Abstract
Aim
To investigate the effects of once‐weekly subcutaneous (s.c.) semaglutide 2.4 mg on gastric emptying, appetite, and energy intake in adults with obesity.
Materials and Methods
A double‐blind, parallel‐group trial was conducted in 72 adults with obesity, randomized to once‐weekly s.c. semaglutide (dose‐escalated to 2.4 mg) or placebo for 20 weeks. Gastric emptying was assessed using paracetamol absorption following a standardized breakfast. Participant‐reported appetite ratings and Control of Eating Questionnaire (CoEQ) responses were assessed, and energy intake was measured during ad libitum lunch.
Results
The area under the concentration–time curve (AUC) for paracetamol 0 to 5 hours after a standardized meal (AUC0–5h,para; primary endpoint) was increased by 8% (P = 0.005) with semaglutide 2.4 mg versus placebo at week 20 (non‐significant when corrected for week 20 body weight; P = 0.12). No effect was seen on AUC0–1h,para, maximum observed paracetamol concentration, or time to maximum observed paracetamol concentration. Ad libitum energy intake was 35% lower with semaglutide versus placebo (1736 versus 2676 kJ; estimated treatment difference −940 kJ; P <0.0001). Semaglutide reduced hunger and prospective food consumption, and increased fullness and satiety when compared with placebo (all P <0.02). The CoEQ indicated better control of eating and fewer/weaker food cravings with semaglutide versus placebo (P <0.05). Body weight was reduced by 9.9% with semaglutide and 0.4% with placebo. Safety was consistent with the known profile of semaglutide.
Conclusions
In adults with obesity, once‐weekly s.c. semaglutide 2.4 mg suppressed appetite, improved control of eating, and reduced food cravings, ad libitum energy intake and body weight versus placebo. There was no evidence of delayed gastric emptying at week 20, assessed indirectly via paracetamol absorption.
Keywords: appetite, control of eating, energy intake, food craving, gastric emptying, GLP‐1 analogue, glucagon‐like peptide‐1, obesity, randomized trial, semaglutide
1. INTRODUCTION
Obesity is a growing global health crisis placing substantial burden on healthcare systems, with excess weight contributing to a range of detrimental effects, including increased risk of type 2 diabetes (T2D), cardiovascular disease, and mortality. 1 , 2 Despite the importance of weight loss in improving health outcomes for patients with overweight/obesity, 1 , 2 relatively few pharmacotherapies are approved for weight management. 3
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) were initially developed for improvement of glycaemic control in T2D. 4 Following the observation of weight reductions in T2D, 4 GLP‐1RAs were studied in patients with overweight or obesity, 5 , 6 and a single agent (liraglutide 3 mg) is currently approved for weight management. 7 , 8 While liraglutide provided clinically relevant reductions in body weight of 5.4% relative to placebo in a pivotal study in overweight/obese patients, 6 there remains an unmet need for those patients for whom weight loss ≥10% is recommended. 1 , 2 Furthermore, other available antiobesity agents fail to achieve ≥10% weight loss and some are associated with safety concerns, 3 , 9 highlighting the need for effective, well‐tolerated treatments.
Semaglutide is a GLP‐1RA approved for the treatment of T2D as a once‐weekly subcutaneous (s.c.) injection at doses up to 1.0 mg, and as a once‐daily oral tablet (up to 14 mg), which is the first oral formulation of a GLP‐1RA. 10 , 11 , 12 , 13 In phase 3 studies in patients with T2D, s.c. semaglutide 1.0 mg reduced body weight from baseline by up to 6.5 kg (at timepoints ranging from 30 to 104 weeks), with two to three times greater reductions than with other studied GLP‐1RAs. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Semaglutide lowers body weight by reducing appetite and hunger, increasing satiety, reducing food cravings, altering food preferences and reducing energy intake. 24 , 25 An initial phase 2 dose‐ranging study in patients with obesity demonstrated clinically relevant weight loss with s.c. semaglutide, when given as once‐daily doses of up to 0.4 mg. 5 Once‐weekly s.c. semaglutide is now in clinical development for weight management in patients with overweight/obesity, within the phase 3 Semaglutide Treatment Effect in People with obesity (STEP) trial programme, which is investigating the efficacy of once‐weekly s.c. semaglutide 2.4 mg. 26
In addition to their effects on regulation of energy intake and body weight, GLP‐1RAs have been associated with delayed gastric emptying, 4 , 27 , 28 which has the potential to affect the absorption of concomitantly administered oral agents. 7 , 8 , 10 , 11 , 12 , 13 A 12‐week study with semaglutide 1.0 mg in subjects with obesity indicated a delay in first hour gastric emptying. 29 We therefore conducted the present phase 1 trial in adults with obesity, with two main objectives: the primary objective was to investigate the effect of once‐weekly s.c. semaglutide 2.4 mg on gastric emptying; the secondary objective was to investigate the effect of the 2.4 mg dose on appetite and energy intake, to provide further insight into the weight‐reducing mechanism of action of semaglutide in obesity.
2. MATERIALS AND METHODS
2.1. Trial design
A single‐centre, randomized, double‐blind, placebo‐controlled, parallel‐group, phase 1 trial was conducted in Germany (NCT03842202). The trial consisted of a 20‐week treatment period (including 21 doses of study drug) and a 7‐week follow‐up (Figure 1). The trial adhered to the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines, and was approved by the relevant institutional, ethical and regulatory bodies.
FIGURE 1.
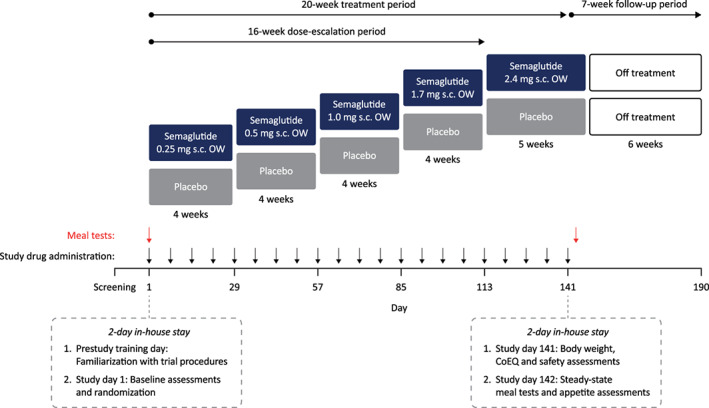
Trial design. OW, once weekly; s.c. subcutaneous
2.2. Trial population
Participants were men and women, aged 18 to 65 years, with body mass index (BMI) of 30.0 to 45.0 kg/m2. Informed consent was required before trial‐related activities. Exclusion criteria included: clinically significant body weight change (≥5%) or dieting attempts in the prior 90 days; use of medications in the prior 14 days (other than contraceptives, occasional paracetamol or acetylsalicylic acid, or stable doses of antihypertensives or lipid‐lowering drugs); use of weight‐lowering drugs or drugs that may cause weight gain within the prior 12 months; presence of gastrointestinal disorders or symptoms of such disorders that may affect absorption of drugs or nutrients; prior obesity surgery or presence of gastrointestinal implant; and glycated haemoglobin (HbA1c) ≥48 mmol/mol or fasting glucose ≥7.0 mmol/L.
2.3. Interventions
Participants were randomized equally to once‐weekly s.c. semaglutide 2.4 mg (initially undergoing a 16‐week dose‐escalation consisting of 0.25, 0.5, 1.0 and 1.7 mg once weekly for 4 weeks each, followed by 2.4 mg for five doses; 21 doses in total over 20 weeks) or volume‐matched placebo (with matching dose‐escalation procedure; Figure 1). The randomization schedule was generated by the sponsor before the trial, and participants were assigned randomization numbers in ascending numerical order at the trial site. Participants were instructed to inject their allocated study drug on the same day each week (any time of day, irrespective of meals).
2.4. Endpoints
The primary endpoint compared the effect of once‐weekly s.c. semaglutide 2.4 mg and placebo on gastric emptying assessed by the paracetamol absorption method at week 20, using the area under the concentration–time curve (AUC) for paracetamol 0 to 5 hours after a standardized meal (AUC0–5h,para). Paracetamol is commonly used as an indirect marker for gastric emptying, 30 and its use provided an approach consistent with a previous study of s.c. semaglutide 1.0 mg. 29 Secondary endpoints related to gastric emptying included paracetamol AUC from 0 to 1 hour after a standardized meal (AUC0–1h,para), maximum observed paracetamol concentration (Cmax,para) and time to maximum observed paracetamol concentration (tmax,para).
Energy intake during the ad libitum lunch was compared between semaglutide and placebo at week 20 as a secondary endpoint.
The effect of semaglutide compared with placebo on appetite was assessed using mean postprandial participant‐reported visual analogue scale (VAS) appetite ratings following a standardized breakfast meal at week 20, focusing on hunger, fullness, satiety, prospective food consumption and overall appetite suppression score (secondary endpoints). Additional exploratory endpoints included assessment of fasting and mean postprandial change from fasting ratings for VAS items measuring thirst, nausea and well‐being following a standardized breakfast. Participant‐reported control of eating was evaluated as an exploratory endpoint using the Control of Eating Questionnaire (CoEQ), 31 completed at week 20.
2.5. Procedures and assessments
Following screening, eligible participants attended a 2‐day in‐house stay at the study centre. The first day of the in‐house stay was a training day before the start of the treatment period, during which participants were familiarized with the study tasks and received an ad libitum meal (no data were collected). A 5‐hour standardized meal test was performed on day 1 of the study (prior to initiating treatment [baseline timepoint]) and during a return visit on day 142 (the day after administration of the final dose of study drug, at the end of the 20‐week treatment period; Figure 1). The meal test consisted of a breakfast meal of approximately 600 kcal (macronutrient composition of ~30 energy percentage [E%] fat, ~15 E% protein and ~55 E% carbohydrate), which participants were required to ingest within 15 minutes. A yoghurt containing paracetamol 1500 mg was included as part of the meal. Blood was sampled for paracetamol concentration using a venous catheter before the start of the meal (baseline), and at regular timepoints thereafter, for up to 5 hours postprandially, and participants completed several VAS 1 to 3 minutes prior to blood sampling. These VAS assessed appetite (hunger, satiety, fullness and prospective food consumption), thirst, well‐being and nausea. A 100 mm scale was used, with the ends indicating the most extreme sensation the participants had ever experienced. The participants subsequently received an ad libitum lunch meal in excess, approximately 5 hours after the scheduled completion of the breakfast meal at baseline and after 20 weeks, and food consumption (kJ) was recorded. The participants were instructed to eat until they were pleasantly satiated.
The participants completed the CoEQ on day −1 (baseline for this analysis) and day 141, based on their experience over the prior 7 days, with ratings for each question recorded on a 100 mm VAS. The questionnaire included 19 questions relating to control of eating, intensity, frequency and type of food craving, appetite/hunger sensations and mood.
Body weight was recorded at baseline and after 20 weeks, and during the follow‐up period. Safety assessments included adverse event (AE) reporting, assessment of vital signs and laboratory tests (biochemistry, haematology and glucose metabolism).
2.6. Statistical analysis
A sample size of 29 completers per treatment group was required to provide 90% power to detect a half‐width of the 95% confidence interval (CI) for the log‐transformed treatment ratio of 0.15 for the primary endpoint, assuming a standard deviation (SD) of 0.25 (based on a previous trial). 24 Assuming an estimated drop‐out rate of 20%, 36 participants were planned to be randomized per group.
Statistical comparisons between groups were conducted using two‐sided tests and at a 5% significance level. For the primary endpoint (AUC0–5,para), data were log‐transformed and analysed using an analysis of covariance (ANCOVA) model, with baseline as covariate and treatment as factor, and results presented as treatment ratio with 95% CI. Secondary endpoints were analysed in a similar way to the primary endpoint, but without log‐transformation for those relating to ad libitum energy intake and appetite VAS. Mean postprandial values were calculated as the AUC for VAS ratings over 30 to 300 minutes after the standardized breakfast, divided by 270 minutes. For the appetite VAS, the overall appetite suppression score was calculated as the average of the four components: (satiety + fullness + [100 – hunger] + [100 – prospective food consumption]) / 4. Descriptive statistics were used for exploratory endpoints (except for CoEQ), changes in body weight and safety assessments.
The exploratory CoEQ endpoint was analysed using ANCOVA models for each CoEQ question, with change from baseline as response, baseline value of the respective question as covariate and treatment as factor. This approach differed from the prespecified methodology, which did not account for the baseline value. Additional post hoc analyses included analysis of the primary endpoint using an ANCOVA model, with log‐transformed body weight at week 20 as an additional covariate, and analysis of percentage change in ad libitum energy intake from baseline to week 20 using an ANCOVA model with energy intake at baseline as covariate and treatment as factor.
3. RESULTS
3.1. Trial population
Seventy‐two participants were enrolled between February and April 2019, and randomized to once‐weekly s.c. semaglutide 2.4 mg (n = 36) or placebo (n = 36; Figure S1). Almost all participants (97.2%) completed the study; one participant in the semaglutide group withdrew consent before study end, and one participant in the placebo group was withdrawn following an AE (colonic abscess). Demographics and baseline characteristics were generally comparable between the groups; the majority of participants were men (61.1%), the mean age was 42.8 years, the mean body weight was 105.5 kg and the mean BMI was 34.4 kg/m2 (Table 1).
TABLE 1.
Demographics and baseline characteristics
| Semaglutide s.c. 2.4 mg (N = 36) | Placebo (N = 36) | Total (N = 72) | |
|---|---|---|---|
| Age, years | 40.7 (12.2) | 45.0 (9.5) | 42.8 (11.1) |
| Sex, n (%) | |||
| Male | 24 (66.7) | 20 (55.6) | 44 (61.1) |
| Female | 12 (33.3) | 16 (44.4) | 28 (38.9) |
| Race, n (%) | |||
| Black or African American | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| White | 35 (97.2) | 36 (100.0) | 71 (98.6) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| Body weight, kg | 106.2 (16.2) | 104.9 (14.0) | 105.5 (15.0) |
| BMI, kg/m2 | 34.2 (3.0) | 34.6 (3.1) | 34.4 (3.0) |
Note: Data are mean (standard deviation), unless otherwise stated.
BMI, body mass index; s.c., subcutaneous
3.2. Gastric emptying
The AUC0–5h,para was 8% higher in the s.c. semaglutide 2.4 mg group compared with the placebo group at week 20 (estimated treatment ratio [ETR] 1.08; P = 0.0054). The difference in AUC0–5h,para between groups was no longer statistically significant when adjusted for body weight at week 20 in a post hoc analysis (ETR 1.05; P = 0.1218). No differences were found between semaglutide and placebo for other endpoints, including AUC0–1h,para (unadjusted ETR 0.99 [P = 0.8474]; body‐weight‐adjusted ETR 0.94 [P = 0.3069]), Cmax,para (unadjusted ETR 0.94 [P = 0.3299]; body‐weight‐adjusted ETR 0.90 [P = 0.1464]) and tmax,para (unadjusted ETR 1.02 [P = 0.7540]; body‐weight‐adjusted ETR 1.02 [P = 0.7861]; Table S1; Figure S2). Median tmax,para was 0.50 hours in both the semaglutide and placebo groups at week 20.
3.3. Ad libitum energy intake
The estimated mean ad libitum energy intake during lunch at week 20 was 35% lower in the s.c. semaglutide 2.4 mg group (mean 1736 kJ) compared with the placebo group (mean 2676 kJ; Figure 2A [see Table S2 for kcal values]). Relative to baseline, this represented a reduction of 1577 kJ at week 20 in the semaglutide 2.4 mg group compared with 637 kJ in the placebo group (estimated treatment difference [ETD] −940 kJ; P <0.0001; Figure 2B). When analysed in terms of the percentage change from baseline to week 20, estimated mean energy intake was reduced by 47.1% with semaglutide versus 18.6% with placebo (ETD 28.5%; P = 0.0001; post hoc analysis; Figure S3).
FIGURE 2.
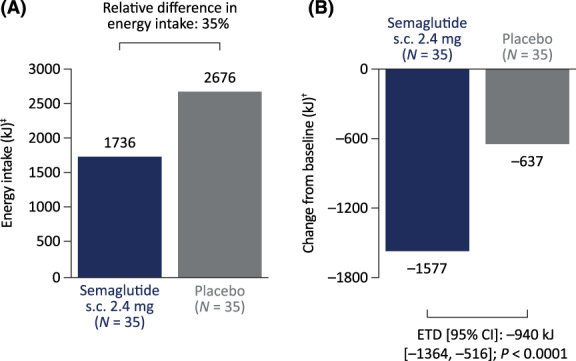
Ad libitum lunch energy intake at week 20 (A) and change from baseline in ad libitum lunch energy intake at week 20 (B). Estimates were calculated from analysis of covariance (ANCOVA) models using baseline energy intake of 3313 kJ, which corresponds to the average baseline value for all participants (semaglutide and placebo groups) who contributed to the analysis. ‡Obtained from an ANCOVA model with energy intake at baseline as a covariate and treatment as a factor. †Obtained from an ANCOVA model with change from baseline value to week 20 as response, energy intake at baseline as a covariate and treatment as a factor. CI, confidence interval; ETD, estimated treatment difference; s.c., subcutaneous
3.4. Appetite
After a standardized breakfast, hunger and prospective food consumption VAS ratings were reduced, and fullness and satiety increased, with s.c. semaglutide 2.4 mg versus placebo (P <0.02 for all; Figure 3). The overall postprandial appetite suppression score after the standardized breakfast was higher with semaglutide versus placebo (ETD 13 mm; P = 0.001 [Figures 3 and S4]).
FIGURE 3.
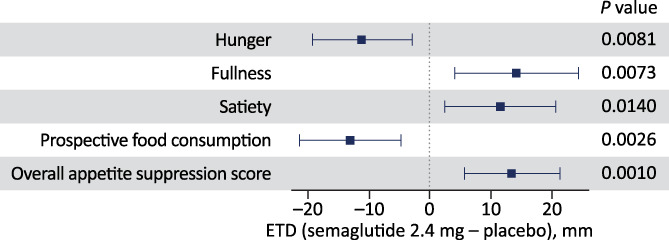
Postprandial appetite ratings after standardized breakfast at week 20. Overall appetite suppression score calculated as: (satiety + fullness + [100 – hunger] + [100 – prospective food consumption]) / 4. Each endpoint was analysed using the analysis of covariance model with baseline value of the respective endpoint as covariate and treatment as factor. The figure shows the estimated treatment difference for semaglutide versus placebo (boxes) and 95% confidence interval (whiskers). CI, confidence interval; ETD, estimated treatment difference; VAS, visual analogue score
Ratings for thirst were similar in the s.c. semaglutide 2.4 mg group and placebo group at week 20. Overall, the mean ratings for nausea at week 20 were low in both groups and mean well‐being ratings were high (exploratory endpoints; data not shown).
3.5. Control of eating and food cravings
Participants' CoEQ scores at week 20 showed lower hunger with s.c. semaglutide 2.4 mg compared with placebo, better control of eating, and fewer and weaker food cravings, including reductions in both desire and craving for savoury foods, desire for sweet foods and craving for dairy foods (P <0.05 for all; Figure 4). In addition, fullness and contentment appeared increased with semaglutide compared with placebo (P <0.05).
FIGURE 4.
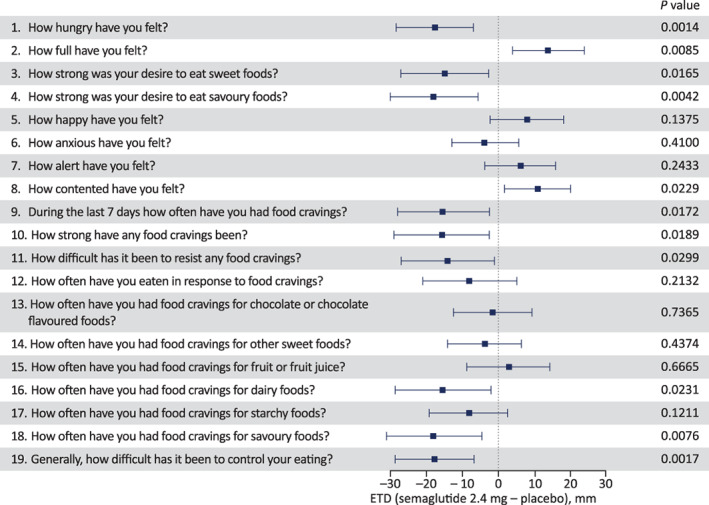
Control of eating and food cravings evaluated by the Control of Eating Questionnaire visual analogue scale at week 20. The Control of Eating Questionnaire was completed by participants at the end of the 20‐week treatment period (day 141), based on their experience over the prior 7 days. Individual scores for each question were analysed using separate analysis of covariance models with change from baseline as response, baseline value of respective question as a covariate and treatment as factor (post hoc analysis methodology). The figure shows the estimated treatment difference (ETD) for semaglutide versus placebo (boxes) and 95% confidence interval (whiskers)
3.6. Body weight
By week 20, body weight was reduced from baseline by a mean (SD) of 10.4 kg (6.3) with s.c. semaglutide 2.4 mg and 0.4 kg (2.6) with placebo (descriptive statistics only), representing relative reductions from baseline of 9.9% and 0.4%, respectively.
3.7. Safety
The number of participants reporting AEs was broadly similar in the s.c. semaglutide 2.4 mg group (29 participants [80.6%]) and placebo group (33 participants [91.7%]; Table S3). All AEs were mild or moderate in severity, with the exception of a single severe, serious AE (colonic abscess) in the placebo group, which led to trial withdrawal. One serious AE was reported in the semaglutide group (injury‐related after a motorcycle accident).
Decreased appetite was the AE reported by the greatest number of participants, and occurred in more participants with semaglutide than placebo (Table S3). Gastrointestinal AEs were reported more frequently in the semaglutide group (25 participants [69.4%]) compared with the placebo group (14 participants [38.9%]), with nausea and diarrhoea most commonly reported. Such events were all mild or moderate in severity and generally of short duration.
4. DISCUSSION
This trial investigated the effect of once‐weekly s.c. semaglutide 2.4 mg on gastric emptying, appetite and energy intake in participants with obesity. Using paracetamol absorption as an indirect measure for gastric emptying, we found no evidence of delayed gastric emptying with semaglutide 2.4 mg at week 20. Meal tests showed a reduction in appetite and energy intake with semaglutide relative to placebo, together with better control of eating, fewer and weaker food cravings, and clinically meaningful reductions in body weight.
Prior studies of GLP‐1RAs on gastric emptying, energy intake and appetite have typically been of shorter durations and therefore often used crossover designs to reduce within‐participant variability, 24 , 28 , 29 as used in a prior 12‐week trial of s.c. semaglutide 1.0 mg in subjects with obesity. 24 In contrast, the present study used a parallel‐group design, given that a 20‐week treatment period was required to allow gradual dose‐escalation to the 2.4 mg dose. In this study we instead accounted for within‐participant variation by including a baseline evaluation and integrating this within the statistical analyses. In addition, baseline demographics and clinical characteristics were similar overall between the two groups.
Delayed gastric emptying would be anticipated to slow paracetamol absorption, and paracetamol absorption is therefore generally accepted as an indirect measure for gastric emptying. Semaglutide 2.4 mg was associated with a statistically significant 8% increase in paracetamol AUC0–5h versus placebo, which might be partially explained by substantially lower body weight in the semaglutide group compared with placebo at week 20 (greater body weight is associated with reduced paracetamol absorption rates and increased clearance 32 , 33 ). This was confirmed by a post‐hoc analysis, which found that the difference in AUC0–5h,para between semaglutide and placebo was no longer statistically significant after adjusting for week 20 body weight. No differences were found between semaglutide and placebo for other paracetamol endpoints, with or without adjustment for week 20 body weight. We observed no reduction in paracetamol absorption (AUC0–5h, AUC0–1h or Cmax) with once‐weekly s.c. semaglutide 2.4 mg versus placebo at week 20, and no effect on tmax,para. In contrast, the 12‐week crossover study of once‐weekly s.c. semaglutide 1.0 mg reported a delay in paracetamol‐assessed gastric emptying over the first postprandial hour, but similarly found no significant difference in overall gastric emptying when assessed as paracetamol AUC 0 to 5 hours postprandially. 29 Similar effects were observed with oral semaglutide in another crossover study in participants with T2D, also using the paracetamol absorption test. 34 Our parallel‐group study used a higher dose (2.4 mg once weekly) of semaglutide than that in the s.c. semaglutide 1.0 mg crossover study, and included a longer treatment period than both of these earlier studies (20 vs. 12 weeks). The absence of delayed paracetamol‐assessed gastric emptying in our trial may therefore relate to more pronounced tachyphylaxis, arising from the longer treatment duration; such tachyphylaxis has previously been reported with long‐acting GLP‐1RAs. 4 , 28 , 35
The present study provides further insights into the mechanisms through which semaglutide mediates body weight loss. Subcutaneous semaglutide 2.4 mg suppressed postprandial appetite, including a reduction in hunger and prospective food consumption, and an increase in satiety and fullness, with participants reporting that they were able to control their eating with less difficulty relative to placebo. CoEQ scores suggested an effect in terms of reduced intensity of desire for sweet and savoury foods, and reduced frequency of craving for dairy and savoury foods. These results are consistent with the prior study of once‐weekly s.c. semaglutide 1.0 mg in participants with obesity, which similarly reported appetite suppression, improved control of eating and reduced food craving (particularly for savoury foods [craving for dairy and desire for sweet/savoury food were not assessed in that study]). 24 In animal studies, the anorexigenic actions of semaglutide have been shown to arise from effects on the central nervous system, mediated by GLP‐1 receptors in the hypothalamus and hindbrain. 25 , 36 Such studies suggest that semaglutide directly activates brain areas that are accessible to the molecule and also causes indirect secondary modulation of neuronal activity in other brain areas, including those involved in appetite regulation, food intake, food preference, reward and meal termination, such as the lateral parabrachial nucleus. 25
Once‐weekly s.c. semaglutide 2.4 mg reduced body weight by 9.9% (10.4 kg) from baseline to week 20, in the absence of structured dietary and exercise intervention, compared with almost no change in the placebo group (0.4% [0.4 kg]). This reduction appears consistent with that seen in the phase 2 study with once‐daily s.c. semaglutide 0.4 mg in obesity (when taking into account the additional contribution of structured dietary and physical activity counselling in the phase 2 study), 5 and is twice as great as the 5 kg reduction seen over 12 weeks in the study of once‐weekly s.c. semaglutide 1.0 mg. 24 Our results demonstrate clinically relevant weight loss with once‐weekly s.c. semaglutide 2.4 mg in participants with obesity during a relatively short 20‐week treatment period (body weight assessment performed after only 4 weeks on the 2.4 mg dose). A greater weight loss may be achievable with longer‐term treatment, which is being investigated in phase 3 studies with semaglutide 2.4 mg in adults with obesity. 26
The body weight‐lowering effects of once‐weekly s.c. semaglutide 2.4 mg are likely to be related to reduced energy intake in response to effects on hedonic and homeostatic control of eating, manifesting as decreased appetite, increased satiety, reduced hunger, better control of eating and reduced food cravings. Mean ad libitum energy intake during lunch was 35% lower with s.c. semaglutide 2.4 mg versus placebo at week 20. Energy intake reductions were also found with once‐weekly s.c. semaglutide 1.0 mg versus placebo in the crossover study in subjects with obesity, with reductions of 18% to 35% reported across ad libitum meals (lunch, evening meal and snack box). 24 A direct comparison of the energy intake results between these two studies is not possible due to key differences in study design (parallel vs. crossover; 20 vs. 12 weeks' treatment) and analysis methodology (adjustment for baseline in the present study, but not in the s.c. semaglutide 1.0 mg trial), and the potential for between‐study differences in placebo effect. 24 It should be noted that the ad libitum lunch test used in the present study represents an isolated assessment of energy intake and may not capture the overall treatment difference in energy intake throughout the day, including other meals.
While it has been proposed that delayed gastric emptying could hypothetically contribute to reduced energy intake and weight loss with GLP‐1RAs, the lack of notable effects of long‐acting GLP‐1RAs on gastric emptying renders this an unlikely mechanism for such agents. 37 We did not identify a role for gastric emptying in weight loss with semaglutide 2.4 mg, based on the paracetamol absorption test.
Overall, the incidence and nature of AEs was consistent with the known safety and tolerability profile of semaglutide, 5 , 14 , 24 with no new safety findings.
Key strengths of the present study include the fact that it was performed at a single centre, thereby reducing the potential for variations in study procedures, and the inclusion of a placebo group. While we did not use the "gold standard" scintigraphy‐based approach for assessing gastric emptying, the paracetamol absorption test is widely used and is the methodology adopted in most prior studies of GLP‐1RAs, 28 and enabled comparison with the results of the prior study of paracetamol‐assessed gastric emptying with semaglutide 1.0 mg in obesity. 29 As an indirect measure, paracetamol absorption has been suggested to have limitations, particularly regarding its ability to reflect gastric emptying of solids, and the potential for short‐term delays in gastric emptying to be missed if paracetamol absorption is only assessed several hours after administration. 28 In the present study, we attempted to mitigate these limitations by administering paracetamol as part of a semi‐solid food (yoghurt; consistent with the semaglutide 1.0 mg study), 29 and by evaluating paracetamol concentration within the first hour post‐dose and over a 5‐hour period post‐dose, as well as assessing the magnitude and timing of peak concentration (Cmax,para and tmax,para). At week 20, none of these assessments indicated a delay in paracetamol absorption with semaglutide 2.4 mg versus placebo, and there was no flattening of the paracetamol concentration–time curve, which, if present, would have suggested a delay in gastric emptying.
In conclusion, in adults with obesity, once‐weekly s.c. semaglutide 2.4 mg suppressed appetite, improved control of eating, reduced the frequency and strength of food cravings, lowered ad libitum energy intake and was associated with clinically meaningful reductions in body weight versus placebo at week 20, with no evidence of delayed gastric emptying as measured indirectly through paracetamol absorption.
CONFLICT OF INTEREST
M.F., S.T. and D.S. are employees and shareholders of Novo Nordisk, the sponsor of this trial; A.W. is an employee of Novo Nordisk. A.B. is an employee of Parexel International GmbH; Parexel International GmbH was paid by Novo Nordisk for assistance in conducting the study.
AUTHOR CONTRIBUTIONS
Study design: A.B., M.F. and S.T. contributed to the study design and collection of data; A.W., D.S., M.F. and S.T. contributed to data analysis and interpretation. All authors contributed to the development of the manuscript and provided final approval for submission.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14280.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This study was funded by Novo Nordisk A/S, Søborg, Denmark. The authors thank the participants, investigators and study‐site personnel involved in the trial. Under direction of the authors, medical writing and editorial support was provided by Paul Barlass of Axis, a division of Spirit Medical Communications Group Limited, funded by Novo Nordisk A/S.
Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754–762. 10.1111/dom.14280
Funding information Novo Nordisk A/S
DATA AVAILABILITY STATEMENT
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymised format.
REFERENCES
- 1. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. [DOI] [PubMed] [Google Scholar]
- 2. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association . 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S89‐S97. [Addendum: Diabetes Care. 2020;43(8):1980‐1980]. [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Meier JJ. Are all GLP‐1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181(6):R211‐R234. [DOI] [PubMed] [Google Scholar]
- 5. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet. 2018;392(10148):637‐649. [DOI] [PubMed] [Google Scholar]
- 6. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 7. Saxenda® Prescribing Information. Revised March 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/206321s011lbl.pdf. Accessed May 1, 2020.
- 8. Saxenda® Summary of Product Characteristics. Revised January 2020. https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf. Accessed May 1, 2020.
- 9. Bessesen DH, Van Gaal LF. Progress and challenges in anti‐obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237‐248. [DOI] [PubMed] [Google Scholar]
- 10. Ozempic® Prescribing Information. Revised January 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf. Accessed May 2, 2020.
- 11. Ozempic® Summary of Product Characteristics; Revised February 2019. https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf. Accessed March 20, 2020.
- 12. Rybelsus® Prescribing Information. Revised January 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213182s000,213051s001lbl.pdf. Accessed July 27, 2020.
- 13. Rybelsus® Summary of Product Characteristics. Revised June 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf. Accessed June 5, 2020.
- 14. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once‐weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1‐7 trials. Diabetes Metab. 2019;45(5):409‐418. [DOI] [PubMed] [Google Scholar]
- 15. Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once‐weekly semaglutide versus daily canagliflozin as add‐on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double‐blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834‐844. [DOI] [PubMed] [Google Scholar]
- 16. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once‐weekly semaglutide 1.0mg vs once‐daily liraglutide 1.2mg as add‐on to 1‐3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100‐109. [DOI] [PubMed] [Google Scholar]
- 17. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 18. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 19. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 20. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 21. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 23. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 24. Blundell J, Finlayson G, Axelsen M, et al. Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38(7):1263‐1273. [DOI] [PubMed] [Google Scholar]
- 28. Maselli DB, Camilleri M. Effects of GLP‐1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol. 2021;1307:171‐192. [DOI] [PubMed] [Google Scholar]
- 29. Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first‐hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256‐2262. [DOI] [PubMed] [Google Scholar]
- 31. Dalton M, Finlayson G, Hill A, Blundell J. Preliminary validation and principal components analysis of the control of eating questionnaire (CoEQ) for the experience of food craving. Eur J Clin Nutr. 2015;69(12):1313‐1317. [DOI] [PubMed] [Google Scholar]
- 32. Lee WH, Kramer WG, Granville GE. The effect of obesity on acetaminophen pharmacokinetics in man. J Clin Pharmacol. 1981;21(7):284‐287. [DOI] [PubMed] [Google Scholar]
- 33. Abernethy DR, Divoll M, Greenblatt DJ, Ameer B. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783‐790. [DOI] [PubMed] [Google Scholar]
- 34. Dahl K, Blundell J, Gibbons C, et al. Oral semaglutide improves postprandial glucose and lipid metabolism and delays first‐hour gastric emptying in subjects with type 2 diabetes. Abstract 50. Presented at the 55th Annual Meeting of the European Association for the Study of Diabetes, September 16–20, 2019, Barcelona, Spain. https://www.easd.org/virtualmeeting/home.html#!resources/oral-semaglutide-improves-postprandial-glucose-and-lipid-metabolism-and-delays-first-hour-gastric-emptying-in-subjects-with-type-2-diabetes. Accessed May 13, 2020.
- 35. Tong J, D'Alessio D. Give the receptor a brake: slowing gastric emptying by GLP‐1. Diabetes. 2014;63(2):407‐409. [DOI] [PubMed] [Google Scholar]
- 36. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metab. 2018;27(4):740‐756. [DOI] [PubMed] [Google Scholar]
- 37. Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymised format.


