Abstract
Background and purpose
Elevated cerebrospinal fluid (CSF) total protein in patients with acute ascending paresis is indicative of Guillain–Barré syndrome (GBS). Recent studies showed that the outdated, but still widely used upper reference limit (URL) for CSF total protein of 0.45 g/L leads to false‐positive results, mainly as a result of lack of age‐adjustment. The objective of this study was to assess the frequency of increased CSF total protein in adult GBS patients according to a new age‐dependent URL.
Methods
Patients with GBS treated at the Medical University of Innsbruck between 2000 and 2018 were included in this study. Demographic, clinical, electrophysiological and CSF data were obtained from patients' medical charts. Frequency of increased CSF total protein depending on disease duration was compared using the conventional URL of 0.45 g/L and the age‐dependent URL.
Results
Ninety‐seven patients with GBS aged 57 ± 18 years, comprising 38% women, underwent CSF sampling within a median of 6 days after symptom onset. The median CSF total protein concentration was 0.65 g/L and correlated with disease duration. Overall, 74% of patients had elevated CSF total protein levels using the conventional URL, as opposed to 52% applying the age‐dependent URL. At 0–3, 4–7, 8–14 and >14 days after disease onset, elevated CSF total protein was found in 46%, 84%, 78% and 100% of patients using the conventional URL, and in 32%, 53%, 65% and 64% of patients using the age‐dependent URL. In multivariate analysis, significant predictors of elevated CSF total protein were disease duration and the demyelinating GBS variant. Similar results were obtained for CSF/serum albumin quotient (Qalb).
Conclusion
Fewer true‐positives for CSF total protein and Qalb must be considered in suspected GBS, especially in the early disease course.
Keywords: age, albumin, cerebrospinal fluid, diagnosis, disease duration, Guillain–Barré syndrome, sensitivity, total protein
Cerebrospinal fluid (CSF) total protein and CSF/serum albumin quotient are less frequently elevated in patients with Guillain–Barré syndrome than previously reported, as a result of novel age‐dependent upper reference limits that reduce false‐positives and avoid over‐interpretation of slightly elevated protein levels in the elderly.
INTRODUCTION
Guillain–Barré syndrome (GBS) is an acute‐onset inflammatory demyelinating polyneuropathy that is characterized by rapidly progressive, symmetrical weakness of the extremities, sensory disturbances and, in some patients, autonomic dysfunction and respiratory insufficiency. GBS is a potentially life‐threatening disease and might be difficult to diagnose in some cases [1]. Besides nerve conduction studies, lumbar puncture (LP) is recommended to rule out GBS mimics, particularly infectious diseases, for example, those caused by cytomegalovirus or human immunodeficiency virus [1]. The typical finding in the cerebrospinal fluid (CSF) of patients with GBS is a combination of elevated protein levels and normal white blood cell count, which is termed “cytoalbuminologic dissociation” [2]. The frequency of these CSF abnormalities in GBS was investigated by prior studies dependent on disease duration [3, 4, 5]. Although it is well known that CSF protein levels increase with the patient's age under physiological conditions, the applied upper reference limits (URLs) for CSF total protein in these studies did not adjust for patient age and are outdated [6, 7, 8]. Furthermore, reports on CSF/serum albumin quotient (Qalb) in GBS are scarce [9] and CSF data in longitudinally collected samples are still lacking.
The objective of the present study was to assess the frequency of increased CSF total protein and Qalb levels in adults with GBS according to a new age‐dependent URL and disease duration, to investigate the longitudinal evolution of CSF total protein and Qalb, and to identify predictors of elevated protein levels in a multivariate analysis.
METHODS
A total of 105 patients with GBS who were treated at the Medical University of Innsbruck between 2000 and 2018 were eligible for this retrospective study. Demographic, clinical, electrophysiological and CSF data were obtained from medical records. Patients were included in the analysis only if the red blood cell count in CSF was <500 per μl, in order to avoid artificially increased CSF protein levels by traumatic LP [10], resulting in a total of 97 patients. All patients fulfilled the diagnostic criteria for GBS [11] or Miller–Fisher syndrome (MFS) [12]. Patients with GBS were electrophysiologically classified according to the criteria proposed by Hadden et al. [13]. All patients with MFS had positive serum anti‐GQ1b antibodies. Disability at nadir of the disease was assessed using the Hughes Grading Scale (HGS) [14]. Disease duration was defined as the time in days between symptom onset and LP.
Laboratory assays
Determination of CSF variables was performed for routine diagnostic purposes at the Neuroimmunology Laboratory of the Department of Neurology, Medical University of Innsbruck, on the day of LP. CSF white and red blood cells were counted in a Fuchs‐Rosenthal chamber, which has a volume of 3.2 μl [15]. Division by 3.2 allowed reporting of cell counts per μL according to the International System of units. CSF total protein was determined by spectrophotometry after incubation of centrifuged CSF with 3% trichloroacetic acid [16]. Albumin and the immunoglobulins (Ig) G, A and M in CSF and serum were measured by nephelometer (Beckman Coulter GmbH, Brea, CA, USA) as previously reported [17]. Qalb was calculated as the ratio of CSF albumin/serum albumin [15]. Intrathecal syntheses of IgG, IgM and IgA were determined by Reiber formula [18].
Reference limits for CSF total protein and Qalb
The frequency of increased CSF total protein concentration was assessed using the conventional URL of 0.45 g/L [15] and a recently published age‐dependent URL [6]. This URL, which adjusts for the patient's age (in years), was determined in a large control group comprising more than 6000 CSF samples using the following equation [6]:
CSF total protein lim = 0.124 + 0.0284 × age − 7.08 × 10−4 × age2 + 8.23 × 10−6 × age3 − 3.35 × 10−8 × age4.
Frequency of elevated Qalb levels was assessed by applying the age‐dependent URL as previously published [7]:
Qalb lim = 0.0435 × age + 7.9249.
Statistical analysis
Statistical analysis was performed using spss 26.0 (SPSS Inc., Chicago, IL, USA). Distribution of data was assessed by Kolmogorov–Smirnov test and data were displayed as mean ± SD, or as median and interquartile range (IQR) or 5th to 95th percentile. Spearman's coefficient was used for correlation analysis. The Kruskal–Wallis test was used for group comparisons and Wilcoxon's test for analysis of paired samples. To assess the longitudinal evolution of CSF total protein and Qalb the absolute as well as the relative change was calculated, the latter as (XFollow‐up−XBaseline)/XBaseline × 100. Linear regression was employed to identify predictors of increased CSF total protein and Qalb, including age, sex, HGS score, GBS variant and disease duration. The dependent variables (CSF total protein and Qalb) and the covariate disease duration were log‐transformed to achieve normal distribution. A p value < 0.05 was considered statistically significant.
Ethics
The study was approved by the Ethics Committee of the Medical University of Innsbruck (approval number 1319/2019). Informed consent was not needed because this was an anonymous retrospective analysis of existing data that were obtained in routine diagnostic procedures.
RESULTS
A total of 97 patients with GBS, whose mean age was 57 years and who included 38% women, underwent CSF sampling within a median (IQR) of 6 (3–12) days after symptom onset. Detailed clinical, electrophysiological and CSF data are summarized in Table 1.
Table 1.
Demographic, clinical, electrophysiological and cerebrospinal fluid data of patients with Guillain–Barré syndrome
| Total number of patients | 97 |
|---|---|
| Mean ± SD age, years | 57 ± 18 |
| Women, n (%) | 37 (38) |
| GBS subtype, n (%) | |
| AIDP a | 67 (69.1) |
| AMAN/AMSAN a | 15 (15.5) |
| Equivocal a | 5 (5.2) |
| MFS b | 10 (10.3) |
| Median (range) HGS score | 2 (1–5) |
| CSF red blood cell count, per μl | 0 (0–192) |
| CSF white blood cell count, per μl | 2 (0–8) |
| CSF total protein, g/L | 0.65 (0.25–1.85) |
| CSF albumin, g/L | 0.37 (0.15–1.01) |
| Serum albumin, g/L | 40 (27–48) |
| Qalb | 9.9 (3.8–25.7) |
| Intrathecal IgG synthesis, n (%) | 1 (1) |
| Intrathecal IgA synthesis, n (%) | 4 (5) |
| Intrathecal IgM synthesis, n (%) | 4 (5) |
| Disease duration, days | 6 (1–29) |
| GM1 antibodies, n (%) | |
| AIDP | 7 (11.5) |
| AMAN/AMSAN | 8 (57.1) |
| GQ1b antibodies, n (%) | |
| MFS | 10 (100%) |
Data are shown as median (5th–95th percentile), unless otherwise specified.
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor‐sensory axonal neuropathy; CSF, cerebrospinal fluid; GBS, Guillain–Barré syndrome; HGS, Hughes Grading Scale; Ig, immunoglobulin; MFS, Miller–Fisher syndrome; Qalb, CSF/ serum albumin quotient.
Patients with GBS were classified as demyelinating variant (AIDP), axonal variant (AMAN/AMSAN) or as “equivocal” according to the Hadden criteria.
All patients with MFS were positive for anti‐GQ1b antibodies in serum.
Cerebrospinal fluid total protein
The median (5th–95th percentile) CSF total protein concentration was 0.65 (0.25–1.85) g/L and was correlated with disease duration (r = 0.391, p < 0.001). Overall, 72 patients (74%) had elevated CSF total protein levels using the conventional URL, whereas this number was 50 patients (52%) when applying the age‐dependent URL. When LP was performed after a disease duration of 0–3, 4–7, 8–14 and >14 days, increased CSF total protein concentrations were found in 46%, 84%, 78% and 100% of patients using the conventional URL (Figure 1A), and in 32%, 53%, 65% and 64% of patients using the age‐dependent URL (Figure 1B). The median CSF total protein concentrations within these periods were 0.44, 0.65, 0.80 and 0.89 g/L.
FIGURE 1.
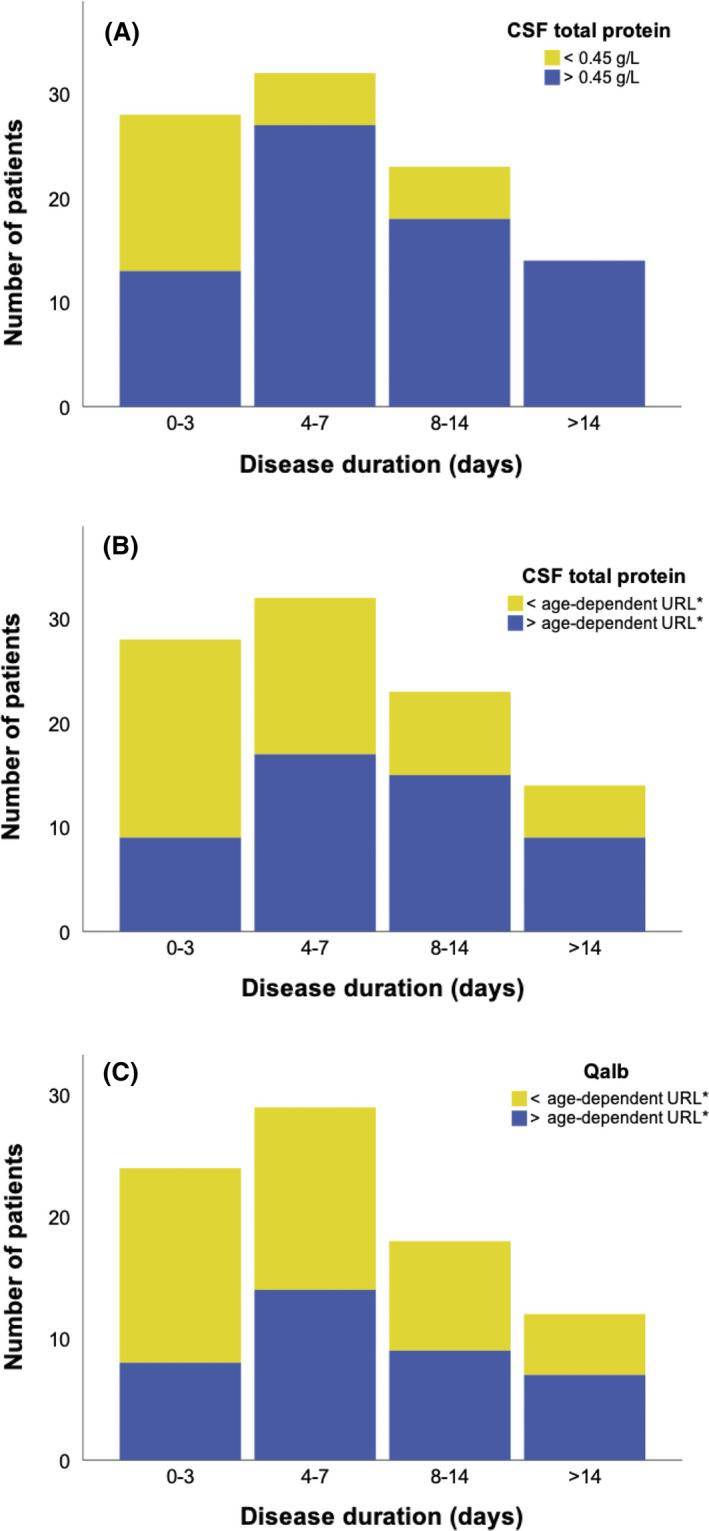
Cerebrospinal fluid (CSF) total protein and CSF/serum albumin quotient (Qalb) dependent on disease duration. The number of patients with elevated CSF total protein concentration using (A) the conventional upper reference limit (URL; 0.45 g/L) and (B) the age‐dependent URL, as well as the number of patients with elevated Qalb according to age‐dependent URL (C) are shown for different disease duration. *For definition of the age‐dependent URL see Methods section
Cerebrospinal fluid/serum albumin quotient
Cerebrospinal fluid/serum albumin quotient (Qalb) was available in 83 of 97 patients (86%). The median (5th–95th percentile) Qalb was 9.9 (3.8–25.7) and correlated with disease duration (r = 0.305, p = 0.005). Overall, 38 of 83 patients (46%) showed elevated Qalb. When LP was performed after a disease duration of 0–3, 4–7, 8–14 and >14 days, Qalb was increased in 33%, 48%, 50% and 58% of patients (Figure 1C). The median Qalb levels within these periods were 6.7, 9.9, 10.3 and 11.5.
Correlation between CSF total protein and Qalb
Cerebrospinal fluid total protein significantly correlated with Qalb (r = 0.936, p < 0.001). Concordance between elevated Qalb and elevated CSF total protein occurred in 76 of 83 patients (92%) using the age‐dependent URL, but in only 58 patients (70%) using the conventional URL for CSF total protein.
Predictors of elevated CSF protein levels
Out of the 97 patients with GBS, 67 were classified as having acute inflammatory demyelinating polyneuropathy, 15 as having acute motor axonal neuropathy or acute motor‐sensory axonal neuropathy, five as being equivocal, and 10 as having MFS. In order to investigate the impact of age, sex, disease duration, HGS score and GBS subtype on CSF total protein and Qalb levels, a linear regression was performed (Table 2). An impact on CSF total protein and Qalb was observed for disease duration; furthermore, higher values of CSF total protein and Qalb were found in acute inflammatory demyelinating polyneuropathy (Figure 2). The impact of age was further assessed by comparing the frequency of false‐positive CSF total protein results between the age‐dependent and conventional URLs after adjustment for the other significant covariates: disease duration and GBS variant (Figure 3). False‐positive CSF total protein results increased with age and were 26.6%, 37.5% and 39.5% in patients younger than 40 years, patients aged between 40 and 60 years and patients older than 60 years, respectively.
Table 2.
Regression analysis identifying predictors for (a) CSF total protein and (b) CSF/serum albumin quotient
| (a) CSF total protein | |||||
|---|---|---|---|---|---|
| Ln (CSF total protein) | Coefficient | SE | p | 95% CI | |
| Sex | −0.016 | 0.107 | .881 | −0.230 | 0.197 |
| Age (years) | −0.002 | 0.003 | .538 | −0.008 | 0.004 |
| Disease subtype (reference: 3 = MFS) | |||||
| 1 = AIDP | 0.565 | 0.170 | .001 | 0.227 | 0.903 |
| 2 = AMAN/AMSAN | 0.396 | 0.206 | .059 | −0.015 | 0.807 |
| HGS (reference: 5) | |||||
| 1 | −0.111 | 0.200 | .580 | −0.510 | 0.287 |
| 2 | 0.044 | 0.174 | .799 | −0.303 | 0.391 |
| 3 | −0.053 | 0.199 | .791 | −0.449 | 0.343 |
| 4 | 0.081 | 0.201 | .687 | −0.318 | 0.481 |
| Ln (disease duration [days]) | 0.207 | 0.062 | .001 | 0.083 | 0.331 |
| Constant | −1.123 | 0.270 | <.001 | −1.660 | −0.586 |
| (b) Qalb | |||||
|---|---|---|---|---|---|
| Ln (Qalb) | Coefficient | SE | p | 95% CI | |
| Sex | 0.124 | 0.133 | .356 | −0.142 | 0.391 |
| Age (years) | 0.000 | 0.004 | .978 | −0.007 | 0.008 |
| Disease subtype (reference: 3 = MFS) | |||||
| 1 = AIDP | 0.491 | 0.209 | .022 | 0.074 | 0.908 |
| 2 = AMAN/AMSAN | 0.333 | 0.263 | .210 | −0.193 | 0.859 |
| HGS (reference: 5) | |||||
| 1 | −0.663 | 0.257 | .012 | −1.176 | ‐0.149 |
| 2 | −0.356 | 0.229 | .124 | −0.813 | 0.100 |
| 3 | −0.403 | 0.263 | .131 | −0.928 | 0.123 |
| 4 | −0.466 | 0.269 | .088 | −1.003 | 0.072 |
| Ln (disease duration [days]) | 0.280 | 0.079 | .001 | 0.123 | 0.438 |
| Constant | −5.143 | 0.332 | <.001 | −5.806 | −4.479 |
Covariates predicting (a) CSF total protein and (b) Qalb levels are shown. Statistically significant results are marked bold. CSF total protein and Qalb, respectively (dependent variable) as well as disease duration (covariate) were logarithmized (ln) to achieve normal distribution.
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor‐sensory axonal neuropathy; CSF, cerebrospinal fluid; HGS, Hughes Grading Scale; MFS, Miller Fisher Syndrome; Qalb, CSF/serum albumin quotient.
FIGURE 2.
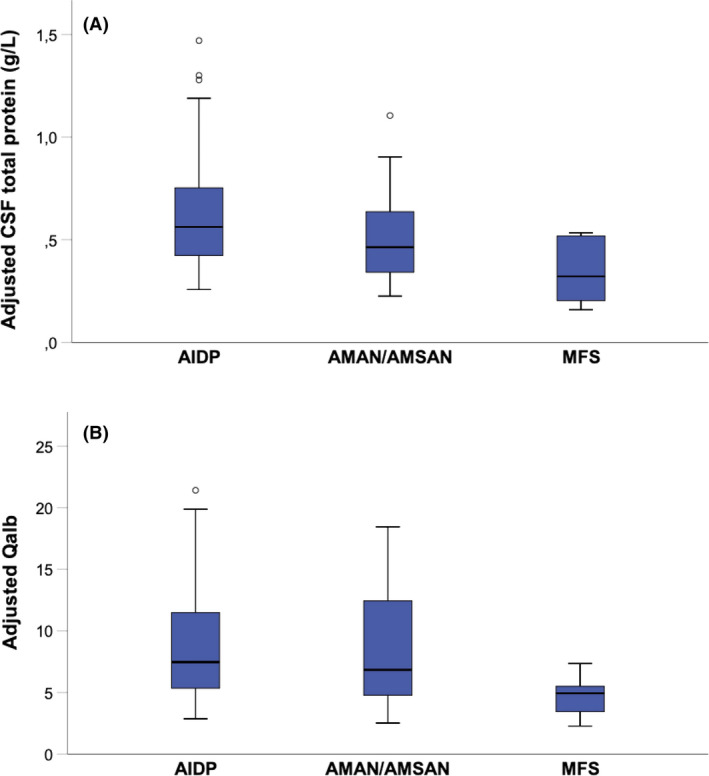
Cerebrospinal fluid (CSF) total protein and CSF/serum albumin quotient (Qalb) differ between Guillain–Barré syndrome (GBS) subtypes. (A) CSF total protein and (B) Qalb levels are shown in different GBS subtypes. As disease duration has a significant impact on CSF total protein and Qalb, correction for its estimated impact on both variables was made. Therefore, in this plot adjusted CSF total protein and adjusted Qalb for a fixed disease duration of 1 day are shown. AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor‐sensory axonal neuropathy; MFS, Miller–Fisher syndrome
FIGURE 3.
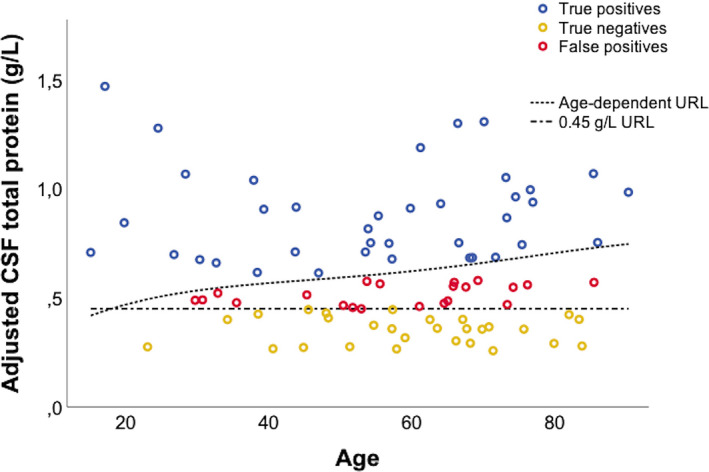
False‐positive cerebrospinal fluid (CSF) total protein results increase with patients' age. CSF total protein dependent on age and with reference to both the conventional URL of 0.45 g/L and the age‐dependent upper reference limit (URL) are shown. As disease duration and Guillain–Barré syndrome (GBS) variant have a significant impact on CSF total protein, correction for their estimated impact was made. Therefore, in this plot adjusted CSF total protein for a fixed disease duration of 1 day and for the demyelinating GBS variant (acute inflammatory demyelinating polyneuropathy) are shown. Qalb, CSF/serum albumin quotient
Longitudinal evolution of CSF total protein and Qalb
A subset of 27 patients had a follow‐up CSF sample. In these patients, the baseline sample was collected after a median (IQR) of 5 (2–7) days' disease duration and the follow‐up sample after median (IQR) of 19 (8–32) days' disease duration. Using the age‐dependent URL, at baseline 17 patients (63%) had normal CSF total protein. Eleven of these 17 patients (65%) showed increased CSF total protein at follow‐up, corresponding to an absolute increase of 0.33 g/L and a relative increase of 60%. Considering the results of both LPs, diagnostic sensitivity increased from 37% to 78% (Figure 4A).
FIGURE 4.
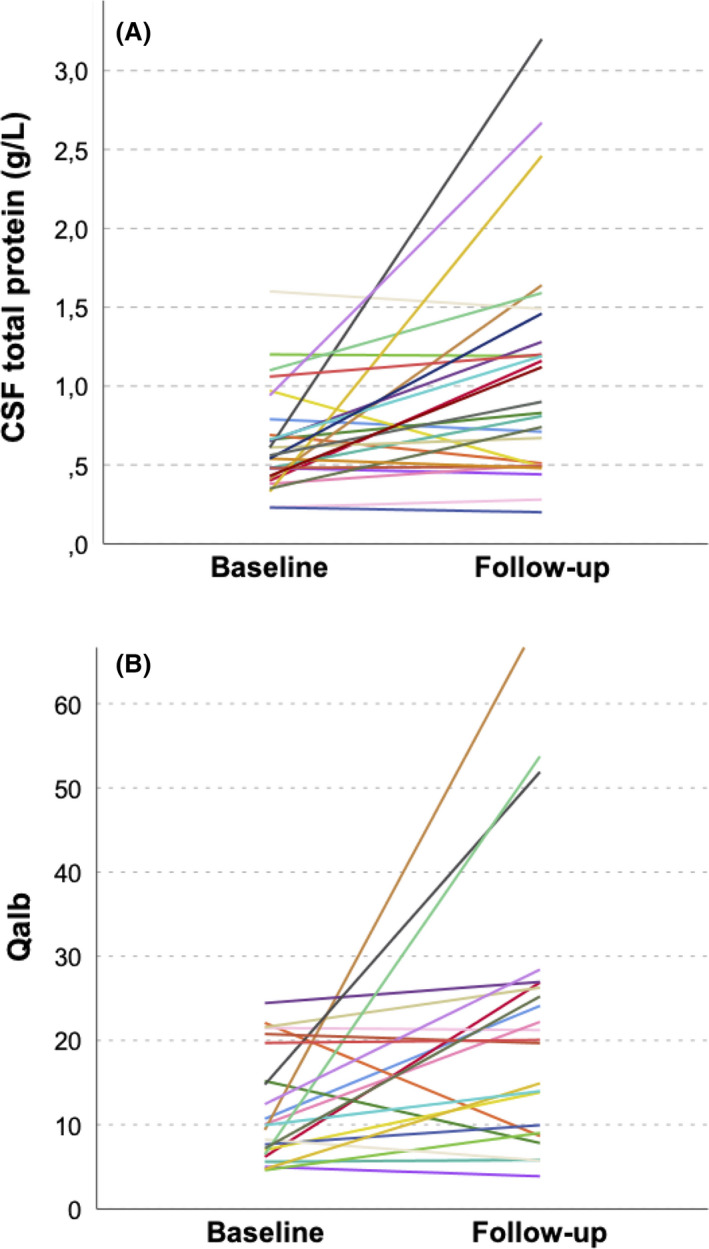
Longitudinal evolution of cerebrospinal fluid (CSF) total protein and CSF/serum albumin quotient (Qalb). (A) CSF total protein and (B) Qalb significantly increased from baseline until follow‐up (Wilcoxon test: p = .002 and .006, respectively)
In 23 patients, Qalb was also determined in a follow‐up CSF sample after a median (IQR) of 23 (11–37) days. Normal Qalb levels were observed in 12 patients (52%) at baseline. Seven of these 12 patients (58%) showed increased Qalb at follow‐up, corresponding to an absolute increase of 8.5 and a relative increase of 112% resulting in an increase in diagnostic sensitivity from 48% to 78% (Figure 4B).
DISCUSSION
In the present study, we showed the diagnostic sensitivities of CSF total protein and Qalb in patients with GBS according to a new age‐dependent URL and disease duration. Furthermore, for the first time, to the best of our knowledge, we identified predictors of elevated CSF protein levels in multivariate analyses and assessed protein dynamics in a subset of GBS patients followed longitudinally.
For CSF total protein, a URL of 0.45 g/L was proposed in 1938 by Merritt and Fremont‐Smith [19], frequently cited in subsequent textbooks, widely adopted by hospital laboratories (in over 85% of clinical centers worldwide) [20] and quoted in clinical practice guidelines [15]. In several studies in patients with GBS, either this reference limit was applied to determine elevation of CSF total protein [5], or center‐specific fixed cut‐off values were used up to approximately 0.6 g/L [3, 4]. However, apart from a recent report [21], none of these studies applied age‐dependent URLs, although it is well known that CSF protein levels increase with age under physiological conditions [22, 23, 24]. We used the age‐dependent URL according to two recent studies on more than 6000 CSF samples [6, 7] as well as a recent systematic review [8].
In the present study, we reaffirm that CSF total protein depends on disease duration in patients with GBS as described in several previous observational studies [3, 4, 5, 21]. These studies using the conventional URL reported a diagnostic sensitivity of CSF total protein within the first week of disease onset of approximately 50% to 70%, increasing to 100% after more than 2 weeks [3, 4, 5, 21]. In contrast, we observed that the use of an age‐dependent URL results in lower sensitivities: 32% within the first 3 days, and 43% within the first week, up to a maximum of 65% after 2 weeks. Bourque et al. [21] observed similar percentages, in the early disease phase, of 32% and 45%. The main benefit of using an age‐dependent URL for CSF total protein is the increase in diagnostic specificity. Using the conventional limit of 0.45 g/L leads to a high rate of false‐positive results [7, 8] probably contributing to misdiagnosis especially in early disease when costly and potentially harmful treatment decisions have to be made. Several other diseases can mimic GBS, especially disorders affecting the neuromuscular junction, for example, Lambert–Eaton myasthenic syndrome or botulism, which typically show normal CSF protein levels [25, 26, 27]. In the present cohort, between 25% and 40% of patients, depending on age, showed false‐positive results, a percentage already previously reported [6, 7]. Notably, the previous use of fixed but higher cut‐off values (eg, 0.6 g/L) also resulted in lower sensitivity (and therefore higher diagnostic specificity), but the lack of age‐adjustment ignores the physiological changes with age and, furthermore, might even lead to false‐negative results in young patients.
For interpretation of Qalb, CSF guidelines indeed recommend considering patient's age, but do not specify a certain URL [15]. We applied an age‐dependent URL that has been recently established in a well‐characterized control cohort [7]. Based on age‐dependent URL, classification of elevated levels of Qalb and CSF total protein were concordant in the vast majority of patients, but in a substantially lower proportion of patients when using the conventional URL for CSF total protein. This underlines the higher validity of an age‐dependent URL for CSF total protein.
In addition to the cross‐sectional analyses, in line with prior studies, we included a subgroup of 27 patients with longitudinally collected CSF samples. This allowed us to investigate CSF protein evolution without the potential bias of unknown patient characteristics, a confounder that might have led to earlier or later LP in the cross‐sectional approach. We found a relative increase of approximately 60% for CSF total protein and 110% for Qalb at follow‐up after a median of 19 days. This profound increase is the rationale for repeating LP in case of diagnostic uncertainty if the initial CSF findings are inconclusive.
Apart from the diagnostic value, previous studies attempted to correlate CSF protein levels with GBS subtype or disease severity. There is some evidence that CSF total protein is higher in GBS than in MFS [3, 28], that it is highest in the demyelinating GBS subtype [28], and that CSF total protein correlates with disease severity [28]. However, all these studies obtained these results with univariate analysis, not correcting for covariates. In the present study, we applied a multivariate linear regression and identified longer disease duration and the demyelinating GBS subtype as significant independent predictors of increased CSF total protein and Qalb, whereas age and sex had no significant impact, confirming previous findings. Why higher CSF total protein levels are associated with demyelinating variants and with longer disease duration is unclear but probably results from two main mechanisms: release of myelin proteins from spinal nerve roots into the CSF due to inflammation and/or disruption of the blood–nerve barrier.
A limitation of the present analysis, similar to other studies, is the lack of data with which to assess diagnostic specificity. This would require a systematic hospital chart review to capture all patients in whom GBS was suspected at the time of LP and finally excluded. However, we screened 105 patients with GBS within the last 18 years. Given an incidence of GBS of approximately one to two per 100,000 inhabitants per year and the catchment area of the Medical University of Innsbruck as a tertiary referral center of Western Austria with approximately 700,000 inhabitants, we most likely included the majority of GBS patients in this region. Furthermore, demographic, main clinical characteristics including CSF and electrophysiological findings were comparable to other typical GBS cohorts in Europe [4, 29].
At this point, we would like to emphasize that, even after applying URLs with higher specificity, elevated CSF protein levels (the so‐called cytoalbuminologic dissociation) can also be found in various other conditions, such as spinal stenosis, and do not prove, but support the diagnosis of GBS in the context of typical clinical symptoms and suitable electrophysiological findings. Furthermore, there is still controversy regarding whether CSF total protein or Qalb should be used to detect cytoalbuminologic dissociation. Both variables are altered dependent on blood–CSF barrier function or CSF flow. CSF total protein might also be influenced by several other factors including protein concentration in blood or an intrathecal protein synthesis. In contrast, albumin in the CSF is exclusively produced outside the central nervous system and, after considering its concentration in serum, the CSF/serum albumin quotient (Qalb) is an ideal variable to reflect all the effects on the passage of proteins into the CSF compartment including diffusivity (predominantly across the blood–CSF barrier) and CSF flow [15, 30, 31]. Based on these considerations, one might argue that Qalb should be preferably used to determine cytoalbuminologic dissociation. Nevertheless, determination of CSF total protein is usually part of CSF emergency diagnostics, as it is a technically simple and reproducible measurement which has been used routinely in neurological diagnosis for the past century [30]. As the concordance between Qalb and CSF total protein is acceptable (>90%), CSF total protein might be used when determination of Qalb is not available.
In conclusion, the present study highlights that CSF total protein and Qalb have low diagnostic sensitivities for GBS, particularly in the first week after disease onset. Realistic cut‐off values for elevated CSF protein levels are important, particularly in uncertain cases, in which alternative diagnoses should be considered.
DISCLOSURE OF CONFLICTS OF INTEREST
H.H. has participated in meetings sponsored by and received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi‐Genzyme, Siemens and Teva, and has received honoraria for acting as consultant for Biogen and Teva. F.L. has nothing to disclose. G.B. has participated in meetings sponsored by and received speaker honoraria or travel funding from Biogen, Celgene, Merck, Novartis, Sanofi‐Genzyme and Teva, and has received honoraria for consulting Biogen, Roche and Teva. M.A. has received speaker honoraria and/or travel grants from Biogen, Novartis, Merck and Sanofi. K.B. has participated in meetings sponsored by and received travel funding from Roche. F.D.P. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi‐Genzyme, Teva, Celgene and Roche. J. Walde has nothing to disclose. J. Wanschitz has participated in meetings sponsored by Sanofi‐Genzyme and Takeda (Shire Austria GmbH) and received speaker honoraria or travel funding from Sanofi‐Genzyme, Astellas, Pfizer, Celgene and Takeda (Shire Austria GmbH). A.Z. has participated in meetings sponsored by and received speaking honoraria or travel funding from Biogen, Merck, Sanofi‐Genzyme and Teva. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Almirall, Alexion, Biogen, Celgene, Genzyme‐Sanofi, Merck, Novartis Pharma, Roche and TEVA Ratiopharm. His institution has received research grants from Biogen and Genzyme Sanofi. He is section editor of the Multiple Sclerosis and Related Disorders journal.
AUTHOR CONTRIBUTIONS
H. Hegen has participated in the conception and design of the study, statistical analysis of the data, and in drafting the manuscript. F. Ladstätter has participated in acquisition of data and reviewing the manuscript for intellectual content. Gabriel Bsteh has participated in reviewing the manuscript for intellectual content. Michael Auer has participated in reviewing the manuscript for intellectual content. Klaus Berek has participated in reviewing the manuscript for intellectual content. Franziska Di Pauli has participated in reviewing the manuscript for intellectual content. Janette Walde has participated in statistical analysis of the data, and in reviewing the manuscript for intellectual content. Julia Wanschitz has participated in reviewing the manuscript for intellectual content. Anne Zinganell has participated in reviewing the manuscript for intellectual content. F. Deisenhammer has participated in reviewing the manuscript for intellectual content.
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
References
- 1. van den Berg B, Walgaard C, Drenthen J, et al. Guillain‐Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nature Publishing Group. 2014;10:469‐482. 10.1038/nrneurol.2014.121 [DOI] [PubMed] [Google Scholar]
- 2. Illes Z, Blaabjerg M. Cerebrospinal fluid findings in Guillain‐Barré syndrome and chronic inflammatory demyelinating polyneuropathies. Handb Clin Neurol. 2017;146:125‐138. 10.1016/B978-0-12-804279-3.00009-5 [DOI] [PubMed] [Google Scholar]
- 3. Wong AHY, Umapathi T, Nishimoto Y, et al. Cytoalbuminologic dissociation in Asian patients with Guillain‐Barré and Miller Fisher syndromes. J Peripher Nerv Syst. 2015;20:47‐51. 10.1111/jns.12104 [DOI] [PubMed] [Google Scholar]
- 4. Fokke C, van den Berg B, Drenthen J, et al. Diagnosis of Guillain‐Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33‐43. 10.1093/brain/awt285 [DOI] [PubMed] [Google Scholar]
- 5. Nishimoto Y, Odaka M, Hirata K, Yuki N. Usefulness of anti‐GQ1b IgG antibody testing in Fisher syndrome compared with cerebrospinal fluid examination. J Neuroimmunol. 2004;148:200‐205. 10.1016/j.jneuroim.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 6. McCudden CR, Brooks J, Figurado P, Bourque PR. Cerebrospinal fluid total protein reference intervals derived from 20 years of patient data. Clin Chem. 2017;63:1856‐1865. 10.1373/clinchem.2017.278267 [DOI] [PubMed] [Google Scholar]
- 7. Hegen H, Auer M, Zeileis A, Deisenhammer F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: implications for increased clinical specificity. Clin Chem Lab Med. 2016;54:285‐292. 10.1515/cclm-2015-0253 [DOI] [PubMed] [Google Scholar]
- 8. Breiner A, Moher D, Brooks J, et al. Adult CSF total protein upper reference limits should be age‐partitioned and significantly higher than 0.45 g/L: a systematic review. J Neurol. 2019;266:616‐624. 10.1007/s00415-018-09174-z [DOI] [PubMed] [Google Scholar]
- 9. Brettschneider J, Claus A, Kassubek J, Tumani H. Isolated blood‐cerebrospinal fluid barrier dysfunction: prevalence and associated diseases. J Neurol. 2005;252:1067‐1073. 10.1007/s00415-005-0817-9 [DOI] [PubMed] [Google Scholar]
- 10. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914‐1922. 10.1212/WNL.0b013e3181c47cc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Ann Neurol. 1990;27:S21‐S24. 10.1002/ana.410270707 [DOI] [PubMed] [Google Scholar]
- 12. Wakerley BR, Uncini A, Yuki N, et al. Guillain‐Barré and Miller Fisher syndromes–new diagnostic classification. Nature Publishing Group. 2014;10:537‐544. 10.1038/nrneurol.2014.138 [DOI] [PubMed] [Google Scholar]
- 13. Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain‐Barré syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain‐Barré Syndrome Trial Group. Ann Neurol. 1998;44:780‐788. 10.1002/ana.410440512 [DOI] [PubMed] [Google Scholar]
- 14. Hughes RA, Newsom‐Davis JM, Perkin GD, et al. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2:750‐753. 10.1016/s0140-6736(78)92644-2 [DOI] [PubMed] [Google Scholar]
- 15. Deisenhammer F, Bartos A, Egg R, et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13:913‐22. 10.1111/j.1468-1331.2006.01493.x [DOI] [PubMed] [Google Scholar]
- 16. Meulemans O. Determination of total protein in spinal fluid with sulphosalicylic acid and trichloroacetic acid. Clin Chim Acta. 1960;5:757‐761. [DOI] [PubMed] [Google Scholar]
- 17. Auer M, Hegen H, Zeileis A, Deisenhammer F. Quantitation of intrathecal immunoglobulin synthesis ‐ a new empirical formula. Eur J Neurol. 2016;23:713‐721. 10.1111/ene.12924 [DOI] [PubMed] [Google Scholar]
- 18. Reiber H. Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood‐CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189‐203. [DOI] [PubMed] [Google Scholar]
- 19. Merritt HH, Fremont‐Smith F. Chemistry and Pathologic Physiology. The Cerebrospinal Fluid. Philadelphia: W.B. Saunders Company; 1938. [Google Scholar]
- 20. Bourque PR, Breiner A, Moher D, et al. Adult CSF total protein: Higher upper reference limits should be considered worldwide. A web‐based survey. J Neurol Sci. 2019;396:48‐51. 10.1016/j.jns.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 21. Bourque PR, Brooks J, McCudden CR, et al. Age matters: Impact of data‐driven CSF protein upper reference limits in Guillain‐Barré syndrome. Neurol Neuroimmunol Neuroinflamm. 2019;6:e576 10.1212/NXI.0000000000000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37:397‐401. 10.1080/00365517709091498 [DOI] [PubMed] [Google Scholar]
- 23. Blennow K, Fredman P, Wallin A, et al. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol. 1993;33:129‐133. 10.1159/000116919 [DOI] [PubMed] [Google Scholar]
- 24. Garton MJ, Keir G, Lakshmi MV, Thompson EJ. Age‐related changes in cerebrospinal fluid protein concentrations. J Neurol Sci. 1991;104:74‐80. [DOI] [PubMed] [Google Scholar]
- 25. Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types A and B food‐borne botulism. Ann Intern Med. 1981;95:442‐445. 10.7326/0003-4819-95-4-442 [DOI] [PubMed] [Google Scholar]
- 26. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain‐Barré syndrome in ten steps. Nature Publishing Group. 2019;15:671‐683. 10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ropper AH. Unusual clinical variants and signs in Guillain‐Barré syndrome. Arch Neurol. 1986;43:1150‐1152. 10.1001/archneur.1986.00520110044012 [DOI] [PubMed] [Google Scholar]
- 28. Bourque PR, Brooks J, Warman‐Chardon J, Breiner A. Cerebrospinal fluid total protein in Guillain‐Barré syndrome variants: correlations with clinical category, severity, and electrophysiology. J Neurol. 2019;10:469‐476. 10.1007/s00415-019-09634-0 [DOI] [PubMed] [Google Scholar]
- 29. Hughes RAC, Cornblath DR. Guillain‐Barré syndrome. Lancet. 2005;366:1653‐1666. 10.1016/S0140-6736(05)67665-9 [DOI] [PubMed] [Google Scholar]
- 30. Tumani H, Hegen H. CSF Total Protein In Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H, eds. Cerebrospinal Fluid in Clinical Neurology. Cham, Switzerland: Springer; 2015: 107‐110. [Google Scholar]
- 31. Tumani H, Hegen H. CSF Albumin: Albumin CSF/Serum Ratio In Deisenhammer F, Sellebjerg F, Teunissen C, Tumani H, eds. Cerebrospinal Fluid in Clinical Neurology. Cham, Switzerland: Springer: 111‐114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


