Abstract
Background
Photodamage creates changes within the skin layers known as solar elastosis. This presents as fragmentation of collagen and elastin fibers, decreases in the extracellular matrix (ECM) ground substance, as well as hyaluronic acid decrease in the thinning epidermis. Traditional immunohistochemistry (IHC) staining has failed to differentiate degenerated elastin from new elastin fibers generated with various topical strategies.
Aims
A combination of stains that can offer a regenerative narrative distinguishing newly formed collagen and elastin from that of degenerated protein, thus distinguishing “good” vs “bad” elastin.
Methods
A series of stains were explored based on their ability to identify early regenerative changes within epidermal, dermal, and ECM areas to examine consistency of outcomes and reliability.
Results
A combination of Movat, fibrillin, elafin, and versican for elastogenesis and reversal of solar elastosis. CD44 for HA status (mainly epidermal) and Herovici stain for identifying early collagenesis in the ECM provides a comprehensive range of stains for identifying new elastin and collagen
Conclusion
This suggested stain combination appears to offer an ideal collection of stains for identifying regenerative events within the skin layers.
Keywords: collagen, elastin, histology, photodamage
1. INTRODUCTION
It is irrefutable that ultraviolet (UV) light exposure to the skin, also known as photodamage, presents the greatest impact on skin homeostasis. This is very evident not only on a subjective and clinical level, but more particularly at a molecular and histologic level. However, the difficulty arises with the contrast in intrinsic chronological aging responses to that of extrinsic UV light exposure. Histologically, intrinsic aging is characterized by a general decrease in the extracellular matrix with reduced elastin and the disintegration of elastic fibers whereas photodamaged skin shows the accumulation of dystrophic elastotic material in the reticular dermis, a process referred to as solar elastosis. 1 This thick elastin‐rich material in the reticular dermis is mainly represented by tropoelastin core protein with a lack of fibrillin fine fibers in the papillary dermis that normally cascade upwards in candelabra‐like fashion to the dermo‐epidermal junction (DEJ). 2 It is this contradiction—a lack of elastin occurring with aging, contrasting with an accumulation of thickened elastin—that creates major challenges when trying to define the status of skin health in relation to elastin. More to the point, can we differentiate “good” elastin from “bad” elastin?
By combining a number of stains reflecting the status of the extracellular matrix and cellular components within the dermis and the epidermis, it may be possible to create a histologic narrative that can point to elastin regeneration rather than degeneration, thus differentiating this important sequence.
Where does this accumulated tropoelastin originate? Normally, keratinocytes are not considered to be elastin‐producing cells but under the influence of UV light, it has been shown that keratinocytes, like fibroblasts, also produce tropoelastin. 1 In healthy skin, elastic fibers are continuous from DEJ to the deep dermis. Core protein elastin stores energy and drives recoil, while fine fibrillin (oxytalan) fibers are involved in cell signaling via TGFβ interactions ensuring cellular‐extracellular homeostasis. 3 Photodamage directly affects this homeostasis with almost immediate loss of fibrillin fibers in the papillary dermis extending to the DEJ. Thus, attempted reversal of solar elastosis often begins with the recognition of increased fibrillin‐1 production by both keratinocytes and particularly by dermal fibroblasts in an effort to re‐establish the integrity of the microfibrillar network that extends from the DEJ into the papillary dermis. 2
Photoaged skin contains fewer fibrillin oxytalan fibers, and dermal elastic fibers are replaced with dystrophic elastotic materials. Included among these dystrophic elastotic materials is the large chondroitin sulfate proteoglycan versican. Versican has double binding sites binding fibulin‐1 and fibulin‐2 of elastin microfibers on one end and hyaluronic acid (HA) via its N‐terminal region on the other end. HA links elastic fibers to the ground substance. 4 Therefore, by binding to fibrillin microfibrils and HA, versican likely provides viscous properties to the elastin fibers. 4 MMP‐12, released by keratinocytes and fibroblasts in response to UV light, abolishes the HA binding ability of versican. Thus, the elastotic material includes accumulated versican that has lost its HA binding capacity, probably depriving elastin of its viscous surrounding with resultant faint staining of HA in regions of solar elastosis. 4 This degradation of versican by MMP‐12 could represent a possible mechanism of the aging process of the ECM. 4 In young skin, HA is found where collagen and elastin fibers intersect—these connections with HA disappear in aged skin possibly decreasing hydration with diminished tonicity and increased skin wrinkling. 2
Aged skin, with loss of volume and lacking the plumpness of youthful skin, is characterized by decreased levels of HA particularly epidermal HA which diminishes almost completely as opposed to dermal levels that remain relatively stable. 2 The fact that HA is synthesized in the epidermis as well as the dermis likely accounts for this discrepancy in findings. HA binds to an ubiquitous and functionally important family of cell surface receptors, CD44, present on the surface of keratinocytes. 5 UV radiation decreases the expression of CD44 receptor and also HA in the epidermis. 5 , 6
Thus, photoaged skin has been shown to be characterized by reduced levels of epidermal HA and its receptor CD44, and elevated levels of chondroitin sulfate proteoglycan, versican.
Acute UV irradiation induces MMP‐12 expression in macrophages and fibroblasts, cleaving the HA binding sites on versican, reducing the viscous milieu of elastin. Significant amounts of MMP‐12 protein are expressed in association with elastotic materials in the upper dermis of photoaged skin, suggesting that MMP‐12 may play an important role in the development of solar elastosis. 1 MMPs, including MMP‐12, may degrade tropoelastin and fibrillin proteins causing the loss of elastic fibers and the accumulation of elastotic materials in photoaged skin. 1 In addition to sunscreen, new strategies to block heat‐induced skin aging (thermal aging) need to be developed to effectively prevent this process. 1 , 7
Ultraviolet irradiation upsets the balance of elastin fibers—excess elastin and insufficient lysyl oxidase (LOXL1) enzyme result in an accumulation of nonfunctional elastin. In addition, UV rays also induce elafin synthesis by fibroblasts which accumulates in the degenerated elastic fibers of photodamaged skin. 8 UV‐mediated elafin interacts with elastin and the cross‐linked elafin‐elastin complex protects elastic fibers from elastolytic degradation, leading to the accumulation of elastic fibers in the solar elastosis of sun‐damaged skin. 9
In aged skin, the intersection of the epidermis and dermis, the DEJ, flattens with a diminished connecting surface area. This loss of DEJ surface area may contribute to the increased fragility of aged skin and may also lead to reduced nutrient transfer between the dermal and epidermal layers. 2 Type VII collagen comprises anchoring fibrils that attach the basement membrane zone to the underlying papillary dermis. UV radiation decreases the expression of type VII collagen in keratinocytes which likely contributes to wrinkles due to the weakened connection between dermis and epidermis. 2 , 10
Anti‐aging strategies are aimed at halting the degradation of the skin's three primary structural constituents, collagen, elastin, and HA, since all three components are known to decline with age. Although no products provide these key skin components directly, some products do promote the natural synthesis of these substances. 2 , 11 , 12
1.1. Histology platform for solar elastosis
Combining the background information above, a strategy for defining histologic changes in photodamaged skin can be developed. Rather than focusing on one aspect of elastin as is traditionally performed using immunohistochemistry and other special stains for defining elastin, a set of stains can be used that will create a scientific narrative with the capacity to distinguish degenerative changes from regenerative ones. This is particularly relevant to elastin where accumulated core protein may stain powerfully positive but, in many cases, does not distinguish between degenerative solar elastosis and regenerative new elastin (Figure 1).
FIGURE 1.
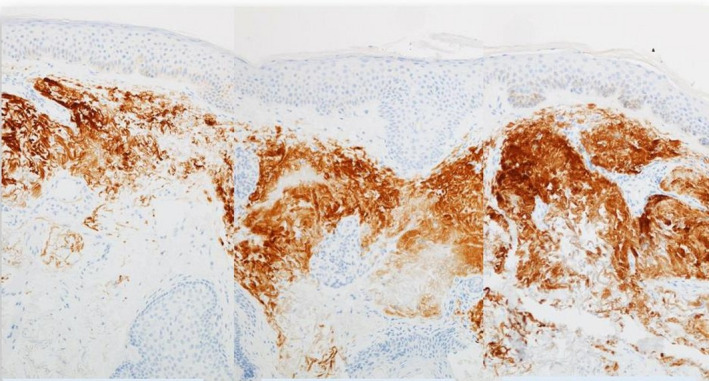
Tripeptide/Hexapeptide (Regenerating Skin Nectar with TriHex Technology®, Alastin Skincare, Inc); histologic changes observed after 3 and 8 wk of treatment; IHC 100× Magnification, 61‐y‐old male face
2. MATERIALS AND METHODS
The pathologic specimens were examined by an experienced dermatopathologist to evaluate staining pattern. Five‐micron‐thick tissue sections were stained with routine H and E stains, in addition to Movat pentachrome, and Herovici histochemical stains. Immunohistochemical staining for CD44, fibrillin, elafin, and versican was performed by an automated stainer (Ventana Benchmark Ultra). All immunohistochemical staining was performed with corresponding positive and negative controls.
2.1. Solar elastosis—immunohistochemistry stain (IHC)
As seen above, IHC will stain elastin very efficiently and we suspect that thicker aggregated areas represent solar elastosis. We also see that with time, when using topical products, there is improved distribution of elastin within the dermis but there is no way of knowing which is mature, elastotic material and which is possibly newly generated elastin.
One better way of demonstrating changes would be to concentrate on the papillary dermis with specific stains that will demonstrate new fibers in the region (Figure 2). Movat's pentachrome stains collagen and reticulin in yellow, glycosaminoglycans in light blue, muscle in red, elastic fibers in dark purple, and nuclei in black. 13 We have found this stain very useful to identify new elastin/fibrillin fibers in the papillary and upper reticular dermis (Figure 2).
FIGURE 2.
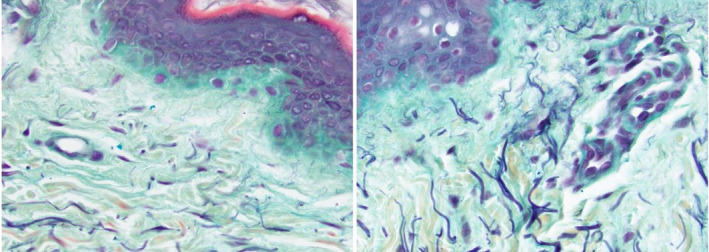
Right forearm—40‐y‐old female pretreatment (A) and 2 wk postapplication of Tripeptide/Hexapeptide (INhance with TriHex Technology®, Alastin Skincare, Inc) (B) Movat stain 40X‐Left panel compared to right: 90% increase in elastic fibers
In addition, newly regenerated elastin is well represented by intracellular fibrillin within fibroblasts (Figure 3).
FIGURE 3.

Forty five‐years‐old female pretreatment (A) and 2 wk postapplication of Tripeptide/Hexapeptide (INhance with TriHex Technology®). (B) Fibrillin stain 10X—Left panel compared to right panel: fibrillin staining within fibroblasts is increased approximately 4‐fold
As noted above, solar elastosis is accompanied by an increase in elafin, a protease inhibitor, that inhibits elastin breakdown. Under normal circumstances, this would be protective of elastin; however, when thick aggregates of elastin form, elafin aggravates the situation by preventing the dissolution of these aggregates. Thus, solar elastosis is characterized by increased levels of dermal elafin and regenerative changes would be represented by an initial decrease in elafin levels during clearance of the ECM and subsequent regeneration of elastin. 9 , 11 This is a subtle change that needs to be examined closely in the ECM which appears “smudgy,” with elafin staining homogenously distributed within the ECM. After topical treatment, the ECM appears “cleaned” and freshened without the background ECM staining but with more intracellular localization of the stain which would be in keeping with elafin secretion as a protector of new elastin (Figure 4).
FIGURE 4.
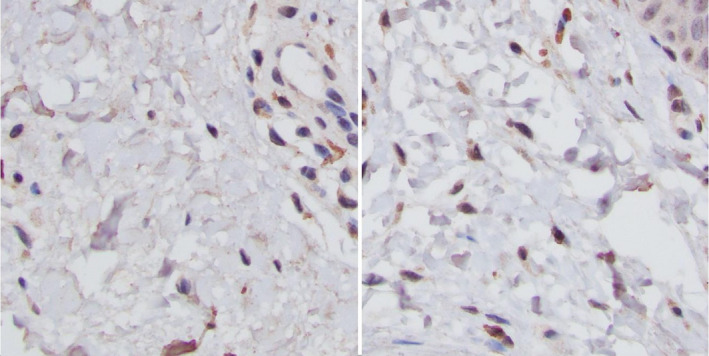
Sixty‐year‐old male, untreated eyelid (A), and 4 wk postapplication of Tripeptide/Hexapeptide (Regenerating Skin Nectar with TriHex Technology®). (B) Elafin staining 40X—note the homogenous extracellular staining of the dermis on L panel as opposed to more dominant intracellular deposition on the R panel after treatment representing clearance of the ECM of elafin and newly formed elafin within fibroblasts that will protect new elastin generation. This is more easily discernable with magnification in panels below
The accumulation of versican occurs with solar elastosis, and correspondingly, the treatment of solar elastosis should create a decrease in dermal versican levels (Figure 5).
FIGURE 5.
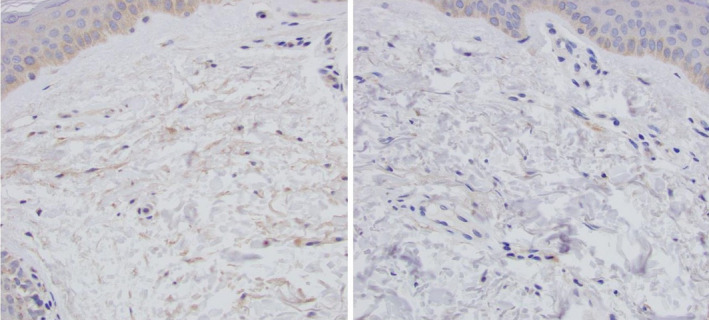
Sixty four‐year‐old male, untreated forearm (A), 3 wk post‐Tripeptide/Hexapeptide (Regenerating Skin Nectar with TriHex Technology®) application. (B) Versican staining 40X—note the clearance of the versican in the dermal ECM after topical therapy
One of the reasons for HA decrease is the effect of UV light on the HA binding site of versican which results in the release of MMP12 which directly affects this binding site. Thus, solar elastosis results in collections of MMP12 in the dermis associated with increased versican and elafin. Although versican and elafin levels may be easy to identify, matrix metalloproteinases work in sporadic releases when needed. Thus, it is a challenge to pinpoint histologic changes due to these timing issues. In contrast, however, CD44 receptor which is normally present on the surface of keratinocytes demonstrates reduced expression with aging and photodamage and can be re‐stimulated using topical active agents (Figure 6).
FIGURE 6.
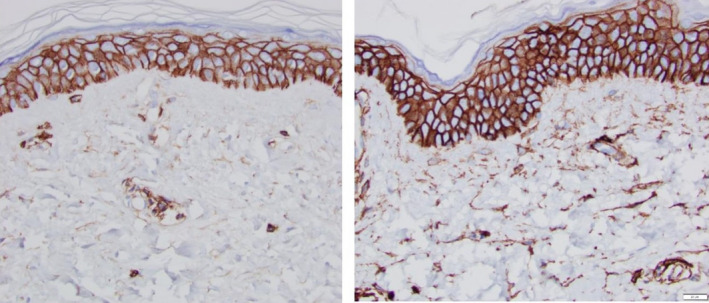
Fifty five‐year‐old female, untreated volar forearm (A), 12 wk post‐Tripeptide/Hexapeptide (TransFORM Body Treatment with TriHex Technology®, Alastin Skincare, Inc) treatment volar forearm (B)
3. DISCUSSION
Elastin regeneration has often been cited as the “holy grail” when considering management or halting of skin aging. In particular, the reversal of solar elastosis is keenly desired and difficult to observe. A great part of that problem revolves around identifying and differentiating new regenerated “good” elastin from that of degenerated “bad” elastin pathognomonic of solar elastosis.
Some topical products have been developed aimed principally at changing the milieu of the ECM and removing the “waste products” of cellular metabolism and aging, creating newly synthesized regenerated structural proteins and ground substance. One such product, containing “TriHex Technology” (Alastin Skincare Inc), has been developed with the aim of recycling the ECM and producing new collagen and elastin. 11 , 12 In vitro tests and clinical studies have validated the changes sought in skin consistency, hydration, healing, and tonicity. At a cellular level, the current standard of histologic stains fails to garner enough information related to the ECM component regeneration and, in particular, differentiating the “good” vs the “bad” elastin elucidated above.
To that end, a combined collection of stains serves the narrative of either solar elastosis or regeneration by painting a more comprehensive picture of molecular events. Thus, solar elastosis can be defined by a thickened dystrophic core protein of elastin—added to that, versican, MMP12, elafin, decreased fibrillin and HA all serve to accentuate the picture (Table 1). Conversely, changes in the levels of these components of the ECM can represent efficacy of a product as described above. The changes described above have been seen to take place as early as 10 days to 2 weeks following topical application of product. 12 , 14 Although these early changes have been documented, the traditional accepted time interval between topical product exposure and histologic changes has been around 12 weeks. This longer time period has the added advantage of seeing the actual gross skin changes from product application which often lags behind the histologic picture that appears to take place earlier.
TABLE 1.
Characteristics of solar elastosis vs healthy skin
| Solar elastosis | Good skin | Details |
|---|---|---|
| Tropoelastin—dystrophic | Thick in reticular dermis, little in papillary dermis | |
| Fibrillin Oxytalan | Fine fibers in upper papillary dermis terminating at DEJ | |
|
Versican MMP 12 |
HA (CD44) | Versican binds fibrillin and HA—broken by MMP12 with photodamage; MMP12 is a good marker of UVA1 exposure. Increased CD44 denotes HA replacement |
| Elafin | Elafin accumulates in degenerated elastic fibers of photodamaged skin; expressed by dermal fibroblasts with UV damage, linked with elastin preventing breakdown, accumulation of poor elastin—solar elastosis | |
| Type V11 Coll | Decreased in keratinocytes UV damage; anchoring fibrils at the dermal‐epidermal junction, secreted from keratinocytes into ECM | |
| Type XVII collagen (COL17) | Transmembranous protein that is mainly expressed in the epidermal basal keratinocytes (not ECM); niche for hair follicle and basal stem cells, to regulate proliferation in the interfollicular epidermis |
In addition to the solar elastotic, elastin formation, other changes within the ECM regarding regenerated collagen are also extremely useful for assessing the regenerative potential of these same products. To that end, Herovici stain has been particularly useful to demonstrate new collagen formation by staining newly laid down mucopolysaccharide components denoting early collagen regeneration (Figure 7). Again, this serves to establish the regenerative changes in the ECM that would be sought with products initiating anti‐aging changes in the skin.
FIGURE 7.
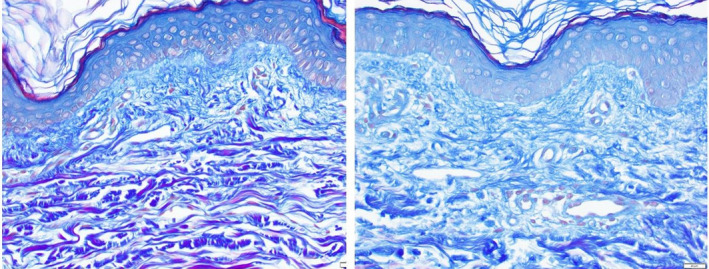
Thirty five‐year‐old female, untreated forearm (A), and 2 wk post‐Tripeptide/Hexapeptide (INhance with TriHex Technology®) treatment (B)—Herovici staining 40X demonstrating increased mucopolysaccharide levels in the papillary dermis. Left panel papillary dermis thickness is 0.0625 mm. Right panel papillary dermis thickness is 0.1 mm (60% increase). Left panel basement membrane zone is somewhat disrupted right panel basement membrane zone is more defined and continuous
4. CONCLUSION
A combination of stains can be used to definitively confirm neoelastogenesis, neocollagenesis, and reversal of solar elastosis. Rather than reliance on a single elastin stain, this combination validates the series of regenerative changes taking place when active topical agents are applied to the skin. In our experience, the ideal combination is Movat, fibrillin, elafin, and versican for elastogenesis and reversal of solar elastosis, CD44 for HA status, and Herovici stain for identifying early collagenesis. Using some or all of these combinations provides a far more comprehensive histologic picture than prior efforts.
Widgerow AD, Napekoski K. New approaches to skin photodamage histology—Differentiating 'good' versus 'bad' Elastin. J Cosmet Dermatol.2021;20:526–531. 10.1111/jocd.13865
Widgerow and Napekoski both authors have contributed to this publication.
Dr Widgerow is Chief Medical Officer for Alastin Skincare, Inc
Funding information
The only funding applied for this study was that required for dermatopathology preparation and analysis of biopsies—which was funded by Alastin Skincare Inc Carlsbad, CA.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen Z, Seo JY, Kim YK, et al. Heat modulation of tropoelastin, fibrillin‐1, and matrix metalloproteinase‐12 in human skin in vivo. J Invest Dermatol. 2005;124(1):70‐78. [DOI] [PubMed] [Google Scholar]
- 2. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241‐251. [DOI] [PubMed] [Google Scholar]
- 3. Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009;31(4):305‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasegawa K, Yoneda M, Kuwabara H, et al. Versican, a major hyaluronan‐binding component in the dermis, loses its hyaluronan‐binding ability in solar elastosis. J Invest Dermatol. 2007;127(7):1657‐1663. [DOI] [PubMed] [Google Scholar]
- 5. Bourguignon LY. Matrix hyaluronan‐activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol. 2014;184(7):1912‐1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayer J, Griffiths CEM. CHAPTER 1. Photoaging in Caucasians In: Griffiths C, Watson R (Eds.). Cutaneous photoaging. Cambridge CB4 0WF, United Kingdom: Royal Society of Chemistry; 2019;1‐30. [Google Scholar]
- 7. Chen Z, Shin MH, Moon YJ, et al. Modulation of elastin exon 26A mRNA and protein expression in human skin in vivo. Exp Dermatol. 2009;18(4):378‐386. [DOI] [PubMed] [Google Scholar]
- 8. Pain S, Berthélémy N, Naudin C, Degrave V, André‐Frei V. Understanding solar skin elastosis‐cause and treatment. J Cosmet Sci. 2018;69(3):175‐185. [PubMed] [Google Scholar]
- 9. Muto J, Kuroda K, Wachi H, Hirose S, Tajima S. Accumulation of elafin in actinic elastosis of sun‐damaged skin: elafin binds to elastin and prevents elastolytic degradation. J Invest Dermatol. 2007;127(6):1358‐1366. [DOI] [PubMed] [Google Scholar]
- 10. Kon A, Takeda H, Ito N, Hanada K, Takagaki K. Tissue‐specific downregulation of type VII collagen gene (COL7A1) transcription in cultured epidermal keratinocytes by ultraviolet A radiation (UVA) and UVA‐inducible cytokines, with special reference to cutaneous photoaging. J Dermatol Sci Suppl. 2005;1(2):S29‐S35. [Google Scholar]
- 11. Widgerow AD, Cohen SR, Fagien S. Preoperative skin conditioning: extracellular matrix clearance and skin bed preparation, a new paradigm. Aesthetic Surg J. 2019;39(Supplement_3):S103‐S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Widgerow AD, Fabi SG, Palestine RF, et al. Extracellular matrix modulation: optimizing skin care and rejuvenation procedures. J Drugs Dermatol. 2016;15(4s):S63‐S71. [PubMed] [Google Scholar]
- 13. Doello K. A new pentachrome method for the simultaneous staining of collagen and sulfated mucopolysaccharides. Yale J Biol Med. 2014;87:341‐347. [PMC free article] [PubMed] [Google Scholar]
- 14. Widgerow AD, Jacob C, Palm MD, Garruto JA, Bell M. Developing a topical adjunct to injectable procedures. J Drugs Dermatol. 2020;19(4):398‐404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


