Abstract
The COVID‐19 pandemic has unfolded to be the most challenging global health crisis in a century. In 11 months since its first emergence, according to WHO, the causative infectious agent SARS‐CoV‐2 has infected more than 100 million people and claimed more than 2.15 million lives worldwide. Moreover, the world has raced to understand the virus and natural immunity and to develop vaccines. Thus, within a short 11 months a number of highly promising COVID‐19 vaccines were developed at an unprecedented speed and are now being deployed via emergency use authorization for immunization. Although a considerable number of review contributions are being published, all of them attempt to capture only a specific aspect of COVID‐19 or its therapeutic approaches based on ever‐expanding information. Here, we provide a comprehensive overview to conceptually thread together the latest information on global epidemiology and mitigation strategies, clinical features, viral pathogenesis and immune responses, and the current state of vaccine development.
Keywords: COVID‐19, immunity, SARS‐CoV‐2, therapy, vaccines
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- Ad5
human serotype 5 adenovirus
- ADE
antibody‐dependent enhancement of disease
- AMs
alveolar macrophages
- ARDS
acute respiratory distress syndrome
- ChAd
chimpanzee‐derived adenovirus
- COVID‐19
the coronavirus disease 2019
- CT
computed tomography
- DCs
dendritic cells
- NAb
neutralizing antibodies
- RBD
receptor‐binding domain
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TII
trained innate immunity
1. INTRODUCTION
In December 2019, a cluster of patients with unexplained viral pneumonia was identified in Wuhan, China. 1 To identify the causative agent of this disease, a large number of tests were conducted, which ruled out several etiological agents that may cause similar symptoms, including the severe acute respiratory syndrome coronavirus (SARS‐CoV), Middle East respiratory syndrome coronavirus (MERS‐CoV), and other common respiratory pathogens. Finally, researchers identified the cause being a novel coronavirus termed as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 With a rapid increase in the number of infected people, on March 11th the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID‐19) as a pandemic 2 (Figure 1). SARS‐CoV‐2 has infected over 100 million individuals and has killed over 2.15 million people worldwide as of January 28th, 2021 and the numbers continue to grow. 3 Since COVID‐19 is new to mankind, it is imperative to develop safe and effective vaccine strategies to successfully control the pandemic and return to normalcy. Although COVID‐19‐related reviews continue to capture the ever‐expanding information, almost all of them focus only on a particular aspect with few conceptually linking together the clinical aspects, immunity, and vaccines. This review intends to provide a general overview of the latest information on epidemiology and mitigation strategies, clinical features, viral pathogenesis and immunological responses, and vaccine development for COVID‐19.
FIGURE 1.
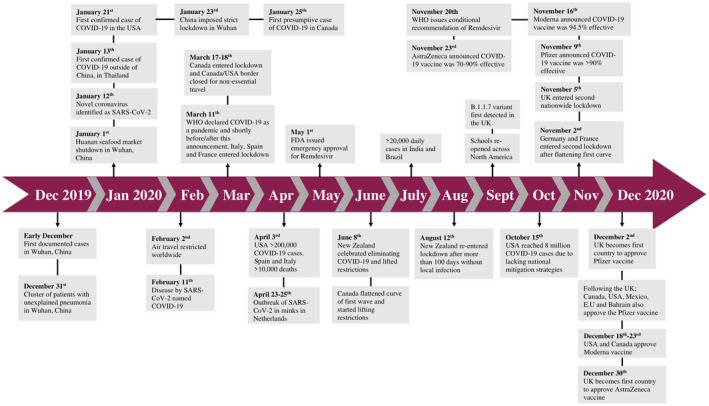
A timeline of the major global events of the COVID‐19 pandemic. The first reported cases were in December 2019 in Wuhan, China. In the following 12 months, there have been more than 100 million cases and 2.15 million deaths worldwide
2. CLINICAL FEATURES
2.1. Epidemiology
COVID‐19 has spread rapidly and becomes a pressing global crisis. It began with the first five documented cases in Wuhan, China between December 18th and 29th of 2019 (Figure 1). 1 By January 22nd of 2020 there were 571 cases in 25 Chinese provinces and by January 30th of 2020 the case numbers grew to be 7734 in China with 90 cases reported in multiple other regions including Taiwan, Thailand, Sri Lanka, Nepal, Japan, Singapore, South Korea, UAE, United States, India, and Canada. 4 Italy was one of the hardest hit countries during the first wave of the pandemic with 110 574 cases and had one of the highest mortality rates from COVID‐19 with 13 155 deaths by April 1st, 2020 (Figure 1). 5 The emergence of an infectious disease comprises three vital elements: infectious source, route of transmission, and a susceptible population. 6 SARS‐CoV‐2‐infected individuals are the source of virus transmission as they produce large quantities of virus in the upper respiratory tract during the pre‐symptomatic period. 7 The majority of infected individuals remain asymptomatic and continue to carry out routine activities, leading to rapid and undetected spread of infection. The viral load detected in asymptomatic individuals is similar to that of symptomatic patients, indicating that asymptomatic infections have the potential for transmission. 7 , 8 The basic reproductive number (R0) of SARS‐CoV‐2, which is used to describe the transmissibility of an infectious agent, was 2.97 at the beginning of the outbreak. 9 , 10 Person‐to‐person transmission of SARS‐CoV‐2 primarily occurs through aerosol droplets, close contact, and potentially fecal‐oral transmission. 11 While SARS‐CoV‐2 viral RNA was found to be stable on the surface of plastic and stainless steel in an experimental setting, 12 real‐life studies investigating the infectious potential of inanimate material and patient fomites showed that they were not contaminated by viable virus, suggesting that contact transmission is unlikely to occur via contaminated surfaces. 13 , 14 The extent of severity and mortality rates among patients with SARS‐CoV‐2 infection are less than those with SARS and MERS, but the prevalence and transmissibility of SARS‐CoV‐2 are much greater than SARS and MERS. 15 As a result, the number of COVID‐19 cases globally has reached over 100 million in over 200 countries and territories, 3 and at the time of writing, many countries/cities around the globe are being hard‐hit by the second wave of the pandemic with lockdowns and/or curfews re‐imposed (Figure 1).
2.2. Risk factors
All age groups are susceptible to SARS‐CoV‐2 infection, but the elderly and those with certain pre‐existing health conditions are particularly prone to severe disease. 16 A systematic review of current literature has found that children account for 1%‐5% of all COVID‐19 cases. 17 Death and incidence of severe disease has been extremely rare among children (Figure 2). For instance, incidences of severe pneumonia, lymphocytopenia, and increase of inflammatory markers were found to be scarce in children. 17 While the mechanisms accounting for milder disease and lack of development of pneumonia in children remain unclear, it has been recently found that angiotensin‐converting enzyme 2 (ACE2) and/or cellular serine protease TMPRSS2, two key receptors in SARS‐CoV‐2 pathogenesis, are differentially expressed between adults and children. Recent studies examining the expression of these two receptors found that, children expressed significantly lower levels of ACE2 and/or TMPRSS2 in the upper and lower airways when compared to adults, hinting at a possible explanation for differential disease outcomes in these two age groups 18 , 19 (Figure 2). Furthermore, adults who smoked or had COPD were found to have significantly higher levels of ACE2 and TMPRSS2 expression in the airways than healthy non‐smoking adults, and the patients with hypertension were found to have significantly higher levels of ACE2 and TMPRSS2 expression in PBMCs. 18 Furthermore, patients with asthma had significantly higher levels of TMPRSS2 but not ACE2 in bronchial epithelial tissue. 18 This increased expression may provide some explanation for the worsened disease outcomes among patients with pre‐existing chronic cardiovascular and/or respiratory conditions (Figure 2).
FIGURE 2.
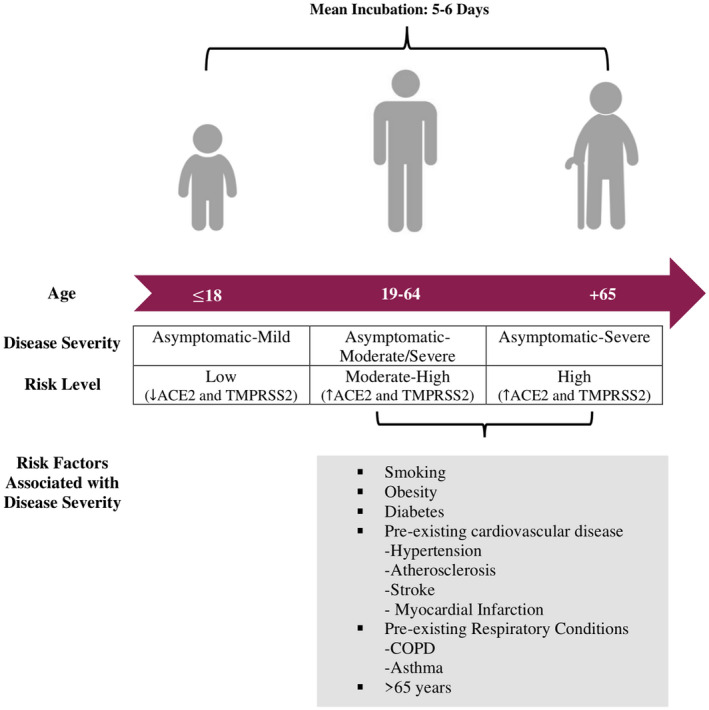
Clinical features of COVID‐19. Typical risk factors, disease severity, and risk level associated with disease severity in three distinct age groups: under 18, 19‐64, 65+. The mean incubation period of SARS‐CoV‐2 is 5‐6 days and can reach up to 24 days. Children tend to be asymptomatic, whereas older people are at higher risk of more severe disease, particularly those with identified co‐morbidities
The mean incubation period of SARS‐CoV‐2 is 5‐6 days but can reach up to 24 days making screening for infection rather difficult. 15 , 20 Since the majority of the population is susceptible to SARS‐CoV‐2 infection, exposure history is one of the most important risk factors. This may represent another reason why fewer children become infected compared to adults as young children are more likely to have regimented schedules and less likely to be at social gatherings. It is important to note that the risk of contracting SARS‐CoV‐2 is not equally associated with poor disease outcomes. Various factors, such as socio‐economic determinants, pre‐existing health conditions, and exposure levels play an important role in determining the extent of disease that exposed individuals may experience. 21 , 22 Other risk factors associated with poor disease outcomes include being >65 years of age, having hypertension and/or obesity (Figure 2). 15 , 23 Higher circulating levels of LDH, D‐dimer, C‐reactive protein, and IL‐6 are also correlated with severe SARS‐CoV‐2 disease. 23 , 24 SARS‐CoV‐2 patients that suffer pre‐existing concurrent cardiovascular or cerebrovascular diseases and hyperglycemia are associated with a higher risk of mortality from COVID‐19. 25
2.3. Mitigation measures
It has been a challenge to governments at all levels to minimize deaths and hospitalizations due to COVID‐19. 26 Various mitigation strategies have been undertaken in different countries at different times to prevent hospitals from being overwhelmed and reduce COVID‐19 morbidity and mortality while attempting to keep the economy ongoing. 26 Mitigation measures enforced by many countries encompass mask‐wearing and hand‐washing, physical distancing, cancelations of social and mass gathering events, partial/complete lock‐downs including border closures and travel restrictions, and/or testing/contact‐tracing/quarantine/isolation. 26 , 27
Since no vaccine exists, China announced risk mitigation measures in Wuhan including quarantine requirements, city lockdown, and isolating infected populations which controlled the spread of the virus 27 (Figure 1). Hong Kong, Taiwan, South Korea, and Mongolia implemented even more stringent risk mitigation measures such as travel restrictions, isolation of travelers from Wuhan, closure of schools and hygienic measures. 27 Soon after, two regions in Italy were severely impacted by the virus as there was a huge surge in cases forcing the entire country to enter lockdown (Figure 1). 28 Other European countries also experienced an increase in COVID‐19 cases shortly after and implemented similar mitigation strategies to control the spread of the virus. 27
Around the globe, each country has adopted various measures in response to COVID‐19, to slow down transmission and prevent oversaturation of health‐care systems. These measures in some cases have drastically varied from country to country, from travel restrictions to social distancing, and complete lockdowns. In early stages of the pandemic there was quite some confusion, particularly in western countries, about the protective effects of facial masks and physical distancing until the established evidence surfaced. 29 , 30 To this point, several restrictions levied have proven effective in slowing down the spread of COVID‐19, but the greatest effect is obtained by applying a combination of measures. 27 , 31 Interestingly, during the 1918‐19 Spanish Flu pandemic similar travel restrictions and quarantine measures were implemented in many countries. It is estimated that implementation of these measures during the 1918‐19 pandemic resulted in a reduction of death rates by 50%. 32 A study comparing the hospitalization rates in two Canadian provinces during the first wave of COVID‐19 has revealed that the curve of hospitalization rates in the province of British Columbia, but not of Ontario, was flattened with the implementation of social distancing and limitations on social gatherings. 33 At the present time, while the true efficacy of these mitigation measures in controlling the current COVID‐19 pandemic remain to be fully established, mathematical modeling provides insight into what is expected to occur or what may have transpired. 34 , 35 , 36 Mathematical modeling of COVID‐19 transmission in Wuhan showed that the implementation of quarantine measures resulted in the R0 value decreasing from 2.65 to 1.98. This study also predicted that implementing lockdown 7 days earlier would have resulted in a 72% decrease of infected individuals. 37 Overall, it is apparent that the extent of COVID‐19 control has been closely associated with the stringency of mitigation measures undertaken in various regions of the world (Figure 1). For instance, while many Asian and European countries as well as Canada had successfully flattened the curve of the first wave and lifted, to varying extents, the initially imposed mitigation measures to revive the economy until the second wave struck, countries such as the United States lack a national mitigation strategy and have never flattened the first wave, now meeting with huge daily cases and deaths with an over‐taxation of health‐care systems 38 (Figure 1).
Nonetheless, while mitigation measures are necessary for the control of COVID‐19, they bear serious economic consequences. 39 , 40 , 41 Goldman Sachs predicted that the United States gross domestic product would shrink by 5% in the second quarter of 2020 largely due to the pandemic, loss of productivity, and the implementation of control measures. 42 Along with the economic impact, there has been a sentiment of resistance to government imposed COVID‐19 restrictions, quite prominently seen in the United States, where people have organized anti‐mask and anti‐quarantine rallies. 43 This sentiment has spread to other countries as well, with rallies being organized in many cities such as the Canadian cities of Toronto and Montreal. 44 , 45 The balance between effective mitigation measures to control the spread and protecting national economies is extremely complex. In fact, modeling and investigation into hospital admission rates associated with mitigation methods, varying lockdown scenarios, times, and duration, indicate that lockdown measures outperform less stringent restrictions in reducing cumulative deaths and pressure on health‐care system. 46 , 47 , 48 It was also projected that lockdown at the early stage of transmission would have saved more lives. The short‐term lockdowns, so‐called circuit breaker intervention in the United Kingdom were found to have the biggest impact when the infection rate is low. 48 Imposing mitigation methods at the national or regional levels thus needs to be carefully chosen considering the transmission rate and health‐care infrastructure, but the benefit of such measures needs to be weighed against the socioeconomic impacts.
2.4. Clinical characteristics
A wide range of clinical manifestations are seen in SARS‐CoV‐2 patients, ranging from mild/moderate to severe, rapidly progressive, and fulminant disease. Symptoms of SARS‐CoV‐2 are non‐specific and disease presentation can range from asymptomatic to severe pneumonia. Incidence of asymptomatic SARS‐CoV‐2 cases ranges from 1.6% to 51.7% and these people do not present typical clinical symptoms or signs and do not present apparent abnormalities in lung computed tomography (CT). 49 , 50 , 51 , 52 , 53 , 54 The most common symptoms of COVID‐19 are fever, cough, myalgia, or fatigue, which are similar to those of SARS and MERS; atypical symptoms include sputum, headache, hemoptysis, vomiting, and diarrhea. 55 , 56 Some patients may present with sore throat, rhinorrhea, headache, and confusion a few days before the onset of fever, indicating that fever is a critical symptom, but not the initial manifestation of infection. 56 Furthermore, some patients experience loss of smell (hyposmia) or taste (hypogeusia), which are now being considered early warning signs and indications for self‐isolation. 57 , 58 Diagnosed patients may also present with lymphopenia, thrombocytopenia, and leukopenia. 55 , 59 , 60 Most of the patients had elevated levels of C‐reactive protein; less common were elevated levels of alanine aminotransferase, aspartate aminotransferase, creatine kinase, and D‐dimer. 55
The clinical course of SARS‐CoV‐2‐induced pneumonia displays a broad spectrum of severity and progression patterns. Around 6.5%‐31.7% of hospitalized patients required admission to the ICU and roughly 29% of these patients developed acute respiratory distress syndrome (ARDS) 8 days following symptom onset. 21 , 56 , 61 Of the hospitalized patients, a small percentage ranging from 2% to 20% required invasive mechanical ventilation 62 which indicates poor course of disease and can lead to progression of multiple organ dysfunction (MODS) and mortality. 21 , 56 The most common comorbidities were hypertension, obesity, and diabetes, in which severe illness and death were more prevalent among older patients (Figure 2). 21 , 63 The clinical characteristics presented in these cases represent the more severe end of confirmed SARS‐CoV‐2 cases with respiratory distress and pneumonia and are not the same as mild or asymptomatic cases.
Furthermore, the persistence of symptoms in recovered patients is an emerging and pressing issue. A study of 143 recovered patients found that 87.4% of them were still experiencing at least one symptom of COVID‐19 up to a month following discharge. The most common symptoms among these patients were fatigue 53.1%, dyspnea 43.4%, joint pain 27.3%, and chest pain 21.7%. 64 Recovered patients with persistent symptoms even months after the initial infection have been described as “long‐haulers” or “long COVID,” and due to the lack of comprehensive and large scale studies the incidence rate of these long‐haulers ranging from 10% to 35% remains to be fully established. 65 , 66 , 67 , 68 , 69 , 70 While the underlying mechanisms for these long‐term effects still remain incompletely understood, persistent lung injuries have been seen in COVID‐19‐recovered patients (detailed in Section 4 below). Some of the long‐term symptoms may also be related to invasive treatments used and lasting pathologies at tissue sites other than the lung 65 , 69 and mitochondrial pathways. 71 As our knowledge in long COVID‐19 cases continues to emerge, it is likely that the care of such patients entails a multidisciplinary approach.
Timely and accurate diagnostic SARS‐CoV‐2 testing is a crucial step in managing the pandemic. Currently, diagnostic testing for COVID‐19 is undertaken via a two‐pronged approach: direct detection of the viral RNA or immune‐based tests to detect viral antigens or antibodies. 72 The most common test detects viral RNA following reverse transcription and DNA amplification by PCR accompanied with real‐time results (rRT‐PCR). 72 The genes S, N, and E are used as targets in the rRT‐PCR assay in combination with the open reading frame 1 (ORF1) and the RNA‐dependent RNA polymerase. 73 There are now many rapid antigen detection tests available for SARS‐CoV‐2 detection. Both WHO and FDA have granted emergency use listings and authorization, respectively, for rapid antigen tests. WHO has listed two and FDA seven rapid antigen tests. 74 , 75 Antibody testing can provide a complementary role along with RT‐PCR in the diagnosis of COVID‐19, and these tests also allow for the characterization of individual humoral immune responses to current and previous infections. Hundreds of these tests exist, targeting IgG, IgA, and IgM responses, and are currently being used in clinical settings. 72 While rRT‐PCR is performed on nasopharyngeal and throat swabs, antigen‐antibody based assays use blood or serum samples. Thus, antigen‐antibody tests are suggested to be used as a complementary diagnostic tool for rRT‐PCR.
2.5. Treatment
At present, active symptomatic support remains the key treatment for mildly to moderately ill patients, such as maintaining hydration, nutrition, and controlling fever and cough. For patients with severe infection or those critically ill with respiratory failure, hospitalization is required and oxygen inhalation through a mask, high nasal oxygen flow inhalation, non‐invasive ventilation, or mechanical ventilation is indicated. 76 , 77 Extracorporeal membrane oxygenation (ECMO) may be implemented if all the above methods do not work. 78 Additionally, antibiotics and antifungals may also be required. A study demonstrated the potential benefits accruing from low‐dose corticosteroid treatment in a subset of critically ill patients with SARS‐CoV‐2 infection. 79 Many potential approaches have been suggested based on the progress of SARS‐CoV‐2 research, including inhibition of SARS‐CoV‐2 fusion/entry, disruption of SARS‐CoV‐2 replication, suppression of excessive inflammatory responses, convalescent plasma (CP) treatment, and the use of vaccines. 80 , 81 , 82
Arbidol and chloroquine phosphate have been added to the list of potential treatment options for COVID‐19. Arbidol was shown to prevent multiple enveloped viruses by inhibiting virus entry/fusion of viral membranes with cellular membranes. 83 Chloroquine, a traditional antimalarial drug, was shown to be effective against SARS‐CoV‐2 infection in vitro and was more effective in inhibiting exacerbation of pneumonia than control treatment. 84 Although the specific mechanisms of hydroxychloroquine and chloroquine phosphate in the treatment of SARS‐CoV‐2 infection remain unclear, it is likely to increase the pH of endosomes, which are required for viral cell entry, and impair the glycosylation of ACE2. 85 A recent large‐scale trial study of 1561 hospitalized patients treated with hydroxychloroquine and 3155 hospitalized patients receiving usual care found that those receiving hydroxychloroquine did not exhibit a lower death incidence‐rate 28 days following treatment. 85 A third update published on December 1 of a previously published living systematic review that focused on treatment of COVID‐19 with hydroxychloroquine has concluded that it is becoming increasingly unlikely that in‐hospital use of hydroxychloroquine will yield beneficial effects. 86 However, it reports that the outpatient use of hydroxychloroquine is promising. 86
Many antiviral agents have been developed against viral proteases, polymerases, MTases, and entry proteins. Remdesivir (GS‐5734) is a mono‐phosphoramidate prodrug of an adenosine analog, which can incorporate nascent viral RNA and inhibit the RNA‐dependent RNA polymerase. 87 Remdesivir effectively inhibited SARS‐CoV‐2 infection in vitro, and improved the first confirmed case of COVID‐19 in the United States without any noticeable adverse effects. 87 , 88 Recent findings from a double‐blind, randomized, placebo control trial of 1062 patients found that those treated with Remdesivir had shortened recovery times compared to those given the placebo. Patients given Remdesivir also exhibited a lower incidence of lower respiratory tract infection and on May 1st 2020 the FDA authorized emergency approval for usage of Remdesivir against COVID‐19 (Figure 1). 89 On November 20th, however, as part of a living guideline on clinical care for COVID‐19, the WHO announced a conditional recommendation against the use of Remdesivir in hospitalized patients, based on a comprehensive analysis of all available data. 90 With the global spread of SARS‐CoV‐2, vaccination is the most efficient and cost‐effective means to prevent and control COVID‐19. 91 In order to better understand COVID‐19 and develop safe, effective vaccination strategies, it is of utmost importance to increase our knowledge in the natural immunological responses to SARS‐CoV‐2.
3. IMMUNOLOGICAL RESPONSES
3.1. Origin and genomics of SARS‐CoV‐2
SARS‐CoV‐2 is a beta‐coronavirus in the Coronaviridae family and the order of Nidovirales. Coronaviruses have the largest genome of all RNA viruses and typically contain six open reading frames (ORFs). 92 SARS‐CoV‐2 has a unique N‐terminal fragment with a spike protein and the genes occur in a 5′ to 3′ order with key structural proteins encoded in ORFs 10 and 11, at the 3′ terminus end. 92 SARS‐CoV‐2 shares 79% and 50% genome sequence identity with SARS‐CoV and MERS‐CoV, respectively. 93 SARS‐CoV‐2 shows higher sequence identity with other bat coronaviruses, including RaTG13, bat‐SL‐CoVZC45, and bat‐SL‐CoVZXC21. 93 Interestingly, the virus also has a high genome sequence identity with pangolin coronavirus, especially in the receptor‐binding domain (RBD), where SARS‐CoV‐2 shows some differences from RaTG13. 93 , 94 This sequence identity at the RBD is a key factor for some groups postulating a recombination event that may have occurred in pangolins, or other animal species as an intermediate species, before jumping to humans. 94 Information surrounding the genome has allowed for the characterization of the SARS‐CoV‐2 structure and has revealed mechanisms of cell entry and pathogenesis of the virus. It has also allowed rapid development of COVID‐19 diagnostics.
Since the emergence of the pandemic, surfacing of SARS‐CoV‐2 mutants has been a concern. As early as February 2020, a substitution in the spike protein of SARS‐CoV‐2 was detected and named D614G variant. 95 By June of 2020, this variant had become prominent globally and was suggested to exhibit increased infectivity with comparable disease severity to wild‐type strain D614. 95 More recently, another variant of a greater global concern has surfaced in United Kingdom, recorded on September 20th and sequenced in early October and named as B.1.1.7. (Figure 1), encompassing 17 mutations, 8 of which are in the spike protein. One of these eight mutations, N501Y, has also been found on another variant of the virus isolated in South Africa. 96 While much more remains to be discovered about these new variants, the B.1.1.7. variant is 0.4 to 0.7 more transmissible than its parental SARS‐CoV‐2 strains. At the time of writing, the emerging evidence suggests that some of these recent variants can evade both natural and vaccine‐induced humoral immunity in testing tubes while it remains to be fully established whether and to which extent they may lead to reduced vaccine efficacy. 97
3.2. Structure of SARS‐CoV‐2
SARS‐CoV‐2 is a spherical, enveloped, positive sense single‐stranded RNA virus that consists of four main structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). 92 The E protein is expressed during replication and although its specific role for SARS‐CoV‐2 is not clear, recombinant viruses lacking the E protein have exhibited reduced viral titers and abrogated viral maturation, suggesting that this protein is important for viral replication and maturation. 98 The M glycoprotein is the most abundant structural protein, spanning the membrane bilayer three times and leaving a NH2‐terminal domain outside the virus as well as a long COOH‐terminus inside the virion. 92 The N protein is important for RNA packaging and viral release following infection of host cells. 99 The S protein is of great importance as it mediates the cell entry and initiation of pathogenesis. S is a type I membrane trimer glycoprotein with an S1 domain comprising the RBD, which is required for receptor‐binding and an S2 domains responsible for cell membrane fusion. 100 S is further cleaved by host proteases including furin and TMPRSS2, at the S2′ site activating proteins necessary for membrane fusion. 101 Overall, there seems to be key similarities in regard to the S protein and RBD between SARS‐CoV‐2 and SARS‐CoV suggesting similar mechanisms of viral cell entry.
3.3. Viral cell entry and life cycle
Current evidence indicates that SARS‐CoV‐2 utilizes S to recognize the hACE2 receptor to facilitate viral entry into host cells. Importantly, it has been shown that after engaging the cell membrane, either viral RNA enters the cytosol or the ACE2/SARS‐CoV‐2 complex is endocytosed with the entire virus and the viral membrane fuses with the luminal side of the endosome allowing for viral RNA transfer to the cytosol. 102 Various groups have demonstrated that SARS‐CoV‐2 is able to infect cell lines expressing ACE2 more effectively than those lacking ACE2 expression. 103 , 104 Another protein that plays a role in SARS‐CoV‐2 entry is the cellular serine protease, TMPRSS2, which primes the S protein and is essential for viral spread and pathogenesis. 104 Recent cell culture work has provided evidence that heparan sulfate is a necessary co‐factor for SARS‐CoV‐2 infection and that it interacts directly with the RBD changing it to an open confirmation to facilitate binding with ACE2. 105 ACE2 is present in various tissues including the lung, heart, kidney, brain, and testes, which speaks to the ability of SARS‐CoV‐2 to cause pathology at these anatomical sites in some patients. ACE2 expression was recently analyzed via sc‐RNA‐seq comparative data between humans, non‐human primates, and mouse models showing that ACE2 and TMPRSS2 are co‐expressed in lung type II pneumocytes, gut enterocytes, and nasal goblet cells. 106
Once the S protein binds the ACE2 receptor, it undergoes conformational changes which stimulate viral envelope fusion with the cell membrane. SARS‐CoV‐2 then releases viral RNA into the host cell and begins the process of replication. 107 Viral genomic RNA first becomes translated into pp1a and pp1b, which are key viral replicase polyproteins, and these polyproteins are then cleaved into small products by viral proteinases. Through discontinuous transcription via polymerase activity a series of subgenomic mRNAs are produced and ultimately translated into key viral proteins. Subsequently, in the host endoplasmic reticulum and Golgi, viral protein, and genomic RNA are assembled into virions and transported out of the cell in vesicles and released into the cytoplasm. 107
3.4. Innate immune response
Some of the key innate immune cell types involved in the innate immune response to SARS‐CoV‐2 are alveolar macrophages (AMs), neutrophils, monocytes, and dendritic cells (DCs). 108 Mounting evidence suggests that SARS‐CoV‐2 can suppress the innate immune activation in early stages of infection (Figure 3). AMs are the most abundant cell type located in the airways and are the first line of defense against most pathogens invading the respiratory mucosa. 109 Detection of damage‐associated molecular patterns (DAMPS) and pathogen‐associated molecular pattern (PAMPs) by AMs leads to the induction of an inflammatory cascade vital for viral control but may also contribute to tissue injury. 109 Detection of the virus is an important step required for the activation of an immune response. Toll‐like receptors (TLRs) and RIG‐I‐like receptors (RLRs) are the two main classes of receptors that recognize viral PAMPs. 110 TLRs 3,7, and 8 located on the endosome of host cells are capable of recognizing the single‐stranded RNA genome of SARS‐CoV‐2, activating TRIF or MyD88, leading to downstream upregulation of the NF‐κB pathway and inflammatory genes pertinent to the immune response. 110 RLRs are cytosolic RNA receptors that primarily recognize nucleic acids of RNA viruses, leading to a similar downstream pathway feeding into the activation of NF‐κB and IFN production. 111 Following initial recognition and upregulation of interferon‐stimulated genes (ISGs), they are able to act in an autocrine and paracrine manner to stimulate IFN signaling through the JAK‐STAT pathway. 110 Type I and III IFN production by AMs signal for intracellular antiviral defense in proximate epithelial cells and secretion of IL‐6 and IL‐1β prompts the recruitment of neutrophils and cytotoxic T cells to the site of infection. 112 Chronic exposure to type I or III IFNs can lead to various interferonopathies. Recent work in various mouse models has suggested that these IFNs disrupt tissue repair by reducing the epithelial cell proliferation and may be an important consideration for immunotherapies in the treatment and management of severe COVID‐19 disease. 113 , 114
FIGURE 3.
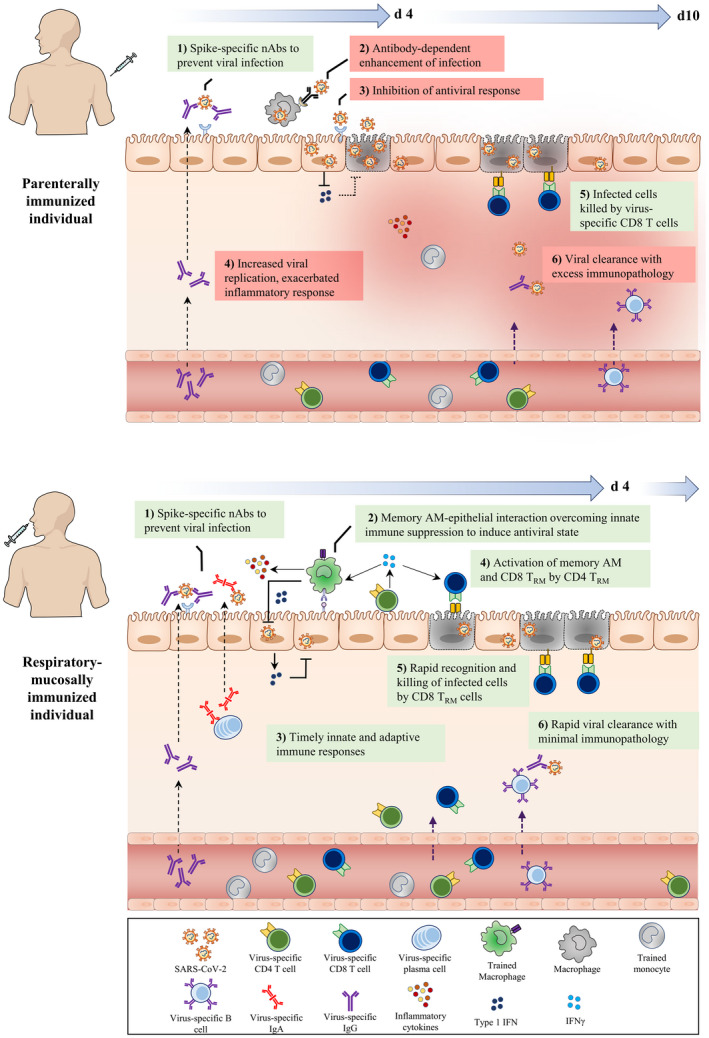
Deduced early immunological events at respiratory mucosa of parenterally or mucosally vaccinated hosts upon SARS‐CoV‐2 exposure. Following the parenteral route of immunization (top panel), circulating monocytes undergo systemic innate immune training through the priming of hematopietic monocyte progenitors in the bone marrow. Furthermore, SARS‐CoV‐2‐specific neutralizing IgG antibodies and T cells are produced and present in the circulation. However, often only the IgG antibodies are transported to the respiratory mucosal surfaces, whereas T cells and trained monocytes remain trapped within the pulmonary vasculature, resulting in an incomplete establishment of respiratory mucosal immunity. Upon SARS‐CoV‐2 infection, although the neutralizing IgG Abs (nAbs) on the surface of the respiratory mucosa bind to the incoming viruses and block its interaction with ACE2 receptor, it can be insufficient due to suboptimal levels of nAbs and weakly nAbs. However, in some hosts this mechanism may be adequate for protection. On the one hand, escaping virions may gain entry to alveolar macrophages (AM) via Ab and FcγR interaction and on the other hand, the virus infects airway epithelial cells, suppresses innate immune responses by inhibiting antiviral pathways, and delays adaptive CD8+ T cell responses. During this critical time gap (d4‐d10), poorly controlled viral replication leads to viral dissemination, dysregulated inflammatory cytokine, and inflammatory monocyte responses, resulting in excessive tissue immunopathology. In comparison, the respiratory mucosal route of immunization (bottom panel), particularly with live attenuated or viral‐vectored vaccines amenable for respiratory mucosal (RM) immunization, induces a holistic RM immunity consisting of trained alveolar macrophages (AM), mucosal IgA and IgG Abs, and lung tissue‐resident memory T (TRM) cells. RM immunization also induces a level of systemic immune protection by inducing circulating virus‐specific Abs and T cells. Upon SARS‐CoV‐2 infection, the holistic mucosal immunity overcomes the initial virus‐mediated innate immune suppression and quickly clears viral infection within the first few days (d4) via a coordinated mucosal immune response by trained (memory) AM, neutralizing mucosal Abs and TRM. nAbs (IgA/IgG) block viral entry to the epithelial cells. In dealing with the viruses that escaped from neutralization, memory AM interact with epithelial cells to overcome viral‐imposed innate immune suppression, enhancing antiviral state to inhibit viral replication. Moreover, CD4+ TRM further activate memory AM and CD8+ TRM which kill infected epithelial cells and probably infected AM. Together, such concerted immune responses lead to not only a timely control of viral infection but also prevention of excessive immunopathology and pneumonia
Monocytes and macrophages express ACE2 and macrophages also express furin and TMPRSS2 which play a role in SARS‐CoV‐2 cell entry and pathogenesis. 115 Recent ex vivo evidence has suggested that elevated glucose levels in diabetic individuals enhance viral replication and cytokine expression in monocytes. 116 This is deemed to be mediated by a change in the metabolic activity of the cell to favor glycolysis via the HIF‐1ɑ axis and co‐culture work has revealed that SARS‐CoV‐2‐infected monocytes can directly reduce the T cell response and inhibit epithelial cell survival, potentially contributing to immunopathology. 116 While previous studies have shown that human macrophages and DCs are susceptible to infection by SARS‐CoV, the virus was unable to productively replicate within these cells but triggered the production of pro‐inflammatory cytokines. 117 , 118 It remains unclear whether the same holds true for SARS‐CoV‐2. 115 Although monocytes and macrophages play an important role in the initial immune response, it has been suggested that overactivation or sustained pro‐inflammatory state in these cells may contribute to a dysregulated immune response leading to disease severity in COVID‐19 patients. 115
NK cells are able to induce lysis of virus‐infected cells which is vital to control viral infection and reduce tissue damage. 119 Peripheral blood from COVID‐19 patients has exhibited decreased NK cell counts which is associated with increased disease severity. 119 Ex vivo analysis of NK cells from the peripheral blood of COVID‐19 patients showed reduced intracellular expression of granzyme B, CD107a, IFN‐γ, TNF‐α, and other important markers of functionality. 120 Furthermore, NK cells and CD8+ T cells from COVID‐19‐infected patients have exhibited increased expression of the inhibitory receptor NKG2A, suggesting a decrease in functionality. 120 A recent study looking at the acute phase immune cell profile in PBMCs of COVID‐19 patients found that patients exhibited reduced frequencies of DCs, monocytes, NK cells, and T cells. 121 An impairment in the functionality and reduction in the frequency of DCs in the acute phase may delay T cell responses leading to heightened infection and worsened disease outcomes (Figure 3). 121 Overall, the mechanisms by which PRRs and innate immune cells act are vital for recognition of SARS‐CoV‐2 and induction of an innate immune response to infection.
3.5. Adaptive immune response
Due to innate immune suppression in early stages of SARS‐CoV‐2 infection, the activation of adaptive immune responses is impeded particularly in those who developed severe disease (Figure 3). Current reports on the T cell compartment of COVID‐19 patients suggests that moderate and severe disease patients exhibit lymphopenia with lower levels of CD4+ and CD8+ T cells. 122 Patients with mild disease outcome have reported increases in T cell numbers, but overall the mechanisms of lymphopenia in moderate to severe patients remains elusive. 119 The overreactive cytokine milieu seen in severe patients may have an effect on lymphopenia in the peripheral blood, given that lymphopenia seems to be correlated with serum levels of IL‐6, IL‐10, and TNF‐α. 119 , 123 A timely Th1 response induced by CD4+ T cells and direct killing of virally infected cells by CD8+ T cells is a key part of antiviral immunity (Figure 3). CD4+ T cells contribute to the induction of humoral immunity which is also important for antiviral immunity. 119 Zheng et al, have proposed that under conditions of severe COVID‐19 disease, CD8+ T cells primarily secrete IFN‐γ while virus‐specific CD4+ T cells secrete anticipated levels of Th1 cytokines IFN‐γ, TNF‐α, and IL‐2. Severe COVID‐19 patients also displayed a reduced proportion of multifunctional CD4+ T cells, which were positive for more than two of IFN‐γ, TNF‐α, and IL‐2, when compared to patients with mild or moderate disease. 120 Given these circumstances, it is evident that patients with severe COVID‐19 disease exhibit a dysregulated and insufficient T cell response. Furthermore, T cells from peripheral blood of COVID‐19 patients tend to express high levels of PD‐1 and Tim‐3, markers of exhausted T cells, and has been positively associated with disease severity. 123
However, a recent study examining T cell responses to S, M, and N proteins has suggested that robust T cell compartment responses are not associated with ameliorated disease outcomes, and in fact a robust T cell response in critical patients may contribute to hyperreactivity and immunopathogenesis. 124 This speaks further to the importance of the timing of both natural immune responses and vaccine‐induced immunity at the site of infection 125 (Figure 3). Another important consideration regarding the T cell compartment is pre‐existing cross‐reactive memory CD4+ T cells in humans unexposed to SARS‐CoV‐2. Approximately 20%‐50% of people possess pre‐existing cross‐reactive CD4+ T cells although the source of these cell subsets remains speculated. 126 A group mapped 142 T cell epitopes revealing that many of the CD4+ T cells reacting to SARS‐CoV‐2 epitopes also cross‐react with corresponding homologous sequences from circulating common coronaviruses. 126 This pre‐existing memory in some individuals may provide a potential explanation for the varying COVID‐19 disease outcomes in different populations. 126 Current knowledge regarding phenotype and functional alterations in the T cell compartment following SARS‐CoV‐2 infection is still limited and further investigation will provide critical information for vaccine design. 125
As seen in the T cell compartment, B cells in peripheral blood of patients with moderate/severe disease were markedly decreased and the number of B cells was negatively correlated with viral burden. 127 B cells in circulation are restored to normal levels in recovered COVID‐19 patients and the infusion of plasma from these convalescent patients has exhibited the potential for effective therapeutic treatment in severe COVID‐19 cases. 128 These results support that virus‐specific antibodies produced by the humoral immune response to SARS‐CoV‐2 are important for resolving the infection. In a study of 58 convalescent patients with mild disease, only 30% generated relatively low titers of neutralizing antibodies, suggesting an increased risk of reinfection. 129 The authors found that severity of disease correlated with the induction of a stronger immune response and those recovered from severe infection may be more likely to be protected against reinfection. 129 Patients have shown antibody seroconversion as early as day 7, with most seroconverting by day 14. 130 By day 7‐10, many patients display an increase in IgG and IgM specific for N and RBD which was followed by a gradual decrease in viral load, further supporting the protective effects of humoral immunity against the virus. 130 In some patients S‐specific IgA was detected as early as 6‐8 days following disease onset and was maintained over a 6‐week observational period. Overall the IgA response in this longitudinal study was more robust than IgM. 131
Various studies have been looking into the longevity of these anti‐SARS‐CoV‐2 antibodies in recovered patients. This is of great importance as considerations for the potential of developing herd immunity against SARS‐CoV‐2 greatly depends on the capacity of neutralizing antibodies to reduce transmission. 132 Furthermore, persistence of these antibodies can provide insight into the likelihood and extent of reinfection in convalescent individuals. A study conducted by Isho et al, examining S‐specific antibody responses in saliva and serum of convalescent patients found antigen‐specific IgG in both biofluids in most samples 16‐30 days post‐symptom onset and these levels did not drastically decline at 110‐115 days post‐symptom onset in most samples. 133 Although these results indicate antigen‐specific IgG may be long‐lasting following SARS‐CoV‐2 infection, other studies have found contradicting results. A study consisting of 343 North American convalescent COVID‐19 patients found that although RBD‐specific IgG titers did not decrease 75 days post‐symptom onset, RBD‐specific IgA and IgM titers significantly decreased 71 and 49 days post‐symptom onset. 134 A large‐scale community study of 365 000 adults in the United Kingdom found a significant decline in the proportion of the population with detectable antibodies over three‐rounds of testing 12, 18, and 24 weeks following the peak of COVID‐19 cases. 135 The results of this study suggest that antibody‐immunity against SARS‐CoV‐2 may be waning within the first 6‐12 months. However, it is important to note that as this large‐scale study was conducted via participants self‐pricking and reporting, the results remain to be verified via further investigation. A case study has reported re‐infection by a genetically different SARS‐CoV‐2 which led to more severe disease than the first, indicating that first exposure might not guarantee immunity in all cases. 136
In an infectious model study of rhesus macaques, two studies have shown that primary infection with SARS‐CoV‐2 provides protection against a subsequent re‐challenge. 137 , 138 More studies into the potential re‐infection, protective capacity and longevity of immunity are critical to furthering our understanding of the pathophysiology of this disease and natural immunity.
While generation of antiviral antibodies is generally thought of as a positive, there is some concern about antibodies worsening disease outcome via antibody‐dependent enhancement (ADE). 129 Antibody‐dependent enhancement is when antibodies interact with the immune system to promote pathology. The ADE phenomenon has been documented for dengue, SARS‐CoV, and other viruses. Neutralizing antibodies in the case of SARS‐CoV engage Fc receptors on immune cells, particularly macrophages, which may then promote inflammation and result in tissue injury due to overactivation of these cells. 139 Many important details have yet to be established regarding the role of humoral immunity following SARS‐CoV‐2 infection.
4. IMMUNOPATHOLOGICAL PROCESSES
4.1. Inflammatory cytokine overdrive
Dysregulation of the immune system has been identified as one of the major mechanisms for the worsening of disease outcome in severe COVID‐19 patients. The role of CD8+ T cells and NK cells in the antiviral response is of utmost importance in order to mediate effective killing of virally infected cells. 120 As previously mentioned, CTLs and NK cells isolated from the peripheral blood of severe COVID‐19 patients were significantly reduced and exhibited increased expression of PD‐1, Tim‐3, or NKG2A, suggesting functional exhaustion. Functional exhaustion of antiviral lymphocytes may contribute to worsened conditions and immunopathology following SARS‐CoV‐2 infection. 120 Furthermore, severe COVID‐19 patients exhibited increased levels of neutrophils compared to those with moderate/mild disease. 120 Although neutrophils are an important cell type in the innate immune response, they can exacerbate immunopathology. Unbalanced neutrophil extracellular trap (NET) production, increased production of pro‐inflammatory cytokines, and the release of reactive oxygen species by neutrophils can worsen inflammation in the lung tissue, further exacerbating the cytokine storm and contributing to the development of ARDS. 140
Timing of antiviral immune responses, particularly when viral load is low and patients are still in an asymptomatic/pre‐symptomatic stage is key 125 (Figure 3). Swift activation of type I and III IFN responses as well as timely non‐excessive production of key pro‐inflammatory cytokines allows the host an opportunity to reduce viral load and limit tissue injury. 125 However, SARS‐CoV‐2, just like MERS‐CoV and SARS‐CoV, is armed with mechanisms to dampen these key initial responses. This results in a dysregulation of the host's immune response, characterized by excessive infiltration of inflammatory immune cells and overproduction of pro‐inflammatory cytokines. 125 Dysregulation of the immune response paired with an increasing viral burden can lead to detrimental viral and immune‐mediated pathology (Figure 3).
Overproduction of pro‐inflammatory cytokines is a major contributor to the immunopathology seen in severe COVID‐19 patients. Elevated serum levels of cytokines including IL‐1β, IL‐2, IL‐7, IL‐8, IL‐9, IL‐10, IL‐17, G‐CSF, GM‐CSF, IFN‐γ, TNF‐ɑ, IP‐10, MCP‐1, MIP‐1ɑ, and MIP‐1β have been associated with the development of ARDS leading to pulmonary edema, lung failure and in some cases cardiac, hepatic, and renal injury. 56 , 122 , 141 Patients in the ICU have exhibited higher levels of IL‐2, IL‐7, IL‐10, G‐CSF, IP‐10, MCP‐1, MIP‐1ɑ, and TNF‐ɑ when compared to non‐ICU patients. 122 , 141 Both IL‐1β and TNF‐ɑ promote vascular leakage and permeability as well as Th17 responses. 141 Skewing of T cell responses toward Th17 was reported to worsen immunopathology and inflammation. Th17 cells produce IL‐17 which has broad pro‐inflammatory effects, G‐CSF contributing to granulopoiesis and neutrophil recruitment, and IL‐22 which may contribute to life‐threatening edema by upregulation of mucins and fibrinogens. 141 It is evident that the immune system may contribute to immunopathology through a variety of mechanisms. However, the details and timeline of these maladaptive interactions still remain to be unraveled.
4.2. Lung pathology
The lung tissue is the main site of immune and virus‐mediated pathology induced by SARS‐CoV‐2 infection. In the case of all three recent coronavirus epidemics/pandemics the primary lung pathology is diffuse alveolar damage which accounts for the ground glass opacity on chest x‐rays. 142 During the early/mild phase of disease, SARS‐CoV‐2 does not seem to result in the formation of hyaline membranes in the lung. However, by the later/more severe phase the extensive hyaline membrane formation and desquamation of pneumocytes occurs. Significant alveolar damage and progressive respiratory failure lead to the onset of severe disease and subsequent death. 142 , 143 Furthermore, there seems to also be persistent damage in the lungs of COVID‐19 recovered patients. A study noted that 6 weeks following hospital discharge 88% of the participants had some visible damage on their lung CT and by 12 weeks the incidence of visible damage reduced to 56%. 144 A comprehensive long‐term study looking at the long‐term effects of SARS‐CoV infection published earlier this year, found that 15 years after infection 4.6% of the participants still had visible lesions of the lung and 38% displayed reduced diffusion capacity. 145 Furthermore, a prospective observational 3‐month follow‐up study found that although lung high‐resolution computed tomography (HRCT) returned to normal in most of the patients, in 42% of them mild pulmonary abnormalities persisted and 50% suffered from symptoms such as dyspnea, cough, chest tightness, and palpitations on exertion 3 months after discharge. 146 Another study has reported that 35 out of 55 recovered participants exhibited different degrees of radiological abnormalities 3 months after discharge. 147 In some follow‐up patients, lung interstitial thickening and pure ground‐glass opacity were among the most common features on HRCT scans. 147 A study carried out in Austria evaluated long‐term lung damage in recovered patients at 1.5, 3, and 6 months following discharge from the hospital. 148 At the first visit, 56% of recovered patients experienced at least one persistent symptom, mainly breathlessness and coughing, and 88% of patients displayed lung damage on CT scans. 148 At 3‐month visit, while breathlessness had improved with lung damage rates reduced to 56%, coughing persisted in 13 patients. Results from 6‐month visit is still pending from this study. 148 Clearly, such persistent lung injuries in some of recovered patients may underpin some of the clinical symptoms experienced by the “long haulers” and call for increased scientific attention and continuing medical care even after the pandemic is behind us.
4.3. Cardiac pathology
It has become clear that SARS‐CoV‐2 or COVID‐19 has a detrimental effect on tissue organs including the cardiovascular system other than the respiratory system. COVID‐19‐associated cardiac complications include, but are not limited to, heart failure, myocarditis, pericarditis, vasculitis, and cardiac arrhythmias. 149 Around 8%‐28% of COVID‐19 patients exhibit a marked troponin release early in the onset of disease, reflecting cardiac stress, or injury. 149 Whether this injury is caused directly by the SARS‐CoV‐2 virus or a result of heightened immune responses to danger signals from the cardiac system remains to be understood. Since ACE2 and TMPRSS2 are widely expressed throughout the cardiac tissue and if the virus makes its way to the cardiac tissue, the virus could potentially infect and cause injury in the tissue. The initial release of troponin for many patients can foreshadow a poor prognosis, conferring a five times higher risk of requiring ventilation. 149 A few autopsy reports have found viral RNA and mild inflammation in cardiac tissue of deceased patients. 150 , 151 Further investigation is required to understand whether there are long‐term consequences of cardiac damage particularly in those who recovered from severe disease.
4.4. Pathology in renal, hepatic, reproductive, and neural tissues
ACE2 is expressed throughout the renal tissue, namely in the apical brush border and podocytes and SARS‐CoV‐2 is capable of infecting renal tubular epithelium and podocytes. Infection of these cells has been associated with acute kidney injury (AKI) and proteinuria. 152 The incidence of AKI in different COVID‐19 clusters ranges from 0.9% to 29%. The CD147 receptor is also highly expressed in renal tissue, likely playing a role in other renal diseases. 152 Although incidence levels of AKI are lower than other pathologies, renal injury in diabetic patients or others with pre‐existing conditions can result in worsened health outcomes associated with COVID‐19.
Likewise, hepatic endothelial cells also express ACE2 and cell culture work with SARS‐CoV demonstrated that SARS‐CoV‐specific protein 7a was capable of inducing apoptosis in liver cell lines through caspase‐dependent pathways. 153 Whether SARS‐CoV‐2 can induce apoptosis in vivo and via a similar mechanism is unclear at the moment. The incidence of liver injury ranged from 14.8% to 53% in COVID‐19 patients with higher incidence in more severe cases. However, it remains to be understood if this is due to virus/immune‐mediated pathology or due to drugs utilized in treatment against the virus. 153 Although specifics of this interaction are largely unreported at the time of writing, it is worth mentioning that ACE2 is also highly expressed in the bile duct tissue, higher levels than in hepatic tissue, and the bile duct plays a vital role in the immune response and liver regeneration. 153 More studies are needed in these areas.
Similar to renal and hepatic tissues, the reproductive tissue in both males and females has been shown to exhibit ACE2 expression. The adult reproductive tissues, spermatogonia, Leydig, and Sertoli cells, enriched for ACE2 receptor expression, are potential targets for SARS‐CoV‐2 infection. 154 While a few studies have reported on testes‐related clinical manifestations of COVID‐19, the data on gonadal tissue‐associated pathophysiology remains scarce. 155 , 156 , 157 Sex hormone imbalance in the serum has been reported in severe COVID‐19 patients. 158 Notably, SARS convalescent male patients exhibited orchitis as a complication of infection along with decreased spermatogenesis. 159 Another growing concern is about the possibility of sexual transmission of COVID‐19. While the data regarding whether SARS‐CoV‐2 is present in semen of infected or recovering men remains conflicting, most of the studies suggest that it is highly unlikely that the virus is sexually transmitted. 160 Indeed, three studies that examined the sexual transmission of COVID‐19 in women by examining lower genital tract via vaginal swab for the presence of SARS‐CoV‐2 at the time of admission or during the period of hospitalization have reported negative results. 161 , 162 , 163 Similar results were reported with vaginal swabs and breast milk from pregnant women. 164 However, like the male reproductive tissue, ACE2 receptor is expressed in the placenta, ovaries, uterus and vagina, raising a concern about vertical transmission of the virus. 165 , 166 A systematic review of 38 cohort studies found an infection rate of 3.25% neonates from COVID‐19‐infected pregnant women. 167
In comparison, there have been many reports of COVID‐19 patients presenting with neurological abnormalities. ACE2 is primarily expressed in neurons and glial cells of the brain. 168 Similar to SARS‐CoV and MERS‐CoV, SARS‐CoV‐2 may also take a direct transsynaptic route via the olfactory bulb following inhalation without the use of ACE2, which may be one of the reasons for loss of smell. Following invasion into neural tissue, the virus can cause reactive astrogliosis and activation of microglia and concurrently, systemic inflammation can compromise the blood‐brain barrier leading to a disturbance in homeostasis and neuronal cell death. 168 Moreover, some case studies suggest the possibility of direct infection of the CNS tissue based on the presence of SARS‐CoV‐2 RNA in the cerebrospinal fluid. 169 Furthermore, systemic hyperinflammatory responses in severely ill patients could also contribute to some of the neurological manifestations. 170 These events can ultimately lead to the development of acute encephalitis, infectious toxic encephalopathy, or acute cerebrovascular attacks. Thus, as seen in the lung it is likely that if some of the neurological pathologies persist in COVID‐19‐recovered patients, they could be the basis of lingering neurological symptoms such as the “brain fog” experienced by some of these patients. Continuing investigation will be critical to developing clinical management strategies for treating COVID‐19 patients with neurological symptoms in stages of disease and recovery.
5. VACCINE DEVELOPMENT
5.1. Lessons learned from SARS and MERS vaccines
Vaccine candidates attempted for SARS and MERS included RNA‐, DNA‐, recombinant protein‐, viral vector‐, live attenuated virus‐, and inactivated virus‐based platforms which are also currently being used for developing COVID‐19 vaccines. 171 , 172 The development of vaccines for SARS and MERS did not go beyond phase 1 clinical trials due to quickly diminished demand. 171 , 173 , 174 Mice vaccinated with a SARS inactivated viral vaccine produced high amounts of neutralizing antibodies (NAb) against S, N, and M proteins. 175 , 176 In a phase 1 clinical trial, subjects given two doses of an inactivated SARS vaccine developed SARS‐CoV‐specific NAb and the vaccine was well‐tolerated. 173 Mass production of inactivated viral vaccines requires large amounts of virus to be properly inactivated to ascertain safety. 171 , 177
A study examined four candidate SARS vaccines including a virus‐like particle‐, two whole virus‐, and a recombinant DNA S protein‐based vaccines, with or without alum, in animal models. 178 While all four vaccines‐induced NAb and provided protection against SARS‐CoV infection, they also elicited Th2 immunopathology. 178 Recombinant adenovirus (Ad) vector is popular for its safety, well‐characterized genome, and suitability for mucosal administration. 177 Ad‐vectored SARS vaccine delivered via the intramuscular route‐induced T cell responses against the N protein and NAb against the S1 in rhesus macaques and mice. 179 , 180 Although the intramuscular route produced higher levels of NAb in sera, the intranasal route‐induced SARS‐specific IgA antibodies and significantly reduced SARS‐CoV replication in the lungs, suggesting the benefit of mucosal immunity in protection against SARS‐CoV infection. 181 Although recombinant viral‐vectored vaccines can induce humoral and cellular immunity, their potency following the first or repeated immunization can be affected by pre‐existing immunity against the viral backbone 125 and their global production capacity may be limited. 171
Both recombinant DNA‐ and RNA‐based vaccines do not involve infectious viruses; their safety and simplicity makes them an attractive alternative to live vaccines. 171 , 177 A DNA vaccine expressing the S protein was shown to induce T cells and NAb and protective immunity in mice. 182 Similar results were seen in a phase 1 clinical trial where three doses of a plasmid DNA vaccine encoding S protein‐induced NAb and CD4+ T cell responses. 174 However, while most DNA vaccines showed promising results in preclinical models, few made their way into clinical trials due to their weak immunogenicity and requirement for more than two repeated injections. 177
A few studies assessed immunogenicity and protective efficacy of a live attenuated recombinant SARS‐CoV lacking the E gene in mice and hamsters. 183 , 184 , 185 This live attenuated SARS viral vaccine was shown to have reduced ability to replicate in experimental animals. 184 , 185 Hamsters immunized with this vaccine produced high levels of NAb and were protected from SARS‐CoV infection in the upper and lower respiratory tracts. 183 These studies suggest that certain viral structural proteins such as E may be depleted to develop safe and live attenuated SARS‐CoV‐2 vaccines.
5.2. Current vaccine candidates and strategies for COVID‐19
Since COVID‐19 is new to mankind, it is imperative to develop different vaccine platforms and strategies in parallel. Currently, there have been more than 200 vaccine candidates in pre‐clinical and clinical development around the world. 3 , 125 Recently, there have been very encouraging results from ongoing phase III COVID‐19 vaccine trials, indicating high protective efficacies by two mRNA vaccines (Moderna & Pfizer) and one chimpanzee adenoviral‐vectored vaccine (AstraZeneca) and leading to their emergency use authorizations in a number of countries (Figure 1). At the time of writing, these front‐runner vaccines are rolling out and administered to prioritized human populations in these countries while their long‐term safety and efficacy data still remain to be obtained. However, as predicted, 125 due to limited supplies, and different economic status and geopolitical policies in various regions and countries, the type of vaccines, vaccine distribution, and vaccination roll‐out time have been highly uneven and heterogenous. This situation may significant delay the effective global control of the pandemic. Furthermore, with recent emergence of highly transmissible virus variants (Figure 1), the question remains whether the currently approved first‐generation vaccines will remain as efficacious.
The six main COVID‐19 vaccine platforms include live attenuated virus, recombinant viral‐vectored vaccines, inactivated or killed virus, protein subunit vaccines, virus‐like particles, and nucleic‐based (DNA or mRNA) vaccines. The main immunological property of each vaccine platform is thoroughly discussed in a separate review and will not be discussed in detail. 125 Vaccine development against most acute viral infections focuses on mimicking the immune response elicited during natural infection. 186 Due to a high degree of similarities in the genome, structural proteins and surface receptors between SARS‐CoV and SARS‐CoV‐2 the immune responses should be comparable and can, therefore, aid in finding immunogenic determinants for vaccine development. 172
Studies have demonstrated that during SARS‐CoV‐2 infection, CD4++ and CD8++ T cell responses were not only directed to the S protein but also to M and N proteins. 186 This indicates that CD4++ and CD8++ T cells have many potential targets and the next generation of COVID‐19 vaccine candidates should include additional structural antigens to provide a broader immune protective response. 125 This consideration is of particular relevance to the possibility of diminished efficacy of current vaccines against new SARS‐CoV‐2 variants. Considerable apprehension also exists regarding potential candidate vaccines causing ADE or Th2 immunopathology. 187 In previous SARS vaccine studies, young and aged mice exhibited Th2 immunopathology, with predominant eosinophil infiltration, and were not protected against rechallenge. 178 , 187 Animal studies and clinical trials of candidate vaccines for COVID‐19 must be properly assessed for potential immune complications.
Several companies have been developing vaccines that express the SARS‐CoV‐2 S protein and aim to induce both antibody‐mediated humoral and T cell responses. 188 All platforms have unique advantages and disadvantages making it tough to predict which strategy will ultimately be most successful in terms of long‐term safety and protective efficacy although the current information favors the mRNA vaccine platform. Immunogenicity and safety of a DNA vaccine targeting the SARS‐CoV‐2 S protein was tested in different animal models and produced robust T cell responses and neutralizing antibodies blocking the binding of S to ACE2. 189 , 190 The DNA vaccine provided optimal protection with expression of the full‐length S immunogen and significantly reduced viral load in upper and lower respiratory tracts of non‐human primates. 190 Inovio Pharmaceuticals recently started a phase 1 clinical trial to evaluate the DNA vaccine, INO‐4800 via two intradermal injections. Inovio announced positive interim data regarding the INO‐4800 vaccine and was deemed safe and well‐tolerated in participants, a phase 2/3 efficacy study is underway. 191 A purified inactivated virus vaccine candidate, PiCoVacc, protected non‐human primates against SARS‐CoV‐2 infection and no ADE was observed. The safety of this vaccine was evaluated; no clinical signs of illness were observed; no notable changes in pro‐inflammatory cytokines and no pathology. 192 Sinovac Research and Development Co. started phase 1/2 clinical trials testing the candidate vaccine CoronaVac, an inactivated vaccine, in April 2020 and preliminary results of the COVID‐19 vaccine showed good safety and immunogenicity. 193 The phase 3 trial has recently commenced to test the efficacy and safety of CoronaVac.
A few dozen vaccine candidates have moved or will soon progress into clinical development to evaluate safety, immunogenicity, and efficacy in humans. 125 Meissa Vaccines has started phase 1 clinical trials to evaluate safety and immunogenicity of intranasal delivery of a live attenuated respiratory syncytial virus (RSV) vaccine in healthy adults. 194 The MV‐014‐210 vaccine expresses the SARS‐CoV‐2 S protein and is adjuvant free. 194 Many countries in Europe, Australia, and the United States have also begun clinical trials to evaluate the efficacy of intradermal BCG vaccination in health care workers and the elderly. 195 CanSino Biologics developed a recombinant adenovirus type‐5 (Ad5) vectored coronavirus vaccine, expressing the SARS‐CoV‐2 S protein, via intramuscular injection. The Ad5‐vectored COVID‐19 vaccine is tolerable and immunogenic and induces humoral responses (28 days post‐vaccination) as well as T cell responses (14 days post‐vaccination) in healthy adults. 196 In the phase 2 trial, Ad5‐vectored COVID‐19 vaccine was safe and induced significant immune responses following a single immunization. 197 Now that a vaccine dose has been determined, ongoing phase 3 trials will evaluate the efficacy of the vaccine. 197 It is important to note that pre‐existing Ad5 immunity may slow down rapid immune responses and negatively affect the quality of protective immunity.
AstraZeneca/University of Oxford developed a chimpanzee adenovirus (ChAdOx1)‐vectored vaccine, encoding the full‐length S protein, referred to as AZD1222. The ChAdOx1 nCoV‐19 vaccine‐induced humoral and cellular immune responses and reduced viral load in the respiratory tract of vaccinated animals. 198 The immune response was not Th2 dominated and no signs of disease enhancement were observed in lungs of non‐human primates. 198 Preliminary data from a phase 1/2 trial revealed that ChAdOx1 nCoV‐19 is safe and homologous boosting increased antibody responses and induced cellular immune responses. 199 Following two repeated intramuscular injections, the vaccine is recently announced to be 70%‐90% effective based on its ongoing phase III trial (Figure 1). Moderna started clinical testing of intramuscular injection of mRNA‐1273, which is a novel lipid nanoparticle encapsulated mRNA‐based vaccine encoding full‐length S. 200 The study evaluated safety and reactogenicity of mRNA‐1273 and immunogenicity measured by IgG levels to the SARS‐CoV‐2 S protein. Moderna reported positive interim clinical data of the mRNA‐1273 vaccine from the phase 1 trial. 200 The vaccine‐induced seroconversion by day 15 and elicited neutralizing antibodies in all eight participants. In addition, the vaccine was safe, well‐tolerated, and elicited an immune response of the same magnitude of natural infection. The encouraging results supported its phase 3 trial beginning in July 2020. In November 2020, Pfizer/BioNTech announced their mRNA‐based COVID‐19 vaccine candidate, BNT162b2, met all of the study's primary efficacy endpoints, with a efficacy rate of 95%. 201 Shortly after, Moderna announced their mRNA‐1273 vaccine has met statistical criteria with a 94.5% vaccine efficacy (Figure 1). 202 At the time of writing this review, while the Pfizer/BioNTech vaccine has been authorized for emergency use in the United Kingdom, Bahrain, Canada, United States, Mexico, and European Union, 203 , 204 , 205 United States and Canada have also approved the Moderna vaccine for emergency use. 206 , 207 Furthermore, as of December 30th, 2020 the United Kingdom became the first country to provide emergency authorization for the use of the AstraZeneca ChAdOx1 vaccine 208 (Figure 1 ). Novavax has developed a recombinant SARS‐CoV‐2 S subunit vaccine (NVX‐CoV2373) that is constructed from full‐length S. In August 2020, the company announced positive data for the phase 1 portion of the phase 1/2 trial indicating that the NVX‐CoV2373 vaccine was well‐tolerated and safe. 209 It also induced strong neutralizing titers in all participants and the Matrix‐M adjuvant‐induced robust CD4++ T cell responses. 209 In September 2020, the company announced it has initiated its first phase 3 trial in the United Kingdom to evaluate the efficacy, safety, and immunogenicity of NVX‐CoV2373. In addition to Pfizer/BioNTech and Moderna mRNA vaccines, an inactivated vaccine developed by Chinese state‐owned Sinopharm has been approved by United Arab Emirates (UAE) and Bahrain. 210 The approval was based on Sinopharm announcement stating that the two‐dose regimen was 86% effective which included trials in 31 000 people in the UAE and 7700 in Bahrain. However, neither of these involved entities released detailed phase III data used to calculate the effectiveness. Sinopharm's vaccine is also undergoing phase III trials in other countries including Egypt, Jordan, and Argentina. Notably, a double‐blind, randomized, placebo‐controlled phase 1/2 trial designed by the Wuhan Institute of Biological Products Co Ltd and Henan Provincial Center for Disease Control and Prevention has reported that vaccine was well‐tolerated at varying doses with the most common adverse reaction being pain at the injection site in only 15% of subjects. 211 The vaccine effectively induced antibody responses but the longer interval between first and second injections produced stronger antibody responses. Of importance, according to Chinese state media more than 100 countries have pre‐ordered Sinopharm vaccine despite the data gap, as this vaccine formulation is appealing over mRNA vaccines in their multi‐antigenicity, storage conditions, and availability.
5.3. Other considerations for COVID‐19 vaccine development
5.3.1. Animal models
Preclinical evaluation of vaccine candidates for COVID‐19 requires the use of relevant animal models. The safety, immunogenicity and protective efficacy of the vaccine is first evaluated and established in animal models before moving to clinical trials. However, due to the pandemic, the preclinical and clinical stages of COVID‐19 vaccine development are condensed and move forwards in parallel. 125 An animal model should mimic infection seen in humans and requires the pathogen to infect the animal using the same receptor as human host cells and to be able to effectively replicate and cause similar disease. 171 Testing the disease in certain animal models can be challenging. For instance, wild‐type mice are not infected by SARS‐CoV‐2 since its ACE2 does not effectively bind the S1 subunit of the S protein and transgenic mice that express human ACE2 only display mild infection. 171 Other animal models have been utilized to study pathogenicity of the virus including hamsters, ferrets, and non‐human primates. 171 Syrian hamsters are a good model to study pathogenesis, vaccination strategies, and antiviral treatment for COVID‐19 since they are readily available, small and resemble the clinical and pathological characteristics seen in human SARS‐CoV‐2 infection. 212 SARS‐CoV‐2 effectively replicates in the lungs of Syrian hamsters, causing pathological lesions in the lungs, making this model attractive for understanding pathogenesis of lung injury. 213 Syrian hamsters are also able to transmit the disease to naïve hamsters through close contact following SARS‐CoV‐2 infection. 212 This animal model would allow for the rapid evaluation of vaccines at a low cost in comparison to other animal models including ferrets and non‐human primates. 213 While ferrets are highly susceptible to SARS‐CoV‐2 infection, viral RNA, and infectious virus has only been found within the upper respiratory tract and is undetectable in other organs. 214 , 215 SARS‐CoV‐2 can efficiently replicate in the upper respiratory tract and effectively transmit to naïve ferrets through direct contact, suggesting they are a good model for evaluating the efficacy of COVID‐19 vaccines in hindering transmission. 214 , 215 Macaques are permissive to SARS‐CoV‐2 infection, shed virus, and display COVID‐19 like symptoms. 216 SARS‐CoV‐2 was able to efficiently replicate in the upper respiratory tract including the nasal cavity, bronchi, bronchioles, and alveoli and lower respiratory tract of macaques. 137 , 216 In addition, SARS‐CoV‐2 infection provided protection against reinfection in macaques, thus making them a promising animal model to test preventative and therapeutic strategies for COVID‐19. 171
5.3.2. Route of vaccination
Although the selection of vaccine antigens and appropriate animal models is important, the route of vaccination is another key consideration of vaccine design. This is especially important for mucosal pathogens including SARS‐CoV‐2 as optimal protection requires the induction of both humoral and cellular immunity. 125 Since SARS‐CoV‐2 is a respiratory disease, inducing memory responses in the respiratory tract through the intranasal route or inhaled aerosol would be beneficial 217 (Figure 3). The two main vaccination routes include parenteral and mucosal and induce different immune responses. Parenteral vaccination induces IgG antibodies within the respiratory mucosa but is unable to induce mucosal IgA antibodies or TRM cells in the lung. 125 In contrast, respiratory mucosal vaccination is proficient at inducing mucosal antibodies, TRM cells and macrophage‐mediated trained innate immunity 125 (Figure 3). During SARS‐CoV and SARS‐CoV‐2 infection, memory CD4++ T cells in the respiratory tract play a vital role in providing protection. 218 Inducing antibody and cellular responses in the respiratory tract through intranasal vaccination is essential for protecting against SARS‐CoV and MERS‐CoV. 181 , 218 However, there are some challenges with mucosal vaccination including the need for safe/effective mucosal adjuvant and vaccine platform and the resources associated with the delivery method. 125 , 219
Based on available information, there are at least half dozen COVID‐19 vaccine candidates designed for mucosal route of administration including a DNA‐based vaccine developed at the University of Waterloo that can be given via the nasal route, a vaccine by Intravacc that contains a Newcastle disease virus (NDV) vector expressing the SARS‐CoV‐2 S protein, an intranasal vaccine AdCOVID developed by Altimmune, and the CoroFlu nasal vaccine developed by FluGen, University of Wisconsin‐Madison and Bharat Biotech. 219 More recently, MediciNova has begun to develop a recombinant parainfluenza viral‐vectored intranasal COVID‐19 vaccine. 220 BlueWillow Biolgics has been developing an oil‐in‐water nanoemulsion‐adjuvanted spike protein subunit vaccine for intranasal delivery. 221
Mucosal vaccinations for SARS‐CoV produced significantly higher levels of secretory IgA in the lung and neutralizing antibodies. 222 , 223 When comparing mucosal to systemic routes of administration of a recombinant adeno‐associated virus encoding the RBD of SARS‐CoV S protein, intranasal vaccination induced a systemic humoral response of similar strength and shorter duration than intramuscular vaccination but a much stronger local humoral response. 224 The intranasal vaccination induced stronger cytotoxic T cell responses but provided comparable protection against SARS‐CoV infection compared to intramuscular vaccination. 224 The near‐sterilizing protective immunity was seen in a murine model of SARS‐CoV‐2 only by single intranasal, not by repeated intramuscular, route of immunization with a ChAd‐vectored COVID‐19 vaccine expressing the S protein. 225 Furthermore, in a hamster model, a single intranasal or intramuscular immunization with the ChAd‐vectored COVID‐19 vaccine protected Golden Syrian hamsters from developing pneumonia. 226 Intranasal immunization provided higher antibody titers in respiratory passageways and provided a greater degree of protection compared to intramuscular immunization, again speaks to the capacity of local mucosal immunization to provide better protection than traditional systemic routes. 125 , 226 Thus, based on the great potential of ChAd platform, the Imperial College London is conducting clinical trials to evaluate the safety and effectiveness of two COVID‐19 vaccines, Oxford's ChAdox1 nCoV‐19 and Imperial's ssRNA vaccine platform. 227 The two vaccine candidates will be delivered directly to the respiratory tract by inhalation through the mouth via a nebulizer and three doses will be assessed. 227 Furthermore, Beijing Wantai Biological Pharmacy Enterprise with Xiamen University and Hong Kong University will soon begin a phase 1 clinical trial to evaluate a duo‐flu & COVID‐19 vaccine via intranasal spray. 228 It is thus anticipated that the next couple of years may see further increased knowledge in the feasibility, safety and immune potency of mucosal COVID‐19 vaccine strategies from both preclinical and clinical studies.
5.3.3. Trained innate immunity in vaccine design
It has been long thought that only the adaptive immune system exhibits memory. However extensive evidence suggests that innate immune cells (monocytes, macrophages etc) also exhibit memory‐like characteristics. 229 Various microbial components including C albicans, β‐glucan, and live attenuated vaccines such as BCG, measles and smallpox provide non‐specific protection against unrelated pathogens. 109 , 229 , 230 This process of heterologous protection is mediated by innate immune memory cells and has now been termed trained innate immunity (TII). Innate immune memory represents a reset state following exposure to an antigen or microbe, leading to an altered responsiveness to the same or unrelated antigen or microbe. 109 Innate immune cells of TII exhibit key hallmarks of a pro‐defense signature, increased cytokine responses, epigenetic and metabolic reprograming, and enhanced protection to secondary infections. 109 , 231 , 232
Bacillus Calmette‐Guerin (BCG) has been and is currently being studied for its non‐specific protective effects against unrelated pathogens and induction of TII. 233 , 234 In a randomized controlled trial BCG vaccination at birth in low‐birth‐weight infants lowered mortality caused by neonatal sepsis, respiratory infection, and fever. 235 Circulating monocytes isolated from healthy volunteers vaccinated with BCG showed an increase in IFN‐γ, TNF‐ɑ, and IL‐1β in response to unrelated bacterial infection, persisting for up to 3 months. 236 , 237 Furthermore, BCG vaccination protected SCID mice, which lack functional T and B cells, against lethal C albicans infection further supporting the importance of innate immune cells in mediating protection. 237 Various research groups are postulating that BCG vaccination may help to prevent SARS‐CoV‐2 infection and/or reduce disease severity. 234 Phase 1 clinical trials have begun in numerous countries to evaluate the efficacy of BCG vaccination in preventing COVID‐19 by strengthening the innate immune system. Another clinical trial is evaluating the effectiveness of the measles vaccine in decreasing the incidence of SARS‐CoV‐2 infection.
With this new knowledge on innate immune memory and TII, vaccine strategies for COVID‐19 should aim to target both innate and adaptive immune systems in the respiratory tract to best control COVID‐19 in early stages of SARS‐CoV‐2 infection 109 , 125 (Figure 3). In doing so, besides protection from COVID‐19, vaccine‐induced TII may also offer non‐specific protection against other respiratory pathogens. 109
6. CONCLUSIONS
The world is in urgent need of a safe and effective COVID‐19 vaccine. To date globally SARS‐CoV‐2 has infected more than 100 million people and has caused over 2.15 million deaths and counting. Many of the specifics about this virus and COVID‐19 still remain incompletely understood including its high transmissibility, asymptomatic state, disparate susceptibility between the young and elderly, and protective immune correlates. Therefore, it is imperative for us to continue to expand our knowledge on all of these fronts. Such knowledge is critical to developing further improved COVID‐19 vaccine strategies beyond the first‐generation vaccines developed according to a compressed pandemic vaccine scheme. 125
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
R. Singh, A. Kang, and X. Luo with Z. Xing developed the manuscript. R. Singh, A. Kang, and X. Luo wrote and finalized the manuscript and with S. Afkhami and M. Jeyanathan, constructed the figures. M. Jeyanathan, A. Gillgrass, S. Afkhami, and Z. Xing edited the entire manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the support from the Canadian Institutes of Health Research (CIHR) COVID‐19 Rapid Research Program and CIHR Foundation Program.
Singh R, Kang A, Luo X, et al. COVID‐19: Current knowledge in clinical features, immunological responses, and vaccine development. The FASEB Journal. 2021;35:e21409 10.1096/fj.202002662R
Ramandeep Singh, Alisha Kang and Xiangqian Luo are contributed equally to this work.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Coronavirus (COVID‐19) Events as they Happen. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐as‐they‐happen. Accessed November 23, 2020. [Google Scholar]
- 3. WHO . WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.WHO.int/?gclid=Cj0KCQiAk53‐BRD0ARIsAJuNhps3swcAHiNCjnDRXQkz4UQO_qLXXLcFu6qbqUcRvfKLZ4EVgur3snIaAhT7EALw_wcB. Accessed January 28, 2020. [Google Scholar]
- 4. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boccia S, Ricciardi W, Ioannidis JPA. What other countries can learn from italy during the COVID‐19 pandemic. JAMA Intern Med. 2020;180(7):927‐928. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Shang W, Rao X. Facing the COVID‐19 outbreak: what should we know and what could we do? J Med Virol. 2020;92(6):536‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐NCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swerdlow DL, Finelli L. Preparation for possible sustained transmission of 2019 novel coronavirus: lessons from previous epidemics. JAMA. 2020;323(12):1129‐1130. [DOI] [PubMed] [Google Scholar]
- 10. Torres‐Roman JS, Kobiak IC, Valcarcel B, Diaz‐Velez C, La Vecchia C. The reproductive number R0 of COVID‐19 in Peru: an opportunity for effective changes. Travel Med Infect Dis. 2020;37:101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382:1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colaneri M, Seminari E, Piralla A, et al. Lack of SARS‐CoV‐2 RNA environmental contamination in a tertiary referral hospital for infectious diseases in Northern Italy. J Hosp Infect. 2020;105(3):474‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colaneri M, Seminari E, Novati S, et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin Microbiol Infect. 2020;26(8):1094.e1‐1094.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Virol. 2020;127:104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Machhi J, Herskovitz J, Senan AM, et al. The natural history, pathobiology, and clinical manifestations of SARS‐CoV‐2 infections. J Neuroimmune Pharmacol. 2020;15:359‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr Int J Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saheb Sharif‐Askari N, Saheb Sharif‐Askari F, Alabed M, et al. Airways expression of SARS‐CoV‐2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schuler BA, Habermann AC, Plosa EJ, et al. Age‐related expression of SARS‐CoV‐2 priming protease TMPRSS2 in the developing lung. J Clin Invest. 2020. 10.1101/2020.05.22.111187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019‐nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 2020;25(5):2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niedzwiedz CL, O'Donnell CA, Jani BD, et al. Ethnic and socioeconomic differences in SARS‐CoV‐2 infection: prospective cohort study using UK Biobank. BMC Med. 2020;18(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei YY, Wang RR, Zhang DW, et al. Risk factors for severe COVID‐19: evidence from 167 hospitalized patients in Anhui. China. J Infect. 2020;81(1):e89‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID‐19 pneumonia caused by SARSCoV‐ 2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country‐based mitigation measures influence the course of the COVID‐19 epidemic? Lancet. 2020;395(10228):931‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruinen de Bruin Y, Lequarre AS, McCourt J, et al. Initial impacts of global risk mitigation measures taken during the combatting of the COVID‐19 pandemic. Saf Sci. 2020;128:104773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reuters . Timeline: How the Global Coronavirus Pandemic Unfolded. https://www.reuters.com/article/health‐coronavirus‐timeline‐idUSL1N2GN04J. Accessed December 4, 2020. [Google Scholar]
- 29. Ueki H, Furusawa Y, Iwatsuki‐Horimoto K, et al. Effectiveness of face masks in preventing airborne transmission of SARS‐CoV‐2. mSphere. 2020;5(5):e00637‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet. 2020;395(10242):1973‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Q, Xie S, Wang Y, Zeng D. Survival‐convolution models for predicting COVID‐19 cases and assessing effects of mitigation strategies. Front Public Heal. 2020;8:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007;104(18):7582‐7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer CK, Retnakaran R. Rates of COVID‐19‐associated hospitalization in British Columbia and Ontario: time course of flattening the relevant curve. Can J Public Heal. 2020;111(5):636‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neuwirth C, Gruber C, Murphy T. Investigating duration and intensity of Covid‐19 social‐distancing strategies. Sci Rep. 2020;10(1):20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCombs A, Kadelka C. A model‐based evaluation of the efficacy of COVID‐19 social distancing, testing and hospital triage policies. PLoS Comput Biol. 2020;16(10):e1008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tuite AR, Fisman DN, Greer AL. Mathematical modelling of COVID‐19 transmission and mitigation strategies in the population of Ontario. Canada. CMAJ. 2020;192(19):E497‐E505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiu T, Xiao H. Revealing the influence of national public health response for the outbreak of the SARS‐CoV‐2 epidemic in Wuhan, China through status dynamic modeling. SSRN Electron J. 2020. 10.1101/2020.03.10.20032995 [DOI] [Google Scholar]
- 38. Haffajee RL, Mello MM. Thinking globally, acting locally — the U.S. response to Covid‐19. N Engl J Med. 2020;382(22):e75. [DOI] [PubMed] [Google Scholar]
- 39. L'Angiocola PD, Monti M. COVID‐19: the critical balance between appropriate governmental restrictions and expected economic, psychological and social consequences in Italy. Are we going in the right direction? Acta Biomed. 2020;91(2):35‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pak A, Adegboye OA, Adekunle AI, Rahman KM, McBryde ES, Eisen DP. Economic consequences of the COVID‐19 outbreak: the need for epidemic preparedness. Front Public Heal. 2020;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonaccorsi G, Pierri F, Cinelli M, et al. Economic and social consequences of human mobility restrictions under COVID‐19. Proc Natl Acad Sci U S A. 2020;117(27):15530‐15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. BNN Bloomberg . Goldman Sachs Predicts U.S. GDP to Shrink 5% in Second Quarter. https://www.bnnbloomberg.ca/goldman‐sachs‐predicts‐u‐s‐gdp‐to‐shrink‐5‐in‐second‐quarter‐1.1406497. Accessed November 23, 2020. [Google Scholar]
- 43. BBC News . Coronavirus Lockdown Protest: What's Behind the US Demonstrations?. https://www.bbc.com/news/world‐us‐canada‐52359100. Accessed November 23, 2020. [Google Scholar]
- 44. CBC News . Anti‐Mask Protest in Montreal Draws Large Crowd, Propelled by U.S. Conspiracy Theories. https://www.cbc.ca/news/canada/montreal/anti‐mask‐protest‐montreal‐1.5722033. Accessed November 23, 2020. [Google Scholar]
- 45. Toronto Sun . Anti‐Lockdown Protesters Believe COVID Restrictions Threaten Freedom. https://torontosun.com/news/local‐news/anti‐lockdown‐protestors‐believe‐covid‐restrictions‐threaten‐freedom. Accessed November 23, 2020. [Google Scholar]
- 46. Di Domenico L, Pullano G, Sabbatini CE, Boëlle PY, Colizza V. Impact of lockdown on COVID‐19 epidemic in Île‐de‐France and possible exit strategies. BMC Med. 2020;18(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fowler Z, Moeller E, Roa L, et al. Projected impact of COVID‐19 mitigation strategies on hospital services in the Mexico City metropolitan area. PLoS One. 2020;15(11):e0241954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davies NG, Barnard RC, Jarvis CI, et al. Assessment of tiered restrictions and a second lockdown on COVID‐19 deaths and hospitalisations in England: a modelling study. Lancet Infect Dis. 2020;3099(20):2‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25(10):2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS‐CoV‐2 infections in residents of a long‐term care skilled nursing facility — King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect Dis. 2020;94:154‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 53. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382:1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ki M. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019‐nCoV) disease in Korea. Epidemiol Health. 2020;42:e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lao WP, Imam SA, Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS‐CoV‐2 infection. World J Otorhinolaryngol Head Neck Surg. 2020;6:S22‐S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Z, Liu T, Yang N, et al. Neurological manifestations of patients with COVID‐19: potential routes of SARS‐CoV‐2 neuroinvasion from the periphery to the brain. Front Med. 2020;14:533‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;m606 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wunsch H. Mechanical ventilation in COVID‐19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID‐19 and association between concomitant cardiovascular diseases and severity of COVID‐19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(7):567‐571. [DOI] [PubMed] [Google Scholar]
- 64. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Del Rio C, Collins LF, Malani P. Long‐term health consequences of COVID‐19. JAMA. 2020;324(17):1723‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weerahandi H, Hochman KA, Simon E, et al. Post‐discharge health status and symptoms in patients with severe COVID‐19. medRxiv Prepr Serv Heal Sci. 2020. 10.1101/2020.08.11.20172742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cirulli E, Barrett KM, Riffle S, et al. Long‐term COVID‐19 symptoms in a large unselected population. medRxiv. 2020. 10.1101/2020.10.07.20208702 [DOI] [Google Scholar]
- 69. Marshall M. The lasting misery of coronavirus long‐haulers. Nature. 2020;585(7825):339‐341. [DOI] [PubMed] [Google Scholar]
- 70. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5‐6 months after COVID‐19 in non‐hospitalised subjects: a population‐based cohort study. Thorax. 2020. 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS‐CoV‐2 ‘long‐haulers’? Chronic Dis Transl Med. 2020. 10.1016/j.cdtm.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID‐19 tests. Nat Rev Microbiol. 2020. 10.1038/s41579-020-00461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chang MC, Hur J, Park D. Interpreting the COVID‐19 test results: a guide for physiatrists. Am J Phys Med Rehabil. 2020;99(7):583‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. WHO . WHO Emergency Use Listing for In Vitro Diagnostics (IVDs) Detecting SARS‐CoV‐2. 2020. https://www.who.int/publications/m/item/200922‐eul‐sars‐cov2‐product‐list. Accessed January 4, 2021. [Google Scholar]
- 75. FDA . In Vitro Diagnostics EUAs. https://www.fda.gov/medical‐devices/coronavirus‐disease‐2019‐covid‐19‐emergency‐use‐authorizations‐medical‐devices/vitro‐diagnostics‐euas. Accessed December 30, 2020. [Google Scholar]
- 76. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med. 2020;382:2451‐2460. [DOI] [PubMed] [Google Scholar]
- 77. WHO . Clinical Management of COVID‐19. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19. Accessed November 23, 2020. [Google Scholar]
- 78. Hong X, Xiong J, Feng Z, Shi Y. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of severe COVID‐19? Int J Infect Dis. 2020;94:78‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cannalire R, Stefanelli I, Cerchia C, Beccari AR, Pelliccia S, Summa V. Sars‐cov‐2 entry inhibitors: small molecules and peptides targeting virus or host cells. Int J Mol Sci. 2020;21(16):5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu STH, Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID‐19: a propensity score–matched control study. Nat Med. 2020;26(11):1708‐1713. [DOI] [PubMed] [Google Scholar]
- 82. Mitra P. Inhibiting fusion with cellular membrane system: Therapeutic options to prevent severe acute respiratory syndrome coronavirus‐2 infection. Am J Physiol Cell Physiol. 2020;319(3):C500‐C509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad‐spectrum antiviral: An update. Antiviral Res. 2014;107:84‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72‐73. [DOI] [PubMed] [Google Scholar]
- 85. Recovery Collaborative Group . Effect of hydroxychloroquine in hospitalized patients with covid‐19. N Engl J Med. 2020;382:2030‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Update alert 3: hydroxychloroquine or chloroquine for the treatment or prophylaxis of COVID‐19. Ann Intern Med. 2020;173(11):W156‐W157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid‐19 — final Report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hsu J. Covid‐19: what now for remdesivir? BMJ. 2020;371:m4457. [DOI] [PubMed] [Google Scholar]
- 91. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID‐19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS‐CoV‐2. Natl Sci Rev. 2020;7(6):1012‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS‐CoV‐2 associated with the COVID‐19 outbreak. Curr Biol. 2020;7(6):1346‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 Virus. Cell. 2020;182(4):812‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scientific American . The U.K. Coronavirus Mutation Is Worrying but Not Terrifying. https://www.scientificamerican.com/article/the‐u‐k‐coronavirus‐mutation‐is‐worrying‐but‐not‐terrifying/. Accessed December 30, 2020. [Google Scholar]
- 97. Callaway Ewen. Fast‐spreading COVID variant can elude immune responses Nature. 2021;589:m4944500–501. [DOI] [PubMed] [Google Scholar]
- 98. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS‐CoV‐2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527(3):618‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mille JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two‐step, furin‐mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111(42):15214‐15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bayati A, Kumar R, Francis V, McPherson P. SARS‐CoV‐2 uses clathrin‐mediated endocytosis to gain access into cells. bioRxiv. 2020. 10.1101/2020.07.13.201509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Clausen TM, Sandoval DR, Spliid CB, et al. SARS‐CoV‐2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 Is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;123(2):3‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xing Z, Afkhami S, Bavananthasivam J, et al. Innate immune memory of tissue‐resident macrophages and trained innate immunity: re‐vamping vaccine concept and strategies. J Leukoc Biol. 2020;108(3):825‐834. [DOI] [PubMed] [Google Scholar]
- 110. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nikolich‐Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS‐CoV‐2 and COVID‐19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42(2):505‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vardhana SA, Wolchok JD. The many faces of the anti‐COVID immune response. J Exp Med. 2020;217(6):e20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Abassi Z, Knaney Y, Karram T, Heyman SN. The lung macrophage in SARS‐CoV‐2 infection: a friend or a foe? Front Immunol. 2020;11:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Codo AC, Davanzo GG, de Monteiro L, et al. Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1α/glycolysis‐dependent axis. Cell Metab. 2020;32(3):437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cheung CY, Poon LLM, Ng IHY, et al. Cytokine responses in severe acute respiratory syndrome coronavirus‐infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79(12):7819‐7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yilla M, Harcourt BH, Hickman CJ, et al. SARS‐coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 2005;107(1):93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52(6):910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhou R, To KKW, Wong YC, et al. Acute SARS‐CoV‐2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(4):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thieme CJ, Anft M, Paniskaki K, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS‐CoV‐2 proteins is not associated with recovery in critical COVID‐19 patients. Cell Reports Med. 2020;1(6):100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20:615‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mateus J, Grifoni A, Tarke A, et al. Selective and cross‐reactive SARS‐CoV‐2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):761‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ma J, Xia P, Zhou Y, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID‐19. Clin Immunol. 2020;214:108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS‐CoV‐2 infection: review of 3939 COVID‐19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID‐19: why children fare better than adults? Indian J Pediatr. 2020;87:537‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Padoan A, Sciacovelli L, Basso D, et al. IgA‐Ab response to spike glycoprotein of SARS‐CoV‐2 in patients with COVID‐19: a longitudinal study. Clin Chim Acta. 2020;507:164‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fontanet A, Cauchemez S. COVID‐19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS‐CoV‐2 spike antigens in COVID‐19 patients. Sci Immunol. 2020;5(52):eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients. Sci Immunol. 2020;5(52):eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ward H, Cooke G, Atchison C, et al. Declining prevalence of antibody positivity to SARS‐CoV‐2: a community study of 365,000 adults. medRxiv. 2020. 10.1101/2020.10.26.20219725 [DOI] [Google Scholar]
- 136. Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis. 2020;21(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chandrashekar A, Liu J, Martino AJ, et al. SARS‐CoV‐2 infection protects against rechallenge in rhesus macaques. Science. 2020;369(6505):812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Deng W, Bao L, Liu J, et al. Primary exposure to SARS‐CoV‐2 protects against reinfection in rhesus macaques. Science. 2020;369(6505):818‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID‐19. Nat Rev Immunol. 2020;20:339‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Thierry AR, Roch B. SARS‐CoV2 may evade innate immune response, causing uncontrolled neutrophil extracellular traps formation and multi‐organ failure. Clin Sci. 2020;134(12):1295‐1300. [DOI] [PubMed] [Google Scholar]
- 141. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zeng Z, Xu L, Xie XY, et al. Pulmonary pathology of early‐phase COVID‐19 pneumonia in a patient with a benign lung lesion. Histopathology. 2020;77(5):823‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):422‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. European Respiratory Society . COVID‐19 Patients Suffer Long‐Term Lung and Heart Damage but it can Improve with Time. https://www.ersnet.org/the‐society/news/covid‐19‐patients‐suffer‐long‐term‐lung‐and‐heart‐damage‐but‐it‐can‐improve‐with‐time. Accessed November 24, 2020. [Google Scholar]
- 145. Zhang P, Li J, Liu H, et al. Long‐term bone and lung consequences associated with hospital‐acquired severe acute respiratory syndrome: a 15‐year follow‐up from a prospective cohort study. Bone Res. 2020;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liang L, Yang B, Jiang N, et al. Three‐month follow‐up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35(47):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhao Y, Shang Y, Song W, et al. Follow‐up study of the pulmonary function and related physiological characteristics of COVID‐19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Science Daily . COVID‐19 Patients Suffer Long‐Term Lung and Heart Damage but It Can Improve with Time. https://www.sciencedaily.com/releases/2020/09/200906202950.htm. Accessed December 30, 2020. [Google Scholar]
- 149. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation. 2020;142:68‐78. [DOI] [PubMed] [Google Scholar]
- 150. Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID‐19. JAMA. 2020;323(24):2518‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wichmann D, Sperhake J‐P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173:268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98(1):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9(4):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Özveri H, Eren MT, Kırışoğlu CE, Sarıgüzel N. Atypical presentation of SARS‐CoV‐2 infection in male genitalia. Urol Case Reports. 2020;33:101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID‐19. Am J Emerg Med. 2020;38(7):1542.e1‐1542.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome–coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology. 2021;9(1):88–98. 10.1111/andr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Xu J, Qi L, Chi X, et al. Orchitis: A complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Madjunkov M, Dviri M, Librach C. A comprehensive review of the impact of COVID‐19 on human reproductive biology, assisted reproduction care and pregnancy: a Canadian perspective. J Ovarian Res. 2020;13(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Aslan MM, Uslu Yuvacı H, Köse O, et al. SARS‐CoV‐2 is not present in the vaginal fluid of pregnant women with COVID‐19. J Matern Neonatal Med. 2020;16:1‐3. [DOI] [PubMed] [Google Scholar]
- 162. Cui P, Chen Z, Wang T, et al. Severe acute respiratory syndrome coronavirus 2 detection in the female lower genital tract. Am J Obstet Gynecol. 2020;223(1):131‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Qiu L, Liu X, Xiao M, et al. SARS‐CoV‐2 is not detectable in the vaginal fluid of women with severe COVID‐19 infection. Clin Infect Dis. 2020;71(15):813‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jing Y, Run‐Qian L, Hao‐Ran W, et al. Potential influence of COVID‐19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26(6):367‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Valdés G, Neves LAA, Anton L, et al. Distribution of angiotensin‐(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27(2–3):200‐207. [DOI] [PubMed] [Google Scholar]
- 167. de Melo GC, de Araújo KCGM. COVID‐19 infection in pregnant women, preterm delivery, birth weight, and vertical transmission: a systematic review and meta‐analysis. Cad Saude Publica. 2020;36(7):e00087320. [DOI] [PubMed] [Google Scholar]
- 168. Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID‐19. Cureus. 2020;12(5):e8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bodro M, Compta Y, Llansó L, et al. Increased CSF levels of IL‐1β, IL‐6, and ACE in SARS‐CoV‐2‐associated encephalitis. Neurol Neuroimmunol neuroinflammation. 2020;7(5):e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Amanat F, Krammer F. SARS‐CoV‐2 vaccines: status report. Immunity. 2020;52(4):583‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mukherjee R. Global efforts on vaccines for COVID‐19: since, sooner or later, we all will catch the coronavirus. J Biosci. 2020;45(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lin JT, Zhang JS, Su N, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107‐1113. [PubMed] [Google Scholar]
- 174. Martin JE, Louder MK, Holman LSA, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338‐6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xiong S, Wang YF, Zhang MY, et al. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol Lett. 2004;95(2):139‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang CH, Lu JH, Wang YF, et al. Immune responses in Balb/c mice induced by a candidate SARS‐CoV inactivated vaccine prepared from F69 strain. Vaccine. 2005;23(24):3196‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Roper RL, Rehm KE. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8(7):887‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Te TC, Sbrana E, Iwata‐Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zakhartchouk AN, Viswanathan S, Mahony JB, Glaudei J, Babiuk LA. Severe acute respiratory syndrome coronavirus nucleocapsid protein expressed by an adenovirus vector is phosphorylated and immunogenic in mice. J Gen Virol. 2005;86(1):211‐215. [DOI] [PubMed] [Google Scholar]
- 180. Gao W, Tamin A, Soloff A, et al. Effects of a SARS‐associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. See RH, Zakhartchouk AN, Petric M, et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87:641‐650. [DOI] [PubMed] [Google Scholar]
- 182. Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Lamirande EW, DeDiego ML, Roberts A, et al. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden syrian hamsters. J Virol. 2008;82(15):7721‐7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. DeDiego ML, Pewe L, Alvarez E, Rejas MT, Perlman S, Enjuanes L. Pathogenicity of severe acute respiratory coronavirus deletion mutants in hACE‐2 transgenic mice. Virology. 2008;376(2):379‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. DeDiego ML, Álvarez E, Almazán F, et al. A severe acute respiratory syndrome coronavirus that lacks the e gene is attenuated in vitro and in vivo. J Virol. 2007;81(4):1701‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181(7):1489‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Peeples L. Avoiding pitfalls in the pursuit of a COVID‐19 vaccine. Proc Natl Acad Sci U S A. 2020;117(15):8218‐8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Salvatori G, Luberto L, Maffei M, et al. SARS‐CoV‐2 spike protein: an optimal immunological target for vaccines. J Transl Med. 2020;18(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID‐19. Nat Commun. 2020;11(1):2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Yu J, Tostanosk LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science. 2020;369(6505):806‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Inovio Pharmaceuticals, Inc . INOVIO Announces Positive Interim Phase 1 Data for INO‐4800 Vaccine for COVID‐19. http://ir.inovio.com/news‐releases/news‐releases‐details/2020/INOVIO‐Announces‐Positive‐Interim‐Phase‐1‐Data‐For‐INO‐4800‐Vaccine‐for‐COVID‐19/default.aspx. Accessed November 26, 2020. [Google Scholar]
- 192. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Sinovac . Sinovac Commences Phase III Clinical Trials for COVID‐19 Vaccine Candidate in Turkey. http://www.sinovac.com/?optionid=754&auto_id=911. Accessed November 26, 2020. [Google Scholar]
- 194. Meissa Vaccines . Vaccine Pipeline: RSV and COVID‐19 Vaccine Candidates. https://www.meissavaccines.com/vaccine‐pipeline. Accessed December 4, 2020. [Google Scholar]
- 195. Fidel PL, Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with covid‐19 infection? MBio. 2020;11(3):e00907‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396(10249):479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV‐19 vaccine prevents SARS‐CoV‐2 pneumonia in rhesus macaques. Nature. 2020;586:578‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;369(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 — preliminary report. N Engl J Med. 2020;383:1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pfizer . Pfizer and BioNTech Conclude Phase 3 Study of COVID‐19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. https://www.pfizer.com/news/press‐release/press‐release‐detail/pfizer‐and‐biontech‐conclude‐phase‐3‐study‐covid‐19‐vaccine. Accessed November 26, 2020. [Google Scholar]
- 202. Moderna, Inc. Moderna’s COVID‐19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. https://investors.modernatx.com/news‐releases/news‐release‐details/modernas‐covid‐19‐vaccine‐candidate‐meets‐its‐primary‐efficacy. Accessed November 26, 2020. [Google Scholar]
- 203. France 24 . European Commission Approves Pfizer‐BioNTech Covid‐19 Vaccine. https://www.france24.com/en/europe/20201221‐eu‐regulator‐approves‐pfizer‐biontech‐covid‐19‐vaccine. Accessed December 31, 2020. [Google Scholar]
- 204. Fox News . Mexico Becomes 4th Country to Approve Pfizer‐BioNTech Coronavirus Vaccine. https://www.fox29.com/news/mexico‐becomes‐4th‐country‐to‐approve‐pfizer‐biontech‐coronavirus‐vaccine. Accessed December 31, 2020. [Google Scholar]
- 205. BBC News . Covid: FDA Approves Pfizer Vaccine for Emergency Use in US. https://www.bbc.com/news/world‐us‐canada‐55265477. Accessed December 31, 2020. [Google Scholar]
- 206. CBC News . Shipment of Moderna COVID‐19 Vaccine Arrives in Canada One Day After Approval. https://www.cbc.ca/news/politics/moderna‐vaccine‐arrives‐canada‐1.5854649. Accessed December 31, 2020. [Google Scholar]
- 207. CBC News . U.S. Approves Moderna’s COVID‐19 Vaccine for Emergency Use. https://www.cbc.ca/news/health/us‐approves‐moderna‐vaccine‐emergency‐use‐1.5848551. Accessed December 31, 2020. [Google Scholar]
- 208. CBC News . Britain Approves Use of AstraZeneca‐Oxford COVID‐19 Vaccine. https://www.cbc.ca/news/world/astrazeneca‐vaccine‐approve‐1.5857186. Accessed December 31, 2020. [Google Scholar]
- 209. Novavax, Inc . Pipeline is Robust at Novavax. https://www.novavax.com/our‐pipeline#nvx‐cov2373. Accessed November 26, 2020. [Google Scholar]
- 210. Cyranoski D. Arab nations first to approve Chinese covid vaccine. Nature. 2020;588(7389):548. [DOI] [PubMed] [Google Scholar]
- 211. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Chan JFW, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID‐19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71(9):2428‐2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Imai M, Iwatsuki‐Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS‐CoV‐2 infection and countermeasure development. Proc Natl Acad Sci U S A. 2020;117(28):16587‐16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Il KY, Kim SG, Kim SM, et al. Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host Microbe. 2020;27(5):704‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus 2. Science. 2020;368(6494):1016‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID‐19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Padron‐Regalado E. Vaccines for SARS‐CoV‐2: lessons from other coronavirus strains. Infect Dis Ther. 2020;9(2):255‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14(11):1509‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Moreno‐Fierros L, García‐Silva I, Rosales‐Mendoza S. Development of SARS‐CoV‐2 vaccines: should we focus on mucosal immunity? Expert Opin Biol Ther. 2020;20(8):831‐836. [DOI] [PubMed] [Google Scholar]
- 220. Globe News Wire . MediciNova Announces Initiation of Master Virus Seed Stock Production for its Intranasal COVID‐19 Vaccine using BC‐PIV Vector Technology Nasdaq:MNOV. https://www.globenewswire.com/news‐release/2020/11/24/2132592/0/en/MediciNova‐Announces‐Initiation‐of‐Master‐Virus‐Seed‐Stock‐Production‐for‐its‐Intranasal‐COVID‐19‐Vaccine‐using‐BC‐PIV‐Vector‐Technology.html. Accessed January 1, 2021. [Google Scholar]
- 221. Biospace . Medigen and BlueWillow Biologics Partner to Develop Intranasal Vaccine for SARS‐CoV‐2. https://www.biospace.com/article/releases/medigen‐and‐bluewillow‐biologics‐partner‐to‐develop‐intranasal‐vaccine‐for‐sars‐cov‐2/. Accessed January 1, 2021. [Google Scholar]
- 222. Shim BS, Park SM, Quan JS, et al. Intranasal immunization with plasmid DNA encoding spike protein of SARS‐coronavirus/polyethylenimine nanoparticles elicits antigen‐specific humoral and cellular immune responses. BMC Immunol. 2010;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Huang X, Lu B, Yu W, et al. A novel replication‐competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS One. 2009;4(1):e4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Du L, Zhao G, Lin Y, et al. Intranasal vaccination of recombinant adeno‐associated virus encoding receptor‐binding domain of severe acute respiratory syndrome coronavirus (SARS‐CoV) spike protein induces strong mucosal immune responses and provides long‐term protection against SARS‐. J Immunol. 2008;180(2):948‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Hassan AO, Kafai NM, Dmitriev IP, et al. A single‐dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS‐CoV‐2. Cell. 2020;183(1):169‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bricker TL, Darling TL, Hassan AO, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS‐CoV‐2 vaccine protects against pneumonia in hamsters. bioRxiv. 2021;35:e21409 10.1101/2020.12.02.408823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Imperial College London . Landmark Coronavirus Study to Trial Inhaled Imperial and Oxford Vaccines. https://www.imperial.ac.uk/news/203653/landmark‐coronavirus‐study‐trial‐inhaled‐imperial/. Accessed November 26, 2020. [Google Scholar]
- 228. European Pharmaceutical Review . Phase I Trial of Intranasal COVID‐19 Vaccine Spray Approved in China. https://www.europeanpharmaceuticalreview.com/news/129563/phase‐i‐trial‐of‐intranasal‐covid‐19‐vaccine‐spray‐approved‐in‐china/. Accessed January 1, 2021. [Google Scholar]
- 229. Ciarlo E, Heinonen T, Théroude C, et al. Trained immunity confers broad‐spectrum protection against bacterial infections. J Infect Dis. 2019;222(11):1869‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sánchez‐Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Trained immunity‐based vaccines: a new paradigm for the development of broad‐spectrum anti‐infectious formulations. Front Immunol. 2018;9:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634‐1650. [DOI] [PubMed] [Google Scholar]
- 232. Netea MG, Joosten LAB, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Giamarellos‐Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Netea MG, Giamarellos‐Bourboulis EJ, Domínguez‐Andrés J, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS‐CoV‐2 infection. Cell. 2020;181(5):969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low‐birth‐weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245‐252. [DOI] [PubMed] [Google Scholar]
- 236. Kleinnijenhuis J, Quintin J, Preijers F, et al. Long‐lasting effects of bcg vaccination on both heterologous th1/th17 responses and innate trained immunity. J Innate Immun. 2014;6(2):152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette‐Guérin induces NOD2‐dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537‐17542. [DOI] [PMC free article] [PubMed] [Google Scholar]


