Abstract
Advanced systemic mastocytosis is a relatively rare entity where allogeneic stem cell transplantation can lead to the cure of the disease in selected patients. Delayed incomplete responses with graft‐versus‐mastocytosis effect were published in a few cases. In this particular patient's report, we describe the direct evidence and potency of graft‐versus‐mastocytosis effect of donor lymphocyte infusions in a patient with systemic mastocytosis with associated hematological neoplasm (SM‐AHN). In a 53‐year‐old female patient, an allogeneic stem cell transplantation after conventional induction treatment was performed for transformed acute myeloid leukemia (AML) during the course of polycythemia vera. After 6 years of remission period of AML and PV, the patient developed aleukemic mast cell leukemia and JAK2‐positive myeloproliferative neoplasm (SM‐AHN). We were able to achieve a sustained complete remission of SM‐AHN lasting for 6 years with only donor lymphocyte infusions in a status of mixed chimerism. The patient is in a good clinical condition and remission. The potent graft‐versus‐mastocytosis effect in this patient resembles the favorable effect of donor lymphocyte infusions in relapsing chronic myeloid leukemia patients after transplantation. This patient is, to our knowledge, the first case showing the proof of principle of graft‐versus‐mastocytosis effect.
Keywords: donor lymphocyte infusion, graft‐versus‐mastocytosis effect, systemic mastocytosis
1. CASE REPORT
Systemic mastocytosis (SM) is a clonal hematological disease that is characterized by the accumulation of atypical mast cells in one or more organs. Patients with advanced forms of SM, such as aggressive SM, SM with associated hematological neoplasm (SM‐AHN), and mast cell leukemia, have a reduced life expectancy. 1 Therefore, cytoreduction and, in some instances, allogeneic stem cell transplantation (allo‐SCT) are needed. 2 The atypical mast cells in SM appear to be resistant to polychemotherapy and to conditioning regimens for SCT. 3 Patients with SM who received donor lymphocyte infusions (DLIs) after allogeneic SCT showed delayed incomplete responses with a graft‐versus‐mastocytosis (GVM) effect. 2 , 4 Here, we describe the first case with sustained remission of SM after DLI as a consequence of a potent GVM effect.
A 53‐year‐old woman followed with polycythemia vera (PV) since 2005 transformed into acute myeloid leukemia (AML) with a normal karyotype in September 2006. The JAK2V617F mutation was present. At this time, no signs of mast cell infiltration were found in the bone marrow, according to a re‐examination of the bone marrow specimen in 2013. After 3 cycles of 7 + 3 (cytarabine and daunorubicin) treatment, PV, but not AML, was still present, and allogeneic peripheral blood stem cell (PBSC) transplantation was performed in February 2007 (Figure 1). The patient received reduced intensity conditioning (fludarabine, melphalan, cytarabine, and alemtuzumab) according to the local protocol in 2007 because of her age followed by PBSC infusion from a 10/10 HLA‐matched unrelated female donor. The transplantation was successful, and 100% donor chimerism (DC) was obtained.
FIGURE 1.
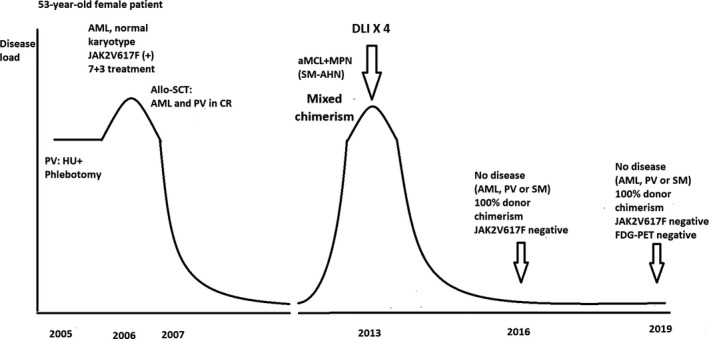
Clinical course. 18FDG‐PET, 18fluorodeoxyglucose positron emission tomography; allo‐SCT, allogeneic stem cell transplantation; aMCL, aleukemic mast cell leukemia; AML, acute myeloid leukemia; DLI, donor lymphocyte infusion; HU, hydroxyurea; MPN, myeloproliferative neoplasia; PV, polycythemia vera; SM‐AHN, systemic mastocytosis associated with hematological neoplasm
Complete remission lasted until July 2013 when various symptoms, including night sweats and weight loss, occurred. The patient had no bone pain. The decreasing DC and increasing JAK2V617F variant allele frequency (VAF) indicated a relapse. The complete blood count, differential blood counts of blood smears, and alkaline phosphatase were within the normal ranges. Two sequential bone marrow examinations of the left posterior iliac crest showed large mast cell aggregates. Mast cells constituted 36% of the nucleated cells in the bone marrow aspiration smears. Cellularity of the bone marrow biopsy specimen was 100%. The mast cells comprised more than 50% of the bone marrow space. The vast majority of mast cells were slightly larger than typical mast cells with a single, round to slightly oval nucleus and round cytoplasm without protrusions. They were well‐granulated with low nuclear/cytoplasmic ratio. Thus, the morphology was in between atypical type 1, immature, and mature mast cells. Less than 5% of mast cells were spindle‐shaped. Blasts constituted 1% of nucleated cells. The mast cells were tryptase positive, CD117 positive, positive for CD2, and negative for CD25 and CD30, as well as for MPO. The areas without mast cell infiltrates were morphologically normal with a cellularity of 50% and normal megakaryocytes without any obvious clustering (Figure 2A).
FIGURE 2.
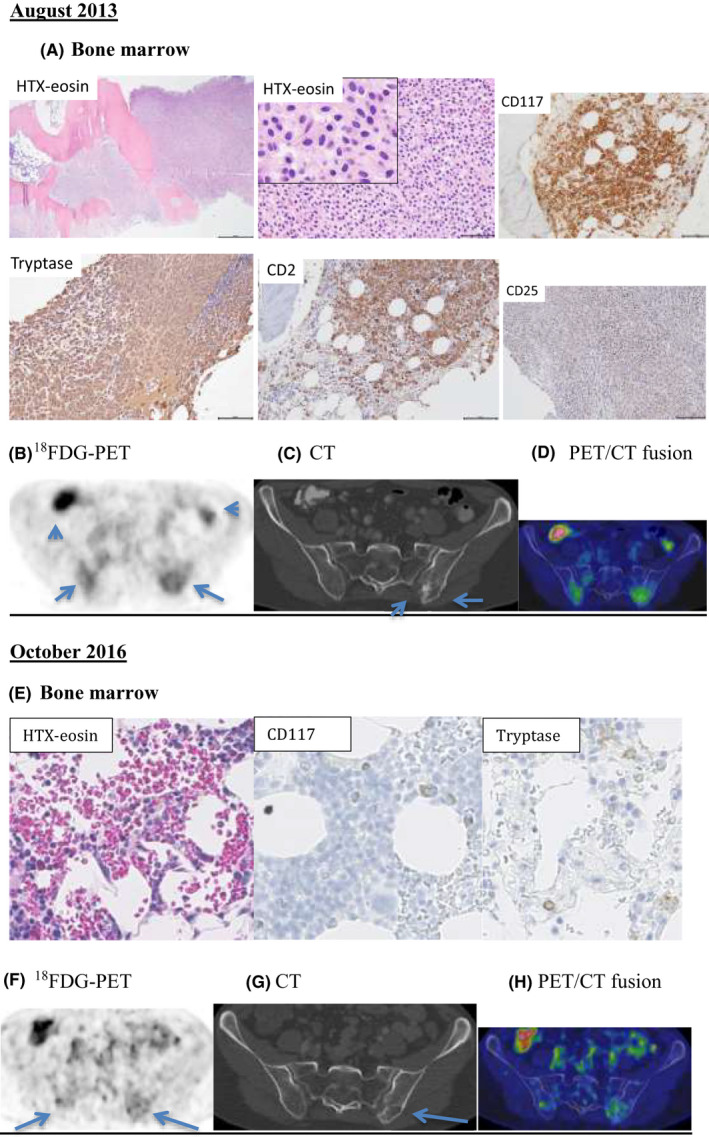
Bone marrow histopathology and 18FDG‐PET/CT examinations. (A) Bone marrow histopathology in August 2013: The bone marrow was heavy infiltrated by dense mast cell infiltrates, broadened bone trabeculae, and only small areas with normal haematopoiesis. The mast cells had a single, round to slightly oval nucleus and moderate amount of cytoplasm (HTX‐Eosin). The mast cells were positive for CD117, tryptase, and CD2 and negative for CD25. The 18FDG‐PET/CT examination in August 2013: (B) Transverse PET image of the pelvis showing diffuse FDG uptake in the posterior part of the os ilium bilaterally with SUVmax values of 8.5 (right) and 8.9 (left). Physiologic bowel uptake of 18FDG (arrowheads) was observed. (C) Transverse CT image of the pelvis showing a sclerotic area (short arrow) and an adjacent lytic region (arrow) in the posterior left os ilium corresponding to the focally increased 18FDG uptake. Notably, the 18FDG uptake in the right os ilium does not correspond to any bone lesions on CT. (D) Transverse PET/CT fusion image from the 18FDG‐PET/CT examination. (E) Bone marrow histopathology in October 2016: Resolution of mast cell infiltration with normal bone marrow findings. The 18FDG‐PET/CT examination in October 2016: (F) Transverse PET image of the pelvis showing that the 18FDG uptake in the posterior part of os ilium decreased bilaterally with SUVmax values of 3.0 (right) and 4.0 (left). (G) Transverse CT image of the pelvis showing partial resolution of the sclerosis and unchanged adjacent osteolysis in the posterior of the left os ilium. (H) Transverse PET/CT fusion image
The serum tryptase level was elevated at 22 ng/mL (>20 ng/mL), which is not a valid minor criterion in this case according to the SM World Health Organization criteria because the patient had SM‐AHN. 3 Cytogenetic analysis revealed t(12;18) (q24;q11) in 5 of 25 metaphases. KIT mutations (exons 8 and 17) from the bone marrow samples were negative. The diagnosis was determined to be SM‐AHN [aleukemic mast cell leukemia (aMCL) associated with JAK2‐positive myeloproliferative neoplasm (MPN)] based on one major criterion (bone marrow mast cell infiltration) and one minor criterion (aberrant CD2 expression on the mast cells).
An 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT) examination performed on August 29, 2013 (Figure 2B‐D) showed sclerotic and osteolytic areas in the pelvis with increased 18F‐FDG maximum standardized uptake value (SUVmax) in the left os ilium and in the right os ilium. These findings were consistent with mastocytosis. The other bones, liver, spleen, and lymph nodes were normal.
Based on the findings of aMCL, MPN, and mixed chimerism, DLI was started without debulking of the mast cell disease burden neither with cladribine nor with midostaurin in September 2013 (Figure 1) with a dose of 1 × 106 and then gradually increased to 10 × 106 CD3+ cells per kg (in total 4 doses) in February 2014. No signs of graft‐versus‐host disease developed after treatment. DC consecutively changed from 83% to 100%. DC in selected CD3+ blood cells remained 100% at all times. Consecutive JAK2V617F VAF went from 11% to undetectable values.
A bone marrow biopsy from the left iliac crest in August 2014 showed complete remission of aMCL and MPN (SM‐AHN). The JAK2V617F mutation was undetectable. The patient had full DC and a reduced tryptase level to 17 ng/mL in August of 2015. In October 2016, a 18F‐FDG PET/CT examination (Figure 2F‐H) showed partial resolution of the sclerotic area in the left os ilium. The corresponding 18F‐FDG uptake decreased with normalization of the SUVmax values as in the right os ilium. In August 2019, 6 years after the DLIs, the patient was in good clinical condition and showed no signs of relapse, with a negative JAK2V617F mutation and full DC.
According to the current recommendations, patients with SM‐AHN should be treated separately for mastocytosis and for the associated disorders. 3 DLI has an excellent graft‐versus‐leukemia effect in MPN. 5 However, in advanced SM patients, the responses observed after DLI developed gradually over the years, suggesting that donor‐derived cells induced a delayed GVM effect. 2 In this case, there was a mixed chimerism after the allogeneic PBSC transplantation that could possibly influence the response to DLI. The 18F‐FDG PET/CT findings were consistent with the bone lesions of SM. Increases in the SUVmax of the bone lesions before DLI probably reflect the osteoblastic activity rather than the mast cell infiltration. 6 We achieved a significant resolution of these lesions as shown by the follow‐up 18F‐FDG PET/CT examination after DLI. Together with this finding, the DC reconstitution, the negative JAK2V617F test, and the negative bone marrow findings for mastocytosis and MPN indicate a potent delayed GVM and MPN effect of DLI alone in this patient.
DLIs were given several years after the allo‐SCT, which was performed to cure the AML developed during the course of PV. The beneficial effect of DLI both for the newly arising aMCL and for the relapsing MPN was delayed in this patient. However, the patient had a very good response, with remissions of both aMCL and MPN without the necessity of debulking the SM component of SM‐AHN. Minimally increased levels of tryptase as in our patient are rare but not absent in advanced SM patients. t(12;18) (q24;q11) is a novel cytogenetic aberration in SM. The effect of DLI and GVM in achieving complete remission of both aMCL and MPN observed in this patient is potent and long lasting with six years of follow‐up.
The clinical course of the patient provides direct evidence of the GVM effect after DLI administration in SM and suggests that a certain period of time is necessary to obtain the best effects of DLI and GVM.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTION
AD and CM treated the patient and provided clinical data; AS analyzed the positron emission tomography/computed tomography examination; BS performed the pathology analyses; HH provided input on the treatment, conceived the study, and wrote the manuscript; ASY and GN wrote and edited the manuscript; and all authors critically reviewed or edited the manuscript, and approved the final version of the manuscript for submission.
Hägglund H, Yavuz AS, Dreimane A, et al. Graft‐versus‐mastocytosis effect after donor lymphocyte infusion: Proof of principle. Eur J Haematol. 2021;106:290–293. 10.1111/ejh.13528
Funding informationThis work was supported and funded by the Swedish Cancer Society.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. 2019;94:363‐367. [DOI] [PubMed] [Google Scholar]
- 2. Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem‐cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264‐3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valent P, Akın C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spyridonidis A, Thomas AK, Bertz H, et al. Evidence for a graft‐versus‐mast‐cell effect after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:515‐519. [DOI] [PubMed] [Google Scholar]
- 5. Merup M, Lazarevic V, Nahi H, et al. Different outcome of allogeneic transplantation in myelofibrosis using conventional or reduced‐intensity conditioning regimens. Br J Haematol. 2006;135:367‐373. [DOI] [PubMed] [Google Scholar]
- 6. Djelbani‐Ahmed CMO, Mekinian A, et al. FDG‐PET/CT findings in systemic mastocytosis: a French multicentre study. Eur J Nucl Med Mol Imaging. 2015;42:2013‐2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


