Abstract
Biomedical application of graphene derivatives have been intensively studied in last decade. With the exceptional structural, thermal, electrical, and mechanical properties, these materials have attracted immense attention of biomedical scientists to utilize graphene derivatives in biomedical devices to improve their performance or to achieve desired functions. Surfaces of graphene derivatives including graphite, graphene, graphene oxide and reduce graphene oxide have been demonstrated to pave an excellent platform for antimicrobial behavior, enhanced biocompatibility, tissue engineering, biosensors and drug delivery. This review focuses on the recent advancement in the research of biomedical devices with the coatings or highly structured polymer nanocomposite surfaces of graphene derivatives for antimicrobial activity and sterile surfaces comprising an entirely new class of antibacterial materials. Overall, we aim to highlight on the potential of these materials, current understanding and knowledge gap in the antimicrobial behavior and biocompatibility to be utilized of their coatings to prevent the cross infections.
Keywords: antimicrobial, biocompatibility, biofilms, biomedical devices, graphene derivatives
Recent advances in the research of biomedical devices with coatings or highly structured polymer nanocomposites composed of graphene derivatives for self‐cleaning surfaces are reviewed. Overall, the authors aim to highlight the potential of these materials as well as the current understanding and knowledge gaps regarding their antimicrobial behavior and biocompatibility for use as biomedical coatings to prevent cross infections.
1. Introduction
Nosocomial infection, which refers to healthcare associated infections (HAI) stands as a one of the major global healthcare concerns since it comprises ∼10 % of all admitted patients with a ∼1 % mortality rate and a ∼3 % mortality contribution to other diseases, amounting to millions of unnecessary deaths worldwide. [1] The Center for Disease Control (USA) reported nearly 2 million of HAI cases and out of that 50–70 % can be attributed to indwelling medical devices. [2] Attributable mortality is highly device‐dependent but can range from <5 % for dental implants and Foley catheters and up to >25 % for mechanical heart valves. [3] The use of biomedical devices such as heart valves, endovascular stents, catheters, joint prostheses, implantable meshes, artificial lenses or cochlear implants, ventricular assist devices, artificial hearts, and deep brain stimulators are being widely used to improve the body functions in case of certain abnormalities. Such devices or materials in human body are always in the risk of microbial colonization and later cause infections, which is commonly known as device associated infections. The use of topical or systemic antibiotics is major treatment strategy for such infections. To address this issue many preventive measures has been explored by scientists using biologically inspired agents such as antimicrobial peptides, bacteriophages, immunomodulators, quorum sensing inhibitors, and even predatory microorganisms. [4] Like traditional antibiotics, each explored bio‐inspired agent prevents microbial growth, colonization and damages the microbial cells by targeting the biological and metabolic factor, which again generates the concern of resistant development towards such agents. In parallel, various nanotechnological approaches are emerging to prevent or combat such cross infections by employing metallic nanoparticles, nanocarbons, nanogels and nanocomposites. The proposed methodologies for such nanocoating's are relatively simple, affordable and tunable compared to other approaches and have shown great potential to be used as antimicrobial coatings / surfaces in biomedical devices. Despite having great potential and possibility, the exploitation of antimicrobial nanomedicine in actual clinical practice is currently very limited. Only silver nanoparticles have been used in several clinical trials and shown clinical success.[ 5 , 6 ] In spite of the partial clinical success of nano‐silver, it is realized that these metallic nanoparticles are not perfect for the substitution of traditional antibiotics. Many evidences suggest the accumulation of these nanomaterials in tissues, local and systemic adverse effect due to the release silver ions, allergic reaction and overall cellular toxicity.[ 7 , 8 ] Furthermore, the antimicrobial phenomenon of silver nanoparticles is primarily based on the ionic interaction with intracellular components and disruption of intracellular biochemical targets like most antibiotics, which again leaves the possibility of resistant development by target microorganisms. On the other hand, graphene derivatives offer excellent antimicrobial behavior which is attained through damaging the cellular envelopes via the combinatorial effect of physiochemical interaction. This unique underlying phenomenon is suggested to overcome or delaying the resistant development ability of microbial cells.
Graphene and its derivatives offer unique characteristics such as, large surface area, high stability in physiological environment, antimicrobial properties, good biocompatibility, easy‐modification, and multifunctional behavior. It is obvious that with these key multifunctional properties, graphene derivatives have shown their potential and became favorite materials for many biomedical scientists, to design new materials or hybrid materials or coatings of these nanomaterials to existing biomedical devices to overcome with the possibility of device associated infections. Since the first observation of antimicrobial behavior of graphene oxide (GO) and reduced graphene oxide (rGO) at 2010, [9] interest towards the antimicrobial coatings of these materials or design of antimicrobial composites in biomedical field is rapidly emerging. As an initial aspect, large number of studies focused into antimicrobial assessment of these materials. Most of the obtained results clearly demonstrated the concentration dependent bacteriostatic and bactericidal activity of these graphene derivatives.[ 10 , 11 ] Later physiochemical interaction of graphene derivatives with the microbial cells was demonstrated as fundamental mechanistic insight for the overall antimicrobial behavior.
Unlike antimicrobial behavior of graphene derivatives in solution, there is controversy regarding the antimicrobial efficiency of these materials on surfaces.[ 12 , 13 ] A number of studies demonstrated the antimicrobial activity graphene derivative coating, composites, membrane and paper like structure.[ 9 , 14 , 15 , 16 ] However other studies did not observe significant antimicrobial properties of graphene coated surfaces.[ 13 , 17 ] There are number of reports suggesting the variation in antimicrobial ability with different graphene derivatives. Majority of studies find GO as most effective by damaging the microbial cells with mechanical interaction of sharp edges as well as by the generating reactive oxygen species (ROS).[ 10 , 18 ] Others found rGO in the form of nano walls as more effective due to the higher hydrophobicity, sharp edges, charge transfer and its interaction with hydrophobic layer of lipid in microbial cell surface. [19] Since the physiochemical interaction of graphene with microbial cells is key to exhibit antimicrobial activity, graphene derivatives in solution apparently have higher affinity or dynamics to interact with free floating microbial cells thus provide strong antimicrobial behavior. When it comes to coatings or development of antimicrobial surfaces or microbial resistant biomedical devices, instant and continuous reaction of surfaces with microbial cells is expected to either prevent the bacterial attachment, inhibit the microbial growth or damage the microbial cells which come in contact to such surfaces. Hence it is crucial to maintain the material exposure, enough roughness (height), density and distribution of graphene derivatives to fully reflect or translate the similar antimicrobial behavior to biomedical surfaces.
In recent years, many reviews have been published mainly covering the antimicrobial properties of graphene materials and fundamental underlying mechanisms. However, there are few reviews covering antimicrobial activity of these materials on surfaces, nanocomposites and membranes. Herein, this review article is an effort towards: (i) antimicrobial potential of graphene derivatives and understanding on mechanistic insight, (ii) current advancement in translation of antimicrobial potential of graphene derivatives on surfaces, nanocomposite surfaces and (iii) approach for understanding of biocompatibility and biosafety. This review also discusses the strength and weakness of graphene to be used or apply in biomedical devices to prevent the possibility of cross‐infection and current knowledge gap on mechanistic behavior of these materials on antimicrobial activity and biocompatibility. Overall, numbers of current challenge on the utilization of these materials in biomedical fields are highlighted. We hope that the roadmap provided here will inspire researchers to move towards the development of graphene based antimicrobial surfaces or biomedical devices and towards an in‐depth analysis of antimicrobial assessment and their biocompatibility for their future use in biomedical fields.
2. Antimicrobial Potential of Graphene Derivatives: Overview
Since the beginning of antimicrobial demonstration of graphene derivatives, large scale of investigations has been carried out to figure out their potential to be utilized in biomedical fields. All the graphene derivatives including nanographite (GnP), monolayer graphene, GO and rGO, are well explored in their different form, concentrations, purity and thickness of materials. Among them, studies conducted on antimicrobial behavior of GO and rGO are significantly more advanced in compared to other derivatives. The first study showed strong antimicrobial behavior of both GO and rGO by deactivating the 98.5 % and 90 % of Escherichia coli (E. coli) DH5α cells. [9] Furthermore, restricted growth of E. coli was observed on freestanding paper fabricated by using GO and rGO. Later Liu et al. [10] compared the time and concentration dependent antimicrobial activity of graphite, graphite oxide, GO and rGO dispersion against E. coli. The highest antibacterial activity was observed with GO, which inactivated 69.3±6.1 % of bacterial cells in comparison to graphite, graphite oxide, and rGO where 26.1±4.8 %, 15.0±3.7 % and 45.9±4.8 % of killing efficiency were observed, respectively. Most of the bactericidal effect was observed in the first four hour of interaction with these graphene derivatives. [10] The generation of oxidative stress was suggested to play a major role on the antimicrobial activity of these materials. It was speculated that graphene materials, which contain a higher density of functional groups, and are smaller in size, have more chances to interact with bacterial cells, resulting in cell disruption. The antimicrobial activity of graphene nanosheets was observed even more effective than standard antibiotic kanamycin. [20] However, antimicrobial efficiency was varying with the different bacterial species. Graphene nanosheets synthesized by hydrothermal method in alkaline condition exhibited excellent antibacterial activity against E. coli, Salmonella typhimuirum (S. typhimuirum), Bacillus subtilis (B. subtilis), and Enterococcus faecalis (E. faecalis). The minimum inhibitory concentration (MIC) values of graphene nanosheets against these bacteria are very low compared with the MIC values of the standard antibiotic, suggesting that graphene can be effectively used as an antibacterial agent. Toxicity of GO and rGO were also tested against the Pseudomonas aeruginosa (P. aeruginosa) and detected the strong inhibitory activity on growth and viability of cells due to the generation of reactive oxygen species, leading to cell death through resulting nuclear fragmentation. [21] Later the toxicity of both GO and rGO were compared against E. coli and realized that GO produce more superoxide anions compared to rGO leading more DNA fragmentation resulting higher toxicity. [22] Afterwards rGO was tested against the few fungal species and found the strong inhibitory activity suggesting the antimicrobial activity of graphene derivatives regardless of type and species of microorganisms. [23] A year later Tu et al, shed light on the underlying mechanism behind the physical disruption of bacterial cells after interaction with graphene sheets. [24] With the computer simulation analysis, the interaction of graphene sheet with lipid membrane of microbial cells and extraction of phospholipid was observed. These simulation results were further validated by using transmission electron microscopy (TEM), where degradation of E. coli cell membrane can be seen clearly. Following the trend interaction of GO and rGO were evaluated against the copper resistant plant pathogens and reveled the significant inhibition in growth and viability of bacterial by disruption of cell membrane and release of cytoplasm. [25] Subsequently, the broad‐spectrum antimicrobial activity of GO was demonstrated by evaluating the antimicrobial activity against the phytopathogen and fungal species. [26] SEM imaging revealed the disruption of bacterial cells and fungal spores suggesting the damage of membrane integrity of these cells by thin GO sheets via perturbation and trapping. The broad‐spectrum antimicrobial activity of GO was further demonstrated by testing its bactericidal effect against dental pathogens including Streptococcus mutans (S. mutans), Fusobacterium nucleatum (F. nucleatum) and Porphyromonas gingivalis (P. gingivalis) causative agents of tooth decay and periodontitis. [27]
In addition to physical perturbation, charge transfer is considered as other dynamics for the generation of oxidative stress to bacterial cells by graphene materials. This was demonstrated by Li et al., where monolayer graphene films fabricated on conductive, semi conductive and non‐conductive substrates and their antimicrobial activity was examined against E. coli and Staphylococcus aureus (S. aureus). [12] Their results showed that antibacterial activity of pristine graphene could only be obtained when conducting substrate is beneath. The electron transfer from bacterial cells membrane to graphene was proposed to damage bacterial cells rather than ROS mediated cell disruption. This phenomenon remains controversial since a year later Dellieu et al., demonstrated that the conductive character of the substrate has no influence on the viability of S. aureus and E. coli bacteria in contact with chemical vapor deposition (CVD) graphene films. [13] Until now the antimicrobial effect of graphene derivatives attributed via various mechanism including membrane stress, generation of oxidative stress, trapping behavior, basal plane and photothermal activity. Fine and sharp edges of graphene derivatives are proven to tear the bacterial cell membrane to inactivate the bacterial cells by storming out of intracellular materials.[ 16 , 28 ] The graphene derivatives mediated oxidative stress is generated via production of ROS, which is known to damage DNA, causes mitochondrial dysfunction followed by the deactivation of bacterial cells. Moreover, when microbial cells get trapped in graphene sheets, get totally isolated from the external environment due to the blockage of gas/ion/nutrient exchange.[ 10 , 29 ] This trapping prevents the proliferation of microbial cells and later loss the viability due to the lack of nutrient and respirational activity. With these proven excellent phenomena, recent research activity is focusing more towards the development of composites by incorporating graphene materials with other antimicrobial materials, polymers, functionalization with bioactive molecules not only to improve the antimicrobial activity but also to stabilize the nanosheets which could be used later to modify or develop antimicrobial biomedical surfaces. There are also efforts going on the method development and optimization for coatings of graphene on the surfaces with enhanced antimicrobial behavior.
3. Graphene‐Based Antimicrobial Surfaces
Due to the multidisciplinary nature of research on graphene based antimicrobial surfaces we must make a few notes about terminology and classification. In this section, by the term ‘nanocomposite’ and'composite’ we refer to materials consisting of at least two phases, whereby one phase, i. e. nanoparticles or particles, are included in bulk phases such as polymers, i. e. matrix. [30] However, the term ‘nanocomposite’ is also used in several publications with an emphasis on the hybrid nature of the nanoparticles, especially GO‐Ag structures, rather than on their inclusion in a bulk phase.[ 31 , 32 ]
The tailoring of graphene based antimicrobial materials relies on the use of suitable procedures in order to obtain structures that allow for the exploitation of graphene and its’ derivatives’ antibacterial potential. Such antibacterial surfaces can be tailored as scaffolds and membranes, surface coatings or from bulk nanocomposites. Antibacterial surfaces can be obtained as e. g. paper, scaffolds and membranes from dispersions using for example drying,[ 15 , 33 ] filtration,[ 34 , 35 ] lyophilization [36] or interfacial self‐assembly. [37] Graphene and graphene derivatives can be attached to other surfaces as coatings for example by electrical and chemical deposition methods,[ 14 , 15 , 16 , 38 , 39 , 40 ] layer‐by‐layer assembly[ 41 , 42 ] or various surface spreading methods.[ 43 , 44 , 45 , 46 , 47 , 48 ] Finally, gel spinning, [49] electrospinning,[ 50 , 51 ] layer‐by‐layer assembly[ 41 , 52 , 53 ] or melt extrusion [54] have been used to create graphene based antibacterial nanocomposites. The method of obtaining the antibacterial surfaces is essential as it dictates the extent to which such surfaces can be tuned in terms of orientation and distribution of the graphene and their upscaling potential.
In addition to the direct use of graphene derivatives, hybrid nanomaterials are also getting attention in scientific community to obtain additive or synergistic antimicrobial activity. Graphene based hybrid nanomaterials are mainly developed by using nano silver, zinc, ferrous and other 2d materials, which are demonstrated to enhance the antimicrobial potential of graphene based antimicrobial surfaces.
3.1. Graphene‐Based Assembled Structures
The assembly of graphene and graphene derivates into self‐sustaining structures in the form of scaffolds, paper and membranes is a rather facile method for screening their antibacterial properties. This is particularly important for graphene derivatives where other particles are attached to e. g. GO. A graphene‐based paper with antimicrobial behavior was developed by Hu et al., suggesting the possibility of fabrication or modification of surfaces having antimicrobial potential by using graphene derivatives. [9] Following that, Pham et al. produced graphene nanofilms with different edge lengths and different angles of orientation of the graphene sheets to compare the antimicrobial behavior with regards to morphology and orientation of nanosheets. These graphene nanofilms exhibited strong but variable bactericidal behavior against P. aeruginosa and S. aureus. The density of the exposed edges of graphene was as a major parameter contributing to the antibacterial behavior of graphene nanosheets. This suggested that the bactericidal activity arises from the formation of pores in the bacterial cell wall, causing a subsequent osmotic imbalance. [55] Furthermore, GO nanowalls reduced by hydrazine were more toxic to the bacteria than the unreduced GO nanowalls due to the better charge transfer between the bacteria and the more sharpened edges of the reduced nanowalls during contact. Another study provided the facile strategy to decorate AgNPs onto rGO by the simultaneous reduction of silver ions and graphene oxide nanosheets within one system, and further to fabricate a dimension‐adjustable rGO/AgNP multi‐layered film by a thermal‐driven self‐assembly process. [37] Previously, titanate nanosheets were incorporated into rGO films to develop free standing hybrid films. This rGO layered titanate films showed enhanced mechanical strength, high surface roughness, chemical stability and hydrophobicity. The prepared composite films of rGO and metal oxide nanosheets exhibited excellent antimicrobial behavior in compared to pure rGO film resulting in the complete sterilization of E. coli O157:H7 (≈100 %) in the very short time, i. e. 15 min. [56] The antimicrobial activity was observed mainly due to the irreversible destruction of E. coli cells by sharp edges nanosheets. Since rGO and copper both antimicrobial behaviors, rGO‐copper free‐standing films were synthesized to boost the biocidal activity. The nanocomposites were further co‐deposited with polydopamine (PDA) onto an ultrafiltration support and tested for antimicrobial potential. The membrane exhibited a strong antibacterial performance with a 97.9 % reduction in viability of E. coli. [35] In another study, different graphene derivatives including zwitterionic graphene nanomaterials were synthesized with defined exposure, in terms of grafted polymer coverage and functionality, and isoelectric points. [54] The synthesized materials had strong bactericidal activity via trapping of bacterial cells as well as piercing of cell membrane due to nano knives effect suggesting that improvement in the solidity of agglomeration and orientation of nanomaterials could enhance the antimicrobial activity. [57] Another study designed the strategy for the synthesis of a polycationic peptide functionalized graphene–silver hybrid nanoparticle structures. The nanocomposite was demonstrated to facilitate enhanced biofilm inhibition and disruption properties to eliminate the biofilm development of gram‐negative bacteria. [32] In another approach, GO sheets and functionalized rGO nanosheets were self‐assembled spontaneously onto solid titanium (Ti), using an evaporation‐assisted electrostatic assembly process and a mussel‐inspired one‐pot assembly process. These assemblies strongly mitigated bacterial adhesion and showed considerable antibiofilm activities. [33] Zou et al developed the wrinkled GO films by vacuum filtration of a GO suspension through a prestrained filter. Where highly wrinkled GO surface observed to bear bactericidal activity with contact‐based mechanism. [34]
3.2. Graphene‐Based Surface Coatings
The application of graphene‐based coatings to arbitrary surfaces has a significant potential for applications to biomedical devices. The coating procedure varies depending on the substrate material, e. g. metals, polymers, and controlling the morphology of the coatings is strongly dependent on the application method.
Polyethyleneimine grafted GO nanosheets incorporated Ni‐P coatings on copper plates depicted to offer surface free energies to have significant influence on bacterial adhesion there by possibility of its use as a preventive measures of bacterial biofilm formation on the surface. [15] In a recent study, antimicrobial activity of GO‐ and rGO coated aluminum plates was tested against E. coli and revealed excellent antibacterial efficacy of the rGO‐coated surface. The intracellular ROS production was observed to play significant role in oxidative damage of the bacterial cells. [58] Graphene in the form of graphene nanowalls coated on stainless steel by electrophoretic deposition exhibited strong antimicrobial activity against both gram positive and negative bacterial cells. The cell membrane damage of bacterial cells due to the direct contact with sharp edges of the nanowalls was clearly demonstrated as mechanism in the bacterial inactivation. [19]
By understanding the interaction of graphene derivatives to microbial cells, recent studies have been more focused towards the practical applications of materials for antimicrobial coating of surfaces and the development of self‐cleaning membranes and films. With the advanced designs, methods of fabrication and coatings have been proven for the development of devices with superior antimicrobial efficiency. Previously GO with different oxidation, hydroxyl, and carbon radical (.C) levels were tested to figure out the role of functional group on antimicrobial potential of this material and found that density of carbon radicals on hydrated GO (hGO) as a major factor to have strong antimicrobial effect with enhanced membrane binding and induction of lipid peroxidation. [43] The hGO coated silicon catheter showed 2.2‐fold of log reduction in the antimicrobial resistant E. coli in compared to non‐coated catheter, suggesting that, such coatings on biomedical devices could potentially be used to deactivate drug resistant bacteria (Figure 1). [43] Yadhav et al., coated synthesized GO by in improved Hummers method and coated to polymeric well plate and examined the antimicrobial potential. The coated well plates exhibited toxicity towards both gram positive and negative bacteria due to the mechanical interaction as well as the generation of ROS. [44] GO coatings on titanium surface was achieved with three kinds of combination types including drop with gravitational effects, electrostatic interaction and electrophoretic deposition. Obtained results showed that the combination of such coatings provides significant numbers of wrinkled areas with sharp edges in compared to single types of coating methods. With the higher exposed sharp edges GO coatings effectively deactivated S. aureus and prevented the gathering of bacterial cells around the coated surface physical disruption as well as by production of ROS. [38] Panda et al, demonstrated the antimicrobial activity of natural shellac‐derived GO coatings on metallic films, such as Zn, Ni, Sn, and steel via electron transfer mechanism and consequent ROS mediated oxidative stress to the bacteria. It was proposed that a synchronous activity of GO acting as an electron pump and subsequent charge transfer from cell membrane to functional oxygen groups on the surface of GO induces ROS production. It was further speculated that the loss of cell membrane integrity is due to the electron transfer at an initial stage and later compromises bacterial metabolism and membrane structure eventually causing cell death. [59] Coatings of silver/hydroxyapatite/graphene hybrid particles on titanium surface exhibited strong antibacterial activity against S. aureus and E. coli after only 3 h of exposure, suggesting the potential of suppressing harmful biofilm formation with no toxic effect on poly nuclear blood cells. [60] In another study, a large CVD grown monolayer graphene covered silver nanowires thin film coating was developed, showing broad spectrum antimicrobial activity by deactivating bacterial and fungal cells. The strong antimicrobial behavior was attributed to the sustained release of Ag+ from the silver nanowires due to graphene coverage. [39]
Figure 1.
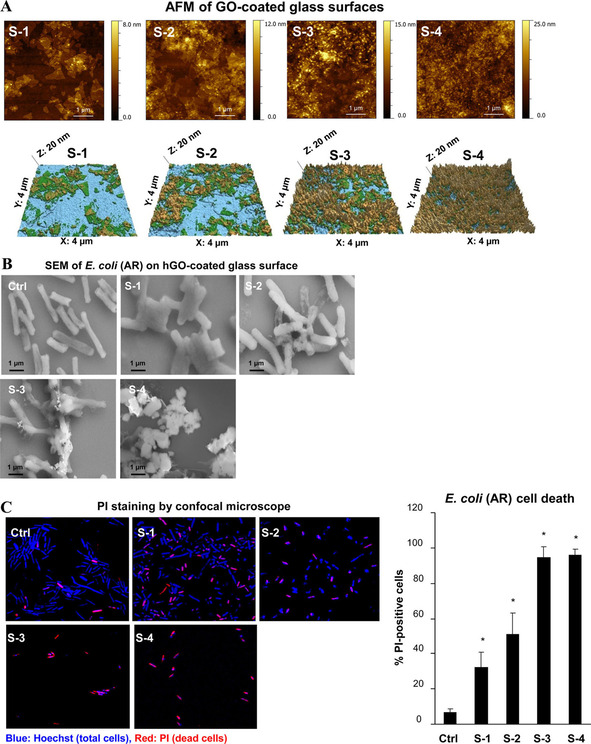
Inhibition of antibiotic resistant (AR) bacteria growth by noncovalently coated hydrated GO (hGO‐2) films on a glass substrate. (A) AFM imaging of hGO‐2 coated substrates. A series of substrates (S‐1, S‐2, S‐3, and S‐4) with different GO coverage and thickness were characterized by AFM. (B) SEM imaging of changes in the bacterial morphology after incubation with the hGO‐2‐coated substrate. (C) Visualization and (D) quantification of bacterial death on hGO‐2 films by confocal microscopy. Following 6 h incubation of AR E. coli with substrates S‐1 to S‐4, the cells were stained with PI, fixed, and washed with 70 % ethanol to determine the percentages of dead cells on substrates. The morphological changes of bacteria were visualized by SEM; *p<0.05 compared to control. Adapted from reference [43] with permission. Copyright 2018 American Chemical Society.
He et al., fabricated hemo‐compatible and antibacterial dual‐layered polymeric membrane by coating a top layer of graphene oxide and sulfonated polyanion co‐doped hydrogel thin film on a bottom membrane substrate. The dual layered membrane exhibited significant antimicrobial activity against E. coli and S. aureus after in situ loading of silver‐nanoparticles by maintaining the sustained release of silver ions. [45] Uniformly distributed silver nanoparticles GO nanosheets through in‐situ reduction of Ag+ subsequently wrapped with a thin layer of type I collagen observed to have synergistic antimicrobial activity on implant surface by rapidly deactivating the 96.3 % and 99.4 % E. coli and S. aureus respectively via physical interaction as well as light inspired photodynamic action. [61] Another study demonstrated the enhanced antimicrobial activity of rGO‐hybridized zinc oxide electrochemically deposited thin film indium‐doped tin oxide (ITO) glass substrate against the S. aureus with photoinactivation. [40] The rGO hybridization with ZnO increased amount of oxygen vacancies cross‐ponding to higher concentration of active sites for ROS formation was postulated as mechanism for antimicrobial behavior. Hui et al developed antimicrobial surface by assembling oppositely charged polyelectrolyte‐stabilized rGO sheets (PEL‐rGO) on a quartz substrate with the layer‐by‐layer (LBL) technique. [62] The PEL‐rGO surface rapidly generated localized heating upon solar irradiation and damages >90 % airborne bacteria, including antibiotic‐tolerant cells on contact. The bactericidal effect is induced likely by permeabilizing their cellular membranes, suggesting the potential use of multilayer PEL‐rGO surface to be utilized on biomedical implant coatings to prevent the microbial colonization. [62] Northan et al, examined the antimicrobial efficiency of rGO coatings on collagen scaffolds. The rGO coatings not only improved the mechanical properties of collagen scaffold but also showed significant inhibitory impact against growth and adhesion of E. coli, S. aureus and Streptococcus pyogenes (S. pyogenes). Going further, scaffolds coated with rGO (400 μg/ml) were shown to enhance the growth and proliferation of cardiomyocytes suggesting their potential use as a cardiac patch. [46] Choudhary et al., developed a rGO‐protein nanoframework by using rGO synthesized from a biosynthetic route. The developed coatings on glass substrates exhibited excellent antimicrobial activity by damaging 94 % of E. coli cells and prevented bacterial colonization. The bactericidal mechanism of rGO involved again mechanical disruption of the cell membrane and later altering transmembrane potential of the cells leading to leakage of cytoplasmic materials. [47] rGO was also shown to induce oxidative stress through intracellular ROS production and inactivates respiratory chain dehydrogenases causing metabolic imbalance in the cells. Arun et al, synthesized magnetic GO paint by incorporating cobalt ferrite and GO along with paint materials via high energy ball milling and deposited the mixture on a galvanized iron substrate, which was then subsequently peeled off. The resulting hybrid coating film significantly inhibited growth and deactivated the E. coli and S. typhimurium cells. The fundamental antimicrobial activity was described by corelating with generation of ROS followed by the mechanical stress to bacterial cell membrane. [48] Zhao et al., prepared novel fabric materials by combining GO sheets onto cotton fabrics firmly by adsorption or by radiation‐induced crosslinking using triallyl isocyanurate (TAIC) as a crosslinker. The GO containing fabrics showed significant antibacterial properties by inactivating 98 % of bacterial cells. This combined fabric materials were able to kill >90 % bacteria even after being washed for 100 times demonstrates the sustainable antimicrobial behavior of materials suggesting its potential to be used to coatings or production of self‐sterilizing fabric based medical products and could potentially reduce the use of antibiotics on such products. [63] In another study, bioinspired polydopamine chemistry was used to fabricate GO‐functionalized membranes via coating and blending. GO coated membranes, where nanosheets are externally exposed, exhibited enhanced biofouling resistance with strong bactericidal effect compared to that of the GO‐blended membrane. This emphasized the importance of GO exposure and interaction with microbial cells for antimicrobial behavior. [64] With the help of coated graphene quantum dots (GQD) with low concentration of H2O2, wound healing band aids were prepared. The developed materials showed excellent antibacterial properties against both E. coli and S. aureus in vitro and vivo with the assistance of low concentration of H2O2 (Figure 2). The ability of GQD was speculated to catalyze H2O2 into 3 OH to exhibit enhanced bactericidal activity. [65] Recently, sulfonated polyetheretherketone (SPEEK) was coated with GO by using simple dip coating method and found a close deposition of GO on the surface due to π‐π stacking interaction between polyetheretherketone (PEEK) and GO. The GO coatings were demonstrated to inhibit biofilm formation and excellent bactericidal efficiency against of E. coli and S. aureus. In addition, GO coatings were observed to accelerate the proliferation and osteogenic differentiation of osteoblast‐like MG‐63 cells, suggesting the potential use of GO coatings on orthopedic implants to prevent the associated infection as well as to enhance the bone integration (Figure 3). [66]
Figure 2.
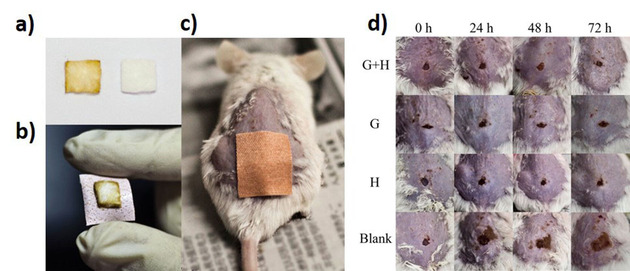
a) The cotton fabric absorbed with (left) and without (right) GQDs. (b) The obtained GQD‐Band‐Aid. c) The mouse treated with GQD‐Band‐Aid. d) Photographs of wound on the mice from the four groups at different times during the therapeutic process. G+H:H2O2+GQD‐Band‐Aid group; G: GQD‐Band‐Aid group; H: H2O2+Blank‐Band‐Aid group; Blank: Saline+Blank‐Band‐ Aid. Adapted from reference [65] with permission. Copyright 2014 American Chemical Society.
Figure 3.
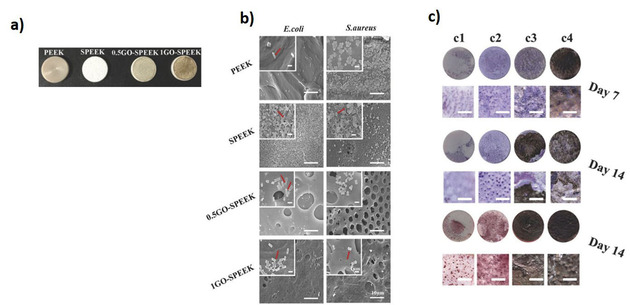
a) The digital pictures of polyetheretherketone (PEEK), sulfonated PEEK (SPEEK), and GO‐SPEEK samples. b) SEM images of bacteria on the substrates. The red arrow shows the morphology of E. coli and S. aureus in high magnification. C) ALP staining and Alizarin Red S staining of different samples. c1) PEEK, c2) SPEEK, c3) 0.5GO‐SPEEK, and c4) 1 GO‐SPEEK. Adapted from reference [66] with permission. Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
In this framework, we initially tested the antimicrobial activity of GO coatings on the titanium surface, which was observed to deactivate E. coli. [14] Later we developed the horizontal and vertical arrays of graphene coatings on metallic and nonmetallic surfaces by using plasma enhanced chemical vapor deposition (PE‐CVD) method. The coatings of pristine CVD graphene observed to be neutral with bacteria similar to results obtained by Dellieu et al, neither deactivated the bacterial cells nor prevented the attachment.[ 13 , 16 ] By contrast, vertical arrays of graphene flakes showed pronounced killing effect and effectively prevented both E. coli and Staphylococcus epidermidis (S. epidermidis) suggesting that the geometry of graphene derivatives on the surface is key parameter deactivate the microbial cells. [16] The vertical array of graphene was observed to damage bacterial cells mainly with physical interaction and no ROS generation was detected. Similarly, Liu et al., explored the antimicrobial activity of GO based on the orientation, where vertically oriented GO flakes on composite surface showed enhanced antimicrobial activity in compared to non‐oriented and randomly oriented GO flakes. [28] Both physical interaction with cells and ROS generation was realized as an underlying mechanism for deactivation of bacterial cells. With excellent antimicrobial behavior and enhancing osteogenic differentiation, such coatings to biomedical implants could provide essence benefit by preventing the possibility of cross infections as well as by enhancing the osseointegration. Another nano‐agent with strong antimicrobial activity was developed by using dopamine‐conjugated polysaccharide sulfate‐anchored and ‐protected Ag‐graphene. The synthesized nanocomposite exhibited robust antibacterial activity against both E. coli and S. aureus in vitro and demonstrated to inhibit S. aureus infection on wounded pig skin without or with NIR laser (Figure 4). [42]
Figure 4.
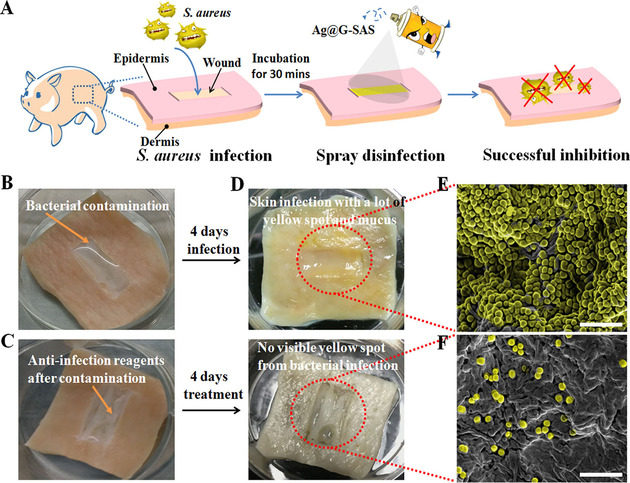
(A) Procedures of S. aureus infection on pig skin and wound disinfection by spraying Ag@G‐SAS (Ag @ graphene‐ sodium alginate sulfate). (B) Photographs of the bacterial contamination procedure for sterilizing pig skin. (C) Photographs of the disinfection‐reagent‐treated wound after bacterial contamination. (D) Photographs of morphologies of the infected wound and uninfected wound, respectively. (E, F) Typical scanning electron microscopy (SEM) images of the infected wound and uninfected wound, respectively. Scale bar: 10 μm. Adapted from reference [42] with permission. Copyright 2017 American Chemical Society.
3.3. Graphene‐Based Nanocomposites
Polymer based graphene nanocomposites have substantial potential applications due to the outstanding properties of graphene and versatility of the polymers. [30] We note here that in the context of nanocomposites, monolayer graphene is rarely used due to the difficulties in obtaining it in sufficient quantities. Thus, graphene derivatives such as graphene nanoplatelets, graphite nanoplatelets, graphene oxide and reduced graphene oxide are most commonly used. Broadly speaking, the most common applications of graphene nanocomposites are mainly directed towards mechanical reinforcement, electrical and thermal conductivity[ 30 , 67 , 68 , 69 ] and gas barrier properties. [70] For most of such applications, some the main challenges revolve around achieving maximal e. g. mechanical, electrical and thermal, improvements with a minimal amount of filler and controlling the morphology, e. g. orientation for gas barrier properties.
Yan et al developed the spray mediated assembly of a bio‐inspired Ag at reduced graphene‐sodium alginate nanocomposite film for effective wound healing. The composite film effectively inactivated the P. aeruginosa, E. coli and Candida albicans (C. albicans) demonstrating the ability of protecting wound from pathogenic microbial infections. This was shown to promote the recovery of wound sites in rats, suggesting the potential use of such composite films for wound healing application. [52] The GO/benzylpenicillin/Mg–Al layered double‐hydroxide hybrid composite film system prepared via solvent evaporation, was shown to maintain the sustained release of antibiotics to achieve enhanced antimicrobial activity suggesting the possible use of graphene derivative composites for efficient delivery of antibiotics/antimicrobial agents. [53] In another work, poly‐lactic acid (PLA) was loaded with cellulose nanocrystal and rGO as reinforcing nanofillers and a membrane was produced through solution casting. The nanocomposite membrane shown to have antibacterial potential against S. aureus and E. coli while being non‐toxic to NIH‐3T3 cells. [71] Recently, multilayer nanofilms were fabricated using a layer by‐ layer technique (LBL) with alternative deposition of GO and lysozyme (Lys) as a novel coating strategy. These nanofilms with Lys as the outmost layer exhibited stronger antibacterial ability against S. aureus and E. coli with enhancing osteogenic differentiation of dental pulp stem cells, through combing the strong bacterial property of Lys and osteogenic profile of GO. [41] In order to develop wound healing patches Lu et al., used electrospinning to prepare chitosan–PVA nanofibers containing graphene. Graphene here was used mainly to obtain the antimicrobial behavior in composite. Indeed, the composite patches were found effective to inhibit E. coli, Agrobacterium, yeast and enhanced the wound healing rate. [50] In another study nanocomposite was developed by using polyethyleneimine (PEI)‐modified and AgNP‐decorated GO. The nanocomposite acquired excellent stability in physiological solutions and electropositivity, showing substantially higher antimicrobial efficacy. The sustainability in antimicrobial efficiency of composite was established by preserving >99 % efficiency against Gram‐negative bacteria, and >95 % efficiency against Gram‐ positive bacteria and fungi even after 1‐week storage. [36] Furthermore, graphene‐polyindole nanocomposite were demonstrated to enhance the antimicrobial potential even against methicillin resistant S. aureus with minimal toxicity towards mammalian cells. The composite demonstrated to heal S. aureus associated skin infection in mice. [72] In another study, GO nanosheets were immobilized to the surface of a polyamide thin film composite forward osmosis membranes. The coatings of these membrane significantly mitigated biofouling with synergistic antibacterial properties (99.9 %) in compared to pristine membrane. [73] A recent study developed laser induced graphene composite as multifunctional surfaces, with great potential to transform polymeric surfaces into antibacterial surfaces, including on biomedical devices. The tailored sandwich composite showed great bactericidal activity and potential for biofilms inhibition. [74] In addition, GO‐poly(methyl methacrylate) (PMMA) nanofibers prepared by pressurized gyration also demonstrated to reduce the viability of E. coli in compared to PMMA nanofibers. [75] Nanocomposites produced by dispersing mechanically exfoliated graphene into a poly vinyl alcohol matrix and then formed into fibers via gel spinning exhibited antimicrobial behavior and were found to be biocompatible. Furthermore, the wounds treated with this type of fibers with 0.3 wt % graphene showed improved wound healing after the 5 days of surgery (Figure 5). [49] Composites containing graphene oxide and quaternary ammonium salt also exhibited antimicrobial activity while being biocompatible to mammalian cells in both in vitro and in vivo analysis (Figure 5). The composites demonstrated the ability to deactivate bacterial cells synergistically with mechanical stress, membrane perforation and generating oxidative stress. The composite was also shown to eradicate multidrug‐resistant bacteria more effectively than conventional antibiotics and rapid wound healing in vivo. [76] Recently, polyurethane and cellulose acetate and GO/Ag electrospun nanocomposite nanofibrous scaffold mats were shown to gain strong antimicrobial activity. The hybrid composite mats were able to deactivate bacterial cells by contact killing. Additionally, the addition of curcumin was found to enhance antimicrobial activity by inactivating 95 % and 100 % of gram positive and gram‐negative bacteria respectively. The hybrid composite mats also demonstrated the ability to significantly promote the wound healing process and regeneration of the epidermis layer supporting the wound healing properties of graphene derivatives. [51]
Figure 5.
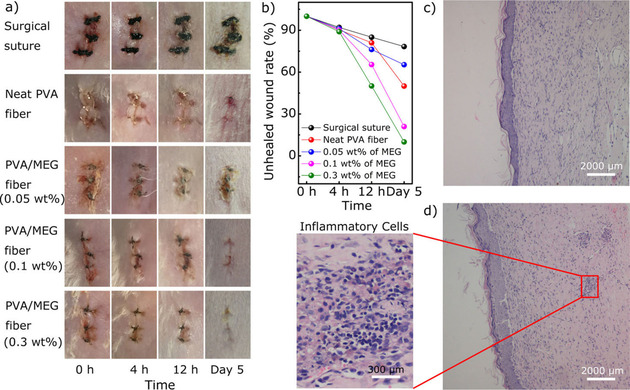
Wound healing evaluation of mice model. a) Representative wounds at different times after surgery, b) unhealed wound rate in five groups during 5 postoperative days. c) Histological examination of nanocomposite fiber with 0.3 wt % of MEG‐treated group and (d) common surgical suture‐treated group. Adapted from reference [49] with permission. Copyright 2018 American Chemical Society.
Recently, we developed the method to prepare antibacterial surfaces from highly structured highly filled polymer nanocomposites based on graphite nanoplatelets [54] which showed strong bactericidal activity by eliminating 99.99 % of bacteria. One should highlight the antimicrobial activity achieved by the graphite nanoplatelets (GNP) and low‐density polyethylene (LDPE) composite is several orders of magnitude stronger than any previously reported using pristine graphene, graphene oxide, and reduced graphene oxide, used either as a coating or in solution.[ 14 , 19 , 55 ] As previously mentioned, the processing stage is crucial for obtaining a microstructure with desired properties in polymer nanocomposites. In the previous research [28] it has been observed that vertical arrays of GO flakes showed pronounced killing effect. Therefore, we have selected extrusion as a processing method from which one can benefit in achieving alignment of the GNP flakes along the polymer flow direction as well as deagglomeration, and efficient dispersion of particles.[ 77 , 78 ] It's worth to highlight that extrusion is one of the most common processing techniques used in the plastic industry. Moreover, selected filler in comparison to lab‐scale produced CVD graphene is easy to manufacture in an economically beneficial and scalable process based on preliminary orientation studies.[ 77 , 78 ] Previous studies by authors revealed also other interesting physical properties that these nanocomposites with aligned GNPs exhibit as: gas barrier properties,[ 79 , 80 ] enhanced mechanical performance, [81] or anisotropic electrical and thermal conductivity.[ 78 , 81 ] A schematic of the manufacturing process is shown on Figure 6, with more details found in our previous study. [54] Moreover, the experimental trials have included two types of LDPE, differing in viscosity by around one order of magnitude. The same kinematic conditions (i. e., apparent shear rate see) were applied. [54] The polymer matrix with the higher viscosity exhibited more consistent deagglomeration and filler orientation and for that reason was chosen for further study. the density of the exposed edges of graphene of is the most important parameters contributing to the antibacterial behavior of graphene nanosheets. [55] The antimicrobial activity of the nanocomposite surfaces was found to be strongly dependent on the density and orientation of sharp edges of graphite nanoplatelets (Figure 7). [54] Overall, the results have the potential as low‐cost and straightforward mass production method of GNP–polymer nanocomposites with outstanding antibacterial properties. We note that due to the unique the manufacturing strategy, the low material costs and overall results, the developed nanocomposites could constitute an entirely new class of high‐performance antibacterial surfaces.
Figure 6.
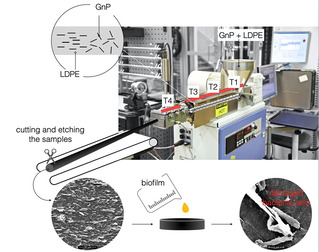
Schematic diagram for the method of extrusion process of graphite nanoplatelets (GNP) ‐LDPE composite with the vertically oriented graphite nanoflakes on the surface.
Figure 7.
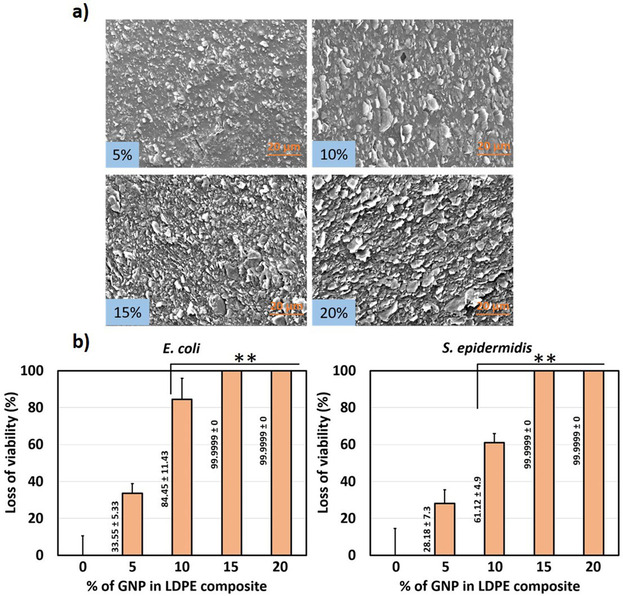
a) SEM images demonstrating the surface morphology of extruded GNP–LDPE polymer composite, b) Loss of viability of E. coli and S. epidermidis by the longitudinal section of GNP–LDPE composite materials compared to control (LDPE). Adapted from reference [54] with permission. Copyright 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Based on the structural analogy with graphene‐like materials, boron nitride (BN) and its derivatives were investigated for their potential antibacterial applications. [82] Boron nitride (BN) exhibits a honeycomb structure similar to graphene, with alternating boron and nitrogen atoms. The results have shown that BN nanoflakes on the extruded BN‐LDPE composite interact with the bacterial cell membrane as anticipated, to cell damage and rupture. Significant loss in viability of E. coli, S. aureus and S. epidermidis was observed compared to the pure LDPE control surface. However, in comparison to GNP‐LDPE nanocomposites the antibacterial effect of BN‐LDPE nanocomposites is weaker. As we noticed BN has higher tendency to agglomerate during processing and as an effect poor orientation of this filler is achieved.
4. Current Understanding on Biocompatibility and Biosafety
The materials must be biocompatible to be used in biomedical devices not only being neutral to the human cells but also be able to cope with in vivo environment and enhanced functional integration to host tissue.[ 83 , 84 ] As like antimicrobial behavior, many studies have evaluated the biocompatibility of graphene derivatives.[ 85 , 86 , 87 , 88 ] The characteristics of graphene materials such as large surface area, mechanical stability and conductivity is believed to endorse the possibility of enhanced extracellular protein adhesion facilitating cell growth and proliferation.[ 14 , 89 , 90 ] Graphene derivatives are demonstrated as biocompatible materials by majority of studies and even suggested the potential use of materials in tissue engineering.[ 91 , 92 , 93 ] Results obtained from in vitro and in vivo suggested enhanced growth and proliferation of mammalian cells resulting the acceleration of bone integration, wound healing and tissue development.[ 49 , 76 , 94 ] In contrast, other studies found adverse effect of these materials such as cytotoxicity, inflammatory cell recruitment and tissue fibrosis suggesting that detail investigation of biocompatibility should be revealed before using such materials in biomedicine either as device coatings or drug delivery.[ 95 , 96 ] The biocompatibility of nanomaterials is generally dependent with the type of materials, size, shape, chemical content, thickness and charge particles. Being 2D material biocompatibility of graphene derivatives have shown to differ with the types (pristine graphene, GO and rGO), number of layers, dynamics of orientation and carbon‐to oxygen atomic ratio. Moreover, any modification and functionalization of these materials for any macroscale applications might have different physiochemical properties in compared to original state and therefore offer distinct biocompatible behavior. [96] Indeed, experimental evidence suggests the distinct interaction with mammalian cells have been observed with different graphene derivatives, morphology and orientation of materials and size of the particles. To address this each type and modification of materials should be tested for the biocompatibility in order to confirm the no adverse effect towards exposed mammalian cells, tissues and organs. Most of the studies regarding the biocompatibility of graphene derivatives mainly evaluated by viability of cells after the few hours to few days of interactions with the materials, which hardly response to question of long‐term toxicity. Currently there is knowledge gap on the changes in molecular dynamics of host cells upon the exposure to graphene derivatives to define or predict the biocompatibility or toxicity in the case of long‐term exposure. There are very few reports on the biocompatibility of these materials with respect to comparison in types of materials, size, and orientation dynamics. Hence still there is no conclusive remark regarding the biocompatibility of graphene derivatives.
Graphene based antimicrobial surfaces are either developed by coatings, impregnation and composites matrices. The biocompatibility of such surfaces is mostly being examined by growing mammalian cells, which possibly be as non‐reactive or even enhance the proliferation of cells. However, possibility of nanomaterials release from the such surfaces cannot be neglected and is the major raising concern. Even though, developed antimicrobial surfaces might show biocompatibility in local area, once the nanomaterials release from the surfaces might show systemic toxicity to various organ and functional systems. Hence the possibility of graphene‐based materials release should be examined carefully while developing antimicrobial surfaces for biomedical applications.
5. Future Perspectives
The orientation dynamics and exposure of graphene derivatives on the surfaces are key parameter to obtain the enhanced antimicrobial and anti‐biofouling efficiency. However, a major challenge is to obtain the desired density of oriented graphene flakes on the surface to efficiently inhibit the biofilm formation. Although few methods have been applied to achieve the right orientation and density of exposed graphene flakes, these existing methods have several drawbacks and cannot be applied to develop coatings or surfaces of arbitrary shapes that could be readily applied to all biomedical devices. Hence, there is a need for the development of simple and scalable methods which could be used to create arbitrary surfaces containing vertically aligned graphene flakes on different types of biomedical devices. Furthermore, the release of graphene particles from such surfaces could be to toxic to other cells in surrounding microenvironment or even show systemic toxicity. The possibility of particle release should be taken seriously while developing graphene based antimicrobial surfaces. Despite having advancement in development of graphene based antimicrobial surfaces, still their knowledge gap on the how graphene derivatives show antimicrobial behavior and biocompatibility in molecular level. Which is due to the lack of studies covering detail investigation of changes in molecular dynamics in the bacterial as well as mammalian cells up on exposure to graphene‐based surfaces. Yet there are not enough reports for translation of in vitro antimicrobial activity of graphene‐based surfaces to in vivo environment. Hence future studies needed to be focused on the in vivo utilization of such graphene based antimicrobial surfaces to revel the actual in vivo antimicrobial performance and biocompatibility.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from SIO‐Grafen, a joint investment of VINNOVA, Formas, and Energimyndigheten, Formas and ÅForsk to RK and IM. Novo Nordisk Foundation grant NNF10CC1016517 and Independent Research Fund Denmark – FTP to IM.
S. Pandit, K. Gaska, R. Kádár, I. Mijakovic, ChemPhysChem 2021, 22, 250.
Contributor Information
Prof. Roland Kádár, Email: roland.kadar@chalmers.se.
Prof. Ivan Mijakovic, Email: ivan.mijakovic@chalmers.se.
References
- 1.World Health Organization. Health care associated infections Fact sheet. World Health Organization 2015, 4.
- 2. VanEpps J. S., Younger J. G., Shock 2016, 46, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstein R. A., Darouiche R.O, Clin. Infect. Dis. 2001, 33, 1567–1572. [DOI] [PubMed] [Google Scholar]
- 4. Karahan H. E., Wiraja C., Xu C., Wei J., Wang Y., Wang L., Liu F., Chen Y., Adv. Healthcare Mater. 2018, 7, e1701406. [DOI] [PubMed] [Google Scholar]
- 5. Fong J., Wood F., Int. J. Nanomed. 2006, 1, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allahverdiyev A. M., Kon K. V., Abamor E. S., Bagirova M., Rafailovich M., Expert Rev. Anti-Infect. Ther. 2011, 9, 1035. [DOI] [PubMed] [Google Scholar]
- 7. Marx D. E., Barillo D. J., Burns 2014, 40, S9. [DOI] [PubMed] [Google Scholar]
- 8. Mao B. H., Chen Z. Y., Wang Y. J., Yan S. J., Sci. Rep. 2018, 8, 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu W., Peng C., Luo W., Lv M., Li X., Li D., Huang Q., Fan C., ACS Nano 2010, 4, 4317. [DOI] [PubMed] [Google Scholar]
- 10. Liu S., Zeng T. H., Hofmann M., Burcombe E., Wei J., Jiang R., Kong J., Chen Y., ACS Nano 2011, 5, 6971–6980. [DOI] [PubMed] [Google Scholar]
- 11. Sun W., Wu F. G., Chem. Asian J. 2018, 13, 3378–3410. [DOI] [PubMed] [Google Scholar]
- 12. Li J., Wang G., Zhu H., Zhang M., Zheng X., Di Z., Liu X., Wang X., Sci. Rep. 2014, 4, 4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dellieu L., Lawarée E., Reckinger N., Didembourg C., Letesson J.-J., Sarrazin M., Deparis O., Matroule J.-Y., Colomer J.-F., Carbon 2015, 84, 310–316. [Google Scholar]
- 14. Zhao C., Pandit S., Fu Y., Mijakovic I., Jesorka A., Liu J., RSC Adv. 2016, 6, 38124. [Google Scholar]
- 15. Shao W., Wu J., Liu H., Dong G., Wang S., Min H., Huang M., RSC Adv. 2016, 6, 46270–46277. [Google Scholar]
- 16. Pandit S., Cao Z., Mokkapati V. R. S. S., Celauro E., Yurgens A., Lovmar M., Westerlund F., Sun J., Mijakovic I., Adv. Mater. Interfaces 2018, 5, 1701331. [Google Scholar]
- 17. Ruiz O. N., Fernando K. A., Wang B., Brown N. A., Luo P. G., McNamara N. D., Vangsness M., Sun Y. P., Bunker C. E., ACS Nano 2011, 5, 8100. [DOI] [PubMed] [Google Scholar]
- 18. Sengupta I., Bhattacharya P., Talukdar M., Neogi S., Pal S. K., Chakraborty S., Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar]
- 19. Akhavan O., Ghaderi E., ACS Nano 2010, 4, 5731. [DOI] [PubMed] [Google Scholar]
- 20. Krishnamoorthy K., Veerapandian M., Zhang L.-H., Yun K., Kim S. J., J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar]
- 21. Gurunathan S., Han J. W., Dayem A. A., Eppakayala V., Kim J.-H., Int. J. Nanomed. 2012, 7, 5901–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurunathan S., Han J. W., Eppakayala A. A., Park V M. R., Kwon D.-N., Kim J.-H., J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar]
- 23. Sawangphruk M., Srimuk P., Chiochan P., Sangsri T., Siwayaprahm P., Carbon 2012, 14, 5156–5161. [Google Scholar]
- 24. Tu Y., Lv M., Xiu P., Huynh T., Zhang M., Castelli M., Liu Z., Huang Q., Fan C., Fang H., Zhou R., Nat. Nanotechnol. 2013, 8, 594. [DOI] [PubMed] [Google Scholar]
- 25. Wang X., Liu X., Han H., Colloids Surf. B 2013, 103, 136–142. [DOI] [PubMed] [Google Scholar]
- 26. Chen J., Peng H., Wang X., Shao F., Yuan Z., Han H., Nanoscale 2014, 6, 1879–1889. [DOI] [PubMed] [Google Scholar]
- 27. He J., Zhu X., Qi Z., Wang C., Mao X., Zhu C., He Z., Li M., Tang Z., ACS Appl. Mater. Interfaces 2015, 7, 5605–11. [DOI] [PubMed] [Google Scholar]
- 28. Lu X., Feng X., Werber J. R., Chu C., Zucker I., Kim J.-H., Osuji C. O., Elimelech M., Proc. Natl. Acad. Sci. USA 2017, 114, E9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jayanthi S., Eswar N. K., Singh S. A., Chatterjee K., Madras G., Sood A., RSC Adv. 2016, 6, 1231–1242. [Google Scholar]
- 30. Ferrari A. C., Bonaccorso F., Fal'Ko V., Novoselov K. S., Roche S., Bøggild P., Borini S., Koppens F. H., Palermo V. and Pugno N., et al., Nanoscale, 2015, 7, 4598–4810. [DOI] [PubMed] [Google Scholar]
- 31. Tan S., Wu X., Xing Y., Lilak S., Wu M., Zhao J. X., Colloids Surf. B 2020, 185, 110616. [DOI] [PubMed] [Google Scholar]
- 32. Parandhaman T., Das S. K., Biomater. Sci. 2018, 6, 3356. [DOI] [PubMed] [Google Scholar]
- 33. Jia Z., Shi Y., Xiong P., Zhou W., Cheng Y., Zhang Y., Xi T., Wei S., ACS Appl. Mater. Interfaces 2016, 8, 17151–17165. [DOI] [PubMed] [Google Scholar]
- 34. Zou F., Zhou H., Jeong D. Y., Kwon J., Eom S. U., Park T. J., Hong S. W., Lee J., ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [DOI] [PubMed] [Google Scholar]
- 35. Zhu J., Wang J., Uliana A. A., Tian M., Zhang Y., Zhang Y., Volodin A., Simoens K., Yuan S., Li J., Lin J., Bernaerts K., Van der Bruggen B., ACS Appl. Mater. Interfaces 2017, 9, 28990–29001. [DOI] [PubMed] [Google Scholar]
- 36. Zhao R., Kong W., Sun M., Yang Y., Liu W., Lv M., Song S., Wang L., Song H., Hao R., ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [DOI] [PubMed] [Google Scholar]
- 37. Zhang P., Wang H., Zhang X., Xu W., Li Y., Li Q., Wei G., Su Z., Biomater. Sci. 2015, 3, 852–860. [DOI] [PubMed] [Google Scholar]
- 38. Qiu J., Liu L., Zhu H., Liu X., Bioact. Mater. 2018, 3, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao C., Deng B., Chen G., Lei B., Hua H., Peng H., Yan Z., Nano Res. 2016, 9, 963–973. [Google Scholar]
- 40. Teh S. J., Yeoh S. L., Lee K. M., Lai C. W., Hamid S. B. A., Thong K. L., J. Photochem. Photobiol. B 2016, 161, 25–33. [DOI] [PubMed] [Google Scholar]
- 41. Li M., Li H., Pan Q., Gao C., Wang Y., Yang S., Zan X., Guan Y., Langmuir 2019, 35, 6752–6761. [DOI] [PubMed] [Google Scholar]
- 42. Fan X., Yang F., Nie C., Yang Y., Ji H., He C., Cheng C., Zhao C., ACS Appl. Mater. Interfaces 2018, 10, 296–307. [DOI] [PubMed] [Google Scholar]
- 43. Li R., Mansukhani N. D., Guiney L. M., Ji Z., Zhao Y., Chang C. H., French C. T., Miller J. F., Hersam M. C., Nel A. E., Xia T., ACS Nano 2016, 10, 10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yadav N., Dubey A., Shukla S., Saini C. P., Gupta G., Priyadarshini R., Lochab B., ACS Omega 2017, 2, 3070–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He C., Shi Z. Q., Cheng C., Lu H. Q., Zhou M., Sun S. D., Zhao C. S., Biomater. Sci. 2016, 4,1431–1440. [DOI] [PubMed] [Google Scholar]
- 46. Norahan M. H., Pourmokhtari M., Saeb M. R., Bakhshi B., Soufi Zomorrod M., Baheiraei N., Mater. Sci. Eng. C 2019, 104, 109921. [DOI] [PubMed] [Google Scholar]
- 47. Choudhary P., Parandhaman T., Ramalingam B., Duraipandy N., Kiran M. S., Das S. K., ACS Appl. Mater. Interfaces 2017, 9, 38255–38269. [DOI] [PubMed] [Google Scholar]
- 48. Arun T., Verma S. K., Panda P. K., Joseyphus R. J., Jha E., Akbari-Fakhrabadi A., Sengupta P., Ray D. K., Benitha V. S., Jeyasubramanyan K., Satyam P. V., Mater. Sci. Eng. C 2019, 104, 109932. [DOI] [PubMed] [Google Scholar]
- 49. Ma Y., Bai D., Hu X., Ren N., Gao W., Chen S., Chen H., Lu Y., Li J., Bai Y., ACS Appl. Mater. Interfaces 2018, 10, 3002–3010. [DOI] [PubMed] [Google Scholar]
- 50. Lu B., Li T., Zhao H., Li X., Gao C., Zhang S., Xie E., Nanoscale 2012, 4, 2978–2982. [DOI] [PubMed] [Google Scholar]
- 51. Esmaeili E., Eslami-Arshaghi T., Hosseinzadeh S., Elahirad E., Jamalpoor Z., Hatamie S., Soleimani M., Int. J. Biol. Macromol. 2020, 152, 418–427. [DOI] [PubMed] [Google Scholar]
- 52. Yan X., Li F., Hu K.-D., Xue J., Pan X.-F., He T., Dong L., Wang X.-Y., Wu Y.-D., Song Y.-H., Xu W.-P., Lu Y., Sci. Rep. 2017, 7, 13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Y., Zhang D., Bao Q., Wu J., Wan Y., J. Mater. Chem. 2012, 22, 23106–23113. [Google Scholar]
- 54. Pandit S., Gaska K., Mokkapati V. R. S. S., Celauro E., Derouiche A., Forsberg S., Svensson M., Kádár R., Mijakovic I., Small 2020, 16, e1904756. [DOI] [PubMed] [Google Scholar]
- 55. Pham V. T. H., Truong V. K., Quinn M. D. J., Notley S. M., Guo Y., Baulin V. A., Al Kobaisi M., Crawford R. J., Ivanova E. P., ACS Nano 2015, 9, 8458. [DOI] [PubMed] [Google Scholar]
- 56. Kim I. Y., Park S., Kim H., Park S., Rouff R. S., Hwang S.-J., Adv. Funct. Mater. 2014, 24, 2288–2294. [Google Scholar]
- 57. Tan K. H., Sattari S., Donskyi I. S., Cuellar-Camacho J. L., Cheng C., Schwibbert K., Lippitz A., Unger W. E. S., Gorbushina A., Adeli M., Haag R., Nanoscale 2018, 10, 9525–9537. [DOI] [PubMed] [Google Scholar]
- 58. Choudhary P., Das S. K., ACS Omega 2019, 4, 387–397. [Google Scholar]
- 59. Panda S., Rout T. K., Prusty A. D., Ajayan P. M., Nayak S., Adv. Mater. 2018, 30(7). [DOI] [PubMed] [Google Scholar]
- 60. Jankovic A., Erakovic S., Vukasinovic-Sekulic M., Miskovic-Stankovic V., Park S. J., Rhee K. Y., Prog. Org. Coat. 2015, 83, 1–10. [Google Scholar]
- 61. Xie X., Mao C., Liu X., Zhang Y., Cui Z., Yang X., Yeung K. W. K., Pan H., Chu P. K., Wu S., ACS Appl. Mater. Interfaces 2017, 9, 26417–26428. [DOI] [PubMed] [Google Scholar]
- 62. Hui L., Auletta J. T., Huang Z., Chen X., Xia F., Yang S., Liu H., Yang L., ACS Appl. Mater. Interfaces 2015, 7, 10511–10517. [DOI] [PubMed] [Google Scholar]
- 63. Zhao J., Deng B., Lv M., Li J., Zhang Y., Jiang H., Peng C., Li J., Shi J., Huang Q., Fan C., Adv. Healthcare Mater. 2013, 2, 1259–1266. [DOI] [PubMed] [Google Scholar]
- 64. Cheng W., Lu X., Kaneda M., Zhang W., Bernstein R., Ma J., Elimelech M., Environ. Sci. Technol. 2020, 54, 517–526. [DOI] [PubMed] [Google Scholar]
- 65. Sun H., Gao N., Dong K., Ren J., Qu X., ACS Nano 2014, 8, 6202–6210. [DOI] [PubMed] [Google Scholar]
- 66. Ouyang L., Deng Y., Yang L., Shi X., Dong T., Tai Y., Yang W., Chen Z. G., Macromol. Biosci. 2018,18, e1800036. [DOI] [PubMed] [Google Scholar]
- 67. Zhan C., Yu G., Lu Y., Wang L., Wujcik E., Wei S., J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar]
- 68. Mohan V. B., Lau K.-t., Hui D., Bhattacharyya D., Composites Part B 2018, 142, 200–220. [Google Scholar]
- 69. Chen W., Weimin H., Li D., Chen S., Dai Z., Sci. Eng. Compos. Mater. 2018, 25, 1059–1073. [Google Scholar]
- 70. Cui Y. B., Kundalwal S. I., Kumar S., Carbon 2016, 3, 313–333. [Google Scholar]
- 71. Pal N., Dubey P., Gopinath P., Pal K., Int. J. Biol. Macromol. 2017, 95, 94–105. [DOI] [PubMed] [Google Scholar]
- 72. Shoeb M., Mobin M., Rauf M. A., Owais M., Naqvi A. H., ACS Omega 2018, 3, 9431–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hegab H. M., ElMekawy A., Barclay T. G., Michelmore A., Zou L., Saint C. P., Ginic-Markovic M., ACS Appl. Mater. Interfaces 2016, 8, 17519–17528. [DOI] [PubMed] [Google Scholar]
- 74. Luong D. X., Yang K., Yoon J., Singh S. P., Wang T., Arnusch C. J., Tour J. M., ACS Nano 2019, 13, 2579–2586. [DOI] [PubMed] [Google Scholar]
- 75. Matharu R. K., Tabish T. A., Trakoolwilaiwan T., Mansfield J., Moger J., Wu T., Lourenço C., Chen B., Ciric L., Parkin I. P., Edirisinghe M., J. Colloid Interface Sci. 2020, 571, 239–252. [DOI] [PubMed] [Google Scholar]
- 76. Liu T., Liu Y., Liu M., Wang Y., He W., Shi G., Hu X., Zhan R., Luo G., Xing M., Wu J., Burns Trauma 2018, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Induchoodan G., Kádár R., Annu. Trans. Nord. Rheol. Soc. 2016, 24, 187–191. [Google Scholar]
- 78. Gaska K., Xu X., Gubanski S., Kádár R., Polymer 2017, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gaska K., Kádar R., Rybak A., Siwek A., Gubanski S., Polymer 2017, 9, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Röding M., Gaska K., Kádár R., Lorén N., ACS Nano 2018, 1, 160–167. [Google Scholar]
- 81. Blinzler B. J., Larsson R., Gaska K., Kádár R., Nanomaterials 2019, 9, 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pandit S., Gaska K., Mokkapati V. R. S. S., Forsberg S., Svensson M., Kádár R., Mijakovic I., RSC Adv. 2019, 9, 33454–33459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kenry, Lee W. C., Loh K. P., Lim C. T., Biomaterials 2018, 155, 236–250. [DOI] [PubMed] [Google Scholar]
- 84. Bullock C. J., Bussy C., Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar]
- 85. Chen G. Y., Pang D. W., Hwang S. M., Tuan H. Y., Hu Y. C., Biomaterials 2012, 33, 418–427. [DOI] [PubMed] [Google Scholar]
- 86. Li N., Zhang Q., Gao S., Song Q., Huang R., Wang L., Liu L., Dai J., Tang M., Cheng G., Sci. Rep. 2013, 3, 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jasim D. A., Lozano N., Bussy C., Barbolina I., Rodrigues A. F., Novoselov K. S., Kostarelos K., FlatChem. 2018, 12, 17–25. [Google Scholar]
- 88. Ryan A. J., Kearney C. J., Shen N., Khan U., Kelly A. G., Probst C., Brauchle E., Biccai S., Garciarena C. D., Vega-Mayoral V., Loskill P., Kerrigan S. W., Kelly D. J., Schenke-Layland K., Coleman J. N., O′Brien F. J., Adv. Mater. 2018, 30, e1706442. [DOI] [PubMed] [Google Scholar]
- 89. Lee W. C., Lim C. H., Shi H., Tang L. A., Wang Y., Lim C. T., Loh K. P., ACS Nano 2011, 5, 7334–7341. [DOI] [PubMed] [Google Scholar]
- 90. Shi X., Chang H., Chen S., Lai C., Khademhosseini A., Wu H., Adv. Funct. Mater. 2012, 22, 751. [Google Scholar]
- 91. Akhavan O., J. Mater. Chem. B 2016, 4, 3169–3190. [DOI] [PubMed] [Google Scholar]
- 92. Peng Z., Zhao T., Zhou Y., Li S., Li J., Leblanc R. M., Adv. Healthcare Mater. 2020, 9, e1901495. [DOI] [PubMed] [Google Scholar]
- 93. Jo S. B., Erdenebileg U., Dashnyam K., Jin G. Z., Cha J. R., El-Fiqi A., Knowles J. C., Patel K. D., Lee H. H., Lee J. H., Kim H. W., J. Tissue Eng. 2020, 11, 2041731419900424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li K., Wang C., Yan J., Zhang Q., Dang B., Wang Z., Yao Y., Lin K., Guo Z., Bi L., Han Y., Sci. Rep. 2018, 8, 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Duch M. C., Budinger G. R., Liang Y. T., Soberanes S., Urich D., Chiarella S. E., Campochiaro L. A., Gonzalez A., Chandel N. S., Hersam M. C., Mutlu G. M., Nano Lett. 2011,11, 5201–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lalwani G., D′Agati M., Khan A. M., Sitharaman B., Adv. Drug Delivery Rev. 2016, 105, 109–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


