Abstract
Glioblastoma multiforme (GBM) is among the most common adult brain tumors with invariably fatal character. Following the limited conventional therapies, almost all patients, however, presented with symptoms at the time of recurrence. It is dire to develop novel therapeutic strategies to improve the current treatment of GBM. Gallic acid is a well‐established antioxidant, presenting a promising new selective anti‐cancer drug, while gold nanoparticles (GNPs) can be developed as versatile nontoxic carriers for anti‐cancer drug delivery. Here, we prepared gallic acid‐GNPs (GA‐GNPs) by loading gallic acid onto GNPs, reduction products of tetrachloroauric acid by sodium citrate, through physical and agitation adsorption. GA‐GNPs, rather than GNPs alone, significantly inhibited the survival of U251 GBM cells, as well as enhanced radiation‐induced cell death. Moreover, GA‐GNPs plus radiation arrested the cell cycle of U251 at the S and G2/M phases and triggered apoptotic cell death, which is supported by increased BAX protein levels and decreased expression of BCL‐2. Thus, GA‐GNPs have great potential in the combination with radiation therapy in future studies for GBM treatment.
Keywords: gallic acid, glioma, gold nanoparticles, radiotherapy
Abbreviations
- GA
gallic acid
- GNPs
gold nanoparticles
1. INTRODUCTION
As the most common intracranial malignancy in adults, glioblastoma multiforme (GBM) is treated with the combination of surgery, radiation, and limited chemotherapy (such as temozolomide). 1 However, most patients with GBM undergo recurrence and succumb to this disease although intensive treatment is given. Importantly, GBM cells in the high‐dose radiation field eventually harbor radioresistant characters, leading to recurrences. 2 Therefore, in order to improve the survival or the well‐being of GBM patients, it is imperative to search for new drugs to augment and sensitize existing radiotherapy.
Some polyphenols occurring in nature have antioxidant, anti‐inflammatory, and antitumor property that might be applied in drug development against cancer. 3 Gallic acid (3,4,5‐trihydroxybenzoic acid), a predominant polyphenol, has been verified to inhibit carcinogenesis in both cancer cell lines in vitro and animal models. Importantly, gallic acid exhibits selective cytotoxicity against a variety of tumor cells, including prostate, lung, colon cancer cells, as well as GBM cells. 4 , 5 A derivative of gallic acid, lauryl gallate, increases activities of caspases and induces cell apoptosis in U87 GBM cells. 6 Gallic acid also leads to DBTRG‐05MG GBM cell death through mitochondrial apoptotic pathways, but spares normal CTX TNA2 rat astrocytes. 7 In addition, other investigations found that gallic acid inhibits cell proliferation and invasion in U87 and T98G GBM cells by decreasing AKT and MAPK pathways. 8 , 9 , 10 On the other hand, gallic acid modulates the expression of genes related to cell cycle, metastasis, and angiogenesis to achieve its antitumor ability. 4 Although gallic acid has been identified to exhibit cytotoxicity in GBM cells, the potential effect of gallic acid on the sensitivity of GBM cells to radiation remains elusive.
Improving drug delivery and duration is crucial for promoting the therapeutic efficacy of a drug and minimizing its side effects. Regarding GBM drug treatment, blood–brain barrier is an another layer of protective barrier abolishing easy passage of therapeutic moieties to brain, which requires drugs to be administered at much higher doses. 11 , 12 , 13 Among the various drug delivery systems, nanoparticles have attracted great attention in the drug delivery for targeting brain disease, as they are capable of accomplishing controlled and site‐specific delivery of a wide variety of drugs. 14 , 15 , 16 One of the key advantages of nanoparticles is their higher surface‐to‐volume ratio, reducing the dose and frequency of drug administration and improving patient compliance. 17 , 18 Gold nanoparticles (GNPs) have large surface area‐to‐volume ratio, enabling their surface to be coated with hundreds of molecules. In addition, GNPs are less toxic to cells and easy to conjugate with bio‐active compounds with high stability. 19 , 20 , 21
In this study, we prepared gallic acid‐gold nanoparticles (GA‐GNPs) and examined the biological effects of GA‐GNPs on the viability and sensitivity to radiation in U251 GBM cells. Our findings indicate that the GA‐GNPs significantly inhibit U251 cell survival and enhance its radiosensitization, suggesting a perspective on GBM theranostics of GA‐GNPs in future investigations.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Human glioma U251 cells were purchased from Suzhou Saiye Biotechnology Co., Ltd. (China). HAuCl4 (chloroauric acid), sodium citrate, and gallic acid were obtained from Aladdin (China). Dulbecco's modified eagle medium (DMEM) and penicillin–streptomycin were obtained from Hyclone, and fetal bovine serum (FBS) was from Biological Industries (Israel). Trypsin–EDTA was purchased from Gibco. MTT and Western blot kit were obtained from Sigma‐Aldrich. Apoptosis detection kit was provided by Invitrogen. Cell cycle detection kit and Coomassie bright blue fast staining solution were purchased from Biyun Sky (Beijing, China).
2.2. Characterization of gold nanoparticles
Gold nanoparticles were synthesized by the Turkevich method. 22 One milliliter 25 mM chloroauric acid (HAuCL4·3H2O) solution was added to 100 ml deionized water in a three‐neck flask (250 ml) with an oil bath at 130°C and stirring speed at 300 rpm. After 2 min, 2.5 ml sodium citrate solution (34 mM) was quickly added in the flask and boiled for 10 min. The color of the solution changed from purple to black, and then to purple red. The solution was cooled to room temperature for storage. The size and morphology of the gold nanoparticles were analyzed by transmission electron microscopy and dynamic light scattering (DLS). The concentration of gold nanoparticles was determined by inductively coupled plasma atomic emission spectroscopy (ICP‐AES). Briefly, The ICP‐AES measurements were done with an Agilent 730 ICP‐OES (Agilent) equipped with an Au ICP touch and a quadrupole mass analyzer by setting axial detector mode and linear calibration type. The following parameters were used in the assay: plasma flow (15 L/min), auxiliary gas flow (1.5 L/min), and nebulizer gas flow (0.75 L/min).
2.3. Generation and characterization of GA‐GNPs
Gold nanoparticle solution (10 ml) was centrifuged at 18,500g for 10 min. The pellet was dissolved in 25 ml deionized water in the dark. Resuspended gold nanoparticle solution (5 ml of each) was mixed with 5 ml of deionized water, 22.4, 44.8, 89.6, 134.4, or 179.2 μg/ml gallic acid solution, respectively, and stirred overnight at room temperature. Finally, the unbound gallic acid was removed by centrifugation at 18,500g for 10 min. The concentration of gallic acid in the supernatants was measured by ultraviolet spectrophotometer to calculate gallic acid:GNPs binding rates. Briefly, a Shimadzu Pharmspec UV 1800 ultraviolet spectrophotometer was used, and the calibration curve was prepared from the above supernatants against clear blank by taking the absorbances of the prepared standard dilutions at indicated wavelengths, and then plotted the result against concentration. The remaining pellets were then resuspended in DMEM media for further biological assays.
2.4. U251 cell culture
U251 cells were cultured in DMEM containing 10% FBS and 1% penicillin–streptomycin. DMEM medium was changed every 3 days. The cells were maintained at 37°C in a humidified incubator with 5% CO2 and passaged when the confluence reached about 90%.
2.5. MTT assay
U251 cells at logarithmic growth stage were inoculated in 96‐well plates (8,500/well) and cultured overnight. Different concentrations of GNPs or GA‐GNPs in the culture medium (0, 100, 150, and 200 μg/ml) were added. After 1, 2, and 3 days, the medium was removed, and the MTT substrate was added to the wells. The plate was then shaken at low speed for 10 min. After incubation in the incubator for 1, 2, or 3 hr, the absorbance of each well at 450 nm was detected by a microplate reader.
2.6. X‐ray irradiation
X‐ray irradiation was given to U251 cells with different doses (0, 2, 4, 6, 8, 10, and 12Gy), respectively (6MV X‐ray, SSD 100 cm, and 1.5 or 10 cm solid water were added to the 96‐well plates above or below the plate).
2.7. Cell cycle and cell death analysis
U251 cells at logarithmic growth stage were inoculated into 6‐well plates at a density of 2 x 105 per well. After treated with control, gallic acid, GNPs, and GA‐GNPs (all groups with radiation treatment) for 48 hr, the cells were detached with trypsin and fixed in ethanol with 70% phosphate buffered saline (PBS). The cells were incubated with propidium iodide (40 μg/ml) and Ribonuclease (RNase) (100 μg/ml) before cells were analyzed with a flow cytometer (BD FACSCalibur System, CA).
Under the same treatment condition, U251 cells were digested with trypsin and washed with PBS to disperse the cells. Annexin‐V dye (5 μL) and propidium iodide (40 μg/ml) were added to 100 μL cell suspension in the dark at room temperature for 15 min. Cell apoptosis was detected by the flow cytometry within 1 hr. CflowPlus software was used to analyze both cell cycle and apoptosis data.
2.8. Western blotting
Total protein of U251 cells in response to indicated treatment was extracted by using RIPA buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP‐40, 1% sodium deoxycholate, and protease inhibitors and phosphatase inhibitor cocktails). The cells were incubated in RIPA buffer on ice for 10 min followed by brief sonication. The extract was further centrifuged for 10 min at 14,000g in a cold microfuge, and the supernatant was harvested as total cell protein lysates. The protein concentration of cell lysates was measured by a BCA protein quantification kit according to manufacturer's instruction. The protein samples were loaded and resolved by 10% SDS‐PAGE, and the gel was transferred onto a PVDF membrane. Subsequently, the membrane was blocked with blocking buffer containing 5% nonfat milk at room temperature for 2 hr, and then incubated with primary antibodies against BAX (Cell Signaling Technology #2772, 1:1000), BCL‐2 (Cell Signaling Technology #15071, 1:1000) and GAPDH (Cell Signaling Technology #5174, 1:1000) overnight at 4°C. After washing three times with 1x TBS‐T, the membrane was incubated with Anti‐rabbit IgG, HRP‐linked Antibody (Cell Signaling Technology #7074, 1:3000, for BAX and GAPDH antibodies) or Anti‐mouse IgG, HRP‐linked Antibody (Cell Signaling Technology #7076, 1:3000, for BCL‐2 antibody) at room temperature for 2 hr, and finally exposed by an automatic imaging system (iBright Western Blot Imaging System, ThermoFisher Scientific) with ECL Western Blotting Substrate (Pierce #32106) according to manufacturers' instruction after washed three times by 1x TBST buffer. The intensity of WB bands was quantitatively analyzed by the iBright Analysis Software (ThermoFisher Scientific).
2.9. Statistical analysis
All experiments were carried out in triplicates, and representative results are shown. Data are presented as the mean ± SEM. Statistical analysis was generally performed using single factor analysis of variance and t‐test. *p < .05, **p < .01, ***p < .001, and ****p < .0001 were considered statistically significant.
3. RESULTS
3.1. Characterization of the acidity of gallic acid solution and gold nanoparticles
As shown in Figure 1a, gallic acid molecule harbors four potential acidic protons, that is, three phenolic OHs with pKa values of, 8.7, 11.4, and 13 and a carboxylic acid with pKa value of 4.0. In order to determine the acidity of gallic acid and avoid the potential effect of the acidity on glioma cell growth, we detected the pH value of gallic acid solutions at the concentrations of 100, 200, and 300 μg/ml. The pH value of the above gallic acid solutions was 7.34 ± 0.041, 7.20 ± 0.0251, and 7.11 ± 0.055 in the 100, 150, and 200 μg/ml group, respectively, while the pH value of PBS solvent was 7.39 ± 0.056 (Figure 1b). Our results indicate that the gallic acid has subtle effect on the solution acidity, which supports that gallic acid is an extremely weak acid. Therefore, it is unlikely that the acidity of gallic acid solution contributes to its biological role in glioma cell growth.
FIGURE 1.
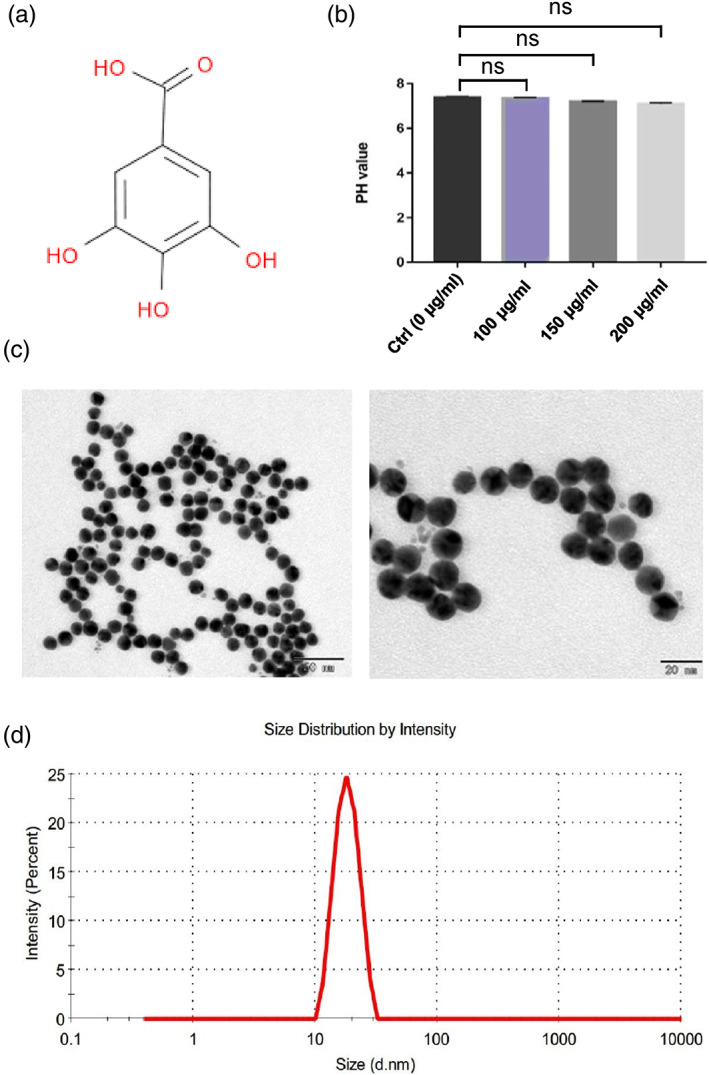
Characterization of gallic acid and gold nanoparticles. (a) Chemical structure of gallic acid. (b) pH values of gallic acid solutions with different concentrations (0, 100, 200, and 300 μg/ml). (c) The morphology of gold nanoparticles was detected by transmission electron microscope. Left panel: low‐magnification image (scale bar = 80 nm). Right panel: high‐magnification image (scale bar = 20 nm). (d) Particle size distribution of GNPs by dynamic light scattering instrument (DLS)
We next examined the characteristics of GNPs by using a transmission electron microscope (TEM), and found that GNPs demonstrated round in shape with relatively uniform in size and shape, with an average diameter of 20 ± 0.65 nm. Furthermore, good dispersion and no obvious fusion adhesion between the particles were observed (Figure 1c). In addition, the GNP physicochemical properties were measured by DLS analysis. The average diameter of GNPs was 23 ± 0.34 nm (Figure 1d), which is slightly larger than the diameter of GNP under TEM due to GNPs encapsulated by water molecules. Consistent with the observation in TEM, the particle size also displayed uniformed distribution. Last, we measured the concentration of GNP solution as 44.80 μg/ml by inductively coupled plasma atomic emission spectroscopy (ICP‐AES), and calculated that the actual gold recovery was 91.24%, which was supported by a previous study. 8 These data imply the GNPs in this study are appropriate for gallic acid carrier due to these characteristics.
3.2. Generation of GA‐GNPs and definition of GA:GNP feed ratio
To further characterize gallic acid solution, we performed ultraviolet spectrophotometer analysis and found that the maximal absorption peak was at 265 nm (Figure 2a). Based on the absorption peak, we further defined a standard curve with linear equation as Y = 0.0419x +0.00552 and the correlation coefficient R 2 = 0.99981 (Figure 2b). Given that the correlation coefficient is higher than 0.999, the linear equation has a good fitting degree with the concentration ranged from 0.1 to 20 μg/ml.
FIGURE 2.
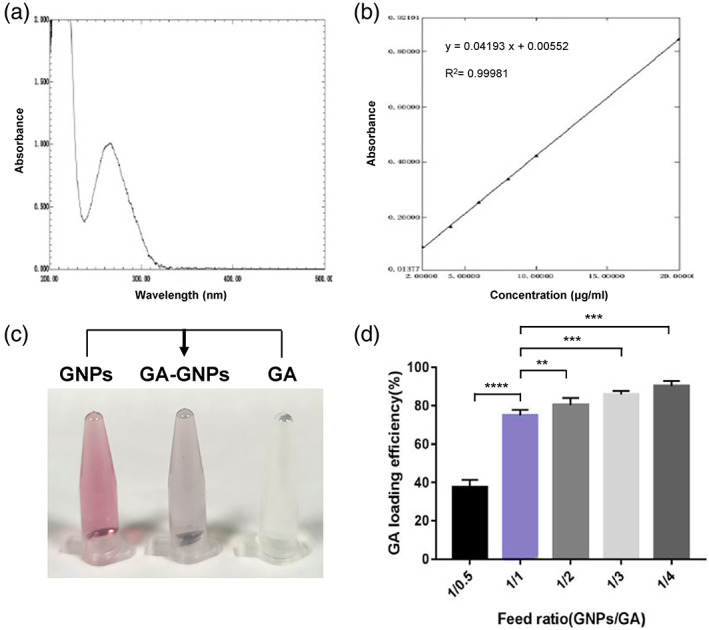
Generation of GA‐GNPs and definition of GA:GNP feed ratio. (a) The ultraviolet absorption peak of gallic acid solution detected by ultraviolet spectrophotometer analysis (~265 nm). (b) The linear equation of the relationship between the concentration of gallic acid solution and the absorbance at wavelength 265 nm. (c) Photographs showing the color change of GNP (pale red) reacted with gallic acid (colorless). The GA‐GNPs sample in the middle demonstrates pale purple. (d) The feed ratio of GA:GNPs was determined by adding different amounts of gallic acid to GNPs (1:1, 2:1, 3:1, and 4:1)
We next generated GA‐GNPs by adding gallic acid to the GNP solution and stirring in the dark for overnight. The 20‐nm‐diameter GNP solution was pale red, while the gallic acid solution showed colorless. When gallic acid was mixed with GNP, we observed a pale purple solution, indicating that gallic acid may conjugate with GNPs (Figure 2c). In order to define the GA:GNP feed ratio, we mixed GNPs and gallic acid at the ratios of 1:1, 1:2, 1:3, or 1:4. When the ratio of GNPs to gallic acid was 1:1, the drug loading almost reached the plateau (Figure 2d), because when the ratios of GNPs to gallic acid continued to increase, the drug loading rate did not show linear elevation (Figure 2d). This might indicate that GA loading under 1/1 ratio has uniformed and even GA distribution on GNPs, which makes GA‐GNPs have stable characteristics. This feed ratio might be attributed to the surface properties of GNPs. Therefore, our generated GA‐GNPs have the feeding ratio 1:1 in our following biological experiments.
3.3. GA‐GNPs demonstrate cytotoxic effect on human glioma U251 cells and lead to sensitization to radiation
We first investigated the toxic effects of GA‐GNPs on U251 cells by using MTT proliferation test. Based on previous publications regarding the cytotoxic concentrations of GA‐GNPs, 23 , 24 we treated U251 cells with increasing concentrations of GA‐GNPs (100, 150, 200 μg/ml) for 24, 48, and 72 hr. The toxic effect was evaluated according to the decrease in survival percentage. As shown in Figure 3a, GA‐GNPs showed a significant cytotoxic effect on U251 cell survival in a time‐dependent manner (day 1 to day 3). These highest values correlated to decrease in surviving cells were 70.25, 70, and 68.75% upon treatment with 100, 150, and 200 μg/ml GA‐GNPs at day 3, respectively. This result validated the cytotoxic effect of GA‐GNPs on glioma cell survival. Intriguingly, we did not observe any significant cytotoxicity of GNPs alone in U251 cells at any individual time point and dose (Figure 3a), indicating that gallic acid on the GA‐GNPs might play a key role in inhibiting U251 cell survival, and GNPs do not abrogate the biological effects of gallic acid.
FIGURE 3.
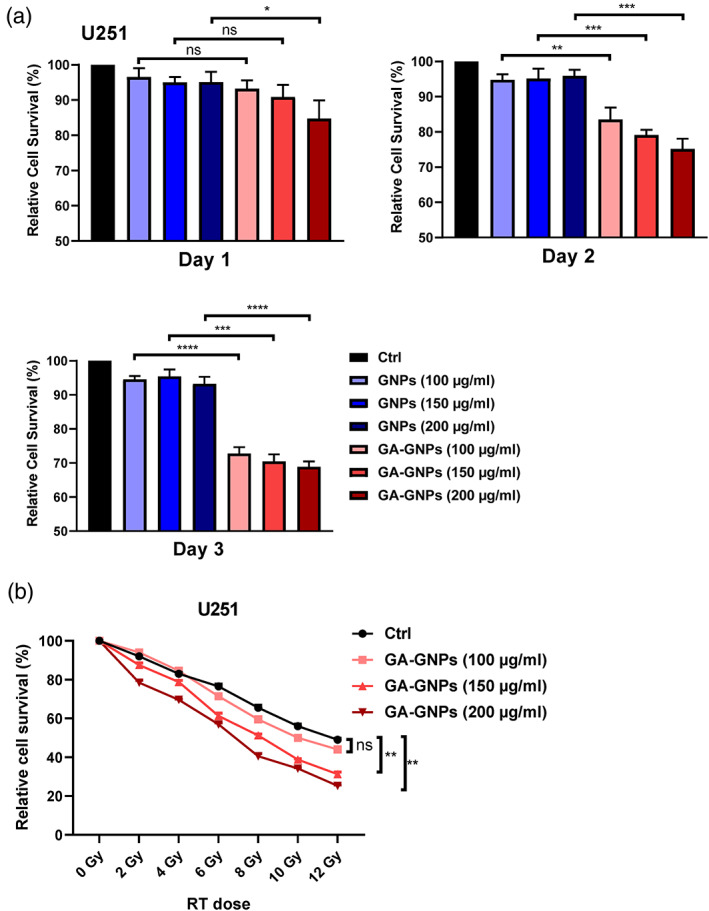
GA‐GNPs demonstrate cytotoxic effect on human glioma U251 cells and lead to sensitization to radiation. (a) U251 cells were treated with different concentrations of GNPs or GA‐GNPs (100, 150, 200 μg/ml) for indicated time points (days 1, 2, and 3). Cell survival was determined by MTT analysis. B. U251 cells were treated with combination of different concentrations of GA‐GNPs (100, 150, and 200 μg/ml) and multiple dose of radiation (0, 2, 4, 6, 8,10, and 12 Gy). After 48 hr, cell viability was determined by MTT analysis
We next evaluated whether GA‐GNPs are able to sensitize glioma U251 cells to radiation, as radiotherapy is usually performed after surgery for high‐grade gliomas. We treated U251 cells by combination of different doses of radiation (0, 2, 4, 6, 8, 10, and 12 Gy) and GA‐GNPs (100, 150, and 200 μg/ml) for 48 hr, and found significant survival differences in the GA‐GNPs plus radiation treatment, relative to radiation alone, especially at higher concentrations of GA‐GNPs (150, 200 μg/ml) (Figure 3b). Therefore, our data that GA‐GNPs render glioma U251 cells sensitive to radiation.
3.4. GA‐GNPs enhance RT‐induced S and G2/M cell cycle arrest and cell death in U251 cells
Given that GA‐GNPs possess radiosensitization ability in glioma U251 cells, we further examined the cell cycle distributions of U251 cells in response to control, gallic acid, GNPs, GA‐GNPs in the presence of radiation. As shown in Figure 4a, both gallic acid and GNPs increased the percentages of S and G2/M cell populations, while GA‐GNPs further induced the S and G2/M cell cycle arrest (with S%, 21.2; G2/M, 8.1). The S and G2/M accumulation of cells was at the expense of a decrease in G1‐phase cell population in the GA‐GNPs treated group. In addition, we performed flow cytometry analysis and observed, upon radiation, a higher increase (32.7%) of U251 cell death when simultaneously treated with GA‐GNPs, compared with control (17.3%), GNPs (24.8%), or gallic acid (28.3%) (Figure 4b). As the Annexin V staining in Figure 4b indicated the early apoptotic cell population, we therefore investigated the expression levels of a crucial pro‐apoptotic cell biomarker BAX as well as an anti‐apoptotic protein BCL‐2 in each treatment. Western blotting analysis demonstrated that GA‐GNPs plus radiation treatment achieved highest BAX and lowest BCL‐2 protein expression in U251 cells (Figure 4c,d). Taken together, our results clearly showed that GA‐GNPs facilitate RT‐mediated S and G2/M cell cycle arrest and cell death in U251 glioma cells.
FIGURE 4.
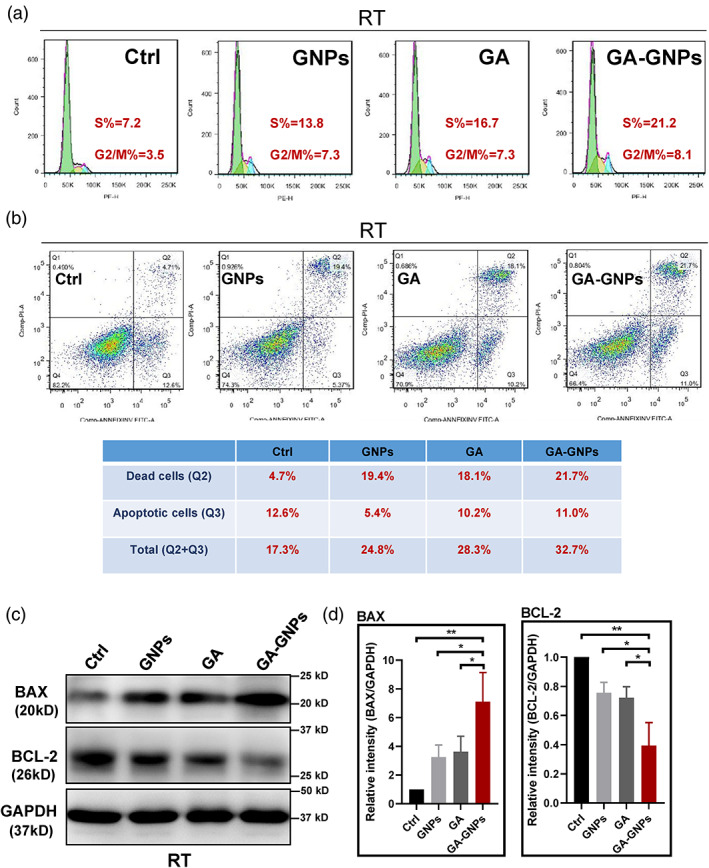
GA‐GNPs enhance RT‐induced S and G2/M cell cycle arrest and cell death in U251 cells. (a) Cell cycle analysis of U251 cells treated with control, gallic acid, GNPs, and GA‐GNPs in the presence of radiation (8 Gy) by flow cytometry. (b) U251 cells were treated with control, gallic acid, GNPs, and GA‐GNPs in the presence of radiation (8 Gy), and the early apoptotic and dead cells were quantitated by annexin V and PI staining in flow cytometry analysis. (c) Immunoblotting analysis to determine the relative expression of BAX and BCL‐2 proteins in U251 cells treated with control, gallic acid, GNPs, and GA‐GNPs in the presence of radiation (8 Gy). (d) Western blots (n = 3) were quantitated for BAX (left panel) and BCL‐2 (right panel) protein levels relative to GPADH in the indicated treatment conditions
4. DISCUSSION
In this study, we have found that GA‐GNPs are able to exert cytotoxic effect on U251 cells and render cells sensitive to radiation. GA‐GNPs enhance radiation‐induced S and G2/M cell cycle arrest and cell death in U251 cells. Given the urgent need of novel therapeutic strategies in GBM treatment, our work provided strong basic evidence for further translational investigation of GA‐GNPs.
GBM is a common tumor of central nervous system, and its incidence in China is significantly higher than the world average with the 5‐year survival rate was less than 10%. 25 , 26 , 27 The European Neuro‐Oncology Society guidelines focus more on radiology based on the 2016 World Health Organization recommendations for the clinical treatment of adult GBM patients. 28 The goal of radiotherapy in GBM is to improve local control at a reasonable risk benefit ratio. In the current clinical treatment, more than 70% of patients with GBM need to receive radiotherapy, while more than 80% of the tumors can significantly improve the local control rate through combined radiotherapy, thus affecting the survival rate and recurrence rate. 29 Therefore, overcoming radioresistence in GBM treatment is crucial for promoting survival and disease‐free benefits in patients.
Gallic acid is one of the important polyphenol candidates for cancer treatment. Gallic acid has demonstrated its potent ability to suppress cell viability, proliferation, invasion, and angiogenesis in the human glioma cell lines. 9 Gallic acid has been also identified to have antioxidant property, which functions as scavengers of reactive oxygen species. 30 Administration of gallic acid prevents radiation‐induced weight loss and mortality in mice by facilitating DNA repair process. 31 Therefore, gallic acid exhibits cytotoxicity in cancer cells but spares normal tissues, which makes gallic acid gain great attention for further drug development.
The rapid development of nanoscience promotes interdisciplinary integration and accelerates the development of anti‐cancer drugs. Up to now, a large number of nanoparticles are synthesized by physical and chemical methods of metals such as silver, copper, platinum, and so forth. Unfortunately, there are often some problems with these nanoparticles, such as high biotoxicity and complex synthesis. GNPs have become the most attractive nano‐system due to their good biocompatibility, small size, low biotoxicity, easy to be synthesized, and can be used as a nano‐carrier for a variety of drugs, as well as the ability to enhance CT and MRI image contrast. 19 , 32 In our work, we generated GNPs by using sodium citrate as stabilizer and reducing agent to react with tetrachloroauric acid in boiling water. The GNPs have excellent particle size dispersion, and the solution of GNPs is generally stable and can be stored for 1 month in the dark at room temperature. Therefore, the GNPs in this study present essential characters as a nano‐carrier of gallic acid.
Although the anti‐tumor effects of GA‐GNPs have been examined in some cancers, the role of GA‐GNPs as a radiosensitizer has not been extensively studied. Our work established that GA‐GNPs enhance radiation‐induced U251 cell death and cell cycle arrest. Future studies will expand this finding to other GBM cell lines, as well as GBM stem cells. In addition, the therapeutic efficacy of combination of GA‐GNPs and radiation needs to be tested in multiple in vivo xenograft mouse models. Taken together, this study revealed GA‐GNPs as a promising anti‐tumor and radiation enhancement reagent for GBM treatment.
AUTHOR CONTRIBUTIONS
Xinjun Wang: Conceived and designed the experiments; wrote the manuscript; read and approved the final manuscript. Xudong Fu: Conceived and designed the experiments; wrote the manuscript; read and approved the final manuscript. Zhou Jing: Performed the experiments and analyzed the data; wrote the manuscript; read and approved the final manuscript. Minghe Li: Performed the experiments and analyzed the data; wrote the manuscript; read and approved the final manuscript. Hongyuan Wang: Performed the experiments and analyzed the data; wrote the manuscript; read and approved the final manuscript. Zhuo Yang: Performed the experiments and analyzed the data; read and approved the final manuscript. Shaolong Zhou: Performed the experiments and analyzed the data; read and approved the final manuscript. Jian Ma: Performed the experiments and analyzed the data; read and approved the final manuscript. Enping Meng: Performed the experiments and analyzed the data; read and approved the final manuscript. Hengwei Zhang: Contributed reagents and materials; read and approved the final manuscript. Wulong Liang: Contributed reagents and materials; read and approved the final manuscript. Weihua Hu: Contributed reagents and materials; read and approved the final manuscript.
ACKNOWLEDGMENTS
This work is sponsored by the grants: Key Research Projects of Henan Higher Education Institutions, China (Grant number: 14A320078); National Natural Science Foundation of China (Grant number: 81874068 and 81972361).
Jing Z, Li M, Wang H, et al. Gallic acid‐gold nanoparticles enhance radiation‐induced cell death of human glioma U251 cells. IUBMB Life. 2021;73:398–407. 10.1002/iub.2436
Zhou Jing, Minghe Li, and Hongyuan Wang should be regarded as joint first authors.
Funding information Key Research Projects of Henan Higher Education Institutions, China, Grant/Award Number: 17A310031; National Natural Science Foundation of China, Grant/Award Numbers: 81874068, 81972361
[Corrections added after first online publication on 29‐Jan‐2021; Funding number 14A320078 modified to 17A310031]
Contributor Information
Xinjun Wang, Email: wangxj@zzu.edu.cn.
Xudong Fu, Email: fxd1064@126.com.
REFERENCES
- 1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: Correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. [DOI] [PubMed] [Google Scholar]
- 3. Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. [DOI] [PubMed] [Google Scholar]
- 4. Verma S, Singh A, Mishra A. Gallic acid: Molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35(3):473–485. [DOI] [PubMed] [Google Scholar]
- 5. Rasool M, Malik A, Manan A, et al. Roles of natural compounds from medicinal plants in cancer treatment: Structure and mode of action at molecular level. Med Chem. 2015;11(7):618–628. [DOI] [PubMed] [Google Scholar]
- 6. Liu CC et al. Lauryl Gallate induces apoptotic cell death through caspase‐dependent pathway in U87 human glioblastoma cells in vitro. In Vivo. 2018;32(5):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu SS, Chou CT, Liao WC, et al. The effect of gallic acid on cytotoxicity, ca(2+) homeostasis and ROS production in DBTRG‐05MG human glioblastoma cells and CTX TNA2 rat astrocytes. Chem Biol Interact. 2016;252:61–73. [DOI] [PubMed] [Google Scholar]
- 8. Lee SH, Kim JK, Kim DW, et al. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochim Biophys Acta. 2013;1830(8):4017–4029. [DOI] [PubMed] [Google Scholar]
- 9. Lu Y, Jiang F, Jiang H, et al. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol. 2010;641(2–3):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paolini A et al. Gallic acid exerts a protective or an anti‐proliferative effect on glioma T98G cells via dose‐dependent epigenetic regulation mediated by miRNAs. Int J Oncol. 2015;46(4):1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arvanitis CD, Ferraro GB, Jain RK. The blood‐brain barrier and blood‐tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banks WA. From blood‐brain barrier to blood‐brain interface: New opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. [DOI] [PubMed] [Google Scholar]
- 13. Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3(6):499–508. [DOI] [PubMed] [Google Scholar]
- 14. Sahni JK, Doggui S, Ali J, Baboota S, Dao L, Ramassamy C. Neurotherapeutic applications of nanoparticles in Alzheimer's disease. J Control Release. 2011;152(2):208–231. [DOI] [PubMed] [Google Scholar]
- 15. Haumann R, Videira JC, Kaspers GJL, van Vuurden DG, Hulleman E. Overview of current drug delivery methods across the blood‐brain barrier for the treatment of primary brain tumors. CNS Drugs. 2020;34:1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendes M, Sousa JJ, Pais A, Vitorino C. Targeted Theranostic nanoparticles for brain tumor treatment. Pharmaceutics. 2018;10(4):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foster C et al. Improved targeting of cancers with Nanotherapeutics. Methods Mol Biol. 2017;1530:13–37. [DOI] [PubMed] [Google Scholar]
- 18. Swain S, Sahu P, Beg S, Babu S. Nanoparticles for cancer targeting: Current and future directions. Curr Drug Deliv. 2016;13(8):1290–1302. [DOI] [PubMed] [Google Scholar]
- 19. Ning L, Zhu B, Gao T. Gold nanoparticles: Promising agent to improve the diagnosis and therapy of cancer. Curr Drug Metab. 2017;18(11):1055–1067. [DOI] [PubMed] [Google Scholar]
- 20. Murphy CJ, Gole AM, Stone JW, et al. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc Chem Res. 2008;41(12):1721–1730. [DOI] [PubMed] [Google Scholar]
- 21. Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir. 2005;21(23):10644–10654. [DOI] [PubMed] [Google Scholar]
- 22. Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110(32):15700–15707. [DOI] [PubMed] [Google Scholar]
- 23. Daduang J, Palasap A, Daduang S, Boonsiri P, Suwannalert P, Limpaiboon T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac J Cancer Prev. 2015;16(1):169–174. [DOI] [PubMed] [Google Scholar]
- 24. Rattanata N, Daduang S, Wongwattanakul M, et al. Gold nanoparticles enhance the anticancer activity of Gallic acid against cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 2015;16(16):7143–7147. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Chen JX, Zhou Q, et al. Statistical report of central nervous system tumors histologically diagnosed in the Sichuan Province of China from 2008 to 2013: A West China glioma center report. Ann Surg Oncol. 2016;23(Suppl 5):946–953. [DOI] [PubMed] [Google Scholar]
- 26. Yang P, Wang Y, Peng X, et al. Management and survival rates in patients with glioma in China (2004‐2010): A retrospective study from a single‐institution. J Neurooncol. 2013;113(2):259–266. [DOI] [PubMed] [Google Scholar]
- 27. Ma X, Lv Y, Liu J, et al. Survival analysis of 205 patients with glioblastoma multiforme: Clinical characteristics, treatment and prognosis in China. J Clin Neurosci. 2009;16(12):1595–1598. [DOI] [PubMed] [Google Scholar]
- 28. Pace A, Dirven L, Koekkoek JAF, et al. European Association for Neuro‐Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 29. Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen HM, Wu YC, Chia YC, et al. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS‐mediated anti‐cancer activity in human prostate cancer cells. Cancer Lett. 2009;286(2):161–171. [DOI] [PubMed] [Google Scholar]
- 31. Nair GG, Nair CK. Radioprotective effects of gallic acid in mice. Biomed Res Int. 2013;2013:953079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


