Abstract
Objectives
The aim of this study was to evaluate thermal effects of ceramic and metal implant drills during implant site preparation using a standardised bovine model.
Material and Methods
A total of 320 automated intermittent osteotomies of 10‐ and 16‐mm drilling depths were performed using zirconium dioxide‐based and stainless steel drills. Various drill diameters (2.0/ 2.2, 2.8, 3.5, 4.2 mm ∅) and different cooling methods (without/ with external saline irrigation) were investigated at room temperature (21 ± 1°C). Temperature changes were recorded in real time using two custom‐built multichannel thermoprobes in 1‐ and 2‐mm distance to the osteotomy site. For comparisons, a linear mixed model was estimated.
Results
Comparing thermal effects, significantly lower temperatures could be detected with steel‐based drills in various drill diameters, regardless of drilling depth or irrigation method. Recorded temperatures for metal drills of all diameters and drilling depths using external irrigation were below the defined critical temperature threshold of 47°C, whereas ceramic drills of smaller diameters reached or exceeded the harmful temperature threshold at 16‐mm drilling depths, regardless of whether irrigation was applied or not. The results of this study suggest that the highest temperature changes were not found at the deepest point of the osteotomy site but were observed at subcortical and deeper layers of bone, depending on drill material, drill diameter, drilling depth and irrigation method.
Conclusions
This standardised investigation revealed drill material and geometry to have a substantial impact on heat generation, as well as external irrigation, drilling depth and drill diameter.
Keywords: ceramic drills, dental implants, heat generation, implant osteotomies, irrigation methods, multiple temperature sensors, standardised testing specimen, thermal osteonecrosis
1. INTRODUCTION
During the past decades, implant‐supported treatment solutions have become a predictable and viable option for prosthetic rehabilitation of toothless and partially edentulous patients. In this respect, titanium dental implants have been well documented over a long period of time and are widely accepted as the gold standard in contemporary dental implantology (Buser et al., 2012; Chiapasco et al., 2020; Chrcanovic et al., 2020; Ducommun et al., 2019; Jung et al., 2012; Pjetursson et al., 2012). Regardless of the reported success rates for dental implants, the implications of implant failure can be both medically and financially challenging (Mardinger et al., 2008). Hence, a variety of factors (biological, iatrogenic, mechanical and patient‐associated complications) resulting in peri‐implant diseases and therefore affecting implant success have been investigated (Esposito et al., 1998a; Lang et al., 2000; Schwarz, 2000). Biological failures have been described as early versus late implant losses, depending on whether failure to achieve or to maintain already established osseointegration was observed (Esposito et al., 1998a). While clinical and diagnostic criteria for peri‐implant diseases have been described in detail in the past (Heitz‐Mayfield, 2008; Renvert et al., 2018), the contribution of factors to early and late implant failures (such as surgical trauma, chronic marginal infection, implant overload and poor bone quality) remains controversial (Esposito et al., 1998b; Piattelli et al., 2003). Recently, discussion on titanium‐based failures due to hypersensitivities and allergies became a focus of attention (Fage et al., 2016; Pigatto et al., 2009; Sicilia et al., 2008; Siddiqi et al., 2011), significance supporting this theory remains still unproven (Javed et al., 2013).
However, greyish peri‐implant soft tissue discolouration with titanium implants may pose a challenge in aesthetically sensitive areas, especially in combination with a mucosal thickness of less than 2 mm (Benic et al., 2017; Cosgarea et al., 2015; Ioannidis et al., 2017; Jung et al., 2008). The rising criticism of titanium and the fact that an increasing number of patients is requesting entirely metal‐free dental reconstructions have led to ceramic oral implants being considered as promising alternative to titanium (Andreiotelli et al., 2009; Sivaraman et al., 2018; Spies et al., 2019). Aluminium oxide (Al2O3, alumina), a ceramic material for dental implants introduced at a similar time as titanium implants (Sandhaus, 1968, 1971; Schulte & Heimke, 1976), was eventually withdrawn from the market due to an increased risk of implant fractures (Ananth et al., 2015; Andreiotelli et al., 2009; Hobkirk & Wiskott, 2009; Kohal et al., 2004). Zirconium dioxide (ZrO2, zirconia), however, shows favourable physicochemical properties (high bending strength and fracture toughness) depending upon its composition and processing (Piconi & Maccauro, 1999; Sivaraman et al., 2018) as well as high biocompatibility similar to titanium (Benic, Thoma, et al., 2017; Bormann et al., 2012; Gahlert et al., 2012; Janner et al., 2018; Roehling et al., 2019). Although long‐term results for zirconia implants are still missing and elevated heat generation was reported in vitro during implant insertion with zirconia implants when compared to titanium implants (Zipprich et al., 2019), promising clinical data investigating the outcome of one‐piece zirconia implants after an observation period of 5 years have been recently obtained (Balmer et al., 2020). Newly developed two‐piece zirconia implants, which are supposed to overcome problems caused by challenging abutment angulations (Wenz et al., 2008), showed a similar screw‐retained stability in vitro (artificial ageing process) when compared to a conventional titanium‐based connection (Joos et al., 2020).
Regardless of the dental implant used, an atraumatic and delicate surgical preparation technique is considered to be a key prerequisite for successful osseointegration (Albrektsson et al., 1981; Eriksson & Adell, 1986). Therefore, thermal bone injury has been discussed in‐depth as a cause for bone tissue necrosis, followed by impaired microcirculation, activation of bone marrow macrophages and consequently implant failure (Augustin et al., 2012; Eriksson & Albrektsson, 1983, 1984; Eriksson et al., 1982; Esposito et al., 1998b; Piattelli et al., 1998; Trisi et al., 2014; Yoshida et al., 2009). Eriksson & Albrektsson identified the temperature threshold for bone survival to be between 44 and 47°C with an exposure time of less than 1 min (Eriksson & Albrektsson, 1983, 1984); however, an exact threshold for osteonecrosis remains still unclear (Augustin et al., 2012; Oliveira et al., 2012; Yoshida et al., 2009). Numerous factors have been reported to influence heat generation during implant bed preparation, such as drill diameter (Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014), drill design and geometry (Cordioli & Majzoub, 1997; Oh et al., 2011; Sannino et al., 2015), drill load (Abouzgia & James, 1997), sharpness and drill wear (Salimov et al., 2020; Scarano et al., 2007), surgical technique used (Frösch et al., 2019; Lajolo et al., 2018; Lucchiari et al., 2016), irrigation (Gehrke et al., 2018; Harder et al., 2013; Strbac et al., 2015) and bone density (Yacker & Klein, 1996). Moreover, drill material has been suggested to affect temperature increase during osteotomy (Hochscheidt et al., 2017; Oliveira et al., 2012; Scarano et al., 2020; Sumer et al., 2011). Conventional rotary preparation with metal burs is still the most commonly used procedure in dental implantology.
However, as a result of the above‐mentioned focus on freedom from metal, the fact that metallic contamination of bone due to drilling procedures (Hobkirk & Rusiniak, 1978) and surface corrosion of steel drills after contact with disinfecting liquids has been reported (Scarano et al., 2019) and the introduction of mixed ceramics with improved mechanical properties (Gaertner et al., 2005; Piconi & Maccauro, 1999) may lead to an increasing use of ceramic burs for implant site osteotomies.
The clinical use of ceramic implant drills is still a topic of discussion, and scientific evidence regarding thermal performance is scarce and controversial. This study aims to investigate temperature changes with different implant drill materials and various irrigation methods in standardised bovine specimens in order to expand knowledge regarding future implant procedures. Our investigation was based on the working hypothesis that ceramic implant drills would provide for beneficial temperature effects when compared to stainless steel drills.
2. MATERIAL AND METHODS
2.1. Bone specimens and implant drills
In the present in vitro study, temperature measurements during drilling procedures were recorded using artificially manufactured bone specimens (BoneSimTM, 1800.35/1300.14 Composite, BoneSimTM, Newaygo, MI, USA) with distinctive cortical (3 mm) and cancellous (15 mm) bone sections (Figures 1 and 2). By simulating human mandibular bone density (type 2 according to Lekholm and Zarb classification), this testing specimen represents a novel standardised bone model for thermal evaluation in bone osteotomies (Abboud et al., 2015; Delgado‐Ruiz et al., 2016, 2018; Lekholm & Zarb, 1985; Strbac et al., 2015; Strbac, Giannis, et al., 2014). A thermal conductivity of 0.3–0.4 W m−1 K−1 ensures a similar thermal conductivity as human bone, thus providing comparable clinical testing conditions (Clattenburg et al., 1975; Davidson & James, 2000). Commercially available surgical twist drills made of stainless steel (stainless martensitic steel DIN Code: 1.4108; 2.2, 2.8, 3.5, 4.2 mm ∅; Straumann PROTM, Straumann®, Basel, Switzerland) and alumina‐toughened zirconia (2.0, 2.8, 3.5, 4.2 mm ∅; Komet CeradrillTM, Komet®, Gebr. Brasseler, Lemgo, Germany) for graduated preparation were used (Figures 3 and 4). Ethics approval was not required for this in vitro study.
FIGURE 1.

Standardised bovine bone specimen (BoneSim, 1800.35/1300.14 Composite, BoneSim, Newaygo, MI, USA) embedded in polystyrene test box
FIGURE 2.

Comparison bone specimen in computed tomography presenting 3 mm cortical and 15 mm cancellous bone sections according to type 2 Lekholm and Zarb classification
FIGURE 3.
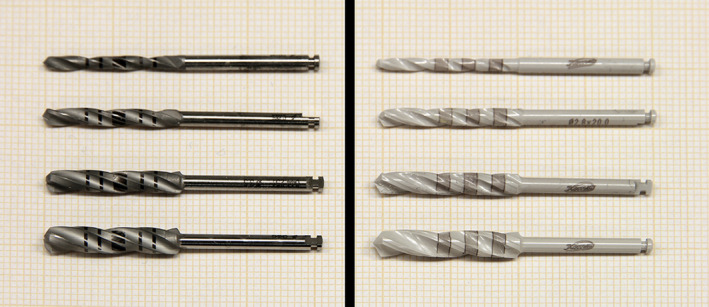
Implant twist drills with respective drill diameters used for the investigation, left image: metal drills (2.2, 2.8, 3.5, 4.2 mm ∅, Straumann PROTM, Straumann®, Basel, Switzerland), right image: ceramic drills (2.0, 2.8, 3.5, 4.2 mm ∅, Komet CeradrillTM, Komet®, Gebr. Brasseler, Lemgo, Germany)
FIGURE 4.
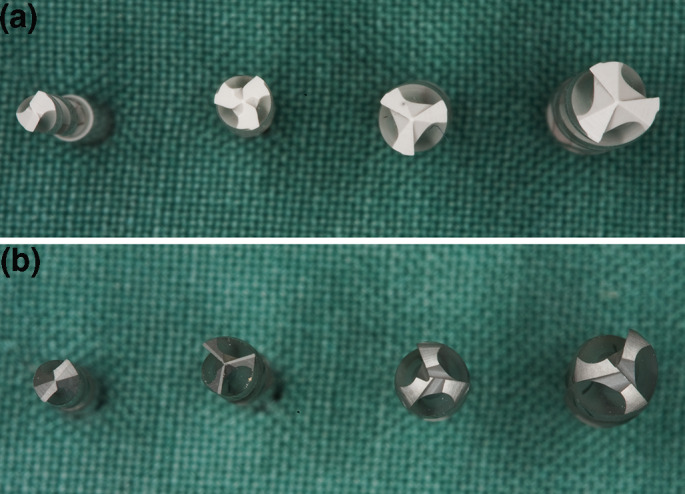
Comparison of drill geometries and drill shapes with increasing drill diameter due to different material properties ((a): ceramic drills, (b): metal drills)
2.2. Experimental set‐up
A customised surgical system (SH‐Surgical Drilling‐Sequence‐Simulator System, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria) with a stepper motor for automated and therefore reproducible drilling cycles was manufactured. Its software program (SSH‐Surgical Drilling‐Sequence‐Software 1.0; Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria) provided for a precise intermittent vertical dislocation of a surgical handpiece (WS‐75 E/KM 20:1, W&H, Bürmoos, Austria) mounted in a computer‐milled clamp (Figure 5). Parameters (drilling/withdrawing feed rate, depth control and dwell time) for two different drilling sequences (10 and 16 mm) were predetermined according to related previous investigations (Strbac et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014). For the 10‐mm drilling depth, the automated osteotomies lasted 27.6 s (drilling 17.3 s, withdrawing 10.3 s) and for the 16‐mm drilling depth 43.5 s (drilling 27.1 s, withdrawing 16.4 s). The drilling and withdrawing feed rate in cortical bone was 0.5 mm/s, in cancellous bone 1 and 5 mm/s during final withdrawing.
FIGURE 5.
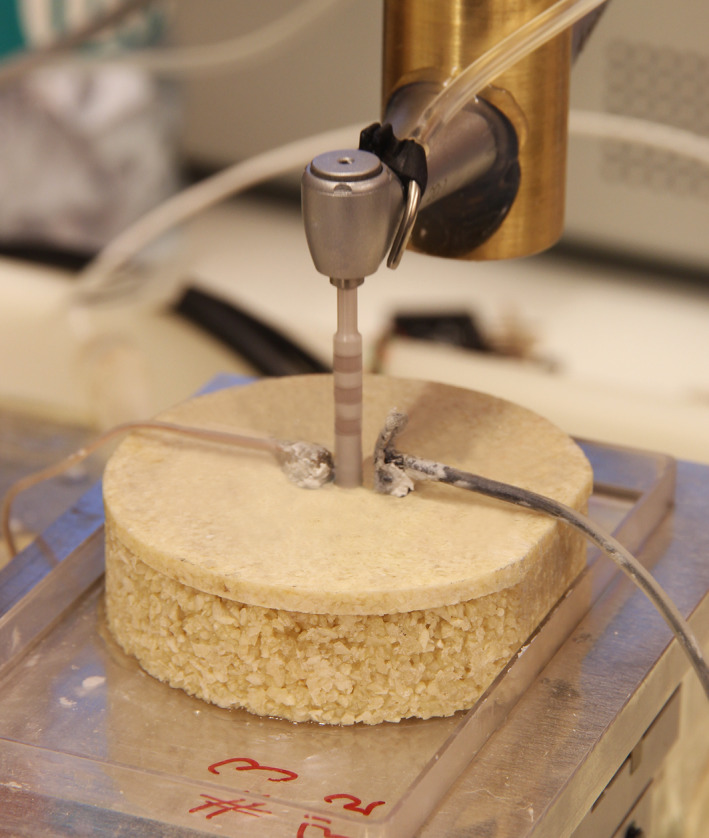
Automated intermittent and graduated preparation sequence (e.g. 4.2 mm ∅ ceramic implant drill, 16‐mm drilling depth, without irrigation) and temperature measurement system (2 thermoprobes with injected heat‐transfer compound)
2.3. Temperature measurement set‐up
Two custom‐built thermoprobes (SHT‐Thermoprobe, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria) with 1.5‐mm diameter and 18‐mm length were used to obtain real‐time temperature changes. These multichannel measuring devices consisted of a 3D printed body with 14 individual temperature sensors (7 sensors per thermoprobe, 0.4 mm ∅, response time >0.2 s, 10 KΩ at 25°C, temperature range −40 to +250°C). The thermoprobes were software designed (NX 5.0.3.2 Unigraphics, PLM Software, Siemens, Cologne, Germany) and manufactured by a rapid prototyping system (Eden350, Objet Ltd., Rehovot, Israel) using photopolymer resin (Objet FullCure720TM, Objet Ltd., Rehovot, Israel). They were planned with predefined notches for the NTC sensors (negative temperature coefficient sensors; Miniature Axial Glass Thermistor, No. GA10KM3499J15, Measurement SpecialtiesTM, Hampton, VA, USA) at depths of 2, 4, 8 and 10 mm for 10‐mm drilling sequence and additionally 11, 13 and 16 mm for 16‐mm drilling sequence (Figure 6). A computer‐aided temperature measurement system (SHTM‐Temperature Measurement System, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria), a measurement amplifier (SHU‐Measurement Amplifier, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria), an ADC‐converter (Analogue‐to‐digital‐converter; NI DAQCardTM‐6062E, National InstrumentsTM, Austin, TX, USA) and a software‐controlled program (DASYLab® Software 5.0; Measurement Computing Corporation, Norton, MA, USA) detected electrical resistance of the NTC sensors, allowing real‐time measurement and recording of temperature after initial calibration against traceable standards (Strbac et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014).
FIGURE 6.
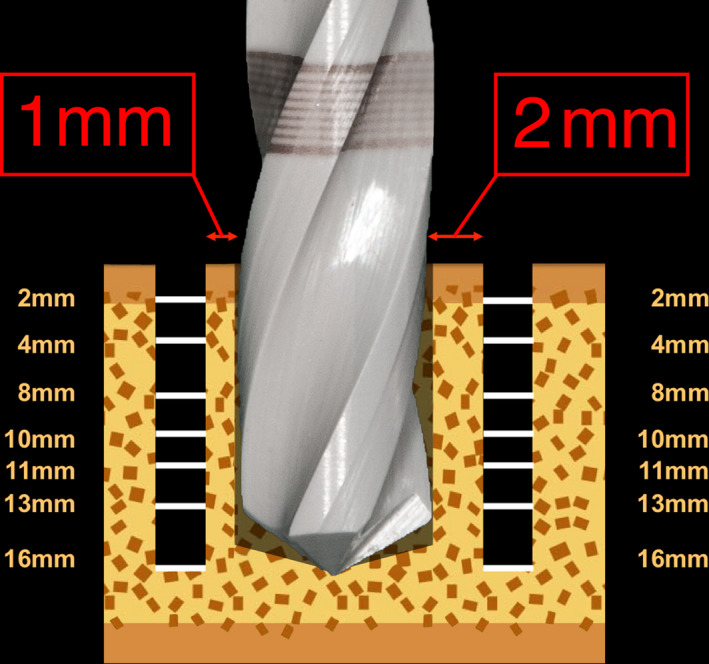
Schematic illustration of real‐time temperature measurement system, 2 thermoprobes in 1‐ and 2‐mm distance to the drilling site, 14 individual temperature sensors and their respective measurement depths
2.4. Experimental protocol
A total of 50 bovine bone specimens were embedded in rectangular polystyrene test boxes (No. 34160‐0101; Bock, Lauterbach, Germany) and bonded with a two‐component epoxy resin adhesive (Loctite® Double BubbleTM, Henkel AG & Co. KGaA, Düsseldorf, Germany), ensuring a stable position throughout the experimental drilling procedure. Individual metal templates for positioning the thermoprobes were CNC‐milled in order to precisely position them 1 and 2 mm from the final preparation site using a twist drill (2 mm ∅, drilling depth 18 mm, 210L20.205.020, Komet®, Gebr. Brasseler, Lemgo, Germany) (Oliveira et al., 2012; Rashad et al., 2011; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014) (Figure 6). Before inserting the two thermoprobes, a heat‐transfer compound (HTCP20S 20 ml, Electrolube®, Leicestershire, UK) was injected in each canal for optimal thermal conductivity (Ercoli et al., 2004; Harder et al., 2013; Strbac et al., 2015). The experimental protocol consisted of two drill materials (ceramic and stainless steel) with 4 diameters each (2.0/ 2.2, 2.8, 3.5 and 4.2 mm ∅), 2 different cooling methods (without/with external saline irrigation), two drilling depths (10 mm/16 mm) and was conducted with 10 repetitions (n = 320 preparations in total) under constant room temperature (21 ± 1°C). A computer‐milled table for horizontal dislocation of the embedded specimen ensured precise automated preparations by the surgical handpiece connected to a surgical motor unit (Implantmed SI‐923; Surgical control S‐N1, W&H, Bürmoos, Austria) with a new and unused drill for each osteotomy (Figure 5). Graduated predrilling according to clinical recommendations was performed prior to measurements. The real‐time temperature at 1‐ and 2‐mm distance was recorded during all osteotomies starting 10 s before drilling and ending 25 s after withdrawing (Figure 7). In case of external irrigation sequences, constant saline cooling of 50 ml/min at room temperature (Ecobag® click, 0.9% NaCl, 5,000 ml, B. Braun Melsungen AG, Melsungen, Germany) was applied throughout the whole preparation period (drilling start to withdrawing end) using an irrigation tubing set (Irrigation set for machinery—80 mm, 32.F0139, Omnia®, Fidenza, Italy) and surgical suction was applied at 1.5 cm from preparation site. All osteotomies were performed according to a standard protocol for an atraumatic preparation at 800 rpm (Gaspar et al., 2013; Koopaie et al., 2019; Oliveira et al., 2012; Scarano et al., 2020; Strbac et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014).
FIGURE 7.
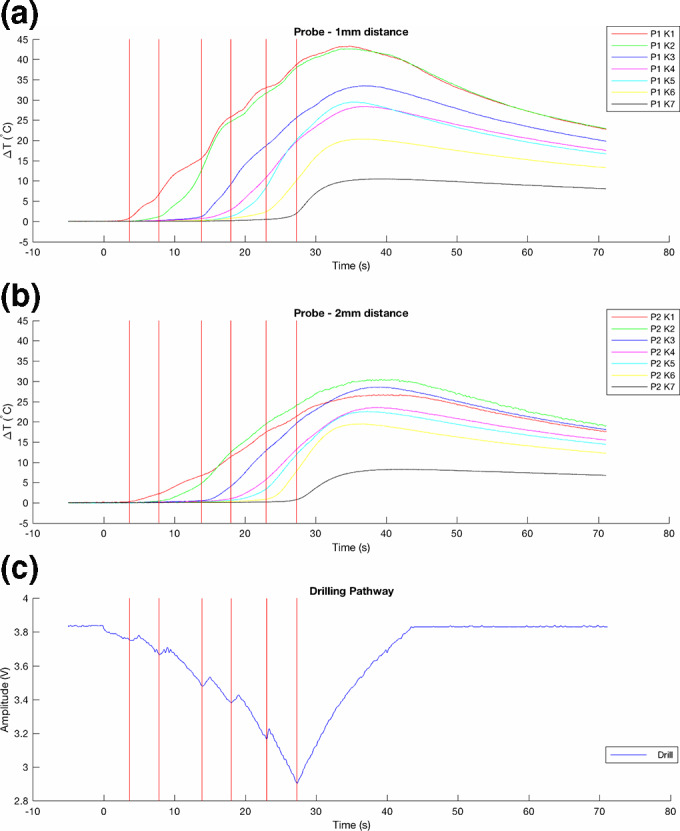
Illustration of multichannel real‐time measurement of temperature changes (∆T) at 16‐mm drilling depth in (a) 1‐ and (b) 2‐mm distance to the osteotomy site, (c) corresponding drilling pathway recorded by external linear motion potentiometer
2.5. Statistical analysis
The obtained experimental data were recorded (ASC file format) for each osteotomy and included real‐time recordings of 14 temperature sensors, a time signature (δ = 0.001 s) and a record of an external linear motion potentiometer (Linear Potentiometer 600 Series, Type 9615R5.1KL2.0, BEI Sensors, Goleta, CA, USA). In order to match temperature variations with each osteotomy and to process the data, a custom analysis software for descriptive statistics was used (MATLAB®, R2016a, MathWorks®, Natick, MA, USA). Temperature changes were calculated [∆T(°C) = Tx‐T0] by subtracting the recorded temperature [Tx] with the bone specimen baseline temperature [T0] before each osteotomy (Abboud et al., 2015; Calvo‐Guirado et al., 2015; Gehrke et al., 2015; Oliveira et al., 2012; Rashad et al., 2011; Sannino et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014). For statistical analysis, temperature and temperature differences were normally distributed and described and tabulated with mean ± standard deviation. Depth of sensor channel with maximum temperature increase was described and tabulated with median, minimum and maximum. For comparison of ceramic and stainless steel drills, a linear mixed model was fitted including the variables material (ceramic/metal), drilling diameter (2.0/2.2, 2.8, 3.5, 4.2 mm), drilling depth (10 mm/16 mm) and irrigation (with/ without). Compound symmetry was assumed for repeated measurements. A four‐way interaction analysis of the four explanatory variables was conducted. In case of significant interactions, subgroup analyses to test for differences in drill materials were performed and corresponding p‐values are presented. Statistical calculations were performed with the statistical software SAS® (Version 9.4, SAS Institute Inc., Cary, NC, USA). All p‐values are two‐sided, and p ≤ .05 was considered statistically significant.
3. RESULTS
Temperature changes during implant site preparation of 320 osteotomies with different drill materials, drill diameters, drilling depths, irrigation methods and 10 repetitions were investigated. The mean bone specimen baseline temperature [T0] before osteotomy procedures was 21.74 ± 1.18°C for the 10‐mm drilling depth and 21.80 ± 1.26°C for the 16‐mm drilling depth. Bone temperatures increased with all the implant drills tested; the distributions of mean differences between drilling and baseline temperatures over all temperature measurement sensors (10‐mm drilling sequence: 2×4 sensors, 16‐mm drilling sequence: 2×7 sensors) are shown in Figure 8 and Table 1.
FIGURE 8.
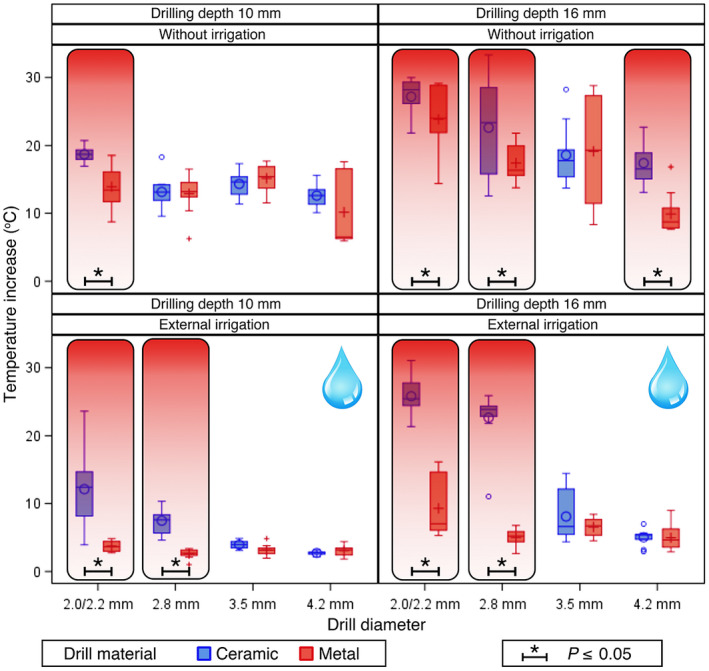
Temperature increase in investigated drill materials (blue colour = ceramic, red colour = metal, *p ≤ .05) of different drill diameters (2.0/2.2, 2.8, 3.5, 4.2 mm ∅), irrigation methods (without/external irrigation) and drilling depths (10/16 mm)
TABLE 1.
Maximum temperature increase [∆T°C mean (SD)] over all temperature sensors (10‐mm drilling sequence: 2×4 sensors, 16‐mm drilling sequence: 2×7 sensors) at different drilling depths with various drill diameters and irrigation methods, testing drill material ceramic (CER) versus metal (MET); P‐values for drill material comparisons are calculated by a linear mixed model after significant four‐way interaction
| Drill diameter | Drill material | Drilling depth 10 mm | Drilling depth 16 mm | ||
|---|---|---|---|---|---|
| Irrigation method | Irrigation method | ||||
| Without | External | Without | External | ||
| 2.0 mm | CER | 18.70 (1.14) | 12.13 (5.68) | 27.20 (2.81) | 25.79 (2.86) |
| 2.2 mm | MET | 13.93 (3.05) | 3.73 (0.73) | 23.85 (4.75) | 9.29 (4.40) |
| p‐value | .002* | <.001* | .025* | <.001* | |
| 2.8 mm | CER | 13.15 (2.35) | 7.47 (1.89) | 22.63 (7.20) | 22.65 (4.23) |
| 2.8 mm | MET | 12.90 (2.93) | 2.59 (0.68) | 17.41 (2.82) | 5.02 (1.17) |
| p‐value | .824 | .002* | <.001* | <.001* | |
| 3.5 mm | CER | 14.33 (1.83) | 3.94 (0.61) | 18.58 (4.50) | 8.09 (3.59) |
| 3.5 mm | MET | 15.16 (2.08) | 3.16 (0.79) | 19.14 (8.15) | 6.55 (1.36) |
| p‐value | .539 | .596 | .708 | .312 | |
| 4.2 mm | CER | 12.61 (1.80) | 2.73 (0.31) | 17.41 (3.13) | 5.01 (1.19) |
| 4.2 mm | MET | 10.17 (5.17) | 3.06 (0.76) | 9.90 (2.99) | 4.97 (1.91) |
| p‐value | .095 | .803 | <.001* | .984 | |
p ≤ .05.
3.1. Highest mean temperature increase
The maximum mean temperature increases for 10‐mm drilling sequences without irrigation were as follows [∆T°C mean (SD)]: 18.70 (1.14) for the 2.0 mm drill (CER) and 13.93 (3.05) for the 2.2 mm drill (MET); 13.15 (2.35) for the 2.8 mm drill (CER) and 12.90 (2.93) for the 2.8 mm drill (MET); 14.33 (1.83) for the 3.5 mm drill (CER) and 15.16 (2.08) for the 3.5 mm drill (MET); and 12.61 (1.80) for the 4.2 mm drill (CER) and 10.17 (5.17) for the 4.2 mm drill (MET; Figure 8, Table 1).
The highest mean temperature increases for 10‐mm drilling sequences with external cooling were as follows [∆T°C mean (SD)]: 12.13 (5.68) for the 2.0 mm drill (CER) and 3.73 (0.73) for the 2.2 mm drill (MET); 7.47 (1.89) for the 2.8 mm drill (CER) and 2.59 (0.68) for the 2.8 mm drill (MET); 3.94 (0.61) for the 3.5 mm drill (CER) and 3.16 (0.79) for the 3.5 mm drill (MET); and 2.73 (0.31) for the 4.2 mm drill (CER) and 3.06 (0.76) for the 4.2 mm drill (MET; Figure 8, Table 1).
The maximum mean temperature increases for 16‐mm drilling sequences without irrigation were as follows [∆T°C mean (SD)]: 27.20 (2.81) for the 2.0 mm drill (CER) and 23.85 (4.75) for the 2.2 mm drill (MET); 22.63 (7.20) for the 2.8 mm drill (CER) and 17.41 (2.82) for the 2.8 mm drill (MET); 18.58 (4.50) for the 3.5 mm drill (CER) and 19.14 (8.15) for the 3.5 mm drill (MET); and 17.41 (3.13) for the 4.2 mm drill (CER) and 9.90 (2.99) for the 4.2 mm drill (MET; Figure 8, Table 1).
The highest mean temperature increases for 16‐mm drilling sequences with external cooling were as follows [∆T°C mean (SD)]: 25.79 (2.86) for the 2.0 mm drill (CER) and 9.29 (4.40) for the 2.2 mm drill (MET); 22.65 (4.23) for the 2.8 mm drill (CER) and 5.02 (1.17) for the 2.8 mm drill (MET); 8.09 (3.59) for the 3.5 mm drill (CER) and 6.55 (1.36) for the 3.5 mm drill (MET); and 5.01 (1.19) for the 4.2 mm drill (CER) and 4.97 (1.91) for the 4.2 mm drill (MET; Figure 8, Table 1).
3.2. Temperature increase and drill material
With regard to implant material, significant temperature differences (p ≤ .05) during implant preparations at drilling depths of 10 and 16 mm with different drill diameters and irrigation methods were observed. Drill materials were compared with respect to corresponding diameter, cooling method and osteotomy depth. The differences in heat generation between the drill materials differed significantly and, whenever significant results were found, ceramic drills invariably showed higher mean temperature increases [∆T°C (SD)] compared with metal drills (Figure 8, Table 1).
3.2.1. Drilling osteotomies of 10‐mm depth
During drilling sequences of 10‐mm depth, significantly higher temperatures were observed using ceramic drills of 2.0‐mm diameter without irrigation (p = .002), with external irrigation (p < .001), as well as using 2.8‐mm ceramic drills with external irrigation (p = .002) (Figure 8, Table 1).
3.2.2. Drilling osteotomies of 16‐mm depth
During drilling osteotomies of 16‐mm depth, significantly higher temperatures were found using ceramic drills of 2.0‐mm diameter without cooling (p = .025), with external cooling (p < .001), using 2.8‐mm ceramic drills without cooling (p < .001), with external cooling (p < .001), as well as using 4.2‐mm ceramic drills without cooling (p < .001) (Figure 8, Table 1).
3.3. Temperature increase and sensor location/ corresponding depth
In order to analyse bone areas affected by the temperature increase, the occurrence of maximum temperature changes at median sensor channel depth [ch (minimum–maximum)] (sensor channel depths: 2, 4, 8, 10 mm for 10‐mm drilling sequence and additionally 11, 13, 16 for 16‐mm drilling sequence) was calculated for 1‐ and 2‐mm measuring distance (Table 2).
TABLE 2.
Location of maximum temperature increase: median sensor channel location [ch (minimum–maximum)] (sensor channel depths: 2, 4, 8, 10 mm for 10‐mm drilling sequence and additionally 11, 13, 16 for 16‐mm drilling sequence) in 1‐ and 2‐mm measuring distance (MD = measuring distance, CER = ceramic, MET = metal)
| Drill diameter | Drill material | MD | Drilling depth 10 mm | Drilling depth 16 mm | ||
|---|---|---|---|---|---|---|
| Irrigation method | Irrigation method | |||||
| Without | External | Without | External | |||
| 2.0 mm | CER | 1 mm | 4 mm (2–4) | 4 mm (4–8) | 4 mm (2–4) | 4 mm (4–11) |
| 2 mm | 4 mm (4–4) | 8 mm (4–8) | 6 mm (4–8) | 8 mm (8–11) | ||
| 2.2 mm | MET | 1 mm | 3 mm (2–4) | 8 mm (4–8) | 4 mm (2–11) | 11 mm (11–13) |
| 2 mm | 4 mm (4–4) | 8 mm (4–8) | 8 mm (4–8) | 13 mm (8–16) | ||
| 2.8 mm | CER | 1 mm | 4 mm (2–4) | 8 mm (4–8) | 6 mm (4–11) | 11 mm (4–11) |
| 2 mm | 4 mm (4–8) | 8 mm (8–8) | 8 mm (8–13) | 10 mm (8–13) | ||
| 2.8 mm | MET | 1 mm | 4 mm (2–4) | 8 mm (8–8) | 4 mm (4–4) | 11 mm (11–11) |
| 2 mm | 4 mm (4–4) | 8 mm (8–8) | 8 mm (4–11) | 13 mm (13–13) | ||
| 3.5 mm | CER | 1 mm | 4 mm (4–4) | 8 mm (4–8) | 9 mm (2–11) | 11 mm (11–13) |
| 2 mm | 4 mm (4–4) | 8 mm (8–8) | 8 mm (4–8) | 13 mm (8–13) | ||
| 3.5 mm | MET | 1 mm | 4 mm (2–4) | 8 mm (4–8) | 4 mm (2–11) | 11 mm (11–11) |
| 2 mm | 4 mm (4–4) | 8 mm (8–8) | 8 mm (4–8) | 13 mm (10–13) | ||
| 4.2 mm | CER | 1 mm | 4 mm (4–4) | 8 mm (4–8) | 4 mm (2–11) | 11 mm (11–11) |
| 2 mm | 4 mm (4–8) | 8 mm (4–8) | 8 mm (8–8) | 13 mm (8–13) | ||
| 4.2 mm | MET | 1 mm | 4 mm (2–4) | 8 mm (4–8) | 4 mm (2–11) | 11 mm (11–11) |
| 2 mm | 4 mm (4–4) | 8 mm (4–8) | 8 mm (8–8) | 13 mm (8–13) | ||
3.3.1. Drill diameter 2.0/2.2 mm
Highest temperature changes using 2.0‐/2.2‐mm drills during 10‐mm drilling osteotomies were found between median sensor channels [ch] of 3 and 4 mm without irrigation and 4 and 8 mm with external irrigation.
During 16‐mm drilling depths, maximum temperature changes using 2.0‐/2.2‐mm drills were observed between median sensor channels [ch] of 4 and 8 mm without irrigation and 4 and 13 mm when external irrigation was used (Table 2).
3.3.2. Drill diameter 2.8 mm
Maximum temperature changes using 2.8‐mm drills during 10‐mm drilling sequences were observed at median sensor channel [ch] of 4 mm without irrigation and 8 mm when external irrigation was used.
During 16‐mm drilling osteotomies, highest temperature changes using 2.8‐mm drills were found between median sensor channels [ch] of 4 and 8 mm without irrigation and 10 and 13 mm with external irrigation (Table 2).
3.3.3. Drill diameter 3.5 mm
Highest temperature changes using 3.5‐mm implant drills during 10‐mm drilling depths were found at median sensor channel [ch] of 4 mm without irrigation and 8 mm when external irrigation was applied.
During 16‐mm drilling sequences, maximum temperature changes using 3.5‐mm drills were observed between median sensor channels [ch] of 4 and 9 mm without irrigation and 11 and 13 mm with external cooling (Table 2).
3.3.4. Drill diameter 4.2 mm
Maximum temperature changes using 4.2‐mm drills during 10‐mm drilling osteotomies were observed at median sensor channel [ch] of 4 mm without irrigation and 8 mm when external cooling was used.
During 16‐mm drilling depths, highest temperature changes using 4.2‐mm drills were found between median sensor channels [ch] of 4 and 8 mm without irrigation and 11 and 13 mm with external irrigation (Table 2).
4. DISCUSSION
As yet, there have been only a few published investigations exploring the performance of ceramic and metal implant drills with regard to intrabony thermal effects. These scientific studies have been performed on a variety of osseous bone models using different temperature measurement systems (various thermocouples or infrared thermography devices) and in vitro study designs. In addition, they have been mainly focused on the temperature correlation between drill material and drill wear (Hochscheidt et al., 2017; Koo et al., 2015; Koopaie et al., 2019; Oliveira et al., 2012; Pires et al., 2012; Scarano et al., 2020).
The purpose of this investigation was to examine metal and ceramic implant twist drills with identical or similar diameters during automated and reproducible drilling osteotomies by using a highly sensitive real‐time multichannel temperature measurement system and a standardised bovine bone model, previously introduced for temperature testing of surgical instruments (Abboud et al., 2015; Delgado‐Ruiz et al., 2016, 2018; Strbac et al., 2015; Strbac, Giannis, et al., 2014). Infrared thermography poses a valid alternative to thermocouple technology and has been successfully used in temperature investigations in the past (Augustin et al., 2012; Benington et al., 2002; Frösch et al., 2019). Doubts concerning its accuracy when recording temperatures at irrigated preparation sites (Benington et al., 1996; Tehemar, 1999) led to further development of real‐time multichannel thermoprobes by the authors in the past (Strbac et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014). This established temperature measurement system was applied in the present investigation as well, taking into account the fact that the comparability of results may be limited when it comes to different study designs (such as varying number of thermocouples, distance to preparation site, number and configuration of temperature sensors).
For overcoming limitations of previous temperature studies and as a result of the fact that temperature increase during implant osteotomies is considered to be a complex interaction of multiple factors (Augustin et al., 2012; Möhlhenrich et al., 2015; Tehemar, 1999), drill wear was consciously excluded as a contributing factor in this investigation by only testing new and unused drills.
The mean temperature increase for metal drills with external irrigation was below the defined critical temperature threshold of 47°C in all drill diameters and drilling depths, thereby confirming the cooling effect of external irrigation on bone temperature using metal implant drills (Augustin et al., 2008; Harder et al., 2013; Oliveira et al., 2012; Rashad et al., 2011; Sener et al., 2009; Strbac, Unger, et al., 2014). One of the most important findings of this investigation was the statistically significant difference in temperature performance between metal and ceramic drills, especially with small drill diameters (2.0/2.2 and 2.8 mm ∅) (Figure 8, Table 1). Recorded temperatures with ceramic drills at 16‐mm drilling depth reached or exceeded the harmful temperature threshold, regardless of whether irrigation was applied or not. Local temperatures at the drilling site can be presumed to be even higher due to the technical measuring distance of 1 and 2 mm to the osteotomy site (Oliveira et al., 2012; Strbac, Giannis, et al., 2014; Yacker & Klein, 1996).
Internal or combined internal and external irrigation may be considered a convenient alternative for overcoming cooling problems with external irrigation alone (Gehrke et al., 2018; Harder et al., 2013; Lavelle & Wedgwood, 1980; Strbac et al., 2015; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014; Tehemar, 1999). However, ceramic drills are not manufactured with internal cooling channels due to the risk of fracture (Pires et al., 2012). Even though our present findings suggest that cooling efficiency of external irrigation using ceramic drills of smaller diameter (2.0/2.8 mm ∅) compared with metal drills should be considered as less effective, external irrigation itself was confirmed to be one of the most influential factors on heat generation (Augustin et al., 2008; Ercoli et al., 2004; Kerawala et al., 1999; Koo et al., 2015; Sener et al., 2009; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014).
Graduated osteotomy technique is believed to reduce friction and consequently temperature by removing a smaller quantity of bone material in each drilling step when compared to single drilling procedures. The observation that predrilling with pilot drills of both drill materials (2.0/2.2 mm ∅), especially in deeper osteotomies, was associated with considerably higher temperatures when compared to expansion drilling procedures (2.8, 3.5, 4.2 mm ∅) and the fact that predominantly lower temperatures both in metal and ceramic drills were recorded with increasing diameter, confirm the beneficial effect of graduated drilling technique (Augustin et al., 2012; Eriksson & Adell, 1986; Lucchiari et al., 2016; Oh et al., 2011; Strbac et al., 2015; Strbac, Giannis, et al., 2014).
As demonstrated by Eriksson & Albrektsson, intrabony thermal effects are not only influenced by temperature generated during preparation, but also influenced by exposure time (Eriksson & Albrektsson, 1983). Previous investigations confirmed that the induced amount of frictional heat correlates with the drilling time (Abouzgia & James, 1995; Grunder & Strub, 1986; Iyer et al., 1997; Sener et al., 2009) and that drilling depth is considered to be a factor influencing temperature generation (Augustin et al., 2012; Cordioli & Majzoub, 1997; Lee et al., 2012; Oliveira et al., 2012; Strbac, Giannis, et al., 2014; Strbac, Unger, et al., 2014). In accordance with these findings, the present study was able to demonstrate that greater drilling depths (10‐ vs. 16‐mm drilling depth) and thus a prolonged exposure time to frictional forces (27.6 s vs. 43.5 s) were almost invariably associated with higher temperatures.
When evaluating temperatures in terms of the respective bone level, temperature increase was recorded at all temperature sensor depths (both in cortical and deeper cancellous layers of bone) for all drill materials, drill diameters, drilling depths and cooling methods. The distribution of maximum temperature for 10‐mm drilling osteotomies indicates that highest temperatures for all drill materials and diameters were mainly observed at subcortical levels (4 mm) without irrigation and in deeper bone sections (8 mm) when external irrigation was used. In 16‐mm drilling depth, maximum temperatures were mostly recorded between 4 and 8 mm for all drill materials and diameters without irrigation and between 8 and 13 mm with external irrigation when using larger drill diameters (2.8, 3.5, 4.2 mm ∅) of both drill materials. However, when comparing ceramic and metal pilot drills (2.0/2.2 mm ∅) of 16‐mm drilling sequence with external irrigation, ceramic drills were found to cause temperature changes in more superficial layers of bone than metal pilot drills (Table 2). These results support earlier investigations, which reported twist drills of small diameters to be associated with higher temperatures (Cordioli & Majzoub, 1997; Strbac et al., 2015; Strbac, Giannis, et al., 2014) and identified maximum temperatures to be located in subcortical or deeper bone sections (Cordioli & Majzoub, 1997; Harder et al., 2013; Misic et al., 2011; Misir et al., 2009; Strbac et al., 2015; Strbac, Unger, et al., 2014; Sumer et al., 2011). Our findings additionally confirmed that external irrigation seems to be associated with temperature increase in deeper layers of bone, thereby verifying the superficial efficiency of external cooling (Cordioli & Majzoub, 1997; Harder et al., 2013; Lavelle & Wedgwood, 1980; Misir et al., 2009; Moshiri et al., 2013).
Comparing the temperature performance of metal and ceramic implant drills, our findings clearly seem to contradict the majority of similar previous investigations, which observed lower temperatures with ceramic drills (Koopaie et al., 2019; Oliveira et al., 2012; Scarano et al., 2020) or did not find any statistically significant differences between the two drill materials (Harder et al., 2013; Koo et al., 2015; Moshiri et al., 2013; Pires et al., 2012). Our investigation was able to confirm findings of Sumer et al., who observed significantly higher temperatures in 3‐mm depth using ceramic drills when compared to stainless steel (Sumer et al., 2011). The observed differences between the two drill materials in our present investigation could mainly be explained by deviating material properties (in particular thermal conductivity) of alumina‐toughened zirconia and stainless steel drills (Gaertner et al., 2005), but also by drill geometry. With regard to the latter, earlier findings suggested drill geometry to be a key factor associated with temperature generation (Ali Akhbar & Yusoff, 2019; Chacon et al., 2006; Oh et al., 2011; Oliveira et al., 2012; Sannino et al., 2015; Scarano et al., 2011; Strbac, Giannis, et al., 2014), given that sharpness and geometry are influencing friction and heat production by having an impact on pressure exerted on the drill bit (Pirjamalineisiani et al., 2016).
However, the present investigation had some limitations. The results of this in vitro study have been obtained using artificial bovine discs with no vital bone experiments or direct simulation of in vivo conditions (such as body temperature or blood flow). Consequently, heat generation of the used instruments and techniques may vary in vivo from the present experimental set‐up. Previous investigations may be regarded as non‐standardised and diverse due to major differences in their study designs (such as bone model, experimental set‐up, drilling speed, temperature measurement system, focus on durability/ drill wear), therefore making comparison between these previously published findings rather difficult.
The aim of this study was, despite of its limitations, to further refine and establish standardised instrument performance testing and to contribute to safety of medical components by avoidance of human or animal experiments on ethical grounds. Future studies could pursue more uniform in vitro testing conditions, facilitating comparison of testing results, especially when it comes to new and hardly investigated treatment options.
In summary, the present study could confirm preceding investigations and reveal drill material and geometry as significant factors for temperature generation, even though recommended saline irrigation, intermittent and graduated drilling were performed. Zirconia and mixed ceramics can be recognised as innovative and promising new dental materials due to their physicochemical and biological characteristics, although further modifications in terms of drill geometry, sharpness, material thickness and properties should be considered and investigated, especially when ceramic drills are being used in bone osteotomies.
To the best knowledge of the authors, this study can be considered as the first fully standardised in vitro investigation using an established uniform bone model for performance testing of ceramic and metal drills under recommended atraumatic clinical conditions.
5. CONCLUSION
This standardised comparative investigation revealed the significant impact of drill material and geometry on intrabony heat generation. Previously published results were mainly obtained using a huge variety of non‐standardised experimental set‐ups including focus on drill wear, impairing the comparability of results. Our findings suggest a beneficial effect of graduated, intermittent preparation technique using external saline irrigation, although major differences in temperature performance between metal and ceramic implant drills could be observed. Furthermore, the results of this study may contribute towards technical modifications of ceramic drills in the future and thus further improve the long‐term clinical results of dental implants, especially considering the use of new upcoming ceramic dental implants.
AUTHOR CONTRIBUTION
Dino Tur: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Investigation (lead); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (lead). Katharina Giannis: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Supervision (supporting); Writing‐review & editing (supporting). Ewald Unger: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (lead); Project administration (supporting); Resources (lead); Software (lead); Supervision (equal); Validation (equal); Visualization (equal); Writing‐review & editing (supporting). Martina Mittlböck: Conceptualization (supporting); Data curation (equal); Formal analysis (lead); Investigation (supporting); Methodology (supporting); Software (supporting); Validation (supporting); Writing‐review & editing (supporting). Xiaohui Rausch‐Fan: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing‐review & editing (equal). Georg D. Strbac: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Funding acquisition (lead); Investigation (equal); Methodology (equal); Project administration (lead); Resources (equal); Supervision (lead); Validation (equal); Writing‐review & editing (equal).
AUTHOR CONTRIBUTIONS
D.T. involved in concept/design, led the experimental investigation and the writing of the manuscript. K.G. and X.R. revised the manuscript critically. M.M. performed statistical analysis of the collected data. E.U. involved in concept/design and supported the investigation technically. GD.S. conceived the ideas, concept/design, revised the manuscript critically, involved in approval of article and funding secured.
ACKNOWLEDGEMENTS
The authors wish to thank Ing. Ewald Unger for his technical support. Furthermore, they would like to acknowledge Dipl.‐Ing. Christoph Kast for his efforts in processing the measured data with the custom analysis software program. The authors were funded by their institutions (University Clinic of Dentistry, Medical University of Vienna; Center for Medical Physics and Biomedical Engineering, Medical University of Vienna; Center for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna) and by the ITI Research Grant 772_2011.
Tur D, Giannis K, Unger E, Mittlböck M, Rausch‐Fan X, Strbac GD. Thermal effects of various drill materials during implant site preparation—Ceramic vs. stainless steel drills: A comparative in vitro study in a standardised bovine bone model. Clin Oral Impl Res.2021;32:154–166. 10.1111/clr.13685
REFERENCES
- Abboud, M. , Delgado‐Ruiz, R. A. , Kucine, A. , Rugova, S. , Balanta, J. , & Calvo‐Guirado, J. L. (2015). Multistepped drill design for single‐stage implant site preparation: Experimental study in type 2 bone. Clinical Implant Dentistry and Related Research, 17, e472–e485. 10.1111/cid.12273 [DOI] [PubMed] [Google Scholar]
- Abouzgia, M. B. , & James, D. F. (1995). Measurements of shaft speed while drilling through bone. Journal of Oral and Maxillofacial Surgery, 53, 1308–1316. 10.1016/0278-2391(95)90590-1 [DOI] [PubMed] [Google Scholar]
- Abouzgia, M. B. , & James, D. F. (1997). Temperature rise during drilling through bone. The International Journal of Oral & Maxillofacial Implants, 12(3), 342–353. [PubMed] [Google Scholar]
- Albrektsson, T. , Brånemark, P. I. , Hansson, H. A. , & Lindström, J. (1981). Osseointegrated titanium implants. Requirements for ensuring a long‐lasting, direct bone‐to‐implant anchorage in man. Acta Orthopaedica Scandinavica, 52, 155–170. 10.3109/17453678108991776 [DOI] [PubMed] [Google Scholar]
- Ali Akhbar, M. F. , & Yusoff, A. R. (2019). Drilling of bone: Effect of drill bit geometries on thermal osteonecrosis risk regions. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine, 233, 207–218. 10.1177/0954411918819113 [DOI] [PubMed] [Google Scholar]
- Ananth, H. , Kundapur, V. , Mohammed, H. S. , Anand, M. , Amarnath, G. S. , & Mankar, S. (2015). A review on biomaterials in dental implantology. International Journal of Biomedical Science, 11(3), 113–120. [PMC free article] [PubMed] [Google Scholar]
- Andreiotelli, M. , Wenz, H. J. , & Kohal, R. J. (2009). Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clinical Oral Implants Research, 20, 32–47. 10.1111/j.1600-0501.2009.01785.x [DOI] [PubMed] [Google Scholar]
- Augustin, G. , Davila, S. , Mihoci, K. , Udiljak, T. , Vedrina, D. S. , & Antabak, A. (2008). Thermal osteonecrosis and bone drilling parameters revisited. Archives of Orthopaedic and Trauma Surgery, 128, 71–77. 10.1007/s00402-007-0427-3 [DOI] [PubMed] [Google Scholar]
- Augustin, G. , Zigman, T. , Davila, S. , Udilljak, T. , Staroveski, T. , Brezak, D. , & Babic, S. (2012). Cortical bone drilling and thermal osteonecrosis. Clinical Biomechanics (Bristol, Avon), 27, 313–325. 10.1016/j.clinbiomech.2011.10.010 [DOI] [PubMed] [Google Scholar]
- Balmer, M. , Spies, B. C. , Kohal, R. J. , Hämmerle, C. H. , Vach, K. , & Jung, R. E. (2020). Zirconia implants restored with single crowns or fixed dental prostheses: 5‐year results of a prospective cohort investigation. Clinical Oral Implants Research, 31, 452–462. 10.1111/clr.13581 [DOI] [PubMed] [Google Scholar]
- Benic, G. I. , Scherrer, D. , Sancho‐Puchades, M. , Thoma, D. S. , & Hämmerle, C. H. (2017). Spectrophotometric and visual evaluation of peri‐implant soft tissue color. Clinical Oral Implants Research, 28, 192–200. 10.1111/clr.12781 [DOI] [PubMed] [Google Scholar]
- Benic, G. I. , Thoma, D. S. , Sanz‐Martin, I. , Munoz, F. , Hämmerle, C. H. F. , Cantalapiedra, A. , Fischer, J. , & Jung, R. E. (2017). Guided bone regeneration at zirconia and titanium dental implants: A pilot histological investigation. Clinical Oral Implants Research, 28, 1592–1599. 10.1111/clr.13030 [DOI] [PubMed] [Google Scholar]
- Benington, I. C. , Biagioni, P. A. , Briggs, J. , Sheridan, S. , & Lamey, P. J. (2002). Thermal changes observed at implant sites during internal and external irrigation. Clinical Oral Implants Research, 13, 293–297. 10.1034/j.1600-0501.2002.130309.x [DOI] [PubMed] [Google Scholar]
- Benington, I. C. , Biagioni, P. A. , Crossey, P. J. , Hussey, D. L. , Sheridan, S. , & Lamey, P. J. (1996). Temperature changes in bovine mandibular bone during implant site preparation: An assessment using infra‐red thermography. Journal of Dentistry, 24, 263–267. 10.1016/0300-5712(95)00072-0 [DOI] [PubMed] [Google Scholar]
- Bormann, K. H. , Gellrich, N. C. , Kniha, H. , Dard, M. , Wieland, M. , & Gahlert, M. (2012). Biomechanical evaluation of a microstructured zirconia implant by a removal torque comparison with a standard Ti‐SLA implant. Clinical Oral Implants Research, 23, 1210–1216. 10.1111/j.1600-0501.2011.02291.x [DOI] [PubMed] [Google Scholar]
- Buser, D. , Janner, S. F. , Wittneben, J. G. , Brägger, U. , Ramseier, C. A. , & Salvi, G. E. (2012). 10‐year survival and success rates of 511 titanium implants with a sandblasted and acid‐etched surface: A retrospective study in 303 partially edentulous patients. Clinical Implant Dentistry and Related Research, 14, 839–851. 10.1111/j.1708-8208.2012.00456.x [DOI] [PubMed] [Google Scholar]
- Calvo‐Guirado, J. L. , Delgado‐Peña, J. , Maté‐Sánchez, J. E. , Mareque Bueno, J. , Delgado‐Ruiz, R. A. , & Romanos, G. E. (2015). Novel hybrid drilling protocol: Evaluation for the implant healing–thermal changes, crestal bone loss, and bone‐to‐implant contact. Clinical Oral Implants Research, 26, 753–760. 10.1111/clr.12341 [DOI] [PubMed] [Google Scholar]
- Chacon, G. E. , Bower, D. L. , Larsen, P. E. , McGlumphy, E. A. , & Beck, F. M. (2006). Heat production by 3 implant drill systems after repeated drilling and sterilization. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons, 64, 265–269. 10.1016/j.joms.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Chiapasco, M. , Tommasato, G. , Palombo, D. , & Del Fabbro, M. (2020). A retrospective 10‐year mean follow‐up of implants placed in ridges grafted using autogenous mandibular blocks covered with bovine bone mineral and collagen membrane. Clinical Oral Implants Research, 31, 328–340. 10.1111/clr.13571 [DOI] [PubMed] [Google Scholar]
- Chrcanovic, B. R. , Kisch, J. , & Larsson, C. (2020). Retrospective evaluation of implant‐supported full‐arch fixed dental prostheses after a mean follow‐up of 10 years. Clinical Oral Implants Research, 31, 634–645. 10.1111/clr.13600 [DOI] [PubMed] [Google Scholar]
- Clattenburg, R. , Cohen, J. , Conner, S. , & Cook, N. (1975). Thermal properties of cancellous bone. Journal of Biomedical Materials Research, 9, 169–182. 10.1002/jbm.820090206 [DOI] [PubMed] [Google Scholar]
- Cordioli, G. , & Majzoub, Z. (1997). Heat generation during implant site preparation: An in vitro study. The International Journal of Oral & Maxillofacial Implants, 12(2), 186–193. [PubMed] [Google Scholar]
- Cosgarea, R. , Gasparik, C. , Dudea, D. , Culic, B. , Dannewitz, B. , & Sculean, A. (2015). Peri‐implant soft tissue colour around titanium and zirconia abutments: A prospective randomized controlled clinical study. Clinical Oral Implants Research, 26, 537–544. 10.1111/clr.12440 [DOI] [PubMed] [Google Scholar]
- Davidson, S. R. , & James, D. F. (2000). Measurement of thermal conductivity of bovine cortical bone. Medical Engineering & Physics, 22, 741–747. 10.1016/s1350-4533(01)00003-0 [DOI] [PubMed] [Google Scholar]
- Delgado‐Ruiz, R. A. , Sacks, D. , Palermo, A. , Calvo‐Guirado, J. L. , Perez‐Albacete, C. , & Romanos, G. E. (2016). Temperature and time variations during osteotomies performed with different piezosurgical devices: An in vitro study. Clinical Oral Implants Research, 27, 1137–1143. 10.1111/clr.12709 [DOI] [PubMed] [Google Scholar]
- Delgado‐Ruiz, R. A. , Velasco Ortega, E. , Romanos, G. E. , Gerhke, S. , Newen, I. , & Calvo‐Guirado, J. L. (2018). Slow drilling speeds for single‐drill implant bed preparation. Experimental in vitro study. Clinical Oral Investigations, 22, 349–359. 10.1007/s00784-017-2119-x [DOI] [PubMed] [Google Scholar]
- Ducommun, J. , El Kholy, K. , Rahman, L. , Schimmel, M. , Chappuis, V. , & Buser, D. (2019). Analysis of trends in implant therapy at a surgical specialty clinic: Patient pool, indications, surgical procedures, and rate of early failures‐A 15‐year retrospective analysis. Clinical Oral Implants Research, 30, 1097–1106. 10.1111/clr.13523 [DOI] [PubMed] [Google Scholar]
- Ercoli, C. , Funkenbusch, P. D. , Lee, H. J. , Moss, M. E. , & Graser, G. N. (2004). The influence of drill wear on cutting efficiency and heat production during osteotomy preparation for dental implants: A study of drill durability. The International Journal of Oral & Maxillofacial Implants, 19(3), 335–349. [PubMed] [Google Scholar]
- Eriksson, A. R. , & Albrektsson, T. (1983). Temperature threshold levels for heat‐induced bone tissue injury: A vital‐microscopic study in the rabbit. The Journal of Prosthetic Dentistry, 50, 101–107. 10.1016/0022-3913(83)90174-9 [DOI] [PubMed] [Google Scholar]
- Eriksson, A. , Albrektsson, T. , Grane, B. , & McQueen, D. (1982). Thermal injury to bone. A vital‐microscopic description of heat effects. International Journal of Oral Surgery, 11, 115–121. 10.1016/s0300-9785(82)80020-3 [DOI] [PubMed] [Google Scholar]
- Eriksson, R. A. , & Adell, R. (1986). Temperatures during drilling for the placement of implants using the osseointegration technique. Journal of Oral and Maxillofacial Surgery, 44, 4–7. 10.1016/0278-2391(86)90006-6 [DOI] [PubMed] [Google Scholar]
- Eriksson, R. A. , & Albrektsson, T. (1984). The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. Journal of Oral and Maxillofacial Surgery, 42, 705–711. 10.1016/0278-2391(84)90417-8 [DOI] [PubMed] [Google Scholar]
- Esposito, M. , Hirsch, J. M. , Lekholm, U. , & Thomsen, P. (1998a). Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. European Journal of Oral Sciences, 106, 527–551. 10.1046/j.0909-8836.t01-2-.x [DOI] [PubMed] [Google Scholar]
- Esposito, M. , Hirsch, J. M. , Lekholm, U. , & Thomsen, P. (1998b). Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. European Journal of Oral Sciences, 106, 721–764. 10.1046/j.0909-8836.t01-6-.x [DOI] [PubMed] [Google Scholar]
- Fage, S. W. , Muris, J. , Jakobsen, S. S. , & Thyssen, J. P. (2016). Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis, 74, 323–345. 10.1111/cod.12565 [DOI] [PubMed] [Google Scholar]
- Frösch, L. , Mukaddam, K. , Filippi, A. , Zitzmann, N. U. , & Kühl, S. (2019). Comparison of heat generation between guided and conventional implant surgery for single and sequential drilling protocols‐An in vitro study. Clinical Oral Implants Research, 30, 121–130. 10.1111/clr.13398 [DOI] [PubMed] [Google Scholar]
- Gaertner, C. , Büchter, A. , Gehrke, S. , & Kleinheinz, J. (2005). Klinischer Einsatz von Knochenfräsen und Implantatbohrern aus Hochleistungsmischkeramik [in German]. Quintessenz, 56(4), 325–332. [Google Scholar]
- Gahlert, M. , Roehling, S. , Sprecher, C. M. , Kniha, H. , Milz, S. , & Bormann, K. (2012). In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clinical Oral Implants Research, 23, 281–286. 10.1111/j.1600-0501.2011.02157.x [DOI] [PubMed] [Google Scholar]
- Gaspar, J. , Borrecho, G. , Oliveira, P. , Salvado, F. , & Martins dos Santos, J. (2013). Osteotomy at low‐speed drilling without irrigation versus high‐speed drilling with irrigation: An experimental study. Acta Medica Portuguesa, 26(3), 231–236. [PubMed] [Google Scholar]
- Gehrke, S. A. , Aramburú Júnior, J. S. , Pérez‐Albacete Martínez, C. , Ramirez Fernandez, M. P. , Sánchez, M. , de Val, J. E. , & Calvo‐Guirado, J. L. (2018). The influence of drill length and irrigation system on heat production during osteotomy preparation for dental implants: An ex vivo study. Clinical Oral Implants Research, 29, 772–778. 10.1111/clr.12827 [DOI] [PubMed] [Google Scholar]
- Gehrke, S. A. , Bettach, R. , Taschieri, S. , Boukhris, G. , Corbella, S. , & Del Fabbro, M. (2015). Temperature changes in cortical bone after implant site preparation using a single bur versus multiple drilling steps: An in vitro investigation. Clinical Implant Dentistry and Related Research, 17, 700–707. 10.1111/cid.12172 [DOI] [PubMed] [Google Scholar]
- Grunder, U. , & Strub, J. R. (1986). Die Problematik der Temperaturerhöhung beim Bearbeiten des Knochens mit rotierenden Instrumenten–eine Literaturübersicht [Problems of temperature elevation during the treatment of bone with rotating instruments–a review of the literature]. Schweizerische Monatsschrift Für Zahnmedizin, 96(8), 956–969. [PubMed] [Google Scholar]
- Harder, S. , Egert, C. , Wenz, H. J. , Jochens, A. , & Kern, M. (2013). Influence of the drill material and method of cooling on the development of intrabony temperature during preparation of the site of an implant. The British Journal of Oral & Maxillofacial Surgery, 51, 74–78. 10.1016/j.bjoms.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. (2008). Peri‐implant diseases: Diagnosis and risk indicators. Journal of Clinical Periodontology, 35, 292–304. 10.1111/j.1600-051X.2008.01275.x [DOI] [PubMed] [Google Scholar]
- Hobkirk, J. A. , & Rusiniak, K. (1978). Metallic contamination of bone during drilling procedures. Journal of Oral Surgery, 36(5), 356–360. [PubMed] [Google Scholar]
- Hobkirk, J. A. , & Wiskott, H. W. A. (2009). Ceramics in implant dentistry (Working Group 1). Clinical Oral Implants Research, 20, 55–57. 10.1111/j.1600-0501.2009.01779.x [DOI] [PubMed] [Google Scholar]
- Hochscheidt, C. J. , Shimizu, R. H. , Andrighetto, A. R. , Moura, L. M. , Golin, A. L. , & Hochscheidt, R. C. (2017). Thermal variation during osteotomy with different dental implant drills: A standardized study in bovine ribs. Implant Dentistry, 26, 73–79. 10.1097/ID.0000000000000535 [DOI] [PubMed] [Google Scholar]
- Ioannidis, A. , Cathomen, E. , Jung, R. E. , Fehmer, V. , Hüsler, J. , & Thoma, D. S. (2017). Discoloration of the mucosa caused by different restorative materials ‐ a spectrophotometric in vitro study. Clinical Oral Implants Research, 28, 1133–1138. 10.1111/clr.12928 [DOI] [PubMed] [Google Scholar]
- Iyer, S. , Weiss, C. , & Mehta, A. (1997). Effects of drill speed on heat production and the rate and quality of bone formation in dental implant osteotomies. Part I: Relationship between drill speed and heat production. The International Journal of Prosthodontics, 10(5), 411–414. [PubMed] [Google Scholar]
- Janner, S. F. M. , Gahlert, M. , Bosshardt, D. D. , Roehling, S. , Milz, S. , Higginbottom, F. , Buser, D. , & Cochran, D. L. (2018). Bone response to functionally loaded, two‐piece zirconia implants: A preclinical histometric study. Clinical Oral Implants Research, 29, 277–289. 10.1111/clr.13112 [DOI] [PubMed] [Google Scholar]
- Javed, F. , Al‐Hezaimi, K. , Almas, K. , & Romanos, G. E. (2013). Is titanium sensitivity associated with allergic reactions in patients with dental implants? A systematic review. Clinical Implant Dentistry and Related Research, 15, 47–52. 10.1111/j.1708-8208.2010.00330.x [DOI] [PubMed] [Google Scholar]
- Joos, M. , Sailer, I. , Filippi, A. , Mukaddam, K. , Rosentritt, M. , & Kühl, S. (2020). Stability of screw‐retention in two‐piece zirconia implants: An in vitro study. Clinical Oral Implants Research, 31, 607–614. 10.1111/clr.13597 [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , Holderegger, C. , Sailer, I. , Khraisat, A. , Suter, A. , & Hämmerle, C. H. (2008). The effect of all‐ceramic and porcelain‐fused‐to‐metal restorations on marginal peri‐implant soft tissue color:A randomized controlled clinical trial. The International Journal of Periodontics & Restorative Dentistry, 28(4), 357–365. [PubMed] [Google Scholar]
- Jung, R. E. , Zembic, A. , Pjetursson, B. E. , Zwahlen, M. , & Thoma, D. S. (2012). Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow‐up of 5 years. Clinical Oral Implants Research, 23, 2–21. 10.1111/j.1600-0501.2012.02547.x [DOI] [PubMed] [Google Scholar]
- Kerawala, C. J. , Martin, I. C. , Allan, W. , & Williams, E. D. (1999). The effects of operator technique and bur design on temperature during osseous preparation for osteosynthesis self‐tapping screws. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 88, 145–150. 10.1016/s1079-2104(99)70108-3 [DOI] [PubMed] [Google Scholar]
- Kohal, R. J. , Weng, D. , Bächle, M. , & Strub, J. R. (2004). Loaded custom‐made zirconia and titanium implants show similar osseointegration: An animal experiment. Journal of Periodontology, 75, 1262–1268. 10.1902/jop.2004.75.9.1262 [DOI] [PubMed] [Google Scholar]
- Koo, K. T. , Kim, M. H. , Kim, H. Y. , Wikesjö, U. M. , Yang, J. H. , & Yeo, I. S. (2015). Effects of implant drill wear, irrigation, and drill materials on heat generation in osteotomy sites. The Journal of Oral Implantology, 41, e19–e23. 10.1563/AAID-JOI-D-13-00151 [DOI] [PubMed] [Google Scholar]
- Koopaie, M. , Kolahdouz, S. , & Kolahdouz, E. M. (2019). Comparison of wear and temperature of zirconia and tungsten carbide tools in drilling bone: In vitro and finite element analysis. The British Journal of Oral & Maxillofacial Surgery, 57, 557–565. 10.1016/j.bjoms.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Lajolo, C. , Valente, N. A. , Romandini, W. G. , Petruzzi, M. , Verdugo, F. , & D'Addona, A. (2018). Bone heat generated using conventional implant drills versus piezosurgery unit during apical cortical plate perforation. Journal of Periodontology, 89, 661–668. 10.1002/JPER.17-0502 [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Wilson, T. G. , & Corbet, E. F. (2000). Biological complications with dental implants: Their prevention, diagnosis and treatment. Clinical Oral Implants Research, 11, 146–155. 10.1034/j.1600-0501.2000.011s1146.x [DOI] [PubMed] [Google Scholar]
- Lavelle, C. , & Wedgwood, D. (1980). Effect of internal irrigation on frictional heat generated from bone drilling. Journal of Oral Surgery, 38(7), 499–503. [PubMed] [Google Scholar]
- Lee, J. , Ozdoganlar, O. B. , & Rabin, Y. (2012). An experimental investigation on thermal exposure during bone drilling. Medical Engineering & Physics, 34, 1510–1520. 10.1016/j.medengphy.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Lekholm, U. , & Zarb, G. A. (1985). Patient selection and preparation In Branemark P.‐I., Zarb G. A., & Albrektsson T. (Eds.), Tissue‐integrated prostheses: Osseointegration in clinical dentistry (pp. 199–210). Quintessence. [Google Scholar]
- Lucchiari, N. , Frigo, A. C. , Stellini, E. , Coppe, M. , Berengo, M. , & Bacci, C. (2016). In vitro assessment with the infrared thermometer of temperature differences generated during implant site preparation: The traditional technique versus the single‐drill technique. Clinical Implant Dentistry and Related Research, 18, 182–191. 10.1111/cid.12246 [DOI] [PubMed] [Google Scholar]
- Mardinger, O. , Oubaid, S. , Manor, Y. , Nissan, J. , & Chaushu, G. (2008). Factors affecting the decision to replace failed implants: A retrospective study. Journal of Periodontology, 79, 2262–2266. 10.1902/jop.2008.080255 [DOI] [PubMed] [Google Scholar]
- Misic, T. , Markovic, A. , Todorovic, A. , Colic, S. , Miodrag, S. , & Milicic, B. (2011). An in vitro study of temperature changes in type 4 bone during implant placement: Bone condensing versus bone drilling. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 112, 28–33. 10.1016/j.tripleo.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Misir, A. F. , Sumer, M. , Yenisey, M. , & Ergioglu, E. (2009). Effect of surgical drill guide on heat generated from implant drilling. Journal of Oral and Maxillofacial Surgery, 67, 2663–2668. 10.1016/j.joms.2009.07.056 [DOI] [PubMed] [Google Scholar]
- Möhlhenrich, S. C. , Modabber, A. , Steiner, T. , Mitchell, D. A. , & Hölzle, F. (2015). Heat generation and drill wear during dental implant site preparation: Systematic review. The British Journal of Oral & Maxillofacial Surgery, 53, 679–689. 10.1016/j.bjoms.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Moshiri, Z. , Roshanaei, G. , Vafaei, F. , & Kadkhodazadeh, M. (2013). Evaluation the effect of drill type on heat generation in implant drilling site. Research Journal of Medical Sciences, 7(5–6), 118–122. [Google Scholar]
- Oh, H. J. , Wikesjö, U. M. , Kang, H. S. , Ku, Y. , Eom, T. G. , & Koo, K. T. (2011). Effect of implant drill characteristics on heat generation in osteotomy sites: A pilot study. Clinical Oral Implants Research, 22, 722–726. 10.1111/j.1600-0501.2010.02051.x [DOI] [PubMed] [Google Scholar]
- Oliveira, N. , Alaejos‐Algarra, F. , Mareque‐Bueno, J. , Ferrés‐Padró, E. , & Hernández‐Alfaro, F. (2012). Thermal changes and drill wear in bovine bone during implant site preparation. A comparative in vitro study: Twisted stainless steel and ceramic drills. Clinical Oral Implants Research, 23, 963–969. 10.1111/j.1600-0501.2011.02248.x [DOI] [PubMed] [Google Scholar]
- Piattelli, A. , Piattelli, M. , Mangano, C. , & Scarano, A. (1998). A histologic evaluation of eight cases of failed dental implants: Is bone overheating the most probable cause? Biomaterials, 19, 683–690. 10.1016/s0142-9612(97)00172-5 [DOI] [PubMed] [Google Scholar]
- Piattelli, A. , Scarano, A. , Favero, L. , Iezzi, G. , Petrone, G. , & Favero, G. A. (2003). Clinical and histologic aspects of dental implants removed due to mobility. Journal of Periodontology, 74, 385–390. 10.1902/jop.2003.74.3.385 [DOI] [PubMed] [Google Scholar]
- Piconi, C. , & Maccauro, G. (1999). Zirconia as a ceramic biomaterial. Biomaterials, 20, 1–25. 10.1016/s0142-9612(98)00010-6 [DOI] [PubMed] [Google Scholar]
- Pigatto, P. D. , Guzzi, G. , Brambilla, L. , & Sforza, C. (2009). Titanium allergy associated with dental implant failure. Clinical Oral Implants Research, 20, 857 10.1111/j.1600-0501.2009.01749.x [DOI] [PubMed] [Google Scholar]
- Pires, L. F. , Tandler, B. , Bissada, N. , & Duarte, S. Jr (2012). Comparison of heat generated by alumina‐toughened zirconia and stainless steel burs for implant placement. The International Journal of Oral & Maxillofacial Implants, 27(5), 1023–1028. [PubMed] [Google Scholar]
- Pirjamalineisiani, A. , Jamshidi, N. , Sarafbidabad, M. , & Soltani, N. (2016). Assessment of experimental thermal, numerical, and mandibular drilling factors in implantology. The British Journal of Oral & Maxillofacial Surgery, 54, 400–404. 10.1016/j.bjoms.2015.09.017 [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Thoma, D. , Jung, R. , Zwahlen, M. , & Zembic, A. (2012). A systematic review of the survival and complication rates of implant‐supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clinical Oral Implants Research, 23, 22–38. 10.1111/j.1600-0501.2012.02546.x [DOI] [PubMed] [Google Scholar]
- Rashad, A. , Kaiser, A. , Prochnow, N. , Schmitz, I. , Hoffmann, E. , & Maurer, P. (2011). Heat production during different ultrasonic and conventional osteotomy preparations for dental implants. Clinical Oral Implants Research, 22, 1361–1365. 10.1111/j.1600-0501.2010.02126.x [DOI] [PubMed] [Google Scholar]
- Renvert, S. , Persson, G. R. , Pirih, F. Q. , & Camargo, P. M. (2018). Peri‐implant health, peri‐implant mucositis, and peri‐implantitis: Case definitions and diagnostic considerations. Journal of Periodontology, 89, S304–S312. 10.1002/JPER.17-0588 [DOI] [PubMed] [Google Scholar]
- Roehling, S. , Schlegel, K. A. , Woelfler, H. , & Gahlert, M. (2019). Zirconia compared to titanium dental implants in preclinical studies‐A systematic review and meta‐analysis. Clinical Oral Implants Research, 30, 365–395. 10.1111/clr.13425 [DOI] [PubMed] [Google Scholar]
- Salimov, F. , Ozcan, M. , Ucak Turer, O. , & Haytac, C. M. (2020). The effects of repeated usage of implant drills on cortical bone temperature, primary/secondary stability and bone healing: A preclinical in vivo micro‐CT study. Clinical Oral Implants Research. Advance Online Publication, 31(8), 687–693. 10.1111/clr.13603 [DOI] [PubMed] [Google Scholar]
- Sandhaus, S. (1968). Tecnica e strumentario dell'impianto C.B.S. (Cristalline Bone Screw) [Technic and instrumentation of the implant C.B.S. (Cristalline Bone Screw)]. Informatore Odonto‐Stomatologico, 4(3), 19–24. [PubMed] [Google Scholar]
- Sandhaus, S. (1971). Wissenschaftlicher Beitrag zum Gebiet der Oralrehabilitation mit Hilfe des Implantationsverfahrens C.B.S. [Oral rehabilitation using implantation method C.B.S]. Zahnärztliche Welt, 80(13), 597–604. [PubMed] [Google Scholar]
- Sannino, G. , Capparé, P. , Gherlone, E. F. , & Barlattani, A. (2015). Influence of the implant drill design and sequence on temperature changes during site preparation. The International Journal of Oral & Maxillofacial Implants, 30, 351–358. 10.11607/jomi.3747 [DOI] [PubMed] [Google Scholar]
- Scarano, A. , Carinci, F. , Quaranta, A. , Di Iorio, D. , Assenza, B. , & Piattelli, A. (2007). Effects of bur wear during implant site preparation: An in vitro study. International Journal of Immunopathology and Pharmacology, 20, 23–26. 10.1177/039463200702001s06 [DOI] [PubMed] [Google Scholar]
- Scarano, A. , Lorusso, F. , & Noumbissi, S. (2020). Infrared thermographic evaluation of temperature modifications induced during implant site preparation with steel vs. zirconia implant drill. Journal of Clinical Medicine, 9, 148 10.3390/jcm9010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarano, A. , Noumbissi, S. , Gupta, S. , Inchingolo, F. , Stilla, P. , & Lorusso, F. (2019). Scanning electron microscopy analysis and energy dispersion X‐ray microanalysis to evaluate the effects of decontamination chemicals and heat sterilization on implant surgical drills: zirconia vs. steel. Applied Sciences, 9, 2837 10.3390/app9142837 [DOI] [Google Scholar]
- Scarano, A. , Piattelli, A. , Assenza, B. , Carinci, F. , Di Donato, L. , Romani, G. L. , & Merla, A. (2011). Infrared thermographic evaluation of temperature modifications induced during implant site preparation with cylindrical versus conical drills. Clinical Implant Dentistry and Related Research, 13, 319–323. 10.1111/j.1708-8208.2009.00209.x [DOI] [PubMed] [Google Scholar]
- Schulte, W. , & Heimke, G. (1976). Das Tübinger Sofort‐Implant [The Tübinger immediate implant]. Quintessenz, 27, 17–23. [PubMed] [Google Scholar]
- Schwarz, M. S. (2000). Mechanical complications of dental implants. Clinical Oral Implants Research, 11, 156–158. 10.1034/j.1600-0501.2000.011s1156.x [DOI] [PubMed] [Google Scholar]
- Sener, B. C. , Dergin, G. , Gursoy, B. , Kelesoglu, E. , & Slih, I. (2009). Effects of irrigation temperature on heat control in vitro at different drilling depths. Clinical Oral Implants Research, 20, 294–298. 10.1111/j.1600-0501.2008.01643.x [DOI] [PubMed] [Google Scholar]
- Sicilia, A. , Cuesta, S. , Coma, G. , Arregui, I. , Guisasola, C. , Ruiz, E. , & Maestro, A. (2008). Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clinical Oral Implants Research, 19, 823–835. 10.1111/j.1600-0501.2008.01544.x [DOI] [PubMed] [Google Scholar]
- Siddiqi, A. , Payne, A. , De Silva, R. K. , & Duncan, W. J. (2011). Titanium allergy: Could it affect dental implant integration? Clinical Oral Implants Research, 22, 673–680. 10.1111/j.1600-0501.2010.02081.x [DOI] [PubMed] [Google Scholar]
- Sivaraman, K. , Chopra, A. , Narayan, A. I. , & Balakrishnan, D. (2018). Is zirconia a viable alternative to titanium for oral implant? A critical review. Journal of Prosthodontic Research, 62, 121–133. 10.1016/j.jpor.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Strbac, G. D. , Giannis, K. , Unger, E. , Mittlböck, M. , Vasak, C. , Watzek, G. , & Zechner, W. (2015). Drilling‐ and withdrawing‐related thermal changes during implant site osteotomies. Clinical Implant Dentistry and Related Research, 17, 32–43. 10.1111/cid.12091 [DOI] [PubMed] [Google Scholar]
- Strbac, G. D. , Giannis, K. , Unger, E. , Mittlböck, M. , Watzek, G. , & Zechner, W. (2014). A novel standardized bone model for thermal evaluation of bone osteotomies with various irrigation methods. Clinical Oral Implants Research, 25, 622–631. 10.1111/clr.12090 [DOI] [PubMed] [Google Scholar]
- Strbac, G. D. , Unger, E. , Donner, R. , Bijak, M. , Watzek, G. , & Zechner, W. (2014). Thermal effects of a combined irrigation method during implant site drilling. A standardized in vitro study using a bovine rib model. Clinical Oral Implants Research, 25, 665–674. 10.1111/clr.12032 [DOI] [PubMed] [Google Scholar]
- Sumer, M. , Misir, A. F. , Telcioglu, N. T. , Guler, A. U. , & Yenisey, M. (2011). Comparison of heat generation during implant drilling using stainless steel and ceramic drills. Journal of Oral and Maxillofacial Surgery, 69, 1350–1354. 10.1016/j.joms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Tehemar, S. H. (1999). Factors affecting heat generation during implant site preparation: A review of biologic observations and future considerations. The International Journal of Oral & Maxillofacial Implants, 14(1), 127–136. [PubMed] [Google Scholar]
- Trisi, P. , Berardini, M. , Falco, A. , Podaliri Vulpiani, M. , & Perfetti, G. (2014). Insufficient irrigation induces peri‐implant bone resorption: An in vivo histologic analysis in sheep. Clinical Oral Implants Research, 25, 696–701. 10.1111/clr.12127 [DOI] [PubMed] [Google Scholar]
- Wenz, H. J. , Bartsch, J. , Wolfart, S. , & Kern, M. (2008). Osseointegration and clinical success of zirconia dental implants: A systematic review. The International Journal of Prosthodontics, 21(1), 27–36. [PubMed] [Google Scholar]
- Yacker, M. J. , & Klein, M. (1996). The effect of irrigation on osteotomy depth and bur diameter. The International Journal of Oral & Maxillofacial Implants, 11(5), 634–638. [PubMed] [Google Scholar]
- Yoshida, K. , Uoshima, K. , Oda, K. , & Maeda, T. (2009). Influence of heat stress to matrix on bone formation. Clinical Oral Implants Research, 20, 782–790. 10.1111/j.1600-0501.2008.01654.x [DOI] [PubMed] [Google Scholar]
- Zipprich, H. , Weigl, P. , König, E. , Toderas, A. , Balaban, Ü. , & Ratka, C. (2019). Heat generation at the implant‐bone interface by insertion of ceramic and titanium implants. Journal of Clinical Medicine, 8, 1541 10.3390/jcm8101541 [DOI] [PMC free article] [PubMed] [Google Scholar]


