/kg GS‐9688 was confirmed in a second woodchuck study. The antiviral response to GS‐9688 did not correlate with systemic GS‐9688 or cytokine levels but was associated with transient elevation of liver injury biomarkers and enhanced proliferative response of peripheral blood mononuclear cells to WHV peptides. Transcriptomic analysis of liver biopsies taken prior to treatment suggested that T follicular helper cells and various other immune cell subsets may play a role in the antiviral response to GS‐9688.
Conclusions
Finite, short‐duration treatment with a clinically relevant dose of GS‐9688 is well tolerated and can induce a sustained antiviral response in WHV‐infected woodchucks; the identification of a baseline intrahepatic transcriptional signature associated with response to GS‐9688 treatment provides insights into the immune mechanisms that mediate this antiviral effect.
Abbreviations
- AST
aspartate aminotransferase
- cccDNA
covalently closed circular DNA
- CD
cluster of differentiation
- cDC
conventional dendritic cell
- CHB
chronic hepatitis B
- CNR
chronically infected antiviral nonresponder
- CR
chronically infected antiviral responder
- CXCL13
chemokine (C‐X‐C motif) ligand 13
- FDR
false discovery rate
- GSEA
gene set enrichment analysis
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IFN
interferon
- IL‐12
interleukin‐12
- MARCO
macrophage receptor with collagenous structure
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- PD
pharmacodynamic
- PK
pharmacokinetic
- RI
replicative intermediates
- SDH
sorbitol dehydrogenase
- TFH
follicular helper T cell
- TLR
toll‐like receptor
- Treg
T regulatory cell
- U
uninfected
- WHsAb
anti‐WHsAg antibody
- WHsAg
woodchuck hepatitis surface antigen
- WHV
woodchuck hepatitis virus
Approximately 260 million individuals are chronically infected with hepatitis B virus (HBV), and over half a million people are estimated to die each year due to liver diseases associated with chronic hepatitis B (CHB), such as cirrhosis and hepatocellular carcinoma (HCC). Immunological control of CHB (“functional cure”) is defined as sustained loss of HBV surface antigen (HBsAg) off treatment, with or without seroconversion to antibody to HBsAg. Several nucleos(t)ide analogs, as well as interferon‐alpha (IFN‐α), are approved for the treatment of CHB. These therapies reduce viremia and improve long‐term outcome but rarely lead to cure. Consequently, there is an urgent need for therapies that induce durable immune control of HBV.
The host immune response to HBV plays a pivotal role in whether infection is cleared or becomes persistent. Individuals who spontaneously resolve HBV infection following an acute infection display vigorous, polyclonal, HBV‐specific CD8+ and CD4+ T‐cell responses.( 1 ) In contrast, CHB is associated with a limited and dysfunctional CD8+ T‐cell response, as well as impaired natural killer (NK) cell antiviral function.( 1 ) Various mechanisms have been identified which may play a role in immune dysfunction in CHB, including persistent antigen presentation leading to T‐cell dysfunction (“exhaustion”), HBsAg‐specific B‐cell dysfunction, and increased activity of immunosuppressive cell types (e.g., regulatory T cells [Tregs] and myeloid‐derived suppressor cells).( 1 , 2 )
Toll‐like receptor 8 (TLR8) and TLR7 are members of the pattern recognition receptor family, whose function is to recognize pathogen‐associated molecular patterns and initiate innate and adaptive immune responses. In contrast to TLR7, which is predominantly expressed in plasmacytoid dendritic cells and B cells, human TLR8 is expressed by conventional (“myeloid”) dendritic cells (cDC1 and cDC2), monocytes, macrophages, neutrophils, and Tregs.( 3 , 4 , 5 ) As a result, TLR8 activation triggers the production of immunomodulatory cytokines (e.g., interleukin‐12 [IL‐12], IL‐18) and proinflammatory cytokines (e.g., tumor necrosis factor alpha, IL‐1β), whereas TLR7 activation predominantly leads to the production of IFN‐α.( 3 ) TLR8 agonists indirectly activate the cytotoxic function of NK cells and trigger production of the antiviral cytokine IFN‐γ from NK cells and mucosa‐associated invariant T cells through IL‐12 and IL‐18.( 6 , 7 ) TLR8 activation also directly induces the maturation of professional antigen‐presenting cells (e.g., cDCs) and stimulates antigen‐specific T‐cell responses, including a CD8+ T‐cell response to HBsAg.( 8 ) In addition, TLR8 agonists enhance CD8+ T‐cell function by directly reversing the immunosuppressive function of Tregs( 4 ) and drive development of a humoral immune response by promoting follicular helper T‐cell (TFH) differentiation.( 9 )
The eastern North American woodchuck (Marmota monax) can be naturally infected with woodchuck hepatitis virus (WHV), a hepadnavirus closely related to human HBV. The woodchuck model of CHB displays many characteristics of human disease and has been used to evaluate the antiviral efficacy of various immunomodulatory agents, including the TLR7 agonist GS‐9620 (vesatolimod).( 10 ) However, the antiviral efficacy of a TLR8 agonist has not been reported. We therefore evaluated the therapeutic potential of GS‐9688 (selgantolimod), a selective small molecule agonist of TLR8, in WHV‐infected woodchucks. We also explored the relationship between systemic pharmacokinetic (PK) and pharmacodynamic (PD) profiles, the baseline intrahepatic transcriptional signature, and the antiviral response to GS‐9688.
Materials and Methods
Ethics Statement
The animal protocol and all procedures involving woodchucks were reviewed and approved by the Northeastern Wildlife IACUC and adhered to the national guidelines of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the American Veterinary Medical Association. Buffy coats from healthy human volunteers were obtained from Stanford Medical School Blood Center (Palo Alto, CA). Consent was obtained from each donor for use of these samples and their derivatives for research purposes using IRB‐approved authorizations.
Investigational drug
GS‐9688 (selgantolimod), a small molecule agonist of TLR8, was manufactured by Gilead Sciences, Inc. (Foster City, CA).
Single dose GS‐9688 studies in uninfected woodchucks
Uninfected adult woodchucks were assigned to treatment groups based on gender and body weight. For each study, woodchucks (n = 3‐6 per dose group) were orally administered a single dose of vehicle or GS‐9688. For all studies, evaluations included clinical observations, clinical pathology (hematology, coagulation and clinical chemistry), pharmacokinetics (PK) and pharmacodynamics (PD).
Repeat dose GS‐9688 efficacy studies in WHV‐infected woodchucks
Study designs are described in Supplementary Fig. 3 and 7. Briefly, woodchucks chronically infected with WHV (n = 5‐14 per group) were dosed orally once per week with vehicle or GS‐9688 for 8‐12 weeks. Woodchucks were monitored at various time points before, during and after treatment for multiple parameters including safety, tolerability, PK, PD, and antiviral efficacy.
RNA‐Seq analysis
Isolation of total cellular RNA and RNA‐Seq was conducted by Expression Analysis (Durham, NC) as described previously.( 10 ) Sequence count normalization, differential gene expression analysis, false discovery rate (FDR) calculation, gene set enrichment analysis (GSEA) and Ingenuity® Pathway Analysis (IPA; Qiagen, Redwood City, CA) were performed as described previously.( 10 , 11 ) All differentially expressed genes (DEGs) had an absolute fold change >2 and FDR <0.05. Cell type enrichment analysis was performed using the xCell method, as described in the Supplementary Materials.
Statistical analysis
Data is expressed as mean ± standard error of the mean (SEM) unless otherwise stated. Adjusted P‐values (i.e. FDR) and unadjusted p‐values are indicated in the text. For all analyses, a value of P < 0.05 was considered significant. Additional details are provided in the Supplementary Materials.
Additional methods are available in the Supplementary Materials and Methods.
Results
Single‐Dose PK and PD of GS‐9688 in Uninfected Woodchucks
We performed a series of in vitro studies which demonstrated that woodchuck TLR8 is functional and selectively activated by GS‐9688 and that the response of woodchuck and human peripheral blood mononuclear cells (PBMCs) to TLR8 activation is comparable (Supporting Figs. S1 and S2). Because these data established the translational value of the woodchuck model of CHB for evaluating TLR8 agonists, we next evaluated the PK, PD, tolerability, and safety of escalating oral doses of GS‐9688 in uninfected adult woodchucks. Plasma GS‐9688 levels were approximately dose‐proportional after a single oral dose of 0.3, 1, 3, and 10 mg/kg GS‐9688 (Fig. 1A). Due to the lack of quantitative assays to measure woodchuck serum cytokines at the time of this initial in vivo study, the PD response was assessed by measuring whole blood macrophage receptor with collagenous structure (MARCO) mRNA levels. Expression of this macrophage‐expressed scavenger receptor was strongly induced by a tool TLR8 agonist in both woodchuck and human PBMCs (Supporting Figs. S1D and S2B). Oral doses of ≥ 3 mg/kg GS‐9688 significantly induced whole blood MARCO mRNA levels, which peaked at 24 hours postdose and returned to near baseline (pretreatment) levels by 48 hours postdose (Fig. 1B). MARCO mRNA induction was comparable at 3 and 10 mg/kg GS‐9688. There was no gender difference in PK and PD responses and no changes in coagulation and clinical chemistry parameters at any dose (data not shown). There were no clinically significant changes in hematology parameters at doses ≤ 3 mg/kg GS‐9688, although a non‐dose‐dependent minimal to mild transient decrease in lymphocytes was observed (Fig. 1C). In contrast, 10 mg/kg GS‐9688 transiently induced a marked (>75%) decrease in both peripheral lymphocyte and platelet counts (Fig. 1C,D). This is comparable to the effect of the TLR7 agonist vesatolimod in woodchucks and may reflect cell trafficking and/or margination.( 10 ) In a subsequent study, we used recently developed woodchuck IL‐12p40 and IFN assays (see Supporting Information) to characterize the systemic cytokine profile of GS‐9688. Oral doses of ≥ 1 mg/kg GS‐9688 significantly induced serum IL‐12p40 levels in uninfected woodchucks, which peaked at 4‐8 hours postdose and returned to baseline levels by 48 hours postdose (Fig. 1E). In contrast, GS‐9688 did not increase serum IFN levels (Fig. 1F). Based on these data, 1 and 3 mg/kg GS‐9688 were selected as the doses to be evaluated in an antiviral efficacy study in woodchucks chronically infected with WHV.
FIG. 1.
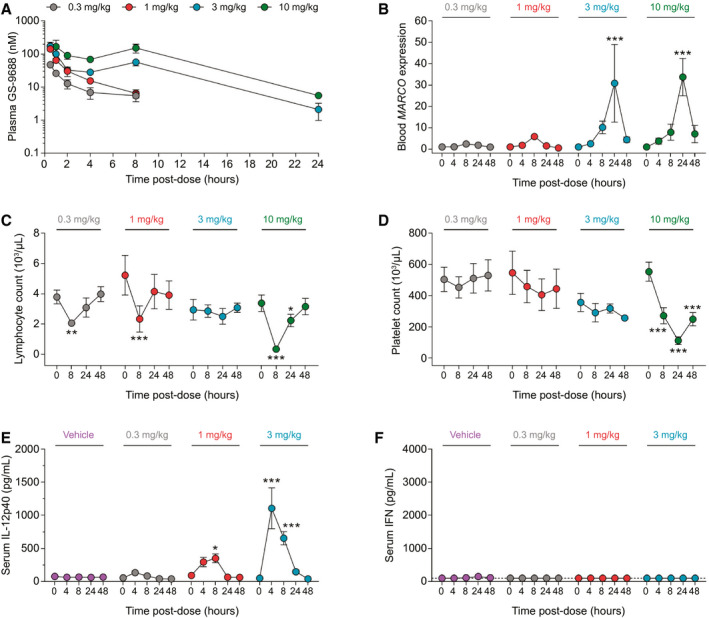
PK and PD response of uninfected woodchucks to oral GS‐9688. (A‐D) Uninfected woodchucks (n = 3‐6/group) were orally administered a single dose of 0.3, 1, 3, or 10 mg/kg GS‐9688. Note that only female animals (n = 3) were administered 1 and 3 mg/kg GS‐9688. (A) Plasma GS‐9688 concentration after dosing. The lower limit of quantification for the assay is 0.1 nM. (B) Whole blood MARCO mRNA levels measured by quantitative real‐time PCR. Data are expressed as fold‐change relative to predose (baseline). (C) Whole blood lymphocyte count. Normal range 3.3‐4.4 (103/μL). (D) Whole blood platelet count. Normal range 506‐642 (103/μL). (E,F) Uninfected woodchucks (n = 4/group) were orally administered a single dose of vehicle or 0.3, 1, or 3 mg/kg GS‐9688. Serum IL‐12p40 and IFN were measured by electro‐chemiluminescence assay and bioassay, respectively. Of note, the IFN bioassay detects woodchuck IFN‐α and IFN‐γ with similar sensitivity. However, because GS‐9688 is a potent inducer of IFN‐γ but not IFN‐α or IFN‐β, any signal detected by the IFN bioassay in the serum of GS‐9688‐treated woodchucks is likely to be predominantly due to IFN‐γ. The lower limit of detection of the IL‐12p40 assay is 4 pg/mL. The lower limit of detection of the IFN assay is 100 pg/mL and is indicated by a dotted line. For all plots, circles indicate the mean and error bars represent the SEM. For (B‐E), statistical significance relative to baseline was calculated by two‐way analysis of variance with Dunnett’s multiple comparison correction. Only P values <0.05 are labeled; *P < 0.05, **P < 0.01, ***P < 0.001.
GS‐9688 Reduced Serum WHV DNA and WHV Surface Antigen Levels in Woodchucks Chronically Infected With WHV
WHV‐infected woodchucks were dosed with vehicle (n = 10), 1 mg/kg GS‐9688 (n = 6), or 3 mg/kg GS‐9688 (n = 6) for 8 weeks and followed for an additional 24 weeks (Supporting Fig. S3). GS‐9688 was administered orally once per week to match the dose route and schedule being evaluated in patients with CHB in ongoing clinical studies. The animals were assessed for antiviral efficacy, PK, PD, and safety/tolerability before, during, and after treatment. Serum WHV surface antigen (WHsAg) and WHV DNA levels in woodchucks dosed with vehicle or 1 mg/kg GS‐9688 did not decrease by ≥ 1 log10 at any time during the study (Fig. 2A‐D). In contrast, 3 mg/kg GS‐9688 substantially reduced serum WHsAg (range 2.2 to >4.6 log10 ng/mL) and serum WHV DNA (range 2.8‐7.4 log10 copies/mL) in four out of six woodchucks (“antiviral responders”) by the end of treatment (Fig. 2E,F). This antiviral response was sustained after treatment cessation for three of the responders (“sustained responders”), whereas the antiviral responder animal with the smallest on‐treatment reduction in serum viral parameters (orange circles) rebounded to baseline levels after treatment was stopped (“transient responder”). There was no reduction in serum viral endpoints at any time during the study in the two remaining woodchucks treated with 3 mg/kg GS‐9688 (“nonresponders”).
FIG. 2.
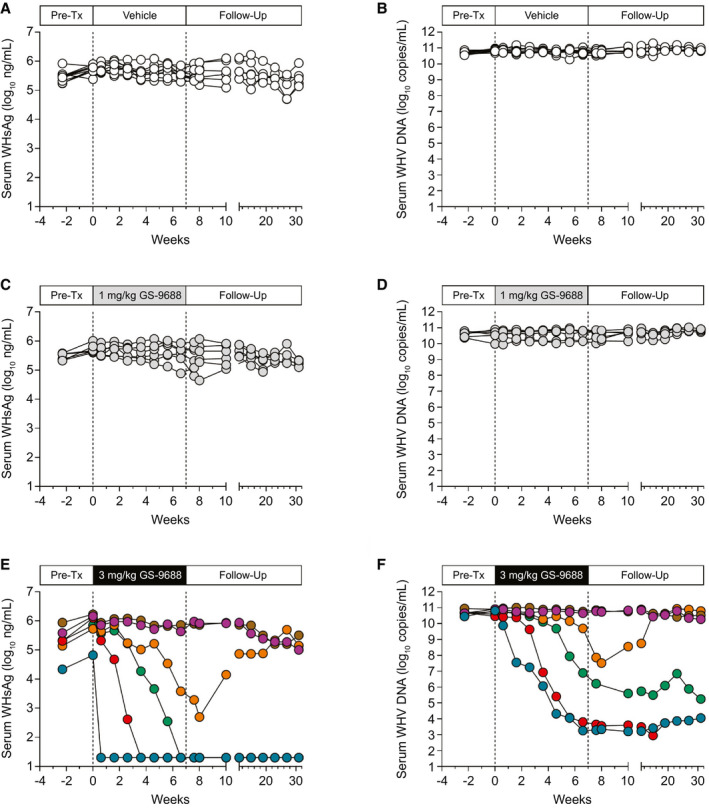
Serum WHsAg and WHV DNA levels in WHV‐infected woodchucks treated with GS‐9688. WHV‐infected woodchucks were dosed orally once per week for 8 weeks with vehicle, 1 mg/kg GS‐9688, or 3 mg/kg GS‐9688 (Supporting Fig. S3). Vertical dotted lines denote the dosing period. (A,C,E) Serum WHsAg levels by dose group. (B,D,F) Serum WHV DNA levels by dose group. Each line represents an individual animal. Abbreviation: Pre‐Tx, pretreatment.
GS‐9688 Reduced Intrahepatic Levels of WHV Nucleic Acids in a Subset of Animals
All woodchucks were assessed for hepatic viral parameters including WHV RNA, WHV DNA replicative intermediates (RI), and WHV covalently closed circular DNA (cccDNA) using liver biopsies obtained before treatment (week –2), 1 week after end of treatment (week 8), and at end of study (week 31). Woodchucks dosed with vehicle or 1 mg/kg GS‐9688 had little to no decrease in intrahepatic viral parameters at either week 8 or week 31 (Fig. 3A). In contrast, treatment with 3 mg/kg GS‐9688 led to a strong decrease in the intrahepatic levels of WHV RNA, DNA RI, and cccDNA (34% to >98% reduction at week 8 and ≥ 96% at week 31) in the three sustained responders (Fig. 3B). There was no reduction at either week 8 or week 31 in either the transient responder or the two nonresponders. These data are consistent with the reduction in antiviral parameters measured in the periphery (Fig. 2E,F).
FIG. 3.
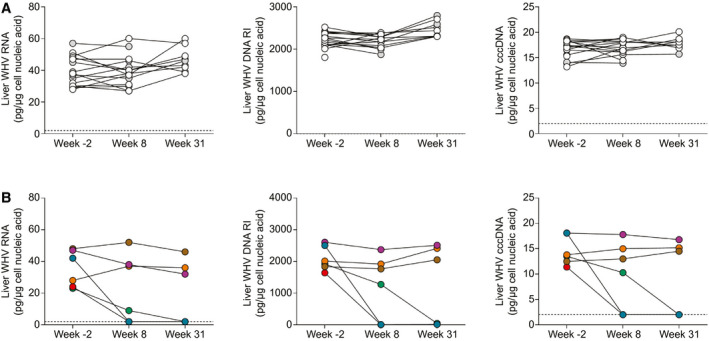
Intrahepatic viral parameters in WHV‐infected woodchucks treated with GS‐9688. WHV‐infected woodchucks were dosed orally once per week for 8 weeks with vehicle, 1 mg/kg GS‐9688, or 3 mg/kg GS‐9688 (Supporting Fig. S3). Liver biopsies were obtained before treatment (week –2), 1 week after treatment (week 8), and at end of study (week 31); and intrahepatic WHV DNA RI, WHV RNA, and WHV cccDNA levels were measured by northern blot (WHV RNA) or Southern blot (WHV DNA RI and cccDNA). (A) Data from woodchucks dosed with vehicle (open circles) and 1 mg/kg GS‐9688 (gray circles). (B) Data from woodchucks dosed with 3 mg/kg GS‐9688. For all plots, each line represents an individual animal; circle colors match those in Fig. 2. The lower limit of detection for the assays is approximately 2 pg/μg cellular nucleic acid and is indicated by a horizontal dotted line.
GS‐9688 Sustained Responders Had Detectable Levels of Anti‐WHsAg Antibodies and Enhanced PBMC Proliferative Response to WHV Peptides
Serum anti‐WHsAg antibody (WHsAb) was not detected in woodchucks dosed with either vehicle or 1 mg/kg GS‐9688 (Fig. 4A,C). In contrast, treatment with 3 mg/kg GS‐9688 induced serum WHsAb levels in the three sustained responder animals, whereas the transient responder and the two nonresponders in the 3 mg/kg dose group did not have detectable serum WHsAb (Fig. 4E). Similarly, treatment with 3 mg/kg GS‐9688 enhanced the proliferative response of PBMCs to WHV peptides in two of the sustained responders (red and blue circles), and there was also a modest increase in the third sustained responder (green circles) (Fig. 4F; Supporting Fig. S4). There was no increase in PBMC proliferative response in the transient responder and the two nonresponders in the 3 mg/kg dose group (Fig. 4F; Supporting Fig. S4). There was also no change in the PBMC proliferative response in woodchucks dosed with either vehicle or 1 mg/kg GS‐9688 (Fig. 4B,D; Supporting Fig. S4).
FIG. 4.
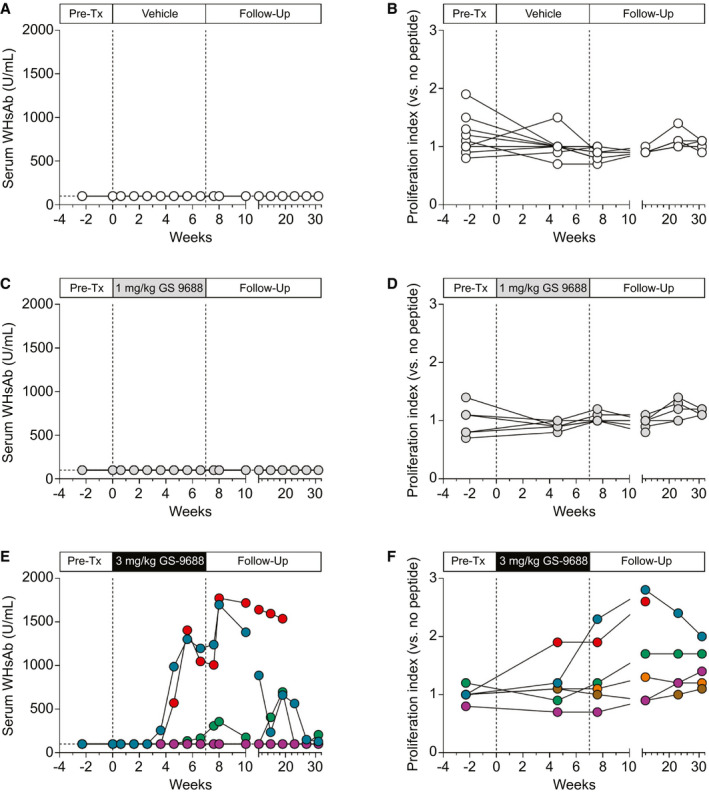
Serum WHsAb levels and PBMC proliferative response to viral peptides in WHV‐infected woodchucks treated with GS‐9688. WHV‐infected woodchucks were dosed orally once per week for 8 weeks with vehicle, 1 mg/kg GS‐9688, or 3 mg/kg GS‐9688 (Supporting Fig. S3). Vertical dotted lines denote the dosing period. (A,C,E) Serum WHsAb levels by dose group. (B,D,F) Proliferative response of PBMCs to WHV core peptides by dose group. A proliferation index of ≥ 2.0 is considered positive in this assay. Each line represents an individual animal; circle colors match those in Fig. 2. Abbreviation: Pre‐Tx, pretreatment.
Tolerability and Safety of Oral GS‐9688 in Woodchucks Chronically Infected With WHV
There were no GS‐9688‐related effects on body weight, temperature, serum chemistry, hematology, and coagulation during the dosing phase. The only finding considered potentially GS‐9688‐related was decreased platelet count in a single woodchuck dosed with 3 mg/kg GS‐9688 (red circles, Figs. 2, 3, 4). This finding was most apparent following doses 3‐5, after which dosing of this animal was interrupted for 1 week (dose 6). Following this 1‐week dose interruption, the platelet count returned to baseline and no additional changes were noted upon readministration of GS‐9688. In addition, the four antiviral responders had a transient, typically modest increase in liver enzymes sorbitol dehydrogenase (SDH) and aspartate aminotransferase (AST) during treatment that temporally correlated with serum WHsAg reduction (Fig. 5). No change in these liver injury biomarkers was observed for other animals, except those that were diagnosed with or died of HCC (Fig. 5; Supporting Fig. S5). Liver histology scores tended to correlate with liver enzyme levels, with only mild liver disease typically being observed in WHV‐infected woodchucks in the absence of HCC (Supporting Table S1). In line with normalization of these liver injury biomarkers during the posttreatment follow‐up period, portal and lobular inflammation (when evaluable) was not observed at the end of study (week 31) in animals with a sustained antiviral response to GS‐9688 (Supporting Table S1).
FIG. 5.
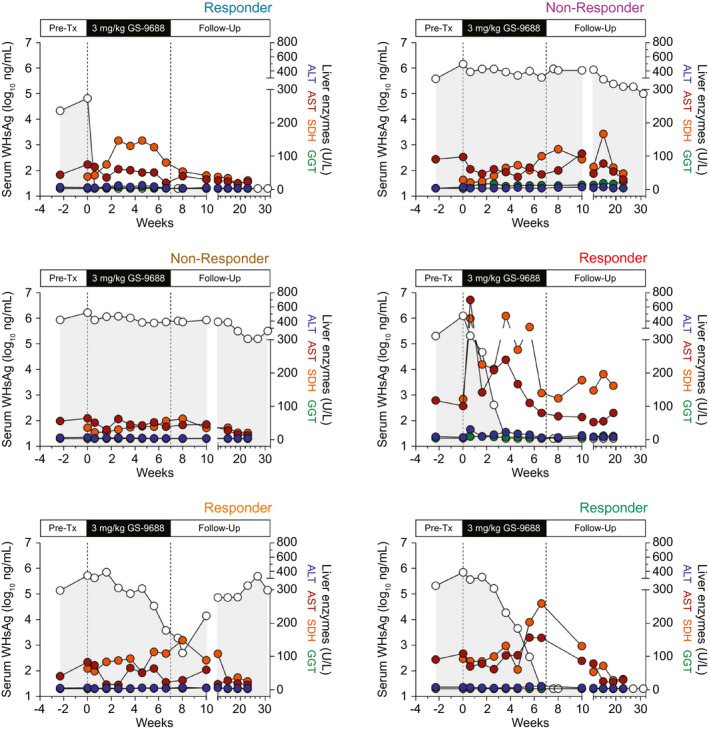
Serum WHsAg and liver enzyme levels in WHV‐infected woodchucks treated with 3 mg/kg GS‐9688. WHV‐infected woodchucks were dosed orally once per week for 8 weeks with 3 mg/kg GS‐9688 (Supporting Fig. S3). Serum WHsAg (open circles) is plotted on the left y‐axis. Serum alanine aminotransferase (blue circles), AST (red circles), SDH (orange circles), and gamma‐glutamyltransferase (green circles) are all plotted on the right y‐axis. Liver enzyme data at week 31 (24 weeks after last dose) were not plotted because some surviving animals had developed HCC by this time. The color of responder/nonresponder labels at the top right of each plot matches the circle color in Fig. 2E,F. Abbreviations: ALT, alanine aminotransferase; GGT, gamma‐glutamyltransferase; Pre‐Tx, pretreatment.
GS‐9688 PK and PD in Woodchucks Chronically Infected With WHV
This efficacy study predated development of the woodchuck IL‐12p40 assay, and therefore, the PD response was assessed by measuring MARCO mRNA levels. Plasma GS‐9688 levels and induction of whole blood MARCO mRNA levels in chronically infected woodchucks were consistent with single‐dose data in uninfected woodchucks (Supporting Fig. S6). There was a trend toward higher mean plasma GS‐9688 maximum concentration (and presumably higher hepatic exposure of GS‐9688) in woodchucks with a sustained antiviral response (Supporting Fig. S6A). In contrast, 3 mg/kg GS‐9688 significantly induced whole blood MARCO mRNA levels, but the degree of induction did not appear to be associated with treatment response (Supporting Fig. S6B). We therefore conducted a second, larger efficacy study to more thoroughly probe the relationship between systemic PK‐PD, the intrahepatic immune profile, and the antiviral response to GS‐9688.
Systemic GS‐9688 and IL‐12p40 Levels Do Not Correlate With Antiviral Response to GS‐9688 in WHV‐Infected Woodchucks
WHV‐infected woodchucks were dosed orally with vehicle (n = 5) or 3 mg/kg GS‐9688 (n = 14) once per week for 12 weeks and followed for an additional 10 weeks (Supporting Fig. S7). Similar to the first efficacy study, treatment with 3 mg/kg GS‐9688 substantially reduced serum WHsAg in a subset of woodchucks (antiviral responders, n = 6) (Fig. 6A). Although there were only two sustained responders in this follow‐up study, WHsAg levels were reduced to below the limit of detection in five of the six antiviral responders. Because this degree of antiviral suppression was only achieved late in the dosing period for the transient responders, extending the treatment duration may have induced sustained response in at least some of these woodchucks. In line with the first efficacy study, the antiviral response temporally correlated with elevations in liver enzymes in some (although not all) animals (Supporting Fig. S8). Baseline serum IL‐12p40 levels were not impacted by infection status, and the magnitude of IL‐12p40 induction by GS‐9688 treatment was comparable in uninfected and chronically infected woodchucks (Fig. 6B). Analogous to blood MARCO mRNA in the first study, the increase in IL‐12p40 levels did not correlate with antiviral response to GS‐9688 (Fig. 6C). While there was a trend toward higher plasma GS‐9688 exposure in woodchucks with sustained antiviral response in the initial efficacy study, plasma GS‐9688 levels were comparable in the responder and nonresponder animals in this larger follow‐up study (Fig. 6D). Unfortunately, due to sampling limitations, only very limited PBMC proliferation data were available for this second efficacy study (data not shown), and no data were available for the sustained responders.
FIG. 6.
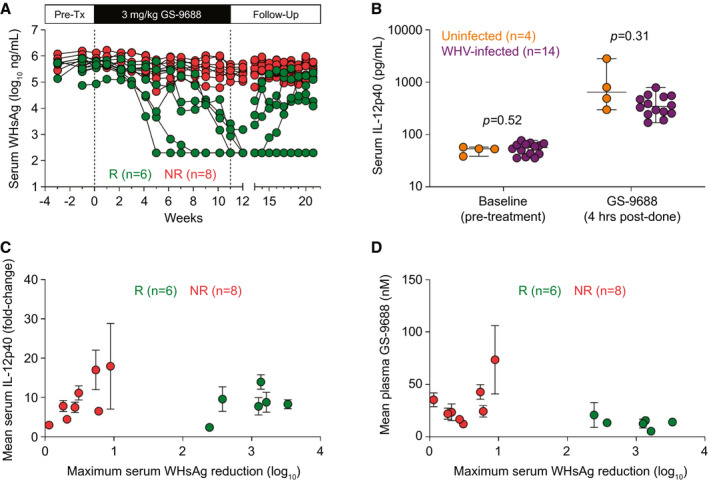
Antiviral response does not correlate with systemic GS‐9688 and IL‐12p40 levels in WHV‐infected woodchucks treated with GS‐9688. WHV‐infected woodchucks were dosed orally once per week for 12 weeks with 3 mg/kg GS‐9688 (Supporting Fig. S7). (A) Serum WHsAg in woodchucks dosed with 3 mg/kg GS‐9688. Vertical dotted lines denote the dosing period. Each line represents an individual animal. Antiviral responders (green circles, n = 6) are defined as animals with a ≥ 1.0 log10 reduction of serum WHsAg during treatment (nonresponders shown as red circles, n = 8). (B) Serum IL‐12p40 levels before and after GS‐9688 treatment in uninfected woodchucks (n = 4, orange circles) and WHV‐infected woodchucks (n = 14, purple circles). Each circle represents an individual animal at a single time point, except for the serum IL‐12p40 levels in WHV‐infected woodchucks after GS‐9688 treatment (each circle represents the mean of weeks 1, 3, 5, 7, 9, and 11 for an individual animal). The horizontal line indicates the median, and error bars represent the range. Statistical significance was calculated by unpaired t test with Welch’s correction. (C) Relationship between serum IL‐12p40 levels at 4 hours postdose (expressed as fold‐change relative to pretreatment) and the maximum reduction in WHsAg during treatment. (D) Relationship between plasma GS‐9688 levels at 4 hours postdose and the maximum reduction in WHsAg during treatment. For (C,D), circles indicate the mean of six time points (weeks 1, 3, 5, 7, 9, and 11), and error bars represent the SEM. Circle colors match those in (A). Abbreviations: NR, nonresponder; Pre‐Tx, pretreatment; R, responder.
Baseline Intrahepatic Transcriptional Profile Differentiates Antiviral Responders and Nonresponders to GS‐9688 Treatment
Recent studies have identified specialized immune cell subsets that are enriched or resident in the liver, highlighting the need to sample this compartment when evaluating the response to immunomodulatory agents.( 2 ) Although we could not serially biopsy the liver of woodchucks during the treatment phase of the study, we were able to characterize the transcriptional profiles of liver biopsies taken from the GS‐9688‐treated and vehicle‐treated animals one or two weeks before dosing was initiated (Supporting Fig. S7). Transcriptional profiling of liver biopsies from uninfected adult woodchucks (n = 9) was included as a comparator. There were substantial differences between the transcriptional profiles of livers from uninfected (U) and chronically infected woodchucks (C), with >1,000 differentially expressed genes (DEGs) associated with persistent WHV infection (data not shown). In line with a previous study,( 12 ) immune cell gene enrichment analysis (xCell), Ingenuity Pathway Analysis, and gene set enrichment analysis (GSEA) collectively demonstrated that chronic WHV infection is associated with an intrahepatic IFN‐γ transcriptional response and that the liver of chronically infected animals is significantly enriched for B‐cell, T‐cell, and neutrophil gene signatures, as well as for markers associated with T‐cell exhaustion (Supporting Fig. S9 and Tables S2 and S3). In contrast, there was no difference in baseline intrahepatic gene expression between the antiviral responder (CR) and nonresponder (CNR) animals based on DEG count, with no individual gene being differentially expressed (absolute fold‐change >2, false discovery rate [FDR] < 0.05) between the groups.
To characterize the intrahepatic transcriptional signatures of responder and nonresponder animals in more detail, we employed analytical approaches that do not depend on arbitrary thresholds to select genes (i.e., GSEA and xCell). In addition, because statistical power was limited by the small number of animals in each response group (n = 6‐8), we compared transcriptional profiles between CR versus U and CNR versus U animals to identify interesting trends in CR versus CNR expression patterns. GSEA identified B‐cell and neutrophil gene signatures as being significantly enriched in the nonresponder versus responder animals (Supporting Table S4). Similarly, there was greater enrichment of pro‐B‐cell, memory B‐cell, activated dendritic cell, and monocyte signatures in the nonresponders by xCell analysis (Fig. 7A). These transcriptional patterns are consistent with the higher expression of various neutrophil and B cell–associated genes in the nonresponder animals (Fig. 7B,C; Supporting Table S5). Conversely, the baseline intrahepatic gene expression profiles of responders were selectively enriched in Treg, plasma cell, and M1 macrophage signatures (Fig. 7A). On an individual gene level, elevated expression of the hallmark transcription factor forkhead box P3 as well as the ubiquitin ligase HECT and RLD domain containing E3 ubiquitin protein ligase family member 6 is consistent with the enriched Treg transcriptional signature in the antiviral responders (Fig. 7D; Supporting Table S5).( 13 ) Increased intrahepatic expression of the gene for butyrophilin subfamily 1 member A1, an inhibitor of T‐cell activation,( 14 ) also suggests that there is negative regulation of T‐cell function in the liver of antiviral responders prior to GS‐9688 treatment (Fig. 7D).
FIG. 7.
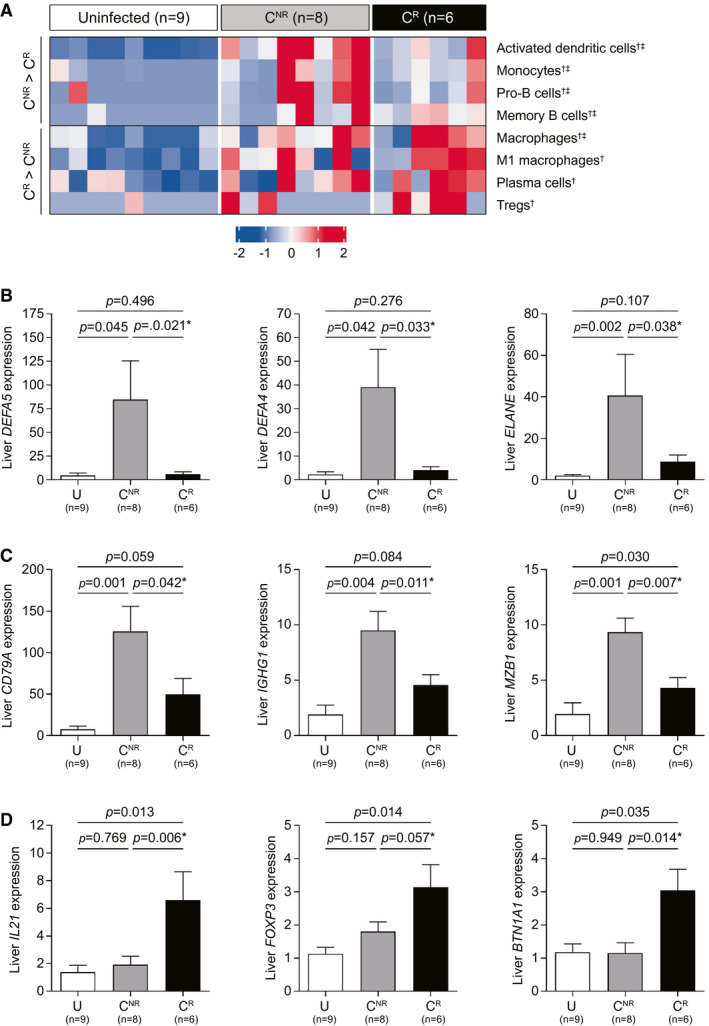
Baseline intrahepatic transcriptional profile in WHV‐infected woodchucks correlates with antiviral response to GS‐9688 treatment. WHV‐infected woodchucks were dosed orally once per week for 12 weeks with 3 mg/kg GS‐9688 (Supporting Fig. S7). Liver biopsies collected from uninfected woodchucks (U, n = 9) and from WHV‐infected woodchucks prior to GS‐9688 treatment (baseline) were analyzed by RNA‐sequencing. The WHV‐infected animals were classified as antiviral responders (CR, n = 6) or nonresponders (CNR, n = 8) to GS‐9688 treatment, as defined in Fig. 6. (A) RNA‐sequencing data were analyzed by xCell. Each column represents a different animal, and each row represents an individual cell type; overexpression (red) and underexpression (blue) are indicated by the scale bar for z‐score values. The mean z score was greater for CNR than CR for the top four rows, whereas the mean z score was greater for CR than CNR for the bottom four rows. Symbols adjacent to the cell type names indicate statistical significance: †unadjusted P value <0.05 for CR versus U, ‡unadjusted P value <0.05 for CNR versus U. The complete xCell analysis (n = 39 cell subsets) is provided in Supporting Fig. S9. (B‐D) RNA‐sequencing data for select genes that were differentially expressed in CR versus CNR. (B) Neutrophil‐associated genes. (C) B cell–associated genes. (D) TFH and Treg‐associated genes. Data are expressed as fold‐change relative to uninfected woodchucks; bar height indicates the mean, and error bars represent the SEM. Adjusted P values (FDR) for CR versus U and CNR versus U are displayed for each gene. No gene reached FDR < 0.05 in the CR versus CNR comparison; unadjusted P values are displayed and are denoted by an asterisk. All statistical analyses were performed using a Wilcoxon rank‐sum test with or without multiple testing correction by the Benjamini and Hochberg method. Abbreviations: BTN1A1, butyrophilin subfamily 1 member A1; DEFA, defensin alpha; ELANE, elastase, neutrophil expressed; FOXP3, forkhead box P3; IGHG1, immunoglobulin heavy constant gamma 1; MZB1, marginal zone B and B1 cell specific protein.
Various studies have identified a potential role for IL‐21 in the immune control of HBV infection,( 15 , 16 , 17 ) and it is therefore striking that baseline intrahepatic IL21 expression was significantly elevated in antiviral responders (Fig. 7D). TFH are major producers of IL‐21, and the expression of other TFH‐associated genes (chemokine [C‐X‐C motif] receptor 5 [CXCR5], inductible T‐cell costimulator, and CD4) was increased in antiviral responders, although not all markers reached statistical significance (Supporting Table S6). The elevated expression of these TFH‐associated genes is also consistent with enrichment of the plasma cell signature in responder animals.( 18 ) In contrast to CD4, intrahepatic CD8A and CD8B levels were higher in CNR versus U compared to CR versus U (Supporting Table S6), suggesting that CXCR5+CD8+ T cells are not the source of the TFH‐type signature in antiviral responders. Higher intrahepatic expression of the costimulatory T‐cell receptor OX40 (tumor necrosis factor receptor superfamily member 4) in CR versus U, but not CNR versus U (Supporting Table S6), is also notable because hepatic OX40+CD4+ T cells play a key role in IL‐21 production by TFH and the development of an effective immune response to HBV in an adoptive transfer mouse model.( 19 )
Collectively, these analyses indicate that the baseline intrahepatic immune profile influences the antiviral response to GS‐9688 and that enrichment of various immune cell subsets (e.g., TFH) may be important for treatment response (Supporting Fig. S10).
Discussion
GS‐9688 is an oral, selective, small‐molecule agonist of TLR8 being developed for the treatment of CHB. The main goal of the current study was to evaluate the antiviral efficacy of GS‐9688 in the woodchuck model of CHB. We first determined that woodchuck TLR8 is functional and that TLR8 activation induces a comparable response in human and woodchuck PBMCs. Collectively, these in vitro data established the translational relevance of the woodchuck model for evaluating small molecule agonists of human TLR8. Consequently, we performed single‐ascending dose studies with GS‐9688 in uninfected woodchucks and then an 8‐week efficacy study in WHV‐infected woodchucks. In the latter, weekly oral dosing with 3 mg/kg GS‐9688 substantially reduced serum and intrahepatic viral endpoints in a subset of WHV‐infected woodchucks. Strikingly, serum WHsAg and intrahepatic cccDNA levels were reduced to below the limit of detection in those animals with a sustained antiviral response to GS‐9688. These animals also developed detectable WHsAb. Interestingly, although these animals had a >5 log10 reduction in viral load, serum WHV DNA was still detectable in the sustained responders throughout the postdosing period. However, the viral load in these animals remained stable and did not rebound through end of study (24 weeks after the last dose of GS‐9688). This suggests that GS‐9688 treatment of chronically infected woodchucks resembles natural resolution of HBV infection in humans; i.e., a small percentage of hepatocytes remain infected, but an ongoing immune response effectively controls the virus.( 1 )
An important observation from the current study is that GS‐9688 did not induce an effective antiviral response in all chronically infected woodchucks. Elucidating the key differences between antiviral responders and nonresponders may identify biomarkers that predict antiviral response and could also help define the immune determinants of viral control. It is therefore notable that antiviral response was not associated with either systemic GS‐9688 or IL‐12p40 levels, whereas there was an enhanced proliferative response of PBMCs to WHV peptides in animals with a sustained antiviral response. Because there was also a temporal correlation between reduction in serum viral endpoints and liver enzyme elevations in some (although not all) animals, these data raise the possibility that virus‐specific, cytotoxic T cells play a role in the antiviral response to GS‐9688. Interestingly, the PBMC proliferative response in sustained antiviral responders was greatest during the treatment follow‐up period, after WHsAg loss and detection of WHsAb. This delay between WHsAg loss and the increase in peripheral immune response may reflect localization of virus‐specific T cells in the liver until clearance of infected hepatocytes is complete.( 20 ) Future studies will therefore focus on characterizing the intrahepatic immune response during GS‐9688 treatment.
Another notable finding from this study is that the baseline intrahepatic transcriptional immune profile of WHV‐infected woodchucks influences antiviral response to GS‐9688 treatment. For example, antiviral responders were enriched in TFH and Treg gene signatures. TFH play a pivotal role in the development of intrahepatic, virus‐specific CD8+ T‐cell and B‐cell responses in various mouse models of HBV infection but can be effectively blocked by Tregs.( 15 , 21 ) Moreover, the frequency of circulating TFH and systemic levels of TFH‐associated cytokines and chemokines (IL‐21, chemokine [C‐X‐C motif] ligand 13 [CXCL13]) are associated with immune control in patients with HBV.( 16 , 17 , 22 ) Because TLR8 agonists promote TFH differentiation through production of IL‐12( 9 ) and directly inhibit Treg function,( 4 ) our data suggest that GS‐9688 may induce effective antiviral immunity by augmenting a preexisting TFH response in the liver (Supporting Fig. S10). In addition to TFH and Tregs, antiviral responders were enriched for an intrahepatic M1 macrophage transcriptional signature. Mouse models have recently identified a central role for hepatic macrophages (Kupffer cells) in effective CD8+ T‐cell priming in the liver.( 23 , 24 ) For example, studies in an adoptive transfer model demonstrated that Kupffer cell–derived CXCL13 facilitates organization of CD8+ T cells, CD4+ T cells (including TFH), and B cells in the liver and that these lymphoid structures support intrahepatic immune priming.( 15 , 23 ) Similarly, the antiviral response to high‐dose TLR7 agonist vesatolimod in HBV‐infected chimpanzees was temporally correlated with induction of intrahepatic CXCL13 expression as well as aggregates of CD8+ T cells, CD4+ T cells (likely including TFH), and B cells.( 11 ) Because human macrophages express TLR8 and GS‐9688 induces CXCL13 production from human PBMCs (Supporting Fig. S11), these data suggest that direct activation of Kupffer cells may play a role in the antiviral immune response to GS‐9688 (Supporting Fig. S10).
Like GS‐9688, the TLR7 agonist vesatolimod also demonstrated antiviral efficacy in WHV‐infected woodchucks.( 10 ) However, vesatolimod had no antiviral effect in patients with CHB. The current study addressed this discrepancy in preclinical versus clinical antiviral response by characterizing the cytokine response to vesatolimod in woodchucks and comparing it to the PD response in humans. This revealed that the efficacious dose of vesatolimod induced a substantially higher systemic cytokine response in woodchucks than was induced in humans at a safe and tolerable dose (Supporting Information and Table S7). Interestingly, our data also suggest that the efficacious dose of vesatolimod may activate TLR8 as well as TLR7 in WHV‐infected woodchucks (see Supporting Information). In contrast, GS‐9688 is a selective agonist of woodchuck TLR8, and the level of serum IL‐12p40 induced by the efficacious dose (3 mg/kg) in WHV‐infected woodchucks (6.1‐fold ± 0.9) is comparable to that induced by 3 mg GS‐9688 in virally suppressed (6.8‐fold ± 0.6) and viremic (6.0‐fold ± 0.4) patients with CHB.( 25 ) Importantly, the 3 mg dose was safe and generally well tolerated in this phase 1 study in patients with CHB and so was selected as the top dose for the ongoing phase 2 studies.
In summary, our data demonstrate that finite, short‐duration treatment with a clinically relevant dose of GS‐9688 was well tolerated and induced a sustained antiviral response in the woodchuck model of CHB. Strikingly, GS‐9688 treatment substantially reduced serum and intrahepatic viral endpoints and induced detectable WHsAb levels, indicating it has potential for inducing functional cure of CHB. Taken together, these data support clinical development of GS‐9688 for the treatment of CHB.
Author Contributions
S.D., S.B., W.E.D., R.M., and S.P.F. conceived the study. S.D., S.B., J.C., J.Z., M.M., W.E.D., R.M., and S.P.F. participated in research design. S.D., R.S., D.R., D.P., S.S., R.C., B.L., S.M., M.H., C.Y., and M.S. performed the experiments. D.K., R.R., L.L., and S.P.F. analyzed the RNA‐sequencing data. All authors analyzed the remaining data. S.D., S.B., J.C., J.Z., W.R., L.L., C.V., S.M., P.C., W.E.D., R.M., and S.P.F. supervised the study. S.D. and S.P.F. wrote the original draft of the manuscript, and all authors reviewed and approved the final manuscript.
Supporting information
Supplementary Material
Acknowledgment
We thank John Nichols for supervising woodchuck antibody development. We also gratefully acknowledge Joanne Song for sample management as well as Anuj Gaggar, Audrey Lau, Tomas Cihlar, Daniel Tumas, Oliver Amin, and Laura Pallett for discussions and support. We also thank the many colleagues whose work we were unable to describe because of space limitations.
Supported by Gilead Sciences, Inc.
Potential conflict of interest: Dr. Daffis is employed by and owns stock in Gilead. Dr. Balsitis is employed by and owns stock in Gilead. Dr. Chamberlain owns stock in Gilead. Dr. Zheng is employed by and owns stock in Gilead. Dr. Santos is employed by and owns stock in Gilead. Dr. Rowe is employed by and owns stock in Gilead. Dr. Ramakrishnan is employed by and owns stock in Gilead. Dr. Pattabiraman is employed by and owns stock in Gilead. Dr. Chu is employed by and owns stock in Gilead. Dr. Kang is employed by and owns stock in Gilead. Dr. Mish is employed by and owns stock in Gilead. Dr. Ramirez is employed by and owns stock in Gilead. Dr. L. Li is employed by and owns stock in Gilead. Dr. B. Li is employed by and owns stock in Gilead. Dr. Ma is employed by and owns stock in Gilead. Dr. Hung is employed by and owns stock in Gilead. Dr. Voitenleitner owns stock in Gilead. Dr. Menne received grants from Gilead. Dr. Delaney is employed by, owns stock in, and holds intellectual property rights with Gilead. Dr. Mackman is employed by and owns stock in Gilead. Dr. Fletcher is employed by and owns stock in Gilead.
References
Author names in bold designate shared co‐first authorship.
- 1. Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64(1 Suppl.):S71‐S83. [DOI] [PubMed] [Google Scholar]
- 2. Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol 2019;16:662‐675. [DOI] [PubMed] [Google Scholar]
- 3. Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005;174:1259‐1268. [DOI] [PubMed] [Google Scholar]
- 4. Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll‐like receptor 8‐mediated reversal of CD4+ regulatory T cell function. Science 2005;309:1380‐1384. [DOI] [PubMed] [Google Scholar]
- 5. Poulin LF, Salio M, Griessinger E, Anjos‐Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR‐1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med 2010;207:1261‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorski KS, Waller EL, Bjornton‐Severson J, Hanten JA, Riter CL, Kieper WC, et al. Distinct indirect pathways govern human NK‐cell activation by TLR‐7 and TLR‐8 agonists. Int Immunol 2006;18:1115‐1126. [DOI] [PubMed] [Google Scholar]
- 7. Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan‐Garcia A, et al. Toll‐like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014;10:e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du J, Wu Z, Ren S, Wei Y, Gao M, Randolph GJ, et al. TLR8 agonists stimulate newly recruited monocyte‐derived cells into potent APCs that enhance HBsAg immunogenicity. Vaccine 2010;28:6273‐6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ugolini M, Gerhard J, Burkert S, Jensen KJ, Georg P, Ebner F, et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat Immunol 2018;19:386‐396. [DOI] [PubMed] [Google Scholar]
- 10. Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the Toll‐like receptor 7 agonist GS‐9620 in the woodchuck model of chronic hepatitis B. J Hepatol 2015;62:1237‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li L, Barry V, Daffis S, Niu C, Huntzicker E, French DM, et al. Anti‐HBV response to toll‐like receptor 7 agonist GS‐9620 is associated with intrahepatic aggregates of T cells and B cells. J Hepatol 2018;68:912‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher SP, Chin DJ, Ji Y, Iniguez AL, Taillon B, Swinney DC, et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology 2012;56:820‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuadrado E, van den Biggelaar M, de Kivit S, Chen YY, Slot M, Doubal I, et al. Proteomic analyses of human regulatory T cells reveal adaptations in signaling pathways that protect cellular identity. Immunity 2018;48:1046‐1059.e1046. [DOI] [PubMed] [Google Scholar]
- 14. Smith IA, Knezevic BR, Ammann JU, Rhodes DA, Aw D, Palmer DB, et al. BTN1A1, the mammary gland butyrophilin, and BTN2A2 are both inhibitors of T cell activation. J Immunol 2010;184:3514‐3525. [DOI] [PubMed] [Google Scholar]
- 15. Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L, et al. IL‐21 is pivotal in determining age‐dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 2011;121:1154‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma SW, Huang X, Li YY, Tang LB, Sun XF, Jiang XT, et al. High serum IL‐21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol 2012;56:775‐781. [DOI] [PubMed] [Google Scholar]
- 17. Yoshio S, Mano Y, Doi H, Shoji H, Shimagaki T, Sakamoto Y, et al. Cytokine and chemokine signatures associated with hepatitis B surface antigen loss in hepatitis B patients. JCI Insight 2018;3 10.1172/jci.insight.122268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL‐21 induces differentiation of human naive and memory B cells into antibody‐secreting plasma cells. J Immunol 2005;175:7867‐7879. [DOI] [PubMed] [Google Scholar]
- 19. Publicover J, Gaggar A, Jespersen JM, Halac U, Johnson AJ, Goodsell A, et al. An OX40/OX40L interaction directs successful immunity to hepatitis B virus. Sci Transl Med 2018;10:eaah5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, et al. Anti‐PD‐1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol 2019;71:900‐907. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Dong Q, Li Q, Li Y, Zhao D, Sun J, et al. Dysregulated response of follicular helper T cells to hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients. Gastroenterology 2018;154:2222‐2236. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Ma S, Tang L, Li Y, Wang W, Huang X, et al. Circulating chemokine (C‐X‐C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL‐21 in patients with chronic hepatitis B virus infection. Hepatology 2013;58:1277‐1286. [DOI] [PubMed] [Google Scholar]
- 23. Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO, et al. Age‐dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 2013;123:3728‐3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benechet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, et al. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature 2019;574:200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gane E, Kim HJ, Visvanathan K, Kim YJ, Nguyen A‐H, Joshi A, et al. Safety, pharmacokinetics and pharmacodynamics of oral TLR8 agonist GS‐9688 in patients with chronic hepatitis B (CHB): a randomized, placebo‐controlled, double‐blind phase 1b study [Abstract]. Hepatology 2018;68:238A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


