ABSTRACT
Organic fluorinated compounds have been detected in various environmental media and biota. Some of these compounds are regulated locally (e.g., perfluorononanoic acid maximum contaminant level in drinking water by the New Jersey Dept. of Environmental Protection), nationally (e.g., perfluorooctanoic acid maximum acceptable concentration in drinking water by Health Canada), or internationally (e.g., Stockholm Convention on Persistent Organic Pollutants). Globally, regulators and researchers seek to identify the organic fluorinated compounds associated with potential adverse effects, bioaccumulation, mobility, and persistence to manage their risks, and, to understand the beneficial attributes they bring to products such as first responder gear, etc. Clarity is needed to determine the best analytical method for the goal of the analyses (e.g., pure research or analysis to determine the extent of an accidental release, monitoring groundwater for specific compounds to determine regulatory compliance, and establish baseline levels in a river of organic fluorinated substances associated with human health risk prior to a clean‐up effort). Analytical techniques that identify organic fluorine coupled together with targeted chemical analysis will yield information sufficient to identify public health or environmental hazards. Integr Environ Assess Manag 2021;17:331–351. © 2020. W.L. Gore & Associates Inc. Integrated Environmental Assessment and Management published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).
Keywords: Perfluoroalkyl, Polyfluoroalkyl, Analytical, Classification, Fluoropolymers
KEY POINTS
This paper provides new and important clarity on fluorine‐containing compounds, their uses, toxicological, chemical, and physical properties in relation to environmental occurrence, analysis, and how the interpretation of the analytical data is critical to effective regulation.
To effectively protect public health and the environment, efforts must focus on those fluorinated compounds that pose the greatest risk, which necessitates an understanding of exposure pathways and determination of the concentration of the hazardous substance therein.
Since national and internationally accepted standards do not always exist, this paper addresses analytical approaches so that fluorinated organic compounds can be identified and quantified appropriately.
INTRODUCTION
In the area of the environmental chemistry of fluorinated substances, and particularly as it applies to setting limits supporting environmental and health and safety regulations, clarity is needed to match the appropriate laboratory analytical method to the stated goal(s) for such analyses. For example, an adequate limit of detection varies depending on the matrix, the compounds of interest, and the intended regulatory purpose or aim of the study. Monitoring for specific purposes, such as emissions from a manufacturing facility or to obtain certain environmental permits, may impact sampling and testing procedures. The capabilities of an analytical chemistry laboratory to measure fluorinated substances in a particular matrix can drive regulatory decision making. On the other hand, regulatory decision making determines how compliance is achieved analytically (i.e., the requirements of an analytical method).
With respect to analytical techniques applied to identify fluorinated compounds, the use of properly validated analytical chemistry measurements is critical to determining the identity and concentration of fluorinated substances. The presence of certain per‐ and polyfluoroalkyl substances (PFAS) in the environment, for example, has become of concern to the regulatory community globally. This paper discusses fluorinated compounds, briefly addressing inorganic fluorine and inorganic fluorinated compounds, then generally focusing on organic fluorinated compounds and more specifically, per‐ and polyfluorinated substances. Fluorinated compounds regulated under the Montreal and Kyoto protocols will be mentioned, as will fluorine‐containing pesticides, pharmaceuticals, and veterinary drugs.
THE UNIVERSE OF FLUORINE‐CONTAINING COMPOUNDS
Inorganic fluorinated compounds
Elemental fluorine, the thirteenth most abundant element in the earth's crust (Jaccaud et al. 2000), almost never occurs in nature except in its inorganic form, fluoride. Fluoride can be released to the environment through natural activities such as volcanic emissions, weathering of minerals, and dissolution mainly due to groundwater and marine aerosols. Some anthropogenic sources of fluorine are the manufacture and use of hydrofluoric acid, and the production of Al, steel, and oil. Historical production or application sites may be sources of fluorine (or the inorganic form fluorine) in the environment.
Common inorganic fluorine compounds include hydrogen fluoride (HF), sodium fluoride (NaF), and naturally occurring minerals such as fluorite (CaF2) (see Table 1). Hydrogen fluoride is an acid used to make refrigerants, pharmaceutical intermediates, fluoropolymers, and metals (Speight 2017). Calcium fluoride is used in the purification of Si, the manufacture of glass, the removal of Pb in flotation waste, the manufacture of clean steel, as pigment for paper with titanium dioxide (TiO2), as a solid lubricant for hot rolling, and to strengthen cement (Kirk et al. 1991). Inorganic fluorine compounds will not be discussed further other than to mention the analytical methods that can detect them.
Table 1.
Examples of fluorinated compounds
| Type | Organic/inorganic | Name | Use | Structure |
|---|---|---|---|---|
| Industrial chemical | Inorganic | NaF | Prevent dental caries, insecticide, wood preservative, cleaning compound, glass making | Na‐F |
| Industrial chemical | Inorganic | HF | Making of refrigerants, pharmaceutical intermediates, fluoropolymers, metals, glass etching and polishing, gasoline production, semiconductor preparation | H‐F |
| Industrial chemical | Inorganic | CaF2 | Purification of Si, glass manufacture, removal of Pb in flotation waste, manufacture of clean steel, pigment for paper with TiO2, solid lubricant for hot rollers, to strengthen cement | CaF2 |
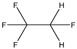
| Industrial chemical | Organic | R134a (1,1,1,2‐tetrafluoroethane) | Refrigerant, organic extraction solvent |
|
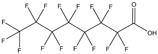
| Industrial chemical | Organic | Perfluorooctanoic acid (PFOA) | Surfactant, fluoropolymer polymerization aid |
|
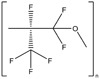
| Industrial chemical | Organic | Krytox (FG26) | Lubricant |
|
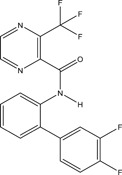
| Pesticide | Organic | Pyraziflumid | Fungicide |
|
| Pesticide | Organic | Fluopyram | Fungicide |
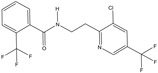
|
| Pesticide | Organic | Broflanilide | Insecticide |
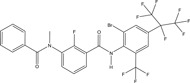
|
| Pesticide | Organic | Fipronil | Insecticide |
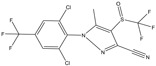
|
| Pharmaceutical | Organic | Lipitor (atorvastatin) | Cholesterol lowering drug |
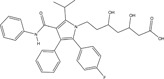
|
| Pharmaceutical | Organic | Prozac (fluoxetine) | Antidepressant, antianxiety drug |
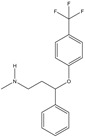
|
| Pharmaceutical | Organic | Celebrex (celecoxib) | Nonsteroidal anti‐inflammatory drug (NSAID) |

|
| Veterinary drug | Organic | Florfenicol | Veterinary antibiotic |
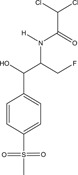
|
| Veterinary drug, pharmaceutical, WHO Essential Medicine | Organic | Isoflurane | Anesthetic |
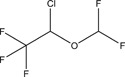
|
PFOA = perfluorooctanoic acid; WHO = World Health Organization.
a For more organic fluorinated compounds used in pharmaceuticals, pesticides, and veterinary drugs, see Cormier et al. (2009).
Organic fluorinated compounds
There are thousands of organic fluorinated compounds, mostly synthetic, which differ in chemical class, uses, and physical, chemical, and toxicological properties. Organic fluorinated compounds include industrial chemicals (e.g., haloalkane refrigerant R‐134a also called 1,1,1,2‐tetrafluoroethane); pesticides (e.g., pyraziflumid, fluopyram, broflanilide); pharmaceuticals (e.g., Lipitor, Prozac, Celebrex, isoflurane); and veterinary drugs (e.g., florfenicol, isoflurane) (Cormier et al. 2009). The addition of fluorine stabilizes an agrochemical and can modify bioactivity through binding to a target site or enzyme, transporting the bioactive molecule from application site to target site, or blocking metabolic activation (Theodoridis 2006; Fujiwara and O'Hagan 2014). The addition of multiple fluorine atoms to pharmaceuticals and veterinary drugs increases lipophilicity and bioavailability of the molecule and can increase its therapeutic index and help optimize pharmacokinetic properties (O'Hagan 2010; Fujiwara and O'Hagan 2014).
To highlight the variety and utility of fluorinated chemicals, Table 1 provides examples of different types of fluorinated chemicals including their names, uses, whether organic or inorganic, and chemical structures. Chemically, these organic fluorinated compounds contrast with the PFAS in that they may have only a few fluorine atoms in their structures, such as terminal CF3. Fluorine has also been found in many industrial or consumer products (e.g., fluorine in liquid crystal displays [LCD], and flat panel televisions, halon fire extinguishers, refrigerants like Freon 12) (Dolbier 2005). Historical production and application sites of fluorinated compounds and products containing them may be sources of fluorine (or the inorganic form fluoride) in the environment.
Per‐ and polyfluoroalkyl substances
Differentiation of organic fluorinated compounds by characterization of functional groups is useful for discussion of analytical methods and interpretation of results. In this paper, PFAS are aliphatic substances for which all or most H atoms have been replaced with fluorine atoms and contain the perfluoroalkyl moiety (CnF2n+1)‐R (Buck et al. 2011; OECD 2018). Broadly, PFAS are either polymers or nonpolymers.
Nonpolymer per‐ and polyfluoroalkyl substances
There are 2 types of nonpolymer PFAS: perfluoroalkyl substances and polyfluoroalkyl substances. Perfluoroalkyl substances are those aliphatic substances for which all of the H atoms attached to C atoms (in the nonfluorinated substance from which they are notionally derived) have been replaced by fluorine atoms (except those H atoms whose substitution would modify the nature of any functional groups present). The polyfluoroalkyl substances are similar to perfluoroalkyl substances with the exception that not all H atoms attached to C atoms have been replaced with fluorine atoms (Buck et al. 2011). Table 2 provides a more detailed comparison of the toxicological, chemical, and physical characteristics of some of the nonpolymer perfluoroalkyl substances (note that this paper will not address the nonpolymer PFAS, such as chlorofluorocarbons [CFCs] and hydrofluorocarbons [HCFCs] that are subject to the Montreal and Kyoto Protocols).
Table 2.
Nonpolymer PFAS toxicity, physical, and chemical properties
| Nonpolymer PFAS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Perfluoroalkyl substances | Polyfluoroalkyl substances | ||||||||
| Description | Perfluoroalkyl acids (PFAA) | Fluorotelomer alcohols | |||||||
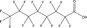
| Substance | PFBA | PFHxA | PFOA | PFBS | PFOS | Hexafluoropropylene oxide (HFPO) dimer acid, ammonium salt | 2,2,3‐trifluoro‐3‐[1,1,2,2,3,3‐hexafluoro‐3‐(trifluoromethoxy) propoxy]propanoic acid | 6:2 FTOH | 8:2 FTOH |
| CAS number | 375‐22‐4 | 307‐24‐4 | 335‐67‐1 | 375‐73‐5 | 1763‐23‐1 | 62037‐80‐3 | 919005‐14‐4 | 647‐42‐7 | 678‐39‐7 |
| Structure |
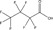
|

|
|

|

|
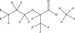
|
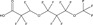
|

|

|
| Chain length | Short | Short | Long | Short | Long | Short | Short | Short | Long |
| Molecular weight | 214.0384 | 314.0534 | 414.069 Da | 300.11 | 500.13 | 347.084 | 378.07 | 364.1 | 464.12 |
| Physical state, description | Liquid | Colorless liquid | White to off‐white powder | Colorless liquid | Liquid | Clear, colorless liqud | Clear, colorless liquid | Clear, colorless to pale yellow liquid | Waxy solid |
| Boiling point | 120 to 121 °C | 157 °C | 192 °C | 210 to 212 °C | 249 °C | 108 °C | 183 °C | 171 °C | NDA |
| Melting point | −17.5 °C | NDA | 54.3 °C | NDA | NDA | 183 to 184 °C | NDA | 78 °C | NDA |
| Vapor pressure | 5866 Pa at 56 °C | 263.9 Pa at 25 °C (est) | 4.21 Pa at 25 °C | 3.57 Pa at 25 °C (est) | 0.2667 Pa at 25 °C | 2910 Pa at 20 °C | 32 Pa at 20 °C | 26.6645 Pa at 25 °C | 31 Pa at 25 °C |
| Stability (hydrolysis, photolysis, oxidation, thermal, biological) | Stable | Concentrations decreased by 0.8% after 106 d of solar irradiation; no other data | Stable | NDA | NDA | Stable | Stable | NDA | NDA |
| Water solubility (USP 2011) | 2.14 × 105 mg·L−1 at 25 °C | In water, 15 700 mg·L−1 at ambient temperature | 9.50 × 103 mg·L−1 at 25 °C | In water, 344 mg·L−1 at 25 °C (est) | 3.2 × 10−3 mg·L−1 at 25 °C (est) | >1000 g·L−1 at 20 °C | 100% Soluble in water | “insoluble” 18.8 mg·L−1 | 140 µg·L−1 at 25 °C |
| Log K OW | Not applicable | 3.48 (est) | 4.81 (est) | 1.82 (est) | 4.49 (est) | NDA | 1.89 | 4.54 | NDA |
| Particle size µm | NDA | NDA | NDA | NDA | NDA | Dried solid 76% >105 microns; 24% < 105 microns | NDA | NDA | NDA |
| Acute Dermal LD50 | NDA | Low (>2000 mg/kg) | Low (>2000 mg/kg) | Low (>2000 mg/kg) | NDA | Low (5000 mg/kg) | Low (5000 mg/kg) | Low (5000 mg/kg) | NDA |
| Skin irritant, Y or N | Y, corrosive | Y | Y | N | N | Y | Y, mild | Y | NDA |
| Eye irritant, Y or N | Y (serious damage) | Y | Y | Y | Y | Y | Y | Y | NDA |
| Skin sensitizer, Y or N | NDA | N | N | N | NDA | N | Y | N | NDA |
| 90‐d animal feeding study (no effect dose) | 6 mg/kg‐day | 50 mg/kg‐day | 0.06 mg/kg‐day | 200 mg/kg‐day | 0.4 mg/kg‐day | 0.1 mg/kg‐day | 10 and 100 mg/kg‐day for males, females | 5 mg/kg‐day | 5 mg/kg‐day |
| Mutagen, Y or N | NDA | N | N | N | N | N | N | N | N |
| Caused tumors in lab rats, Y or N | NDA | N | Y | NDA | Y | Y | NDA | NDA | NDA |
| Half‐life in humans | 69 to 87 h | <28 d | 3.3 to 3.8 y | 24 to 46 d | 5.4 to 5.9 y | 2.3 h | 23 ± 11 d | NDA | NDA |
| References | (Ding and Peijnenburg 2013; NICNAS 2015; ChemSpider 2018; Sigma Aldrich 2018a; ATSDR 2018) | (Savu 1994; USEPA 2012; Environ 2014; Zhao L et al. 2014; ChemSpider 2016; Luz et al. 2019) | (Kennedy et al. 2004; Butenhoff et al. 2004; Yalkowsky et al. 2010; Bhhatarai and Gramatica 2011; USEPA 2012; Haynes 2014) | (Kosswig 2000; Lewis 2012; USEPA 2012; Sigma Aldrich 2015) | (Ashford 1994; Lewis 2012; USEPA 2012) | (Biegel et al. 2001; Caverly Rae et al. 2015; Beekman et al. 2016; Hoke et al. 2016; ECHA 2019b; PubChem. 2019) | (Gordon 2011; Wang et al. 2013; ECHA 2020) | (Daikin 2007, 2009a, 2009b) | (Ladics et al. 2008; Climate and Pollution Agency, Norway 2010) |
PFAS = per‐ and polyfluoroalkyl substances; PFAA = perfluoroalkyl acids; PFBA = perfluorobutanoic acid; PFHxA = perfluorohexanoic acid; PFOA = perfluorooctanoic acid; PFBS = perfluorobutane sulfonic acid; HFPO = hexafluoropropylene oxide; FTOH = fluorotelomer alcohol; NDA = no data available.
Nonpolymer perfluoroalkyl substances, such as perfluoroalkyl acids (PFAAs), include examples as the C8 carboxylic or sulfonic acids perfluorooctanoic acid (PFOA) or perfluorooctane sulfonic acid (PFOS), respectively; the C6 carboxylic or sulfonic acids perfluorohexanoic acid (PFHxA) or perfluorohexane sulfonic acid (PFHxS), respectively; and the C4 carboxylic or sulfonic acids perfluorobutanoic acid (PFBA) or perfluorobutane sulfonic acid (PFBS), respectively. See Table 2 for a comparison of some chemical, physical, toxicological, and environmental fate endpoints for nonpolymer PFAS. Table 2 presents 3 examples of the perfluoroalkyl carboxylic acids (C4, C6, C8), 2 examples of perfluoroalkyl sulfonic acids (C4, C8), 1 per‐ and 1 polyfluoropolyether carboxylic acids (NH4 + salt of hexafluoropropylene oxide dimer acid, and 2,2,3‐trifluoro‐3‐[1,1,2,2,3,3‐hexafluoro‐3‐(trifluoromethoxy) propoxy]propanoic acid, respectively), and 2 fluorotelomer alcohols (C6 and C8). All of these have relatively low molecular weights (≤500 Da). The C8 carboxylic and C8 sulfonic acids appear to be more potent in toxicity and have longer half‐lives, based on the data in Table 2. The PFAAs tend to be water soluble. Stability data are less readily available. In water, PFAAs dissociate into their anions that are highly persistent and a cation such as K+, Li+, and NH4 +. Their global distribution may be through atmospheric transport of acids and/or transport of anions in water (Vierke et al. 2013). Atmospheric transport and transformation of volatile precursors, such as fluorotelomer compounds that degrade to PFAAs, may also be an important mechanism of global distribution (Prevedouros et al. 2006; Wang et al. 2014a, 2014b).
The perfluorinated polyether surfactants, such as hexafluoropropylene oxide dimer acid (HFPO‐DA), are nonpolymer perfluoroalkyl substances, more specifically perfluoroether carboxylic acids (PFECA) (see Table 2). Additional perfluoroalkyl substances include perfluorooctane sulfinic acid (PFOSI) and bis(perfluoryloctyl) phosphinic acid, perfluoroalkane sulfonyl fluorides and sulfonamides, perfluoroalkanoyl fluorides, and perfluoroalkyl iodides and aldehydes (Buck et al. 2011). Refer to Table 2 for more comparisons of the toxicological, chemical, and physical properties of perfluoroalkyl substances.
Table 2 also compares the toxicological, chemical, and physical characteristics of some of the nonpolymer polyfluoroalkyl PFAS. Nonpolymer polyfluoroalkyl substances comprise a large number of compounds including semifluorinated n‐alkanes and alkenes, fluorotelomer‐based compounds (e.g., 6:2 fluorotelomer sulfonamide alkylbetaine, 6:2 fluorotelomer sulfonate, 6:2 fluorotelomer sulfonamide amine, 6:2 fluorotelomer alcohol, 8:2 fluorotelomer alcohol, fluorotelomer iodides, fluorotelomer olefins, fluorotelomer aldehydes) (Moe et al. 2012; D'Agostino and Mabury 2014), polyfluoroalkyl phosphate surfactants (commonly referred to as PAPS), and their thioether derivatives (Trier et al. 2011). Many polyfluoroalkyl substances have the potential to be transformed in the environment into perfluoroalkyl substances (Prevedouros et al. 2006; Wang et al. 2014a, 2014b; Mejia‐Avendaño et al. 2016; D'Agostino and Mabury, 2017) and as such can be PFAA precursors.
Pesticides, pharmaceuticals, and veterinary drugs
As previously mentioned, many pesticides, pharmaceuticals, and veterinary drugs contain at least 1 flourine atom with some containing more (see Table 1). These chemicals represent the many fluorinated chemicals that are beneficial to society that are not typically considered as PFAS as defined by Buck et al. (2011) or OECD (2018) but may be detected by nontargeted methods of analysis.
Polymer per‐ and polyfluoroalkyl substances
There are 3 types of PFAS polymers: fluoropolymers, perfluoropolyethers, and side‐chain fluorinated polymers. The properties of fluoropolymers, such as polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), ethylene tetrafluoroethylene (ETFE), and perfluoroalkoxy (PFA), were extensively discussed by Henry et al. (2018). Fluoropolymers are characterized by very high molecular weights (>100 000 Da), insolubility in water and most organic solvents. See Table 3 for the toxicological, physical, and chemical properties of examples of the polymer PFAS.
Table 3.
Polymer PFAS toxicity, physical, and chemical properties
| Polymer PFAS | |||||
|---|---|---|---|---|---|
| Description | Side‐chain fluorinated polymers | Fluoropolymer | Perfluoropolyether | ||
| Substance | 6:2 Fluorotelomer acrylatea | 6:2 Fluorotelomer methacrylatea | 1H,1H‐perfluorooctyl methacrylatea | Polytetrafluoroethylene (PTFE) | 1,1,1,2,2,3,3‐heptafluoro‐3‐(1,1,2,2,2‐pentafluoroethoxy)propane |
| CAS number | 17527‐29‐6 | 2144‐53‐8 | 3934‐23‐4 | 9002‐84‐0 | 60164‐51‐4 |
| Structure |
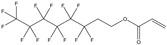
|
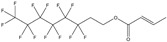
|
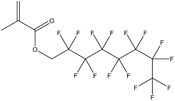
|
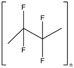
|
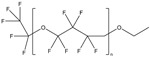
|
| Chain length | Short | Short | Long | N/A | N/A |
| Molecular weight | 418.15 | 432.18 | 468.16 | 389 000–45 000 000 | NDA |
| Physical state/description | Colorless liquid | Yellow liquid | Liquid | Solid | Colorless, viscous liquid |
| Boiling point | 210.2 °C | 210 °C | 70 °C | Not applicable | NDA |
| Melting point | Not applicable for liquids | Not applicable for liquids | Not applicable for liquids | 326 °C | NDA |
| Vapor pressure | 259 Pa at 25 °C | 8.6 Pa at 25 °C | NDA | NDA | NDA |
| Stability (hydrolysis, photolysis, oxidation, thermal, biological) | Unstable | Unstable | NDA | Stable | Stable |
| Water solubility (USP 2011) | 0.185 mg·L−1 at 25 °C | 0.042 mg·L−1 at 20 °C | NDA | Practically insoluble or insoluble (1 × 10−5 mg·L−1) | Insoluble |
| Log K OW | NDA | 5.2 at 23 °C | NDA | NDA | NDA |
| Particle size µm | Not applicable for liquids | Not applicable for liquids | Not applicable for liquids | 100 to 500 µm (powders) | Not applicable for liquids |
| Acute dermal LD50 | NDA | >5000 mg/kg | NDA | NDA | >17 000 mg/kg |
| Skin irritant, Y or N | N | N | Y | N | N |
| Eye irritant, Y or N | N | N | Y | N | N |
| Skin sensitizer, Y or N | N | N | NDA | N | N |
| 90‐d animal feeding study (no effect dose) | NDA | 5 mg/kg‐day | NDA | NDA | NDA |
| Mutagen, Y or N | N | N | NDA | N | N |
| Caused tumors in lab rats, Y or N | NDA | NDA | NDA | N | NDA |
| Half‐life in humans | NDA | NDA | NDA | N/A | NDA |
| References | (Sigma Aldrich 2018b; ECHA 2020a). | (Anand et al. 2012; ECHA 2019a, 2020b; Sigma Aldrich 2020) | (ECHA Substance Infocard 12/21/2019; Sigma Aldrich 2017) | (Henry et al. 2018) | (DuPont SDS 11/2014) |
PFAS = per‐ and polyfluoroalkyl substances; PTFE = polytetrafluoroethylene; NDA = no data available.
Note these data are for the fluorinated side‐chains.
Perfluoropolyethers span a broad range of molecular weight from <500 Da to greater than 10 000 Da (Sianesi et al. 1994). They exist as liquids or greases, and are sold commonly under the trade names of Krytox™, Fomblin®, and Galden® (see Table 3).
Side‐chain fluorinated polymers constitute a diverse type of polymer substances. The fluorinated side‐chain moieties of these materials can be produced by the telomerization or electrochemical fluorination process. Fluorinated side‐chains are attached to the polymer backbone by a “spacer moiety” and a linking group, as shown in Figure 1. Fluorotelomer acrylates manufactured with 8:2 fluorotelomer acrylate and 6:2 fluorotelomer methacrylate side‐chains are 2 examples of this type of polymer PFAS. The linking group can be susceptible to cleavage, depending on its structure, resulting in loss of the fluoroalkyl side‐chain (Russell et al. 2008; Rankin et al. 2014; Russell 2015; Washington et al. 2015; Holmquist et al. 2016). Thus, side‐chain fluorinated polymers can ultimately be a source of perfluoroalkyl acids (PFAAs), such as PFOA, PFOS, perfluorobutanoic acid (PFBA), perfluorohexane sulfonic acid (PFHxS), or perfluorohexane carboxylic acid (PFHxA), and so forth, unless there is stability data to prove otherwise.
Figure 1.
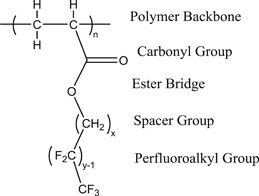
Side‐chain fluorinated polymer structure (adapted from Honda et al. 2005).
ENVIRONMENTAL OCCURRENCE OF PFAS
Matrices
Some PFAS, mostly PFAAs, certain precursors, PFECAs, or perfluoroether sulfonic acids (PFESAs), are quite mobile and have been reported in multiple environmental and biotic matrices (e.g., drinking water, soil, air, surface or groundwater, precipitation, sediment, human blood, human breast milk, urine, aquatic and terrestrial organisms, plants) (Giesy and Kannan 2001; Ahrens and Bundschuh 2014; Wang et al. 2015; Chen et al. 2018; Macheka‐Tendenguwo et al. 2018; Gewurtz et al. 2019; Ghisi et al. 2019). The widespread occurrence of nonpolymer PFAS is due to their diverse chemical and physical properties (see above), which determine their environmental fate and transport.
Due in part to their lower molecular weight, nonpolymer PFAS are more likely to occur in water and air, which leads to their mobility and distribution distant from their point of manufacture, use, or disposal. For example, some of the nonpolymer perfluoroalkyl substances, like PFAAs, exist in their anionic form in water under environmental conditions, making them soluble and mobile in that matrix as demonstrated by their detection in surface water, groundwater, and drinking water (Ahrens and Bundschuh 2014; Chen et al. 2018; Crone et al. 2019; Gewurtz et al. 2019). In addition, per‐ and polyfluorinated substances, especially acids, telomers (acids and alcohols), amides, and amidoethanols, have been measured in the air where they are primarily associated with particles and aerosols (Barton et al. 2006; Chen et al. 2018; Wang et al. 2018; Han et al. 2019). In the atmosphere, some nonpolymer polyfluorinated PFAS, such as fluorotelomer alcohols (FTOHs), can be transformed to PFAAs (Ellis et al. 2004; Styler et al. 2013), and then undergo global transport. Thus, the ability of some nonpolymer PFAS to dissolve in water or enter the atmosphere enables their long‐range transport and global distribution (Dreyer et al. 2009; Cai et al. 2012a, 2012b; Vierke et al. 2013). Water solubility and low molecular weight of the PFAA anions render them more likely to bioaccumulate (Conder et al. 2008; Liu et al. 2011).
The fluoropolymers and perfluoropolyethers generally display a high molecular weight, show no degradation under environmental conditions, little to no water solubility or volatility, and therefore pose a low risk of mobility (note there are some lower molecular weight perfluoropolyethers (<1000 Da) with appreciable vapor pressures at ambient temperatures) (Sianesi et al. 1994). These polymer PFAS types would not be expected to be found in water or air or distant from their point of use or disposal, nor would they be expected to degrade to lower molecular weight PFAS.
The side‐chain fluorinated polymers have high molecular weights, which makes them relatively nonmobile. The polymers themselves would not be expected to be found in water and air or distant from their point of use or disposal. However, unlike other polymer PFAS, they can degrade under environmental conditions to nonpolymer PFAS, which themselves can be mobile and undergo long‐range transport (Prevedouros et al. 2006; Wang et al. 2014a, 2014b).
As discussed previously, water is one of the primary matrices by which the mobile PFAS are transported and how humans are exposed. For this reason, this paper will focus on drinking water and the various regulatory limits for PFAS in this matrix (see Table 4).
Table 4.
Standards and guidance values for PFAS in drinking water; PFAS analyte concentration (µg/L)
| Location | Agency and/or dept. | Standard and/or guidance | Type | PFBA | PFHxA | PFOA | PFBS | PFOS | HFPO NH4 + | |
|---|---|---|---|---|---|---|---|---|---|---|
| CAS number | 375‐22‐4 | 307‐24‐4 | 335‐67‐1 | 375‐73‐5 | 1763‐23‐1 | 3252‐13‐6 | ||||
| US Environmental Protection Agency | ||||||||||
| USEPA | Office of Water | HA | DW | 0.070 | 0.070 | |||||
| US states | ||||||||||
| Alaska (AK) | DEC | Action level | DW/GW/SW | 0.070 | 0.070 | |||||
| California (CA) | SWRCB | NL | DW | 0.0051 | 0.0065 | |||||
| SWRCB | RL (CA) | DW | 0.0100 | 0.0400 | ||||||
| Connecticut (CT) | DPH | AL | DW/GW | 0.070 | 0.070 | |||||
| Massachusetts (MA) | DEP | Drinking water values | DW | 0.020 | 0.020 | |||||
| Michigan (MI) | DEQ | GCC | DW/GW | 0.070 | 0.070 | |||||
| DHHS | Screening levels | DW | 0.009 | 1 | 0.008 | |||||
| Minnesota (MN) | MDH | HRL‐subchronic | DW/GW | 7 | 0.035 | 9 | ||||
| MDH | HRL‐chronic | DW/GW | 7 | 0.035 | 7 | 0.300 | ||||
| MDH | HBV‐subchronic | DW/GW | 3 | 0.015 | ||||||
| MDH | HBV‐chronic | DW/GW | 2 | 0.015 | ||||||
| Nevada (NV) | DEP | BCL | DW | 0.667 | 667 | 0.667 | ||||
| New Jersey (NJ) | DEP | MCL | DW | |||||||
| DWQI | MCL | DW | 0.014 | |||||||
| DWQI | MCL | DW | 0.013 | |||||||
| North Carolina (NC) | DHHS | Health goal | DW | 0.140 | ||||||
| Ohio (OH) | ODH | Action level | DW | 0.070 | 140 | 0.070 | 0.700 | |||
| Rhode Island | DEM | GWQS | DW/GW | 0.070 | 0.070 | |||||
| Vermont (VT) | DEC/DOH | MCL | DW/GW | 0.020 | 0.020 | |||||
| DEC/DOH | HA | DW/GW | 0.020 | 0.020 | ||||||
| International | ||||||||||
| Australia | DOH | Health‐based | DW | 0.560 | 0.070 | |||||
| British Columbia, Canada | Water standard | DW/GW | 0.200 | 80 | 0.300 | |||||
| Canada | HC | DWSV | DW | 30 | 0.200 | 0.200 | 15 | 0.600 | ||
| HC | DWSV | DW | ||||||||
| HC | MAC | DW | 0.200 | 0.600 | ||||||
| Denmark | EPA | Health‐based | DW/GW | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | ||
| Germany | GMH | Health‐based | DW | 0.300 | 0.300 | |||||
| Administrative | DW | 0.100 | 0.100 | |||||||
| Italy | Health‐based | DW | 7 | 1 | 0.500 | 3 | 0.030 | |||
| Netherlands | EPA | Health‐based | DW | 0.530 | ||||||
| Administrative | DW | 0.0053 | ||||||||
| Sweden | Health‐based | DW | 0.090 | |||||||
| Administrative | DW | 0.090 | 0.090 | 0.090 | 0.090 | |||||
| UK | DWI | Health‐based | DW | 10 | 0.300 | |||||
| Admin. level 1 | DW | 0.300 | 0.300 | |||||||
| Admin. level 2 | DW | 10 | 1 | |||||||
| Admin. level 3 | DW | 90 | 9 | |||||||
DEC = Dept. of Environmental Conservation; DEM = Dept. of Environmental Management; DEP = Dept. of Environmental Protection; DEQ = Dept. of Environmental Quality; DHHS = Dept. of Health and Human Services; DOH = Dept. of Health; DPH = Division or Department of Public Health; DWI = Drinking Water Inspectorate; DWQI = NJ Drinking Water Quality Institute; EPA = Environmental Protection Agency; GMH = German Ministry of Health; HC = Health Canada; MDH = Minnesota Department of Health; ODH ‐ Ohio Dept. of Health; SWRCB = California State Water Resources Control Board; AL = private well action level; BCL = basic comparison level; DWSV = Drinking Water Screening Value; GCC = Generic Cleanup Criteria; GWQS = Groundwater Water Quality Standard; HA = lifetime health advisory; HBV = health‐based value; HRL = health risk limit; MAC = maximum acceptable or allowable concentration; MCL = maximum contaminant level; NL = Notification Level; RL = reporting level; RL (CA) = Response Level (California only); PFAS = per‐ and polyfluoroalkyl substances; PFOA = perfluorooctanoic acid (C8); PFOS = perfluorooctane sulfonic acid (C8); PFBA = perfluorobutyric acid (C4); PFBS = perfluorobutane sulfonic acid (C4); PFHxA = perfluorohexanoic acid (C6); HFPO NH4 + = hexafluoropropylene oxide dimer acid, ammonium salt; DW = drinking water; GW = groundwater.
a Based on ITRC (2020), Table 4–1, accessed 2020‐06‐24.
REGULATION OF PFAS
Globally regulators seek to identify, quantify, and characterize the organic fluorinated compounds in various media (e.g., drinking, surface, and groundwater; soil; sediment; air) to facilitate risk management. Regulations currently address only mobile PFAS, not polymeric PFAS. Some organic fluorinated compounds are regulated locally (e.g., perfluorononanoic acid maximum contaminant level [MCL] in drinking water by the New Jersey Dept. of Environmental Protection), nationally (e.g., perfluorooctanoic acid in drinking water maximum acceptable concentration by Health Canada), or internationally (e.g., Stockholm Convention on Persistent Organic Pollutants).
Currently, the most “horizontal” and widest legally binding regulation of PFAS are the listings in the Stockholm Convention on Persistent Organic Pollutants (POPs) with the aim of elimination of production and use of these chemicals: PFOS, its salts, and PFOSF in annex B (UNEP 2009, 2019a); PFOA, its salts, and PFOA‐related compounds in annex A (UNEP 2019b); and PFHxS, its salts, and PFHxS‐related compounds (recommended for listing) in annex A (UNEP 2019c). No other PFAS are under consideration for the Stockholm Convention currently. Note that the POPs definition does not include limit values for any matrix and often is not correct as to terminology although CAS numbers are provided. To establish trends over time using ambient air, surface water, or human milk as core matrices, all POPs listed in the Convention (e.g., PFOS, PFOA) including certain transformation products or precursors shall be included in environmental and human monitoring plans (UNEP 2019d).
The World Health Organization (WHO), in accordance with the WHO Framework for safe drinking water, has included PFOS and PFOA, due to their widespread occurrence and respective health concerns (WHO 2017). The report recommends that the guideline values (GVs) for drinking water are 0.4 µg·L−1 (ppb) for PFOS and 4.0 µg·L−1 (ppb) for PFOA (note that these GVs were derived on the basis tolerable daily intake [TDI] values of the European Food Safety Authority [EFSA] report as of 2008, which stipulated TDIs of 0.15 µg per kg bodyweight per day for PFOS and 1.5 µg per kg body weight and day) (EFSA 2008). The EFSA recommended much lower tolerable intake values in 2018 expressed as tolerable weekly intakes (TWI) of 13 ng per kg bodyweight per week and TWI of 6 ng per kg bodyweight per week for PFOS and PFOA, respectively (EFSA 2018a). A group value TWI of 4.4 ng per kg bodyweight per week was proposed by EFSA in 2020 for PFOS, PFOA, PFHxS, and PFNA (EFSA CONTAM Panel 2020). In contrast to limits set for water, air, or soil, which reflect the concentration of the above mentioned PFAS in that medium, the TDI or TWI is the mass of the PFAS that can be safely consumed in the diet per kilogram of human body weight per day or week; it is a dose. The EFSA used the upper bound 95th percentile human consumption for these limits imparting a high degree of conservatism because 95% of the population will consume this amount or less of these specific PFAS per day or week.
Regarding drinking water intended for human consumption in Europe, the EC modified the 2017 WHO values and recommends sum parameters by including 11 PFAS (PFBS, PFHxS, PFOS, 6:2 fluorotelomer sulfonic acid [6:2 FTSA], PFBA, perfluoropentanoic acid, [PFPeA], PFHxA, perfluoroheptanoic acid [PFHpA], PFOA, perfluorononanoic acid [PFNA], and perfluorodecanoic acid [PFDA]) and recommends values of 0.1 μg·L−1 (ppb) for individual PFAS and 0.5 μg·L−1 (ppb) for PFAS as the sum (EC 2017).
In the United States, regulatory human health‐based guidance values (HBVs) and/or standards have been derived for 16 PFAAs, and/or including 2 polyfluoroalkyl precursors, and 1 fluorinated ether carboxylate (FECA) by state and/or federal agencies in the United States, as of September 2019 (see Table 4 for US and international standards and HBVs for drinking water for a subset of these perfluorocarboxylic acids [PFCAs], PFSAs, and a PFECA). The values for these nonpolymer PFAS vary across programs. Differences can be attributed to: selection and interpretation of different key toxicity studies, choice of uncertainty factors, approaches used for animal‐to‐human extrapolation, the life stage and the percentage of exposure assumed to come from nondrinking water sources, and so forth. The vast majority of regulatory guidance and/or standards available are for PFOA and PFOS (ITRC 2020).
The US Environmental Protection Agency (USEPA) and the Food and Drug Administration (FDA) are the 2 US federal agencies that have the legal authority to regulate PFAS. Currently, both agencies have regulatory or guidance initiatives for PFAAs.
Currently the only USEPA “limit” for a PFAS that exists is the USEPA lifetime health advisory (LHA) of 70 ng/L−1 for PFOA and PFOS (individually or in combination if both are present) in drinking water, which is advisory in nature, not legally enforceable, and is subject to change (USEPA 2016a, 2016b). But, since 2002, the USEPA has utilized use and import regulations under Section 5 of the Toxic Substance Control Act (TSCA) to monitor PFAS through multiple final Significant New Use Rules (SNURs) covering over 250 fluorinated organic substances (polymeric and nonpolymeric) and an SNUR for PFOA and PFOS. The USEPA established an action plan for PFAS in 2019 (USEPA 2019c), while US regulatory HBVs and standards have been primarily focused on PFCAs, PFSAs, and precursors to them, in early 2020, a SNUR and Premanufacture Notice was issued for a fluoropolymer (P‐17‐0400; SNUR 40 CFR 721.11414) involving any new use of a terpolymer of vinylidene fluoride, tetrafluoroethylene, and 2,3,3,3‐tetrafluoropropene, that results in inhalation exposure.
The USEPA has generated data around PFAS in public drinking water through the Unregulated Contaminant Monitoring Rule (UCMR), which included 6 PFAAs in the most recent round of monitoring efforts: PFOS, PFOA, PFNA, PFHxS, PFHpA, and PFBS. The UCMR3 reported limits of between 10 and 90 ng/L depending on the specific PFAAs (USEPA 2017).
To the inexperienced, these limits can appear to be an almost random assortment of numerical values. In actuality, each limit is based in part on definitions, statutes, and risk assessment processes that vary between laws and across regulatory authorities, jurisdictions, and media being regulated (e.g., surface, ground, or drinking water) (see Table 4). The end result is widely varying regulatory limits for the same chemical often in the same matrix. Thus, caution must be used when comparing and interpreting analytical results to different limits across regulatory jurisdictions.
Robust, reproducible, and validated methods to differentiate organic from inorganic fluorinated compounds, as well as between the many types of organic fluorinated compounds, are needed for regulatory purposes. The next section will present some analytical approaches for various types of fluorine and the importance of interlaboratory assessment.
ANALYTICAL METHODS TO IDENTIFY ORGANIC FLUORINATED COMPOUNDS
In this section, we will focus on the organic per‐ and polyfluoroalkyl substances, especially the sulfonic and carboxylic acids, but not on pesticides, pharmaceuticals, or veterinary drugs. The analytical methods discussed here will start with the determination of all fluorine in a sample, briefly cover some parameters, and finish with confirmatory analysis of specific PFAS including identification of certain isomers. This compilation is primarily created to support regulations or controls for the various jurisdictions or attempt to create a common basis for harmonizing reporting of results. Table 5 provides a graphical sketch to categorize fluorine in the different types as they can be determined analytically. Some fluorine‐containing compounds, such as nonpolymer PFAS, can be detected by multiple method types, while others, such as CaF2, can only be detected by total fluorine methods (Koch et al. 2020).
Table 5.
Analytical approaches for PFAS within determination of fluorine
| PFAS | Total fluorine | |||||
|---|---|---|---|---|---|---|
| IF | OF | |||||
| Nonextractable fluorine | Extractable fluorine TOF | |||||
| Quantifiable | Unquantifiable | |||||
| Identifiable | Unknown | |||||
| Nonpolymer ‐ perfluoroalkyl substances | ||||||
| Perfluoroalkyl acids | + | − | − | +[Link], 1 | + | ? |
| Perfluoropolyether carboxylic acids | + | − | − | +1 | + | ? |
| Perfluoroalkyl sulfinic acids | + | − | − | +1 | + | ? |
| Perfluoroalkyl phosphinic acids | + | − | − | +1 | + | ? |
| Perfluoroalkyl sulfonyl fluorides | + | − | − | +1 | + | ? |
| Perfluoroalkyl sulfonamides | + | − | − | +1 | + | ? |
| Perfluoroalkyl iodides | + | − | − | +2 | + | ? |
| Nonpolymer ‐ polyfluoroalkyl substances | ||||||
| Fluorotelomer alcohols | + | − | − | +[Link], 1 | + | ? |
| Fluorotelomer sulfonamides and derivatives | + | − | − | +[Link], 1 | + | ? |
| Fluorotelomer sulfonates | + | − | − | +1 | + | ? |
| Fluorotelomer iodides | + | − | − | +2 | + | ? |
| Fluorotelomer olefins and aldehydes | + | − | − | +2 | + | ? |
| Fluorinated pharmaceuticals and pesticides | + | − | − | +1 | + | ? |
| Polyfluoralkyl phosphate surfactants | + | − | − | +1 | + | ? |
| Semifluorinated n‐alkanes and alkenes | + | − | − | +3 | + | ? |
| Polymer ‐ fluoropolymers | ||||||
| PTFE, FEP | + | − | + | − | − | − |
| Fluoroelastomers | + | − | + | − | − | − |
| Polymer – perfluoropolyethers | ||||||
| Fomblin or Galden materials | + | − | + | −a | −a | −a |
| Polymer ‐ side‐chain fluorinated polymers | ||||||
| Fluorinated acrylate and methacrylate polymers | + | − | + | −b | −b | −b |
| Fluorinated urethane polymers | + | − | + | −b | −b | −b |
PFAS = per‐ and polyfluoroalkyl substances; IF = inorganic F; OF = organic F; TOF = total organic fluorine; PTFE = polytetrafluoroethylene; FEP = fluorinated ethylene propylene.
+ = a PFAS is detectable by the type of analytical method.
− = a PFAS is not detectable by the type of analytical method.
? = substances under this definition can be detected with a given analytical method as unknowns.
Lower molecular weight species (<1000 Da) may be quantifiable, unquantifiable yet identifiable, or unknowns.
Transformation can create other PFAS that are quantifiable, unquantifiable yet identifiable, or unknowns.
Numeric footnotes provide common types of instrumentation used for targeted quantitative analysis.
LC‐MS – (Jahnke & Berger 2009; D'eon et al. 2009; Sun et al. 2011; Lee and Mabury 2017; Robel et al. 2017; ISO 2019; USEPA 2019a; USEPA 2020; Pan et al. 2020).
GC‐MS – (Alzaga & Bayona 2004; Jahnke & Berger 2009; Orata et al. 2009; Bach et al. 2016; Robel et al. 2017).
GC‐ECD (Plassmann et al. 2010).
The ability of PFAS to respond in a method depends on the specific PFAS, extraction method, sample matrix, chromatography conditions (if applicable), and instrument settings. The table also indicates which types of methods are unable to differentiate between PFAS. For example, many approaches only detect fluorine and may not be able to differentiate between nonpolymer and polymer PFAS. By application of certain extraction solvents as used in extractable organic fluorine (EOF), some specification can be obtained. Only after extraction and purification can neutral PFAS be differentiated from ionic forms and specific PFAS be determined (e.g., identified by CAS numbers).
Total fluorine
Total fluorine (TF) in chemical compounds can be defined as the sum of inorganic fluorine (IF) and organic fluorine (OF). Total fluorine in biota, especially blood, can be determined by total mineralization in O or H atmospheres and subsequent absorption of the fluoride in water and detection with fluoride ion‐selective electrodes, such as in the Wickbold Torch method (Čápka et al. 2004). Total fluorine can also be analytically determined by proton induced gamma emission (PIGE), which measures gamma ray emissions from fluorine atoms in a sample in response to an ion beam. The PIGE has been applied to determine the total fluorine in paper and textiles (Robel et al. 2017). Another approach uses inductively coupled plasma mass spectrometer (ICP MS/MS) through the formation of the polyatomic ion BaF+ to enable fluorine analysis (Jamari et al. 2017; Feldmann et al. 2018). While, theoretically, all fluorine, organic and inorganic, can be determined with these methods, the ability to detect polymer PFAS, for example, will depend on sample preparation and material properties. Most fluoropolymers have limited, if any, solubility. Inorganic fluorine, such as sodium fluoride, and fluorinated pharmaceuticals and pesticides, are expected to respond in this ICP MS/MS method.
Total organic fluorine (TOF) or total extractable organic fluorine
In total extractable organic fluorine (TEOF) analysis, the sample is divided whereby the first portion undergoes targeted or confirmatory analysis according to one of the approaches discussed in the Targeted Analysis section. Combustion ion chromatography (CIC) is used on the second portion to determine TF and EOF. It uses an automated combustion unit and an ion chromatography system (Yeung et al. 2009). The ability to detect nonpolymer PFAS, including fluorinated pharmaceuticals and pesticides, will be affected by the extraction conditions, the nature of the sample matrix, and the specific nonpolymer PFAS. The ability to detect polymer PFAS will depend on sample preparation and material properties. In most applications, there was a large amount of fluorine that could not be assigned to any type of fluorinated organic substance; thus, often leaving up to 92% of the fluorine unknown (Kärrman et al. 2019; Koch et al. 2019).
Total oxidizable precursor
Total oxidizable precursor (TOP) analysis uses hydroxyl radicals where all polyfluorinated precursors are oxidized into PFCAs, the final “dead end” oxidation products (Houtz and Sedlak 2012; Houtz et al. 2013). This procedure involves chemical and thermal treatment of the samples and is restricted to aqueous extracts or water samples. Water soluble nonpolymer PFAS are expected to respond in this method. Nonpolymer PFAS with few fluorine atoms, such as a single CF3 group, are expected to have a poor response. Polymer PFAS, such as fluoropolymers and perfluoropolyethers, will not be detected in these methods because of their insolubility in aqueous solvents.
It shall be noted that all of the above‐mentioned approaches can be used for mass balance purposes to quantify fluorine in samples of interest. The approaches are destructive to the organic molecule and do not allow a retrospective identification of specific organic fluorinated compounds. Both the TOF and the TOP approaches are typically complemented by a second chromatographic separation and determination, the targeted analysis to identify the known and often restricted substances.
Nontargeted and targeted analysis for nonpolymer PFAS
Nontargeted analysis explores unknown compounds using mass spectrometry by identifying a peak in a chromatogram. Today, time‐of‐flight (ToF) or Orbitrap instruments are used for nontargeted analysis. In a typical approach, one would be looking at protonated molecules using MS/MS spectra for identification. A nontargeted method would allow detection of both known and unknown chemicals; however, not all peaks are identified and only a limited number of the most abundant ions (50–100) will be monitored over the entire time of the experiment. The largest problem in nontargeted analysis is the data processing. Since the chemicals present in the (injected) sample depend on extraction and purification methods, in practice it is not possible to see all chemicals in a sample (even the unknowns). A recent paper provides an example for the determination of 24 PFAS in firefighting foams using LC/qToF instrumentation (Dubocq et al. 2020).
Targeted analysis means that the analyst is looking for specific chemicals only. For such confirmatory analysis, typically mass spectrometry on selected reaction monitoring (SRM) and the fragmentation profile based on retention time of compounds compared with internal standards are used. The mass spectra and retention times for each compound are known and all targeted chemicals above the noise level will be detected by this approach. The determination is typically optimized for sensitivity and selectivity, and subsequently reduced by limiting the number of analytes. Other chemicals present in the sample will not be seen. Berger et al. (2004) and Liu et al. (2019) provide good reviews of targeted methods for nonpolymer PFAS.
Nonpolymer PFAS can be identified after either gas chromatographic or liquid chromatographic separation followed by mass selective determination. The most used instrumentation for the determination of nonpolymer ionic PFAS is a combination of liquid chromatography (LC) using either HPLC or UPLC columns (high performance or ultrahigh performance liquid chromatography) coupled to a mass selective detector (LC/MS). The most commonly used detector is a triple quadrapole MS (MS/MS) detector in negative ion mode. Time‐of‐flight detectors or Orbitrap, can be used as well; however, the large datasets generated may pose a problem due to limitations in storage size. The LC MS/MS approaches are commonly used for the determination of ionic PFAS in ambient or indoor air. Gas chromatography is used for the neutral volatile PFAS such as certain PFOS precursors such as the perfluorooctane sulfonamides (FOSAs) and the perfluorooctane sulfonamido ethanols (FOSEs), or FTOHs, which are determined with chemical ionization (CI) GC/MS. Fluoroalkyl acids can be analyzed by GC/MS (with negative chemical ionization, GC‐NCI‐MS) approaches after derivatization, thus requiring much greater effort in sample preparation; for examples, see Alzaga and Bayona (2004) and Orata et al. (2009).
Applications of either LC MS/MS or GC/MS instrumentation include the analysis of ambient or indoor air collected with polyurethane foam (PUF), XAD® or solvent impregnated PUFs (SIPs), and solid‐phase cartridges (SPE) or grab samples (Jahnke et al. 2007; Wang et al. 2018). Analyses of aqueous matrices include drinking water, ground or surface waters, as well as waste waters. The most commonly used methods are either ISO or EPA methods or variations thereof (ISO 2019; USEPA 2019b; Shoemaker and Tettenhorst 2020). Ionic and neutral PFAS have also been determined after Soxhlet, pressurized liquid or solid liquid extraction followed by ENVI‐Carb or SPE clean‐up in abiotic solid matrices like sediments, soils, or sludges (Ahrens 2011; Ahrens et al. 2016) as well as in foods of animal or plant origin (Chu et al. 2016; EFSA 2018b, 2020; Sadia et al. 2020). Reviews by various authors or institutions provide examples of approaches of analysis of perfluoroalkyl and PFASs in different sample matrices (Jahnke and Berger 2009; Berger et al. 2011; Liu et al. 2019; USEPA 2019b; Pan et al. 2020; USEPA et al. 2020).
Quality assurance and quality control
Today, commercial suppliers provide a wide spectrum of native and isotopically labeled internal standards for PFAS used at different steps of the analysis (extraction, recovery, quantification). The availability of such standards steadily increases and include: PFCAs, PFSAs, FOSAs, FOSEs, FOSAAs, FTOHs, fluorotelomer acids (FTAs), fluorotelomer sulfonates (FTSs), perfluoroalkylphosphonic acids (PFAPAs), and perfluoroalkylphosphinic acids (PFPis). There are not yet internationally agreed mixtures of PFAS available since the scope of many monitoring and other projects vary. Most research groups prepare their own standard solutions as a mixture of individual compounds to meet the purpose of the investigation.
For quality control purposes, standard reference materials (SRM) are available (for serum and human milk by the US National Institute of Standards and Technology (NIST) (Schantz et al. 2013), and international intercalibration assessments are undertaken (Fiedler et al. 2017; Fiedler et al. 2020) coordinated by UNEP or Quasimeme (Weiss et al. 2013). In the UNEP‐coordinated interlaboratory assessments, between 20 and 30 laboratories participate in these studies. In comparison with the analysis of organochlorine pesticides, these LC MS/MS laboratories generally have better performances. In the UNEP‐coordinated interlaboratory assessments, satisfactory performance is granted to a laboratory's value that is 25% above or below the assigned value (satisfactory results with <2 z‐scores corresponding to ±25% deviation from assigned value); others use ±40%. In general, the more sophisticated laboratories (e.g., having MS detection and isotopically labelled internal standards) perform better than the laboratories with simple instrumentation, for example, electron capture detection (Fiedler et al. 2020). The results have shown that dioxin and PFAS laboratories have higher percentages of satisfactory results than laboratories analyzing organochlorine pesticides. However, it also has to be noted that due to the lack of harmonized regulation, the spectrum of PFAS analyzed and the types of matrices are many, so that often the number of participating laboratories is too small for a statistical evaluation to be performed.
As discussed previously, TOF, TOP, or PIGE spectroscopy methods can be applied to polymer PFAS. Response will depend on sample preparation and material properties. It also shall be noted that on the basis of these sum parameters, environmental fate, transformation and/or metabolism, or toxicity cannot be determined.
It is critically important to remember that chemical bonds that can be broken in an analytical lab do not automatically reflect real world environmental transformations. For example, combustion ion chromatography subjects samples to extreme temperatures not reflective of actual environmental scenarios. In addition, some testing is intended to be destructive to the sample while other testing is not. Finally, since robust analytical data can be the basis for regulation, be mindful of whether the method is analyzing a parent compound or something created or manipulated in the laboratory analysis.
DISCUSSION
In this paper, we summarized a broad spectrum of inorganic and organic fluorinated substances and matched them with analytical approaches and subsequent application in regulatory context. We found widely accepted methods only for a limited number of organic fluorinated substances such as the PFAAs in drinking water while those for other environmental matrices are not yet defined.
In order to address the above mentioned gap, clarity is needed to match the analytical method to the goal of the analyses. Is the analysis to advance pure research or pragmatically to determine the extent of a reported accidental release to a river? Is it monitoring for specific substances in the emissions at a manufacturing facility to determine environmental permit compliance? Is it a survey to determine if organic fluorinated substances are present and then to identify and quantify baseline levels of specific compounds? Knowing the purpose of the analysis and how the results are intended to be interpreted and used (e.g., identify what was released into the river; confirm the facility is in compliance with the permit; no organic fluorine is present) will inform analytical decision making. Analytical techniques that identify organic fluorine coupled together with targeted chemical analysis will yield information sufficient to identify public health or environmental hazards.
Valid analytical chemistry measurements provide critical information on the presence of PFAS in the environment, but cannot by themselves determine sources of PFAS because the measured analyte itself can have multiple sources (e.g., itself as a product, transformation product from nonpolymer PFAS, and degradation from side‐chain fluorinated polymers). In other cases, the analytical method (e.g., PIGE, T(E)OF) does not provide sufficient specificity for PFAS identification, which limits the utility of the measurement in determining sources. Proper source attribution requires an understanding of the method specificity, the environmental fate, transport, and possible precursors of the analyte, as well as location‐specific information on historical use and possible sources. Misattribution of sources may result in undue public alarm, ineffective regulation, and wasted regulatory resources. Similarly, incorrect comparisons between analytical measurements and regulatory limits (e.g., ocean water measurements compared to drinking water standards) can mislead the public as to whether a PFAS in a particular environmental matrix should be a concern. This paper provides guidance on an approach to analyses of drinking water for the presence of organic fluorinated compounds as well as which analytical method is optimal.
Before a regulatory authority sets a limit for a PFAS substance, some clear, unambiguous criteria, usually hazard‐based, should be established. Definitions of hazard differ across and within jurisdictions for different statutes, law, conventions, and so forth, leading to variations in limits for the same substance in the same media (e.g., food, drinking water, soil). The objectives of various laws, conventions, and other regulatory authorities may also vary impacting how limits are set and for which substances. Differences in limits for the same PFAS in the same media across jurisdictions can be attributed to selection and interpretation of different key toxicity studies, choice of uncertainty factors, approaches used for animal‐to‐human extrapolation, the life stage assessed, and the percentage of exposure assumed to come from nondrinking water sources, and so forth. Matrices for PFAS limits differ for water between surface, ground, and drinking water. Some limits are advisory in nature while others are legally enforceable. Even within a jurisdiction, differences in limits for the same substance may be attributed to differing exposure or risk assessment processes using the same scientific data. In the USEPA alone there are MCLs under the Safe Drinking Water Act, water quality standards under the Clean Water Act, the Clean Air Act's technology‐based “maximum achievable control technology” air quality criteria to avoid emission that “may reasonably be anticipated to endanger public health or welfare,” or site cleanup standards under the Superfund Amendments and Reauthorization Act. Some laws are set to protect environmental quality and others human health. Because these different regulatory objectives, endpoints, processes, matrices, and units vary, one must be cautious in interpreting analytical results in terms of specific regulatory values.
CONCLUSIONS
There are many organic fluorinated compounds of varying physical, chemical, and toxicological properties. Some of these compounds are PFAS, but there are other fluorinated organic compounds that do not have the CF3‐(CF2)n moiety. Whether they are PFAS or not, they can be persistent, widespread in the environment, or toxic. To effectively protect public health and the environment, efforts must focus on those fluorinated compounds that pose the greatest risk, which necessitates an understanding of exposure pathways and determination of the concentration of the hazardous substance therein. Adequate samples from potential exposure media must be taken and analyzed appropriately per national and internationally accepted methods. Since these methods do not always exist, this paper addressed analytical approaches so that fluorinated organic compounds can be identified and quantified appropriately. This paper provided an overview of fluorine‐containing compounds, their uses, their toxicological, chemical, and physical properties in relation to environmental occurrence, their analysis, and how the interpretation of the analytical data is critical to effective regulation.
Disclaimer
Todd Kennedy and Barbara J Henry are employees of WL Gore & Associates, Inc, a global manufacturer of products made with fluoropolymers.
Supporting information
This article contains online‐only Supplemental Data.
Supporting information.
Acknowledgment
The authors would like to thank John D Jones, Mary M Maloney Huss, Jan Oberdoerster, Carol A Wilkinson, Amy Calhoun, and Joseph P Carlin of WL Gore & Associates, Inc for their review and comments. We also wish to thank the editors and reviewers for their comments to improve this paper.
Data Availability Statement
Data and associated metadata and calculation tools are available upon request by contacting corresponding author Heidelore Fiedler (Heidelore.Fiedler@oru.se).
REFERENCES
- Ahrens L. 2011. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J Environ Monit 13(1):20–31. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Bundschuh M. 2014. Fate and effects of poly‐ and perfluoroalkyl substances in the aquatic environment: A review. Environ Toxicol Chem 33(9):1921–1929. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Gashaw H, Sjöholm M, Gebrehiwot SG, Getahun A, Derbe E, Bishop K, Åkerblom S. 2016. Poly‐ and perfluoroalkylated substances (PFASs) in water, sediment and fish muscle tissue from Lake Tana, Ethiopia and implications for human exposure. Chemosphere 165:352–357. [DOI] [PubMed] [Google Scholar]
- Alzaga R, Bayona JM. 2004. Determination of perfluorocarboxylic acids in aqueous matrices by ion‐pair solid‐phase microextraction–in‐port derivatization–gas chromatography–negative ion chemical ionization mass spectrometry. J Chromatogr A 1042(1–2):155–162. [DOI] [PubMed] [Google Scholar]
- Barton CA, Butler LE, Zarzecki CJ, Flaherty J, Kaiser M. 2006. Characterizing perfluorooctanoate in ambient air near the fence line of a manufacturing facility: Comparing modeled and monitored values. J Air Waste Manage Assoc 56(1):48–55. [DOI] [PubMed] [Google Scholar]
- Berger U, Kaiser MA, Karrman A, Barber JL, van Leeuwen SP. 2011. Recent developments in trace analysis of poly‐ and perfluoroalkyl substances. Anal Bioanal Chem 400(6):1625–1635. [DOI] [PubMed] [Google Scholar]
- Berger U, Langlois I, Oehme M, Kallenborn R. 2004. Comparison of three types of mass spectrometer for high‐performance liquid chromatography/mass spectrometry analysis of perfluoroalkylated substances and fluorotelomer alcohols. Eur J Mass Spectrom 10(5):579–588. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Xie Z, Moeller A, Yin Z, Huang P, Cai M, Yang H, Sturm R, He J. 2012a. Polyfluorinated compounds in the atmosphere along a cruise pathway from the Japan Sea to the Arctic Ocean. Chemosphere 87(9):989–997. [DOI] [PubMed] [Google Scholar]
- Cai M, Zheo Z, Yin Z, Ahrens L, Huang P, Cai M, Yang H, He J, Sturm R, Ebinghaus R et al. 2012b. Occurrence of perfluoroalkyl compounds in surface waters from the North Pacific to the Arctic Ocean. Environ Sci Technol 46(2):661–668. [DOI] [PubMed] [Google Scholar]
- Čápka Vr, Bowers CP, Narvesen JN, Rossi RF. 2004. Determination of total fluorine in blood at trace concentration levels by the Wickbold decomposition method with direct potentiometric detection. Talanta 64(2004):869–878. [DOI] [PubMed] [Google Scholar]
- Chen H, Yao Y, Zhao Z, Wang Y, Wang Q, Ren C, Wang B, Sun H, Alder AC, Kannan K. 2018. Multimedia distribution and transfer of per‐ and polyfluoroalkyl substances (PFASs) surrounding two fluorochemical manufacturing facilities in Fuxin, China. Environ Sci Technol 52(15):8263–8271. [DOI] [PubMed] [Google Scholar]
- Chu S, Letcher RJ, McGoldrick DJ, Backus SM. 2016. A new fluorinated surfactant contaminant in biota: Perfluorobutane sulfonamide in several fish species. Environ Sci Technol 50(2):669–675. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC. 2008. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol 42(4):995–1003. [DOI] [PubMed] [Google Scholar]
- Cormier E, Das PM, Ojima I. 2009. Approved active pharmaceutical ingredients containing fluorine in fluorine in medicinal chemistry and chemical biology In: Iwao Ojima, editor. Fluorine in medicinal chemistry and chemical biology. Chichester (UK): Blackwell‐Wiley Publishing; p 525–604. [Google Scholar]
- Crone BC, Speth TF, Wahman DG, Smith SJ, Abulikemu G, Kleiner EJ, Pressman JG. 2019. Occurrence of per‐ and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit Rev Environ Sci Technol 49(24):2359–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino LA, Mabury SA. 2014. Identification of novel fluorinated surfactants in aqueous film forming foams and commercial surfactant concentrates. Environ Sci Technol 48(1):121–129. [DOI] [PubMed] [Google Scholar]
- D'Agostino LA, Mabury SA. 2017. Aerobic biodegradation of 2 fluorotelomer sulfonamide‐based aqueous film‐forming foam components produces perfluoroalkyl carboxylates. Environ Toxicol Chem 36(8):2012–2021. [DOI] [PubMed] [Google Scholar]
- Dolbier WR Jr. 2005. Fluorine chemistry at the millennium. J Fluor Chem 126(2):157–163. [Google Scholar]
- Dreyer A, Weinberg I, Temme C, Ebinghaus R. 2009. Polyfluorinated compounds in the atmosphere of the Atlantic and Southern Oceans: Evidence for a global distribution. Environ Sci Technol 43(17):6507–6514. [DOI] [PubMed] [Google Scholar]
- Dubocq F, Wang T, Yeung LWY, Sjöberg V, Kärrman A. 2020. Characterization of the chemical contents of fluorinated and fluorine‐free firefighting foams using a novel workflow combining nontarget screening and total fluorine analysis. Environ Sci Technol 54(1):245–254. [DOI] [PubMed] [Google Scholar]
- [EC] European Commission . 2017. Proposal for a Directive of the European Parliament ad of the Council on the quality of water intended for human consumption (recast). 2018 Feb 1. COM/2017/0753.
- [EFSA] European Food Safety Authority . 2008. Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. Scientific Opinion of the Panel on Contaminants in the Food Chain (CONTAM PANEL). EFSA J 6(7):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [EFSA] European Food Safety Authority . 2018a. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 16(12):5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [EFSA] European Food Safety Authority . 2018b. Risk for animal and human health related to the presence of dioxins and dioxin‐like PCBs in feed and food. EFSA J 16(11):e05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [EFSA] European Food Safety Authority . 2020. Scientific opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):6223–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Sulbaek Andersen MP, Wallington TJ. 2004. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ Sci Technol 38(12):3316–3321. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Raab A, Krupp EMJA, Chemistry B. 2018. Importance of ICPMS for speciation analysis is changing: Future trends for targeted and non‐targeted element speciation analysis. Anal Bioanal Chem 410(3):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler H, van der Veen I, de Boer J. 2017. Bi‐ennial global interlaboratory assessment on persistent organic pollutants—Third round 2016/2017. Geneva (CH): UN Environment Programme. 127 p.
- Fiedler H, van der Veen I, de Boer J. 2020. Global interlaboratory assessments of perfluoroalkyl substances under the Stockholm Convention on persistent organic pollutants. Trends Anal Chem 124:115459. [Google Scholar]
- Fujiwara T, O'Hagan D. 2014. Successful fluorine‐containing herbicide agrochemicals. J Fluorine Chem 167:16–29. [Google Scholar]
- Gewurtz SB, Bradley LE, Backus S, Dove A, McGoldrick D, Hung H, Dryfhout‐Clark H. 2019. Perfluoroalkyl acids in Great Lakes precipitation and surface water (2006–2018) indicate response to phase‐outs, regulatory action, and variability in fate and transport processes. Environ Sci Technol 53(15):8543–8552. [DOI] [PubMed] [Google Scholar]
- Ghisi R, Vamerali T, Manzetti S. 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ Res 169:326–341. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7):1339–1342. [DOI] [PubMed] [Google Scholar]
- Han D, Ma Y, Huang C, Zhang X, Xun H, Zhou Y, Liang S, Chen X, Huang X, Liao H et al. 2019. Occurrence and source apportionment of perfluoroalkyl acids (PFAAs) in the atmosphere in China. Atmos Chem Phys 19(22):14107–14117. [Google Scholar]
- Henry BJ, Carlin JP, Hammerschmidt JA, Buck RC, Buxton LW, Fiedler H, Seed J, Hernandez O. 2018. A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr Environ Assess Manag 14(3):316–334. [DOI] [PubMed] [Google Scholar]
- Holmquist H, Schellenberger S, van der Veen I, Peters GM, Leonards PEG, Cousins IT. 2016. Properties, performance and associated hazards of state‐of‐the‐art durable water repellent (DWR) chemistry for textile finishing. Environ Int 91:251–264. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Higgins CP, Field JA, Sedlak DL. 2013. Persistence of perfluoroalkyl acid precursors in AFFF‐impacted groundwater and soil. Environ Sci Technol 47(15):8187–8195. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sedlak DL. 2012. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ Sci Technol 46(17):9342–9349. [DOI] [PubMed] [Google Scholar]
- [ISO] International Standards Organization . 2019. ISO 21675:2019 Water quality—Determination of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in water—Method using solid phase extraction and liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). [accessed 2020 Sep 15]. https://www.iso.org/standard/71338.html
- [ITRC] Interstate Technology Regulatory Council . 2020. Per‐ and polyfluoroalkyl substances (PFAS) web page. ITRC PFAS water and soil values table. [accessed 2020 Jun 24]. http://pfas-1.itrcweb.org
- Jaccaud M, Faron R, Devilliers D, Romano R. 2000. Fluorine In: Ullmann F, editor. Ullmann's encyclopedia of industrial chemistry. Vol 15. Weinheim (DE): Wiley‐VCH; p 381–395. [Google Scholar]
- Jahnke A, Ahrens L, Ebinghaus R, Berger U, Barber JL, Temme C. 2007. An improved method for the analysis of volatile polyfluorinated alkyl substances in environmental air samples. Anal Bioanal Chem 387:965–975. [DOI] [PubMed] [Google Scholar]
- Jahnke A, Berger U. 2009. Trace analysis of per‐ and polyfluorinated alkyl substances in various matrices—How do current methods perform? J Chromatogr A 1216(3):410–421. [DOI] [PubMed] [Google Scholar]
- Jamari NLA, Dohmann JF, Raab A, Krupp EM, Feldmann J. 2017. Novel non‐target analysis of fluorine compounds using ICPMS/MS and HPLC‐ICPMS/MS. J Analyt Atomic Spectrom 32:942–950. [Google Scholar]
- Kärrman A, Wang T, Kallenborn R, Langseter AM, Grønhovd SM, Ræder EM, Lyche JL, Yeung L, Chen F, Eriksson U et al. 2019. PFASs in the Nordic environment: Screening of poly‐ and perfluoroalkyl substances (PFASs) and extractable organic fluorine (EOF) in the Nordic Environment. Copenhagen (DK): Nordisk Ministerråd. 515 p.
- Kirk RE, Othmer DF, Kroschwitz JI, Howe‐Grant M. 1991. Kirk‐Othmer Encyclopedia of chemical technology. 4th ed. Vol 1 New York (NY): John Wiley and Sons; p 241. [Google Scholar]
- Koch A, Aro R, Wang T, Yeung LWY. 2020. Towards a comprehensive analytical workflow for the chemical characterisation of organofluorine in consumer products and environmental samples. TrAC. Trends Analyt Chem 123:115423. [Google Scholar]
- Koch A, Kärrman A, Yeung LWY, Jonsson M, Ahrens L, Wang T. 2019. Point source characterization of per‐ and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates. Environ Sci Proc Imp 21(11):1887–1898. [DOI] [PubMed] [Google Scholar]
- Liu C, Gin KYH, Chang VWC, Goh BPL, Reinhard M. 2011. Novel perspectives on the bioaccumulation of PFCs—The concentration dependency. Environ Sci Technol 45(22):9758–9764. [DOI] [PubMed] [Google Scholar]
- Liu Y, D'Agostino LA, Qu G, Jiang G, Martin JW. 2019. High‐resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly‐ and per‐fluoroalkyl substances (PFASs) in environmental and human samples. TrAC. Trends Analyt Chem 121:115420. [Google Scholar]
- Macheka‐Tendenguwo LR, Olowoyo JO, Mugivhisa LL, Abafe OA. 2018. Per‐ and polyfluoroalkyl substances in human breast milk and current analytical methods. Environ Sci Pollut Res Int 25(36):36064–36086. [DOI] [PubMed] [Google Scholar]
- Mejia‐Avendaño S, Duy SV, Sauvé S, Liu J. 2016. Generation of perfluoroalkyl acids from aerobic biotransformation of quaternary ammonium polyfluoroalkyl surfactants. Environ Sci Technol 50(18):9923–9932. [DOI] [PubMed] [Google Scholar]
- Moe MK, Huber S, Svenson J, Hagenaars A, Pabon M, Trümper M, Berger U, Knapen D, Herzke D. 2012. The structure of the fire fighting foam surfactant Forafac1157 and its biological and photolytic transformation products. Chemosphere 89(7):869–875. [DOI] [PubMed] [Google Scholar]
- O'Hagan D. 2010. Fluorine in health care: Organofluorine containing blockbuster drugs. J Fluor Chem 131(11):1071–1081. [Google Scholar]
- [OECD] Organisation for Economic Co‐operation and Development . 2018. Environment Directorate, Environment, Health and Safety Division toward a new comprehensive global database of per‐ and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per‐ and polyfluoroalkyl substances (PFASs), 2018 May 4. Paris (FR). 24 p.
- Orata F, Quinete N, Wilken RD. 2009. Long chain perfluorinated alkyl acids derivatisation and identification in biota and abiota matrices using gas chromatography. Bull Environ Contam Toxicol 83:630–635. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang J, Yeung LWY, Wei S, Dai J. 2020. Analysis of emerging per‐ and polyfluoroalkyl substances: Progress and current issues. TrAC. Trends Analyt Chem 124:115481. [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. 2006. Sources, fate at transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44. [DOI] [PubMed] [Google Scholar]
- Rankin K, Lee H, Tseng PJ, Mabury SA. 2014. Investigating the biodegradability of a fluorotelomer‐based acrylate polymer in a soil‐plant microcosm by indirect and direct analysis. Environ Sci Technol 48(21):12783–12790. [DOI] [PubMed] [Google Scholar]
- Robel AE, Marshall K, Dickinson M, Lunderberg D, Butt C, Peaslee G, Stapleton HM, Field JA. 2017. Closing the mass balance on fluorine on papers and textiles. Environ Sci Technol 51(16):9022–9032. [DOI] [PubMed] [Google Scholar]
- Russell MH. 2015. Aerobic degradation of fluorotelomer‐based acrylic polymers. Presentation at Fluoros 2015; 2015 July 12–July 14; Golden, CO.
- Russell MH, Berti WR, Szostek B, Buck RC. 2008. Investigation of the biodegradation potential of a fluoroacrylate polymer product in aerobic soils. Environ Sci Technol 42(3):800–907. [DOI] [PubMed] [Google Scholar]
- Sadia M, Yeung LWY, Fiedler H. 2020. Trace level analyses of selected perfluoroalkyl acids in food: Method development and data generation. Environ Pollut 263(A):113721. [DOI] [PubMed] [Google Scholar]
- Schantz M, Eppe G, Focant J‐F, Hamilton C, Heckert NA, Heltsley RM, Hoover D, Keller JM, Leigh SD, Patterson DG Jr et al. 2013. Milk and serum standard reference materials for monitoring organic contaminants in human samples. Anal Bioanal Chem 405(4):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JA, Tettenhorst DR. 2020. Method 537.1: Determination of selected per‐ and polyflourinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). Washington (DC): US Environmental Protection Agency. 50 p.
- Sianesi D, Marchionni G, De Pasquale RJ. 1994. Perfluoropolyethers (PFPEs) from perfluoroolefin photooxidation: Fomblin® and Galden® fluids In: Banks RE, Smart BE, Tatlow JC, editors. Organofluorine chemistry: Principles and commercial applications. New York (NY): Plenum; p 431–462. [Google Scholar]
- Speight JG. 2017. 3.3.10: Hydrofluoric acid in environmental inorganic chemistry for engineers Oxford (UK): Elsevier; p 111–169. [Google Scholar]
- Styler SA, Myers AL, Donaldson DJ. 2013. Heterogeneous photooxidation of fluorotelomer alcohols: A new source of aerosol‐phase perfluorinated carboxylic acids. Environ Sci Technol 47(12):6358–6367. [DOI] [PubMed] [Google Scholar]
- Theodoridis G. 2006. Chapter 4 fluorine‐containing agrochemicals: An overview of recent developments. Adv Fluorine Sci 2:121–175. [Google Scholar]
- Trier X, Granby K, Christensen JH. 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18(7):1108–1120. [DOI] [PubMed] [Google Scholar]
- [UNEP] United Nations Environment Programme . 2009. Decision SC‐4/17. Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride. In: Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants. Geneva (CH). [accessed 2020 Sep 15]. http://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.4-SC-4-17.English.pdf
- [UNEP] United Nations Environment Programme . 2019a. Decision SC‐9/4: Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride. In: Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants. Geneva (CH). [accessed 2020 Sep 15]. http://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.9-SC-9-4.English.pdf
- [UNEP] United Nations Environment Programme . 2019b. Decision SC‐9/12: Listing of perfluorooctanoic acid (2019), its salts and PFOA‐related compounds. In: Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants. Geneva (CH). [accessed 2020 Sep 15]. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.9-SC-9-12.English.pdf
- [UNEP] United Nations Environment Programme . 2019c. POPRC‐15/1: Perfluorohexane sulfonic acid (PFHxS), its salts and PFHxS‐related compounds. Geneva (CH). [accessed 2020 Sep 15]. http://chm.pops.int/TheConvention/POPsReviewCommittee/Recommendations/tabid/243/Default.aspx
- [UNEP] United Nations Environment Programme . 2019d. Guidance on the global monitoring plan for persistent organic pollutants. Geneva (CH): UNEP/POPS/COP.9/INF/36. 149 p.
- [USEPA] US Environmental Protection Agency . 2016a. Drinking water health advisory for perfluorooctane sulfonate (PFOS). Washington (DC): Office of Water (4304T). Health and Ecological Criteria Division. EPA 822‐R‐16‐004. 88 p.
- [USEPA] US Environmental Protection Agency . 2016b. Drinking water health advisory for perfluorooctanoic acid (PFOA). Washington (DC): Office of Water (4304T). Health and Ecological Criteria Division. EPA 822‐R‐16‐005. 103 p.
- [USEPA] Environmental Protection Agency . 2017. Data summary of the third unregulated contaminant monitoring rule (UCMR 3). EPA 815‐S‐17‐001. [accessed 2020 Aug 20]. https://www.epa.gov/sites/production/files/2017-02/documents/ucmr3-data-summary-january-2017.pdf
- [USEPA] Environmental Protection Agency . 2019a. PFAS action plan. Washington (DC). EPA 823‐R‐18‐004. [accessed 2020 Aug 20]. https://www.epa.gov/pfas/epas-pfas-action-plan
- [USEPA] Environmental Protection Agency . 2019b. Method 533: Determination of per‐ and polyfluoroalkyl substances in drinking water by isotope dilution anion exchange solid phase extraction and liquid chromatographt/tandem mass spectrometry. November 2019. Cincinnati (OH): Office of Water (MS‐140). EPA 815‐B‐19‐020. 52 p.
- [USEPA] Environmental Protection Agency . 2019c. PFAS action plan. February 2019. Washington (DC). EPA 823‐R‐18‐004. [accessed year month day]. https://www.epa.gov/pfas/epas-pfas-action-plan)
- [USP] US Pharmacopeial Convention . 2011. US Pharmacopeia National Formulary 2011. USP 34 NF 29. Rockville (MD). General Notices, Section 5.3.0. 6 p.
- Vierke L, Berger U, Cousins IT. 2013. Estimation of the acid dissociation constant of perfluoroalkyl carboxylic acids through an experimental investigation of their water‐to‐air transport. Environ Sci Technol 47(19):11032–11039. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang P, Meng J, Liu S, Lu Y, Khim Jong S, Giesy JP. 2015. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 129:87–99. [DOI] [PubMed] [Google Scholar]
- Wang X, Schuster J, Jones KC, Gong P. 2018. Occurrence and spatial distribution of neutral perfluoroalkyl substances and cyclic volatile methylsiloxanes in the atmosphere of the Tibetan Plateau. Atmos Chem Phy 18:8745–8755. [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuhler K. 2014a. Global emission inventories for C4 – C14 perfluoroalkyl carboxylic acid homologues from 1951 to 2030, part I: Production and emissions from quantifiable sources. Environ Int 70:62–75. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuhler K. 2014b. Global emission inventories for C4 – C14 perfluoroalkyl carboxylic acid homologues from 1951 to 2030, part II: The remaining pieces of the puzzle. Environ Int 69:166–176. [DOI] [PubMed] [Google Scholar]
- Washington JW, Jenkins TM, Rankin K, Naile JE. 2015. Decades‐scale degradation of commercial, side‐chain fluorotelomer‐based polymers in soil and water. Environ Sci Technol 49(2):915–923. [DOI] [PubMed] [Google Scholar]
- Weiss JM, van der Veen I, de Boer J, van Leeuwen SPJ, Cofino W. 2013. Analytical improvements shown over four interlaboratory studies of perfluoroalkyl substances in environmental and food samples. TrAC. Trends Analyt Chem 43:204–221. [Google Scholar]
- [WHO] World Health Organization . 2017. Drinking Water Parameter Cooperation Project, support to the revision of Annex I Council Directive 98/83/EC on the quality of water intended for human consumption (drinking water directive)—Recommendations. Bonn (DE): WHO Regional Office for Europe. [accessed 2020 Aug 20]. https://ec.europa.eu/environment/water/water-drink/pdf/20171215_EC_project_report_final_corrected.pdf
- Wong F, Shoeib M, Katsoyiannis A, Eckhardt S, Stohl A, Bohlin‐Nizzetto P, Li H, Fellin P, Su Y, Hung H. 2018. Assessing temporal trends and source regions of per‐ and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP). Atmos Environ 172:65–73. [Google Scholar]
- Yeung LW, Miyake Y, Li P, Taniyasu S, Kannan K, Guruge KS, Lam PK, Yamashita N. 2009. Comparison of total fluorine, extractable organic fluorine and perfluorinated compounds in the blood of wild and pefluorooctanoate (PFOA)‐exposed rats: Evidence for the presence of other organofluorine compounds. Anal Chim Acta 635(1):108–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains online‐only Supplemental Data.
Supporting information.
Data Availability Statement
Data and associated metadata and calculation tools are available upon request by contacting corresponding author Heidelore Fiedler (Heidelore.Fiedler@oru.se).


