Abstract
Objective
The burden of underweight remains a major problem in Indonesia, and at the same time, the prevalence of overweight is increasing. Malnutrition is a major determinant of health and has been linked to allergic disorders in children. We examined the relationship between malnutrition and TH2 immune markers in school‐aged children in Makassar, Indonesia.
Methods
A cross‐sectional study was performed in five schools where socio‐demographic characteristics were recorded. Children’s standardised z‐scores of body mass index (z‐BMI) and age‐standardised z‐scores of height (z‐HA) were assessed using WHO child growth standards. Skin prick test (SPT) reactivity was determined to house dust mite allergens. Helminth infection status, (growth) hormones including insulin‐like growth factor (IGF‐1) and TH2 immune markers were measured.
Results
In total, 954 children were included of whom 21.6% were underweight and 14.8% overweight. After controlling for confounders, overweight was positively associated with leptin (GMR 3.55, 95% CI: 2.99–4.23) and IGF‐1 (GMR 1.45, 95% CI: 1.15–1.82), whereas underweight was negatively associated (respectively GMR 0.57, 95% CI: 0.49–0.66 and GMR 0.78, 95% CI: 0.63–0.97). Underweight was associated with a lower eosinophil count (GMR 0.79, 95% CI: 0.64–0.97) but not with total IgE levels or SPT reactivity. Overweight was positively associated with SPT reactivity (adjusted OR 2.68, 95% CI: 1.50–4.78) but no relationship was found with the other TH2 immune markers.
Conclusion
Malnutrition is prominent in school‐aged children in Makassar, with overweight associated with increased SPT reactivity. Therefore, interventions should focus on undernutrition, but also on overweight to prevent the increase of allergic disorders in Indonesia.
Keywords: malnutrition, skin prick test, TH2 immune response, body mass index
Abstract
Objectif
La charge de l'insuffisance pondérale reste un problème majeur en Indonésie et parallèlement, la prévalence du surpoids augmente. La malnutrition est un déterminant majeur de la santé et a été associée à des troubles allergiques chez les enfants. Nous avons examiné la relation entre la malnutrition et les marqueurs immunitaires TH2 chez les enfants d'âge scolaire à Makassar, en Indonésie.
Méthodes
Etude transversale dans cinq écoles où les caractéristiques sociodémographiques ont été enregistrées. Les scores z standardisés de l'indice de masse corporelle (z‐IMC) et les scores z standardisés pour l'âge de la taille (z‐HA) pour les enfants ont été évalués en utilisant les normes de croissance de l'enfant de l'OMS. La réactivité du test cutané (SPT) a été déterminée pour les allergènes d'acariens. L'état de l'infection par les helminthes, les hormones (de croissance), y compris le facteur de croissance analogue à l'insuline (IGF‐1) et les marqueurs immunitaires TH2 ont été mesurés.
Résultats
Au total, 954 enfants ont été inclus, dont 21,6% en insuffisance pondérale et 14,8% en surpoids. Après contrôle des facteurs de confusion, le surpoids était positivement associé à la leptine (GMR 3,55, IC95%: 2,99–4,23) et à l'IGF‐1 (GMR 1,45 ; IC95%: 1,15–1,82), tandis que l'insuffisance pondérale était associée négativement (respectivement GMR 0,57 ; IC95%: 0,49–0,66 et GMR 0,78 ; IC95%: 0,63–0,97). L'insuffisance pondérale était associée à un nombre plus faible d'éosinophiles (GMR 0,79 ; IC95%: 0,64–0,97) mais pas aux taux d'IgE totaux ou à la réactivité du SPT. Le surpoids était positivement associé à la réactivité du SPT (OR ajusté 2,68 ; IC95%: 1,50–4,78) mais aucune relation n'a été trouvée avec les autres marqueurs immunitaires T H 2.
Conclusion
La malnutrition est importante chez les enfants d'âge scolaire à Makassar, avec un surpoids associé à une réactivité accrue du SPT. Par conséquent, les interventions devraient se concentrer sur la dénutrition, mais aussi sur le surpoids pour prévenir l'augmentation des troubles allergiques en Indonésie.
Introduction
Despite great efforts to reduce undernutrition, underweight remains a major problem in Indonesia and almost 10% of school‐aged children remain underweight. Meanwhile, the nutritional transition in Indonesia has triggered a rapid increase in the prevalence of overweight and obesity, as illustrated by the increase in frequency of overweight in children aged 6–13 years from 5.1% in 1993 to 15.6% in 2014 [1]. Both overweight and underweight are forms of malnutrition, a condition that can negatively affect health and cause a great health burden [2]. In parallel to the nutritional transition, allergic disorders are also on the rise in Indonesia, leading to an increase in the prevalence of allergic rhinitis from 5% to 23% per cent in Indonesia [3].
An association between overweight/obesity and the prevalence of allergic disorders has been documented in children [4, 5, 6, 7]. Children with an elevated body mass index (BMI) are at increased risk for wheeze and asthma and a positive association has been observed between BMI and the prevalence of allergic rhinitis and atopic dermatitis [4, 5, 6, 7].
Allergic disorders are mediated by TH2 response characterised by elevated IgE and eosinophils. The TH2 response is thought to have evolved to eliminate extracellular parasites like helminths, but can also be misdirected towards harmless allergens in atopic individuals [8]. Malnutrition influences the functioning of the immune response, including total IgE levels, and might thereby affect existing allergic predispositions and the development allergic disorders [9, 10].
An important factor that affects both nutritional status and allergic disorders is socio‐economic status (SES) [11, 12]. In Indonesian low SES urban and rural populations, infections with helminths are still prevalent [11, 13]. These infections can interfere with child growth and affect nutritional status [14]. Although helminths are known to skew immune responses towards the TH2 response, they are also able to induce strong immunoregulatory responses and thereby suppress allergic disorders [15]. As a result, helminth infections are associated with reduced skin reactivity [16].
In the present study, we aimed to assess the degree of malnutrition in schoolchildren from different socio‐economic backgrounds in Makassar, Indonesia, and explore the relationship between malnutrition and TH2‐mediated responses.
Methods
Study population and design
This cross‐sectional study comprised children attending five primary schools in the urban centre of Makassar, the capital city of South Sulawesi, Indonesia. The data were obtained from three studies with similar study design and research aim, namely exploring differences in allergy, helminths and TH2‐related parameters between high and low SES (see flow chart in Figure S1). These studies were conducted from September 2017 until October 2019 after approval from the Health Research Ethical Committee, Faculty of Medicine, Hasanuddin University. Schools were selected on the basis of the socio‐economic background of its pupils. The primary school SD Mangkura is a public school that is mostly attended by children of high SES background due to its great reputation and will henceforth be referred to as ‘High SES A’. The private school SD Athirah has extensive facilities and is only attended by children from high SES families and will therefore be referred to as ‘High SES B’. The three other primary schools are public schools with limited facilities that are solely attended by children of low SES. Based on alphabetical order, those are referred as ‘Low SES A’ (SD Baraya), ‘Low SES B’ (SD Cambaya) and ‘Low SES C’ (SD Maccini Sombala). Within every school, the grades that could participate were selected based on the age of the children. Within these selected grades, all children were invited to participate in the study. Parents were informed about the study and written consent was obtained for their child to participate in the study prior to sample collection. All children with parental/guardian consent were included in the study. Children who had taken antihistamines or corticosteroids in the week before the study began were excluded.
Questionnaire and measurements
Information regarding demographics and indicators of socio‐economic status was obtained using a questionnaire. Parental educational level was categorised as ‘high’ if a parent had attended an academy or university and ‘low’ if the parent was illiterate or had only completed elementary school or high school. Body weight and height were measured using a Seca scale and stadiometer (Seca GmbH & Co KG, Hamburg, Germany). These were used to calculate the age‐standardised z‐scores of body mass index (z‐BMI) and age‐standardised z‐scores of height (z‐HA), according to WHO guidelines [17]. A high z‐BMI score indicates overweight or obesity and a low z‐BMI score reflects wasting, thinness and underweight [17, 18]. For the purpose of this study, the categorisation of BMI‐for‐age was simplified by classifying all children with a z‐score above 1 as ‘overweight’, all children with a z‐score between 1 and −2 as ‘normal weight’ and all children with z‐scores lower than −2 as ‘underweight’. In this study, a z‐HA score below −2 reflects stunted growth and below −3 severely stunted growth, according to the recommendations of the WHO [17].
Skin prick testing
To determine skin reactivity to common aeroallergens, a skin prick test (SPT) was performed. SPT reactivity to house dust mite (HDM) was tested by using two extracts: Dermatopagoides pteronyssinus and D. farinae (ALK‐Abello BV, Almere, the Netherlands). Saline was used as a negative control, and histamine chloride (10 mg/ml) was the positive control. The SPT was performed on the volar side of the child’s lower arm using skin prick lancets. Fifteen minutes after application, the wheal sizes were measured. Skin test reactivity was considered positive if the longest diameter plus the diameter perpendicular of wheal size divided by two was 3 mm or larger. Only subjects with skin reactivity positive to histamine were included in the analysis.
Leptin, IGF‐1 and total IgE levels and eosinophil count
Blood samples were collected from children’s fingertips using Microvette® CB 300 tubes (Sarstedt Inc, Nümbrecht, Germany) or by venepuncture using BD vacutainer® blood collection tubes (Becton Dickinson and Company, Franklin Lakes, NJ, USA). Total IgE and leptin levels were measured by ELISA as previously described [19]. The levels of IGF‐1 were quantified using Human IGF‐1 Duo Set (R&D System, Abingdon, UK), according to the manufacturer’s guidelines. The results were expressed in International Units (IU/ml), ng/ml and pg/ml for total IgE, leptin and IGF‐1, respectively. A Giemsa‐stained peripheral thin blood smear was read in the Department of Clinical Pathology, Hasanuddin University, Makassar, to assess differential white blood cell counts, resulting in a relative percentage of eosinophils.
Parasitological examination
All children were asked to fill a stool container (Sarstedt Inc, Nümbrecht, Germany) by using an enclosed plastic spatula without contamination with water or urine. Eggs of intestinal helminths such as Ascaris lumbricoides, Trichuris trichiura and hookworm were quantified using Kato‐Katz methods.
Statistical analysis and conceptual framework
Figure 1 shows the conceptual framework for this study. We hypothesised that socio‐economic status and helminth infection status may be important confounding factors for the relationship between nutritional status and TH2 immune markers. Age and sex were considered as a priori confounders. Descriptive statistics were used to examine the characteristics of the whole study population and stratified based on z‐BMI. To obtain approximately normally distributed data, leptin, IGF‐1, total IgE and eosinophil count were log10‐transformed. To examine the association between under‐ and overweight and continuous outcome variables, linear regression models were fitted. The association between z‐BMI and SPT was examined using logistic regression models. Confounding by helminth infection and socio‐economic status were examined and the models were adjusted accordingly. Statistical testing was performed using F‐tests for the linear regression models and likelihood ratio tests for the logistic regression models. All data were analysed using IBM Statistical Package for the Social Sciences Statistics version 24 (IBM‐SPSS Inc., Chicago, IL, USA), and the visualisation was performed using RStudio.
Figure 1.
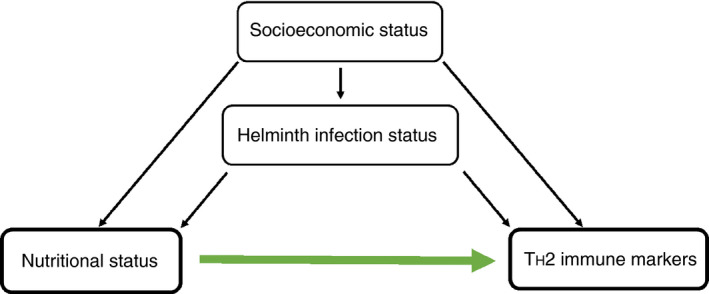
Conceptual framework of the study. Relationship between nutritional status and TH2 immune markers, confounded by an individual’s socio‐economic status (SES) and helminth infection status. [Colour figure can be viewed at wileyonlinelibrary.com]
Results
Characteristics of study participants
A total of 996 school‐aged children were included from five different schools (Table 1). Of these, 260 children attended high SES schools (high SES A: n = 160, high SES B: n = 100) and 736 children attended low SES schools (low SES A: n = 260, low SES B: n = 165 and low SES C = 311). The overall helminth infection prevalence was 25.2% and 12.5% of the children were SPT‐positive for HDM allergens.
Table 1.
Characteristics of children attending primary school in Makassar, Indonesia (N = 996)
|
Total population N = 996 |
|
|---|---|
| Age (in years, mean, SD) | 9.34 ± 1.51 |
| Sex (female %, n/N) | 52.5 (521/993) |
| School (low SES %, n/N) | |
| Low SES A | 26.1 (260/996) |
| Low SES B | 31.2 (165/996) |
| Low SES C | 16.6 (311/996) |
| High SES A | 16.1 (160/996) |
| High SES B | 10.0 (100/996) |
| Helminth infection by microscope (%, n/N) | |
| Any helminth infection | 25.2 (196/773) |
| Only A. lumbricoides | 10.6 (82/773) |
| Only T. trichiura | 7.2 (56/773) |
| Hookworm | 0.0 (0/773) |
| Both A. lumbricoides and T. trichiura | 7.6 (59/773) |
| Education father (high %, n/N) | 23.6 (172/730) |
| Maternal education (high %, n/N) | 20.2 (150/742) |
| Floor material (ceramics %, n/N) | 50.8 (397/782) |
| Wall material (brick or concrete %, n/N) | 57.0 (441/774) |
| Toilet (private inside %, n/N) | 90.1 (702/779) |
| Leptin (ng/m) [geometric mean (95% CI)] | 2.77 (2.58; 2.97) |
| IGF‐1 (pg/ml) [geometric mean (95% CI)] | 0.67 (0.61; 0.74) |
| Eosinophil count (% of total) [geometric mean (95% CI)] | 3.09 (3.78; 4.44) |
| Total IgE (IU/ml) [geometric mean (95% CI)] | 324.0 (297.0; 353.5) |
| Atopy by skin prick reactivity (%, n/N) | |
| Any skin prick reactivity | 12.5 (112/895) |
| Only D. pteronyssinus | 2.2 (20/895) |
| Only D. farinae | 3.8 (34/895) |
| SPT reactive for both D. pteronyssinus and D. farinae | 6.3 (56/895) |
CI, confidence interval; SD, standard deviation; SES, socio‐economic status.
The number of positives (n) of the total number (N).
Distribution of z‐BMI and z‐HA
Information regarding the z‐BMI was available for 954 children. Although the majority of the children had a normal weight (63.6%), a wide range of z‐BMI scores was observed (−7.88 to +4.50). When z‐BMI is plotted based on school, a distinct distribution is shown. The pattern of z‐BMI in children from high SES schools was more towards overweight while the distribution towards underweight was shown in children of low SES schools. With regard to low SES schools, a wider pattern towards underweight was observed in children of low SES A compared to the other 2 low SES schools which presented quite a similar pattern (Figure 2a).
Figure 2.
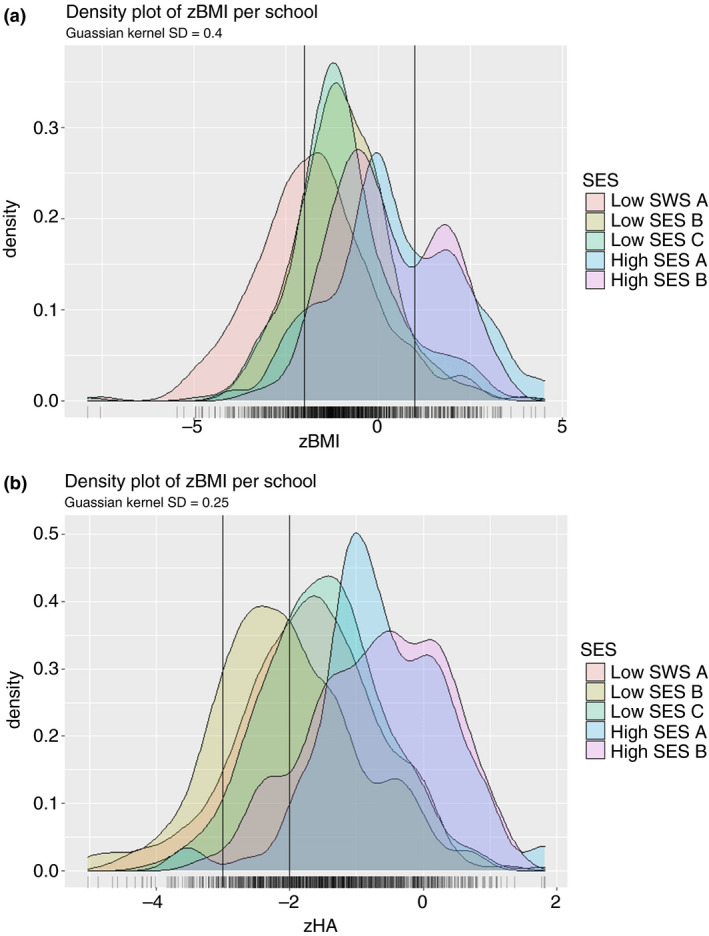
Distribution of z‐BMI (a) and z‐HA (b) depicted in a density plot per school. (a) The leftmost vertical line is at a z‐BMI score of −2, indicating cut‐off between underweight and normal weight and the rightmost vertical line is at a score of +1, indicating the cut‐off between normal weight and overweight. Gaussian Kernel SD = 0.4. (b) The leftmost vertical line is at a z‐HA score of −3, indicating the cut‐off between severely stunted and stunted. The rightmost vertical line is at a z‐score of −2, indicating the cut‐off between stunted and normal height. Gaussian Kernel SD = 0.25. SD: standard deviation. z‐BMI: z‐score of body mass index for age. z‐HA: z‐score of height for age. [Colour figure can be viewed at wileyonlinelibrary.com]
The distribution of z‐HA ranged from −5.02 to +1.81. While the majority of children were of an age‐appropriate length (70.0%), 23.0% of the children were stunted and 7.0% were severely stunted. When type of school is considered, we observed that most children of high SES schools had an age‐appropriate length (88.8% high SES A and 95.5% high SES B). By contrast, the z‐HA pattern in low SES schools tended towards stunted/severely stunted with a proportion of 37.1%, 28.4% and 45.2% of the children attending low SES school A, B and C, respectively (Figure 2b).
Next, the 954 children were categorised based on their z‐BMI, creating three categories: underweight, normal weight and overweight. Children were slightly older in the overweight group, whereas the sex distribution was similar (Table 2). Almost all children with underweight were in low SES schools (93.2%) while more than half of the children with overweight were in high SES schools (61.7%). Fewer helminth infections were detected in children with overweight (12.5%) than in those with underweight (22.6%) or normal weight (28.8%). Children in the overweight group appeared to have more highly educated parents and better housing conditions than children with normal or underweight.
Table 2.
Socio‐demographic characteristics by z‐BMI category of children attending primary school in Makassar, Indonesia (N = 954)
|
Underweight N = 206 |
Normal weight N = 607 |
Overweight N = 141 |
|
|---|---|---|---|
| Age (in years, mean, SD) | 9.19 (1.60) | 9.34 (1.51) | 9.68 (1.36) |
| Sex (female %, n/N) | 46.6 (96/206) | 55.7 (338/269) | 49.6 (70/141) |
| School (High SES %, n/N) | |||
| Low SES A | 51.0 (105/206) | 19.3 (117/607) | 9.2 (13/141) |
| Low SES B | 14.6 (30/206) | 20.8 (126/607) | 6.4 (9/141) |
| Low SES C | 27.7 (57/206) | 35.7 (217/607) | 22.7 (32/141) |
| High SES A | 3.9 (8/206) | 7.7 (47/607) | 23.4 (33/141) |
| High SES B | 2.9 (6/206) | 16.5 (100/607) | 38.3 (54/141) |
| Helminths (infected %, n/N) | 22.6 (36/159) | 28.8 (144/500) | 12.5 (13/104) |
| Education father (high %, n/N) | 13.0 (21/162) | 22.8 (104/457) | 42.0 (42/100) |
| Maternal education (high %, n/N) | 11.0 (18/164) | 18.6 (87/467) | 40.4 (40/99) |
| Floor material (ceramics %, n/N) | 44.7 (76/170) | 47.5 (231/486) | 72.2 (78/108) |
| Wall material (brick or concrete %, n/N) | 51.8 (87/168) | 55.5 (267/481) | 70.1 (75/107) |
| Toilet (private inside %, n/N) | 87.8 (151/172) | 89.6 (432/482) | 97.2 (105/108) |
SD, standard deviation; z‐BMI, z score of body mass index for age.
Underweight is a z‐BMI score < −2, normal weight is a z‐BMI > 1 and >−2, and overweight/obesity is a z‐BMI > 1. The number of positives (n) of the total number within that z‐BMI category (N).
Association between z‐BMI and IGF‐1 and leptin
A strong association was observed between leptin and IGF‐1 levels with the z‐BMI (Table 3). Leptin levels showed an increasing gradient across the z‐BMI with the highest detected levels in the overweight group (GM 11.49, 95% CI: 10.27–12.84). After adjustment for confounders, overweight remained positively associated with leptin (adjusted GMR 3.55, 95% CI: 2.99–4.23), while for underweight, a negative association was found (adjusted GMR 0.57, 95% CI: 0.49–0.66).
Table 3.
Association between underweight or overweight and the level of (growth) hormones and TH2 immune markers
| (Growth) hormones | Crude1 | Adjusted for SES and helminth2 | ||||
|---|---|---|---|---|---|---|
| n/N † | Geometric mean (95% CI) | Geometric mean ratio (95% CI) | Overall P‐value | Geometric mean ratio (95% CI) | P‐value | |
| Leptin | ||||||
| Underweight | 180/877 | 1.29 (1.14; 1.45) | 0.54 (0.47–0.61) | <0.001 | 0.57 (0.49–0.66) | <0.001 |
| Normal weight | 571/877 | 2.56 (2.37; 2.76) | Reference | Reference | ||
| Overweight | 126/877 | 11.49 (10.27; 12.84) | 4.38 (3.76– 5.11) | 3.55 (2.99–4.23) | ||
| IGF‐13 | ||||||
| Underweight | 91/620 | 0.38 (0.31; 0.46) | 0.57 (0.45–0.73) | <0.001 | 0.78 (0.63–0.97) | <0.001 |
| Normal weight | 437/620 | 0.65 (0.58; 0.72) | Reference | Reference | ||
| Overweight | 92/620 | 1.42 (1.10; 1.84) | 1.91 (1.50–2.44) | 1.45 (1.15–1.82) | ||
| TH2 immune markers | Crude1 | Adjusted for SES and helminth2 | ||||
|---|---|---|---|---|---|---|
| n/N † | Geometric mean (95% CI) | Geometric mean ratio (95% CI) | P‐value | Geometric mean ratio (95% CI) | P‐value | |
| Eosinophil count4 | ||||||
| Underweight | 143/532 | 2.20 (1.73–2.76) | 0.71 (0.59; 0.86) | <0.001 | 0.79 (0.64–0.97) | 0.04 |
| Normal weight | 327/532 | 3.41 (2.97–3.91) | Reference | Reference | ||
| Overweight | 62/532 | 4.13 (3.06–5.58) | 1.16 (0.89; 1.50) | 1.10 (0.83–1.47) | ||
| Total IgE | ||||||
| Underweight | 180/878 | 383.4 (314.6; 467.1) | 1.12 (0.90; 1.40) | 0.02 | 0.94 (0.73–1.20) | 0.86 |
| Normal weight | 572/878 | 334.0 (299.3; 372.6) | Reference | Reference | ||
| Overweight | 126/878 | 237.2 (189.5; 296.9) | 0.73 (0.56; 0.94) | 0.98 (0.73–1.32) | ||
| n/N † | % (n/N)‡ | Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
|---|---|---|---|---|---|---|
| Skin prick test (positive) | ||||||
| Underweight | 180/884 | 10.0 (18/180) | 0.94 (0.54–1.65) | <0.001 | 1.57 (0.81–3.07) | 0.004 |
| Normal weight | 575/884 | 10.1 (58/575) | Reference | Reference | ||
| Overweight | 129/884 | 27.1 (35/129) | 3.33 (2.06–5.38) | 2.68 (1.50–4.78) | ||
CI, confidence interval.
1. Adjusted for sex and age (in years). 2. Adjusted for sex, age (in years) and SES as categorical variable and helminth infection status. 3. Data only available for Low SES B, C and High SES A. 4. Data only available for Low SES A, C and High SES B. Leptin is in ng/ml IGF‐1 in pg/ml, eosinophil count in percentage of the white blood cell count and total IgE levels in IU/ml.
The number of children in each z‐BMI category (n) of the total children measured (N).
The number of children with SPT positivity (n) of the number of children within each z‐BMI category.
A similar pattern was seen for IGF‐1 levels, with the lowest IGF‐1 levels in the underweight (GM 0.38, 95% CI: 0.31–0.46) and the highest in the overweight group (GM 1.42, 95% CI: 1.10–1.48). Upon considering confounders, overweight was still positively associated (adjusted GMR 1.45, 95% CI: 1.15–1.82) and underweight was negatively associated with IGF‐1 (adjusted GMR 0.78, 95% CI: 0.63–0.97).
Association between z‐BMI and TH2 immune markers
Children with underweight exhibited a lower eosinophil count (GM 2.20, 95% CI: 1.73–2.76) than normal weight and overweight children (respectively GM 3.41, 95% CI: 2.79–3.91 and GM 4.13, 95% CI: 3.06–5.58). Even in the adjusted model, there was still a significant inverse association between underweight and eosinophil count (adjusted GMR 0.79, 95% CI: 0.64–0.97, overall P‐value = 0.04).
The lowest total IgE levels were seen in overweight children (GM 237.2, 95% CI: 189.5–296.9), which were lower than in the normal weight group and overweight group (respectively, GM 334.0, 95% CI: 299.3–372.6 and GM 383.4, 95% CI: 314.6–467.1). After controlling for confounding, total IgE levels were not associated with overweight (adjusted GMR 0.98, 95% CI: 0.73–1.32) or underweight (adjusted GMR 0.94, 95% CI: 0.73–1.20).
The proportion of SPT reactivity was equal in the underweight and normal weight group (respectively 10.0% and 10.1%), but larger in the overweight group (27.1%). After adjustment for SES and helminth infection status, overweight children evidently had higher odds of SPT reactivity than normal weight children (adjusted OR 2.68, 95% CI: 1.50–4.78), whereas underweight children did not (adjusted OR 1.57, 95% CI: 0.81–3.07).
Discussion
This study aimed to assess the degree of malnutrition and to delineate the effect of z‐BMI on TH2 immune markers in Indonesian children attending high and low SES schools in an urban area. The results show that both underweight and overweight were highly prevalent in these children, with the majority of underweight children attending low SES schools and the majority of overweight children attending high SES schools. Underweight and overweight were, respectively, associated with lower and higher leptin and IGF‐1 levels. In addition, underweight was associated with lower eosinophil count but no association was found with total IgE levels or SPT reactivity. Overweight was positively associated with SPT reactivity, but no relationship was found with other TH2 immune markers.
The high proportion of children with either underweight or overweight in our study illustrates the double burden of malnutrition (DBM) on the population level that Indonesia is currently facing. The double burden of over‐ and undernutrition on a population level can be found in almost all low‐ and middle‐income countries, including many in South‐East Asia [19]. Despite the rapid increase in the prevalence of overweight and obesity, especially in urban populations, reducing underweight is still prioritised by many countries, including Indonesia. Traditionally, Indonesian health policies solely focused on undernutrition, but to tackle the DBM in Indonesia, interventions are needed that address both underweight and overweight as a nutritional problem [20].
The differences between the distribution of the z‐BMI and z‐HA in this study highlight the dissimilarity between malnutrition and stunted growth. In the current study, the lowest z‐scores for both z‐BMI and the z‐HA were seen in low SES schools and highest in the high SES schools. However, most children with underweight were observed in Low SES A, whereas the stuntedness was most prevalent in Low SES B. The differences between the malnutrition and stuntedness in children have also been reported before [17, 21, 22]. As described by the WHO, a low height for age indicates impaired growth (stunting), likely resulting from long‐term nutritional deprivation that can already start in utero, whereas a low weight‐for‐age or BMI‐for‐age implies underweight or wasting, which often due to a recent and severe process of weight loss due to acute undernutrition or severe pathology [17, 22]. A recent study by Scheffler et al. [22] in Indonesian school‐aged children supports the findings of the current study, by showing that malnutrition and growth indicators did not correlate. Although stunting and malnutrition are often used as synonyms, the results of our study suggest that these might be different concepts.
In the current study, a strong association between z‐BMI and (growth) hormones was found, which is in line with previous literature [23, 24, 25]. Leptin is secreted in response to increasing levels of adiposity and elevated leptin levels are seen in overweight and obese children compared to normal weight children [25]. The levels of IGF‐1 are also related to an individual’s nutritional status, since the levels are regulated within the GH/IGF‐1 axis that is highly sensitive to alternations in the nutritional state. Caloric restriction for six days already resulted in significantly lower IGF‐1 levels in children and a positive association was found between a child’s weight and overall IGF‐1 levels [23, 24]. Therefore, our results are in line with the notion that these (growth) hormones might serve as markers for nutritional status in children.
A low z‐BMI was associated with a reduced eosinophil count, but underweight did not appear to affect the odds of SPT reactivity or the total IgE levels. These results are not in line with the study of Mutius et al.,[6] which found no association between eosinophil count and BMI, probably due to the limited number of children with underweight in this study. The absence of an association between underweight and other SPT reactivity in the current study might be related to the severity of underweight. An earlier study by Tanaka et al. [26] showed that the association of asthma prevalence and BMI follows a U‐shaped curve, indicating increased risk of asthma in children with either severe overweight or severe underweight. Only few children with very low z‐BMI values were seen in our study population. Therefore, the results of this study suggest that moderate/light underweight has a limited effect on allergic sensitisation.
Overweight in school‐aged children was positively associated with SPT reactivity, but no association was found with other TH2 immune markers such as total IgE and eosinophilia. Visness et al. [27] showed that obese and overweight children had elevated total and allergen‐specific IgE levels compared to normal weight children, but these associations were only observed in response to food allergens and were solely found in (severely) obese children. In addition, Cibella et al. [7] showed that overweight and obesity are associated with asthma and allergic sensitisation, but that eosinophilic airway inflammation is not involved. Finally, the TH2 immune markers measured in this study, eosinophil count and total IgE levels, are general markers of this response, meaning that involvement of specific elements of the TH2 response cannot be ruled out.
Possible limitations of these studies include the inability to assign causality to the associations and the introduction of bias by missing data due to the self‐administration of questionnaires and limited quantity of samples. Another concern is that the SES was not measured objectively by using standard tools; however, this study includes children with diverse socio‐economic backgrounds within one urban centre making these results generalisable to the urban school‐aged children in Indonesia at large. Finally, we were not able to assess the nutritional status, since only anthropometric measurements were made. Future studies into the correlation between nutritional status and TH2 immune markers should therefore make use of biochemical/biophysical, clinical and dietary methods in addition to anthropometry measurements to properly assess the nutritional status and study its effect on TH2 immune markers.
Conclusion
Both underweight and overweight are prominent in school‐aged children in Makassar, Indonesia, and overweight is positively associated with allergic sensitisation. These findings highlight the need of interventions to tackle not only underweight, but also the problem of overweight to prevent the increase of allergic disorders in Indonesia.
Supporting information
Figure S1. Flow chart of the study.
Acknowledgements
The authors would like to thank all participants involved in this study as well as all students of the LUMC and Hasanuddin University for their efforts on sample collection in participating primary schools in Makassar. We thank Hasanuddin University Medical Research Center (HUM‐RC) for laboratory facilities during sample collections and Hasanuddin University for the support. The study was funded by Leiden University Medical Center, The Netherlands and the Ministry of Research, Technology and Higher Education, Republic of Indonesia (Kemenristekdikti).
Sustainable Development Goals (SDGs): 2.1, 2.2
References
- 1. Oddo VM, Maehara M, Rah JH. Overweight in Indonesia: an observational study of trends and risk factors among adults and children. BMJ Open 2019: 9: e031198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO – Malnutrition. 2018. (Available from: www.who.int). Accessed September 14, 2020.
- 3. Soegiarto G, Abdullah MS, Damayanti LA, Suseno A, Effendi C. The prevalence of allergic diseases in school children of metropolitan city in Indonesia shows a similar pattern to that of developed countries. Asia Pac Allergy 2019: 9: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao TC, Ou LS, Yeh KW, Lee WI, Chen LC, Huang JL. Associations of age, gender, and BMI with prevalence of allergic diseases in children: PATCH study. J Asthma 2011: 48: 503–510. [DOI] [PubMed] [Google Scholar]
- 5. Visness CM, London SJ, Daniels JL et al Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010: 47: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax 2001: 56: 835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross‐sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol 2011: 107: 330–336. [DOI] [PubMed] [Google Scholar]
- 8. Artis D, Maizels RM, Finkelman FD. Allergy challenged. Nature 2012: 484: 458–459. [DOI] [PubMed] [Google Scholar]
- 9. Bartz S, Mody A, Hornik C et al Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab 2014: 99: 2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morishita R, Franco MC, Suano‐Souza FI, Solé D, Puccini RF, Strufaldi MWL. Body mass index, adipokines and insulin resistance in asthmatic children and adolescents. J Asthma 2016: 53: 478–484. [DOI] [PubMed] [Google Scholar]
- 11. Hamid F, Wahyuni S, van Leeuwen A, van Ree R, Yazdanbakhsh M, Sartono E. Allergic disorders and socio‐economic status: a study of schoolchildren in an urban area of Makassar, Indonesia. Clin Exp Allergy 2015: 45: 1226–1236. [DOI] [PubMed] [Google Scholar]
- 12. Syahrul S, Kimura R, Tsuda A, Susanto T, Saito R, Ahmad F. Prevalence of underweight and overweight among school‐aged children and it’s association with children’s sociodemographic and lifestyle in Indonesia. Int J Nurs Sci 2016: 3: 169–177. [Google Scholar]
- 13. World Health Organization . Global Health Observatory (GHO) Data Soil Transmitted Helminthiases. WHO: Geneva, 2017. [Google Scholar]
- 14. Sanchez AL, Gabrie JA, Usuanlele MT, Rueda MM, Canales M, Gyorkos TW. Soil‐transmitted helminth infections and nutritional status in school‐age children from rural communities in honduras. PLoS Negl Trop Dis 2013: 7: e2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Den Biggelaar AHJ, Van Ree R, Rodrigues LC et al Decreased atopy in children infected with Schistosoma haematobium: a role for parasite‐induced interleukin‐10. Lancet 2000: 356: 1723–1727. [DOI] [PubMed] [Google Scholar]
- 16. Van Den Biggelaar AHJ, Lopuhaa C, Van Ree R et al The prevalence of parasite infestation and house dust mite sensitization in gabonese schoolchildren. Int Arch Allergy Immunol 2001: 126: 231–238. [DOI] [PubMed] [Google Scholar]
- 17. de Onis M, Onyango AW, Borghi E et al Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ 2007: 85: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC) . About Child & Teen BMI. Heal Weight. 2015.
- 19. Abdullah A. The double burden of undernutrition and overnutrition in developing countries: an update. Curr Obes Rep 2015: 4: 337–349. [DOI] [PubMed] [Google Scholar]
- 20. Shripton R, Rokx C. The Double Burden of Malnutrition in Indonesia. World Bank Group: Jakarta, 2013. Accessed September 14, 2020. [Google Scholar]
- 21. De Onis M, Blössner M. WHO Global Database on Child Growth and Malnutrition. Program Nutr World Heal Organ: Geneva, 1997. Accessed September 14, 2020. [Google Scholar]
- 22. Scheffler C, Hermanussen M, Bogin B et al Stunting is not a synonym of malnutrition. Eur J Clin Nutr 2019: 74: 527–528. [DOI] [PubMed] [Google Scholar]
- 23. Hosick PA, McMurray RG, Hackney AC, Battaglini CL, Combs TP, Harrell JS. Differences in the GH‐IGF‐I axis in children of different weight and fitness status. Growth Horm IGF Res 2012: 22: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin‐like growth factor‐I (IGF‐I) and IGF‐binding proteins in children and adults. J Clin Endocrinol Metab 1995: 80: 443–449. [DOI] [PubMed] [Google Scholar]
- 25. Ellis KJ, Nicolson M. Leptin levels and body fatness in children: effects of gender, ethnicity, and sexual development. Pediatr Res 1997; 42: 484–488. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka K, Miyake Y, Arakawa M, Sasaki S, Ohya Y. U‐shaped association between body mass index and the prevalence of wheeze and asthma, but not eczema or rhinoconjunctivitis: the Ryukyus Child Health Study. J Asthma 2011: 48: 804–810. [DOI] [PubMed] [Google Scholar]
- 27. Visness CM, London SJ, Daniels JL et al Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 2009: 123: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the study.


