Abstract
Aims
B cell functions in the process of atherogenesis have been investigated but several aspects remain to be clarified.
Methods and results
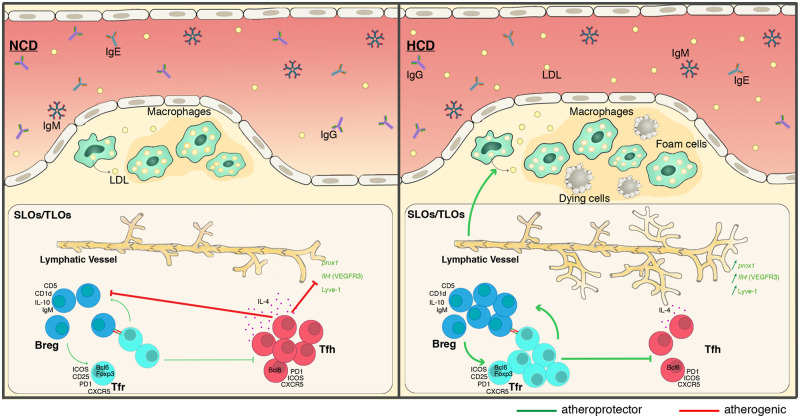
In this study, we show that follicular regulatory helper T cells (TFR) control regulatory B cell (BREG) populations in Apoe−/− mice models on a high-cholesterol diet (HCD). Feeding mice with HCD resulted in up-regulation of TFR and BREG cell populations, causing the suppression of proatherogenic follicular helper T cell (TFH) response. TFH cell modulation is correlated with the growth of atherosclerotic plaque size in thoracoabdominal aortas and aortic root plaques, suggesting that TFR cells are atheroprotective. During adoptive transfer experiments, TFR cells transferred into HCD mice decreased TFH cell populations, atherosclerotic plaque size, while BREG cell population and lymphangiogenesis are significantly increased.
Conclusion
Our results demonstrate that, through different strategies, both TFR and TFH cells modulate anti- and pro-atherosclerotic immune processes in an Apoe−/− mice model since TFR cells are able to regulate both TFH and BREG cell populations as well as lymphangiogenesis and lipoprotein metabolism.
Keywords: Follicular helper T cell (TFH), Follicular regulatory helper T cells (TFR), Regulatory B cell, Lymphangiogenesis, Atherosclerosis
Graphical Abstract
1. Introduction
Cardiovascular diseases (CVD) are the main cause of deaths worldwide. One of the most studied CVD is atherosclerosis. Its development is characterized by the activation of innate and adaptive immune responses to high concentrations of circulating lipids. During this process, T and B cells are significantly involved, although the exact functions of T and B cell subtypes are not fully understood. Foxp3+ regulatory T cells (TREG) assume an immunosuppressive function by reducing experimental atherosclerosis.1 It has been paradoxically demonstrated that atherogenesis is associated with increasing TREG cell populations while their depletion accelerates atherosclerosis and promotes the atherogenic lipoprotein profile.1–3 The recently identified follicular regulatory helper T cell (TFR) populations are a subset of TREG cells localized in germinal centre (GC) where they control the magnitude of the response by suppressing TFH- and B cell activation.4 Since the size and specificity of the GC is modulated by TFR cells, GC responses represent an important axis for atherogenesis regulation.4 It has been demonstrated very recently that follicular helper T cells (TFH) are proatherogenic, and innate-like marginal zone B cells (MZB) participate in their regulation.5,6 The study of B cell subsets indicates that BREG cell populations are up-regulated in Apoe−/− mice and carry atheroprotective functions, confirming that other subsets of B cells could also participate in atherogenesis.7
TREG cells have been shown to modulate cholesterol homoeostasis, and CD4+ T cells have been identified as regulators of lymphatic vessel formation.8,9 Whereas the lymphatic system has long been described to be involved in immune cell trafficking,10 recent studies suggest that it is a prerequisite player in macrophage reverse cholesterol transport (RCT).11 Early atherosclerotic plaque formation is associated to a defect in the capacity of the collecting lymphatic vessel to propel lymph,12 whereas advanced lesion is also associated to a defect in the absorption of lymph from peripheral tissues.11,13 Furthermore, defective lymphatic function appears to have a systemic effect on B cell homing and to impair lipoprotein metabolism that promotes atherosclerosis.14 The present study clearly identifies TFR cells as key regulators of atherosclerosis through decreasing TFH cell populations, thereby inducing expansion of anti-atherogenic BREG cell populations and stimulating lymphangiogenesis.
2. Methods
2.1 Ethical statement
All breeding and experimental protocols and procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Geneva University School of Medicine (protocol number: GE/16/56 and GE/16/131). Animal care and experimental procedures were carried out in accordance with the guidelines of the Institutional Animal Care, Use Committee of the Geneva University School of Medicine and complied with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
2.2 Mice
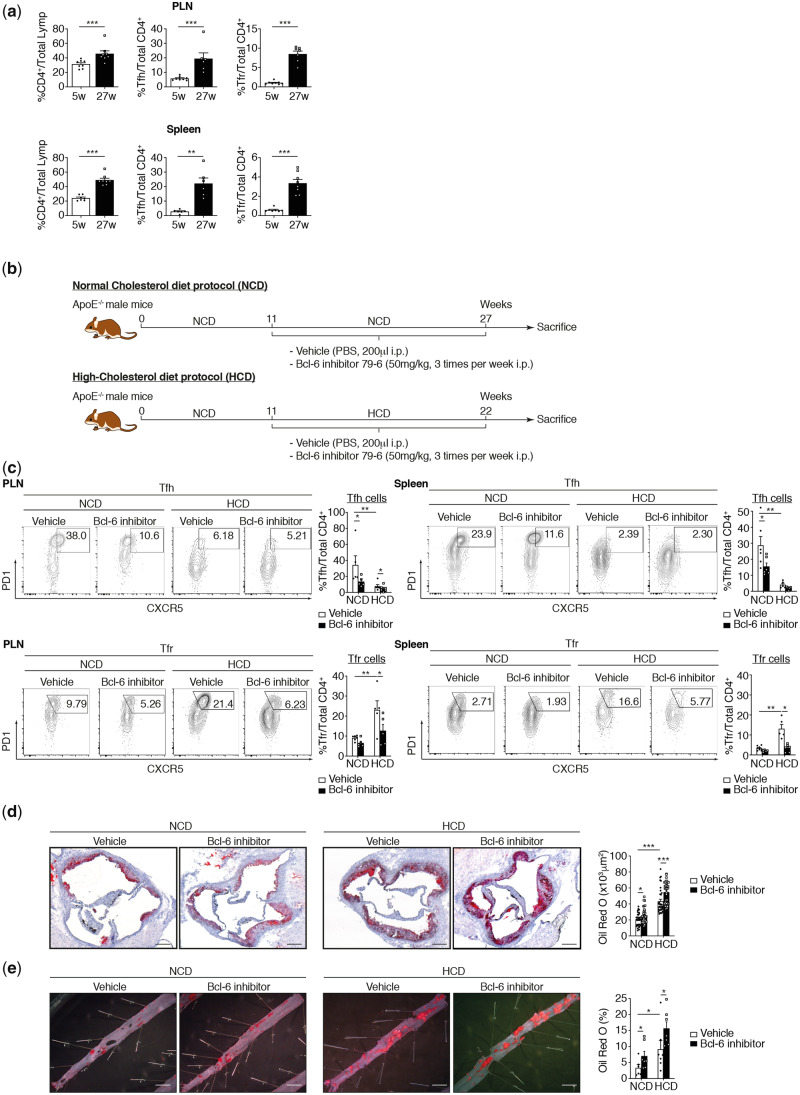
An 11-week-old Apoe−/− C57Bl/6 mice was submitted to two atherogenesis protocols to test the Bcl-6 inhibitor 79-6 (Merck, Germany). In the earlier atherosclerosis protocol, mice were fed a normal chow diet (NCD) for 16 weeks (Figure 1B). In the more advanced atherosclerosis protocol, to accelerate atherogenesis, mice were fed a high-cholesterol diet (HCD) for 11 weeks (20.1% fat, 1.25% cholesterol, Research Diets, Inc., New Brunswick, NJ, USA) (Figure 1B).10 Mice were randomly assigned to receive treatments either with vehicle (10% DMSO) or Bcl-6 inhibitor 79-6. This drug (10 mg/mL, diluted in 200 μL 10% DMSO per injection corresponding to 50 mg/kg) and vehicle (equal volume of 200 μL 10% DMSO) were intraperitoneally injected three times per week before animal euthanasia by exsanguination following the guidelines of the Institutional Animal Care. No analgaesia or anaesthesia was required before these intraperitoneally injections. Mice well-tolerated the treatments and atherosclerosis protocols and no adverse events (such as weight loss and signs of systemic toxicity) were reported. At sacrifice, haematological parameters, serum triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), free fatty acids, and glucose were routinely measured and expressed in mmol/L. Animals were sacrificed by exsanguination after anaesthesia with 4% isoflurane.
Figure 1.
TFR population controls pro-atherogenic TFH population on a high-cholesterol diet. (A) Lymphocytes from spleen and peripheral lymph nodes (PLN) of Apoe−/− mice collected at Weeks 5 and 27 were stained for CD4, CD25, CXCR5, PD1, and Bcl-6. Tabulated results from all mice are presented as percentage of CD4+. (B) Mouse atherosclerosis protocols and treatment schedule (n = 5–8 mice/group). (C) Effect of Bcl-6 inhibitor on TFH and TFR populations on NCD and HCD fed mice (PLN TFH: n = 5–8 mice/group; PLN TFR: n = 5–6 mice/group; spleen TFH: n = 5–6 mice/group; spleen TFR: n = 5–8 mice/group). (D) Quantification of atherosclerotic lipid content and representative microphotographs of aortic root plaques (Oil Red O staining) in Apoe−/− mice treated with vehicle (PBS) or Bcl-6 inhibitor under NCD and HCD (n = 6–7 mice per group/5 slides per mice). Original magnification, ×10. Scale bars, 400 μm. (E) Quantification of atherosclerotic lipid content and representative microphotographs from Oil Red O-stained thoracoabdominal aortas (n = 5–7 mice/group). Scale bars, 500 μm. The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005. All data were represented as mean ± SEM.
2.3 Flow cytometry
For nine-colour immunofluorescence analysis, single-cell suspensions (1 × 106 cells) of spleen and lymph nodes (inguinal, mesenteric, brachial, axillary, and superficial cervical) were incubated with anti-mouse FcRIIB/FcRIIIA mAb (BD Biosciences, MD, USA) to avoid non-specific staining and were subsequently stained with fluorophore-conjugated antibodies (see Supplementary material online, Table S1) at 4°C using predetermined optimal concentrations of mAb for 30 min. Briefly, lymphocytes were stimulated in vitro with PMA (50 ng/mL; Sigma-Aldrich, Germany) and ionomycin (1 μg/mL; Sigma-Aldrich, Germany), in the presence of brefeldin A (1 μL/mL; Sigma-Aldrich, Germany) for 4 h before staining. After staining, cells were washed, fixed, and then permeabilized using the Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences, MD, USA) according to the manufacturer’s instructions. Permeabilized cells were then stained with antibodies against intracellular targets of interest. FACS data will be acquired in a Gallios™ flow cytometer (BD Biosciences, MD, USA) and analysed using FlowJo software (TreeStar, Version 10.0.8r1). For analysis, dead cells and doublets were excluded based on exclusion dye or forward scatter profiles, respectively. TFR cells were gated as CD4+Foxp3+CD25+PD1+CXCR5+, while TFH cells were gated as CD4+Foxp3−CD25−PD1+CXCR5+. Then, cell populations have analysed for their Bcl-6 expression (TFR and TFH cell population) and IL-21 expression (TFH cell population) (see Supplementary material online, Figure S1A). BREG cells were gated as B220+CD43−CD1dhighCD5+, follicular B cells as B220+CD43−CD21+CD23+ and MZB as B220+CD43−CD21+CD23−CD5−. Then, MZ, FO, and BREG cell population have analysed for their IL-10 and IgM expression, respectively (see Supplementary material online, Figure S2A). All positive cells were defined using a ‘fluorescence minus one’ (FMO) sample.
2.4 Aorta single-cell preparation
The aorta was perfused and, then, cut into pieces before being digested using a cocktail of enzymes for 60 min at 37°C (1.2 mg/mL collagenase P and Dispase I, and 180 U/mL DNase I). Then, aorta single-cell suspension has been subjected to flow cytometry protocol.
2.5 Immunohistochemistry
Mouse aortic sinus was serially cut in 5 μm transversal sections, as previously described.15,16 Sections from mouse specimens were fixed in acetone and immunostained with specific antibodies anti-mouse CD68 (macrophages, ABD Serotec, Düsseldorf, Germany), anti-mouse Ly-6G (neutrophils, BD PharmingenTM, CA, USA), anti-mouse MMP-9 (R&D Systems, MN, USA), or LYVE-1 (Abcam, Cambridge, UK). Vector Red alkaline phosphatase substrate: SK-5100; in association with Levamisole solution: SP-5000; which produce a magenta colouration is used for revelations (Vector Laboratories, Inc., CA, USA). Quantifications were performed using the MetaMorph or Definiens software. Results for other parameters were calculated as percentages of stained area on total lesion area, number of infiltrating cells per mm2 of lesion area or number of lymphatic vessels in adventitia.
2.6 Oil Red O staining for lipid content
Five sections per mouse aortic and aortas sinus were stained with Oil Red O, as previously described.15,16 Sections and aortas were counter-stained with Mayer’s hemalun and rinsed in distilled water. Quantifications were performed using the MetaMorph software. Data were calculated as ratios of stained area on total lesion area.
2.7 Quantitative real-time PCR
Total mRNA was prepared by Trizol® (Thermofischer), according to the provider protocol. Reverse transcription was performed using the ImProm-II Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Real-time PCR (StepOne Plus, Applied Biosystems, Waltham, MA, USA) was performed with the SensiFast (LabGene). Real-time duplex qPCR analysis was conducted as described elsewhere.17,18 The levels of mRNA expression were normalized against the expression of a housekeeping gene (hprt) and analysed using the comparative ΔCT method. Probes were purchased from Applied Biosystems. All measurements were conducted in triplicate.
2.8 Adoptive transfer
Adoptive transfers were performed as previously described.19 Briefly, recipient mice received two intraperitoneal injections of single-cell follicular regulatory helper T cell suspensions collected from three donor mice. Briefly, T cells from spleens and lymph nodes (inguinal, mesenterics, brachial, axillary, and superficial cervical) were isolated by passing the tissue through 70 μm cell strainer (BD Biosciences, MD, USA). Erythrocytes were lysed and nucleated cells washed twice, counted, and suspended in a culture medium. T cell suspensions were enriched with CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec, Germany) and sorted with Beckman Coulter MoFlo Astrios (CD4+CD25+PD1+CXCR5+) under sterile conditions (1.5×105/injection/mouse) (see Supplementary material online, Figure S1B). Dead cells and doublets were excluded based on exclusion dye or forward scatter profiles, respectively. T cell purity (>99%) and phenotype were confirmed by flow cytometry. For TFR homing experiments, TFR was stained with Yellow trace™ (Thermofisher, MA, USA) before their intraperitoneal injections.
2.9 In vitro B cell differentiation assay
Follicular regulatory helper T cells from spleens and LN collected from three Apoe−/− donor mice were enriched with CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec) and sorted with Beckman Coulter MoFlo Astrios (CD4+CD25+PD1+CXCR5+) under sterile conditions (see Supplementary material online, Figure S1A). Dead cells and doublets were excluded based on exclusion dye or forward scatter profiles, respectively. Follicular helper T cells were isolated from flow-through from CD4+CD25+ regulatory T cell isolation kit, which was then positively selected and sorted (CD4+CD25−PD1+CXCR5+). Single-cell suspension followed by purification with the Pan B Cell Isolation Kit (Miltenyi Biotec) was performed in order to obtain purified B cells from Apoe−/− mice. In vitro suppression assays were performed as described.20 Briefly, 5 × 104 B cells, 3 × 104 TFH cells, and/or 750–5 × 104 TFR cells were plated in 96-well plates along with 2 μg/mL anti-CD3 (145-2c11, eBioscience) and 5 μg/mL anti-IgM (FFA21, Invitrogen). For analysis, BREG cells were gated as B220+CD43−IgMhighCD1dhighCD5+, follicular B cells as B220+CD43−CD21+CD23+ and MZB as B220+CD43−CD21+CD23−CD5−. Cell supernatants were harvested, diluted twice, and used to treat purified B cells from Apoe−/− mice. Briefly, 5 × 104 B cells were plated in 96-well plates along with 5 μg/mL anti-IgM (FFA21, Invitrogen) and treated with cells supernatant. For analysis, BREG cells were analysed as described above.
2.10 Statistical analysis
Data are presented as mean ± SEM. For clinical scores, significance between groups was analysed using the non-parametric Mann–Whitney U test because values were not normally distributed and/or the population size was too small (n ≤ 8). *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005.
3. Results
3.1 TFR cell population controls the pro-atherogenic TFH cell population in Apoe−/− mice on an HCD
Recent publications have shown that TFH cell populations are important for the TFH-cell germinal-centre response to an HCD.5,6 It has thus been demonstrated that TFH cell populations expand during the development of atherosclerosis while MZB negatively regulate TFH cells. Both studies suggest that TFH cells are a pro-atherogenic cell population. As TFR cells naturally regulate TFH cells, we investigated the potential anti-atherogenic function of TFR cell populations. The gating strategy described in Supplementary material online, Figure S1A, was used to characterize TFH and TFR cell populations. Positive cells were defined using a FMO sample. We initially considered the spontaneous evolution of TFH and TFR cells in Apoe−/− mice from 5 to 27 weeks. While we observed that CD4+ overall and TFH cell populations in particular increase in secondary lymphoid organs in adult Apoe−/− mice on a normal cholesterol diet as previously described,6 we also observed an expansion of TFR cell populations (Figure 1A). The frequency of TFH cell populations is, however, higher than that of TFR cell populations (Figure 1A). To address the role of TFR cells in atherosclerosis, we used two atherogenesis protocols based on earlier and more advanced phases of the disease.21,22 In the earlier atherosclerosis protocol, mice were fed an NCD for 16 weeks to avoid the onset of severe hypercholesterolaemia. To accelerate atherogenesis in the more advanced atherosclerosis protocol, mice were fed a HCD for 11 weeks (Figure 1B). In parallel, we used Bcl-6 transcriptional repressor (Bcl-6 inhibitor 79-6) to inhibit TFR and TFH cell differentiation. In Apoe−/− mice on HCD, TFH cell populations significantly decreased while TFR cell populations increased leading to sustained changes in the ratio between TFR and TFH cells in secondary lymphoid organs (Figure 1C). In this context, Bcl6 inhibitor injected three times per week for the last 16 weeks (NCD) or 11 weeks (HCD) strongly abrogated TFR and TFH cell differentiation (Figure 1C). Bcl6 inhibitor seems to have also some effects on Th2 and Th17 but not on Th1 in secondary lymphoid organs. Indeed, Th2 cell population is decreased in Bcl6 inhibitor-treated mice in both diet while Th17 seems to be decreased only in spleen of HCD-fed mice (see Supplementary material online, Figure S2A). The disruption of TFH and TFR cell populations in peripheral lymph nodes (PLN) and the spleen induced by Bcl6 inhibitor markedly accelerated both early and advanced atherosclerotic lesion sizes in thoracoabdominal aortas and aortic root as compared to untreated mice (Figure 1D and E). These results suggest that the pro-atherogenic role of TFH cells is less essential than the anti-atherogenic function of TFR cells in the process of atherogenesis. It further implies that regulation of TFH cell populations is not the only function of TFR cells.
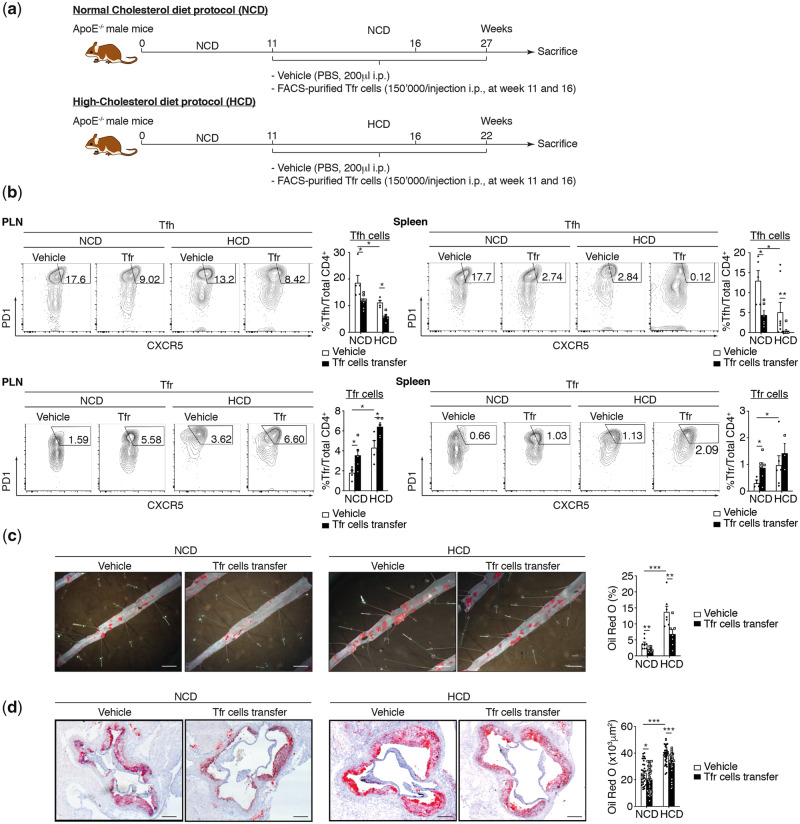
As additional functions of TFR cells have not previously been described, we therefore performed an adoptive transfer approach in order to gain a mechanistic insight. After sorting TFR cells from 27-week-old Apoe−/− mice, we adoptively transferred these cells twice into Apoe−/− recipient mice, at 11 and 16 weeks (Figure 2A and see Supplementary material online, Figure S1B). We observed that TFR cells reach secondary lymphoid organs and aorta at 3 days. They are still present 3-week post-injection (see Supplementary material online, Figure S1C and D). In Apoe−/− mice under NCD and HCD, the increase of TFR cell frequency and the significant reduction of TFH cell populations brought about by adoptive transfer (Figure 2B) abrogates the acceleration both of early and of advanced atherosclerotic lesion size in thoracoabdominal aortas and aortic root as compared to vehicle-treated mice (Figure 2C and D). In both early and advanced atherosclerotic, the adoptive transfer of TFR cells leads also to decrease Th1 and Th2 cell population in spleen while Th2 and Th17 cells are modulated only in lymph nodes(see Supplementary material online, Figure S2B). These data demonstrate that abrogation of both TFH and TFR cell populations leads to the exacerbation of atherosclerotic lesions, whereas TFR cells display atheroprotective properties. This, in turn, means that discarding TFH cell populations diminishes atherogenesis only if TFR cell populations remain present.
Figure 2.
TFR cell population regulates atherogenesis in HCD. (A) Mouse atherosclerosis protocols and adoptive transfer strategy. (B) Effect of TFR cell transfer on secondary lymphoid organs TFH and TFR populations on NCD and HCD fed mice (PLN TFH: n = 4–7 mice/group; PLN TFR: n = 4–5 mice/group; spleen TFH: n = 4–8 mice/group; spleen TFR: n = 3–6 mice/group). (C) Quantification of atherosclerotic lipid content and representative microphotographs from Oil Red O-stained thoracoabdominal aortas in Apoe−/− mice treated with vehicle (PBS) or TFR cells (TFR cell transfer) under NCD and HCD (n = 5–8 mice/group). (D) Quantification of atherosclerotic lipid content and representative microphotographs of aortic root plaques (Oil Red O staining) in Apoe−/− mice treated with vehicle (PBS) or TFR cells (TFR cell transfer) under NCD and HCD (n = 7 or 8 mice per group/5 slides per mice). Scale bars, 500 μm. Original magnification, ×10. Scale bars, 400 μm. The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005. All data were represented as mean ± SEM.
3.2 TFR cells control BREG cell differentiation in presence of TFH cells
TFR cells regulate TFH cell populations while also playing some pleiotropic functions. In this context, TFH cells are known to provide both co-stimulation and stimulatory cytokines for B cell differentiation, leading to the development of GCs.23 In order to determine the additional roles of TFR cells in atherogenesis, we investigated the role of TFR cells in B cell differentiation in an early and advanced atherosclerosis mouse model. Using the gating strategy described in Supplementary material online, Figure S3A, we characterized three potentially important B cell subtypes: follicular B cell (FOB), MZB, and BREG. We observed a marked augmentation of MZB and BREG cell populations in secondary lymphoid organs of mice in advanced atherosclerotic model, while FOB populations and serum IgG1 level decrease (Figure 3A and B and see Supplementary material online, Figure 3B). Bcl6 inhibitors have only a weak effect on FOB and MZB populations in advanced atherosclerosis. In the early atherosclerosis model, Bcl6 inhibitors both diminished and increased the frequencies of FOB and MZB population, respectively (Figure 3A). However, Bcl6 inhibitor induces a strong increase of IgG1, IgM, and IgE in both atherosclerotic models (see Supplementary material online, Figure S3B). Interestingly, Bcl6 inhibitor abrogated BREG cell-augmented frequency brought about by an HCD (Figure 3A) while their ability to produce IL-10 is not modulated neither by an HCD nor by an Bcl6 inhibitor (data not shown). As recent data have indicated that BREG cells may have anti-atherogenic properties,7 we used adoptive transfer of TFR cells (Figure 2A) to evaluate whether BREG cell populations are regulated by TFR cells. We observed that TFR cell transfer induces an expansion of BREG cell populations in advanced atherosclerosis while other B cell subsets are not affected (Figure 3B). TFR cell transfer triggers a significant augmentation of IgM and a diminution of IgE in serum of NCD- and HCD-fed mice (see Supplementary material online, Figure S3C). However, TFR cell transfer increases BREG cells but also FOB populations in early atherosclerosis (Figure 3B). Our data strongly suggest that TFR cells regulate BREG cell populations. We next examined whether TFR cells are also able to regulate the expansion of BREG cell populations in vitro. Following the protocol of Sage et al.,20 we demonstrated that TFR cells do control BREG cell expansion in vitro in presence of TFH cells. BREG cell populations indeed increase proportionally to TFR cell numbers when TFH cells are present (Figure 4A and B), providing direct evidence that TFR cells regulate BREG cell populations. TFR cells also increased FOB populations, although to a lesser extent than BREG cells, but not MZB populations (see Supplementary material online, Figure S4A). The regulation of BREG cell expansion by TFR cells requires direct contact between both of them. In vitro stimulation of B cells with the supernatant from a differentiation assay, in fact, had no effect on BREG cell proliferation or differentiation (Figure 4C). Our results established that TFR cells regulate BREG cell populations both in vivo and in vitro. Our findings further suggest that the ability of TFR cells to trigger BREG cell expansion in advance atherosclerosis contributes to their anti-atherogenic properties.
Figure 3.
TFR cells increase BREG and FOB cell population. (A) Effect of Bcl-6 inhibitor on secondary lymphoid organs B cell populations on an NCD and HCD fed mice (PLN FOB: n = 6 mice/group; PLN MZB: n = 5–7 mice/group; PLN BREG: n = 3–5 mice/group; spleen FOB: n = 5–7 mice/group; spleen MZB: n = 5–8 mice/group; spleen BREG: n = 5–8 mice/group). (B) Effect of TFR cell transfer on secondary lymphoid organs B cell populations on NCD and HCD fed mice (PLN FOB: n = 5–8 mice/group; PLN MZB: n = 5–8 mice/group; PLN BREG: n = 5–7 mice/group; spleen FOB: n = 4–7 mice/group; spleen MZB: n = 4–8 mice/group; spleen BREG: n = 5–7 mice/group). The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005. All data were represented as mean ± SEM.
Figure 4.
TFR cells control BREG cell differentiation in vitro. (A) Representative flow cytometry plots of the effects of dose response of TFR cell number on in vitro BREG cell differentiation in the presence of TFH cells. (B) Quantification of BREG cell differentiation relative to initial BREG cell population and expressed in fold increased (n = 5). (C) Quantification of BREG cell differentiation induced by cell supernatant from in vitro differentiation relative to initial BREG cell population and expressed in fold increase (n = 5). The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05. Data were represented as mean ± SEM.
3.3 TFR cells regulate lymphangiogenesis in advanced atherosclerosis
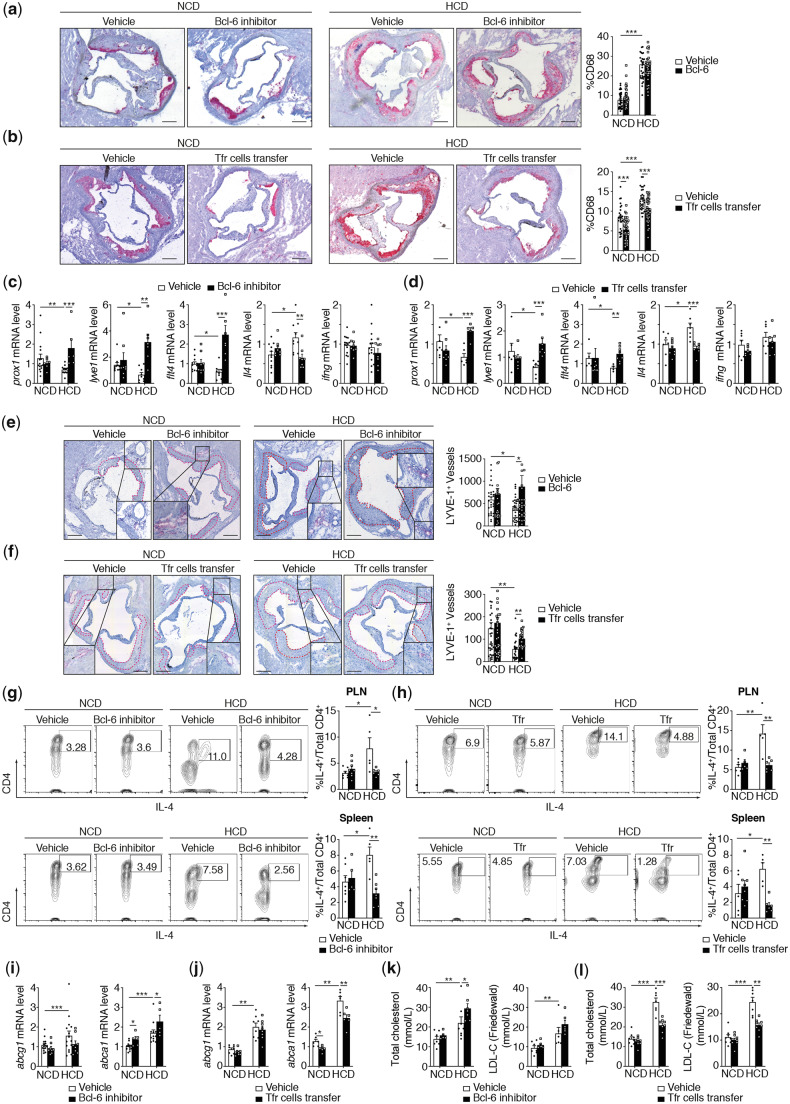
Intriguingly, while the disruption of TFH and TFR cell populations in PLN and the spleen, induced by Bcl6 inhibitors, markedly accelerated both early and advanced atherosclerotic lesion sizes in the aortic root as compared to untreated mice (Figure 1E), the amount of infiltrated macrophages in the aortic root was not affected (Figure 5A). However, the increase of TFR cell frequency and the considerable reduction of TFH cell populations brought about by adoptive transfer (Figure 2B) triggered a significant reduction of infiltrated macrophages (Figure 5B). The relative proportions of macrophage subsets within a plaque could be also an indicator of plaque stability. We thus investigated the M1 and M2 polarization of aortic roots macrophages upon TFR cells adoptive transfer. We have quantified the M1 and M2 subsets population simultaneously based on inducible nitric oxide synthase for M1 macrophages and Arginase-1 for M2 macrophages (see Supplementary material online, Figure S5A).24,25 While M1 macrophages polarization seems to be not affected by HCD, M2 macrophages prominently decreased in HCD-fed mice. TFR adoptive cell transfer do not have significant effects on M1 or M2 macrophages neither in NCD nor in HCD (see Supplementary material online, Figure S5B and C). In this context, the emigration of mononuclear phagocytes or efferocytosis through lymphatic vessels to secondary lymphoid organs, that normally characterizes the resolution of inflammation, is impaired during atherogenesis.26 Furthermore, we observed that lymphatic vessel dysfunction and degeneration present in hypercholesterolaemic mice alters the lipid balance and plasma cholesterol levels.26,27 Finally, it became clear that CD4 T cells and IL-4 regulate lymphatic vessel formation, and that the main producer of IL-4 in GC is the TFH cell population.8,9,28–30 We next considered whether TFR cells control lymphangiogenesis and, in turn, the plasmatic lipid balance by inhibiting TFH cell-dependent IL-4 production. To examine this, we investigated the expression of prospero homeobox 1 (prox1), lymphatic vessel endothelial hyaluronan receptor 1 (lyve1), vascular endothelial growth factor receptor 3 (flt4), and IL-4 (Il4) in the spleens of both Bcl-6-inhibitor and TFR-treated Apoe−/− mice fed with an NCD or an HCD. We observed in advance, atherosclerosis (not in early), a repression of prox1-, lyve1-, and flt4-mRNA expression, is associated to an advances atherosclerotic lesion, thereby demonstrating lymphangiogenesis inhibition in the late stage of the disease (Figure 5C and D). II4 mRNA expression was inversely increased in advance atherosclerosis. Treatment with Bcl-6 inhibitors or TFR cells led to a strong increase in prox1-, lyve1-, and flt4-mRNA expression and concomitantly to an important reduction in Il4-mRNA expression. Although IFNγ could also modulate lymphangiogenesis, its mRNA levels were not affected in advance atherosclerosis (Figure 5C and D). Consistently, the number of LYVE-1+ vessels in adventitia is decreased in advanced lesions while Bcl-6 inhibitors or TFR cell transfer restore the lymphangiogenesis as demonstrated by the increase in the number of LYVE-1+ vessels in adventitia (Figure 5E and F). CD4+IL-4+ cells are clearly and consistently increased by an HCD in secondary lymphoid organs. Bcl-6 inhibitors and TFR cell adoptive transfer inhibit non-Th2-cell CD4+IL-4+ populations (Figure 5G and H and see Supplementary material online, Figure S6A and B).
Figure 5.
TFH cells control lymphangiogenesis through IL-4 secretion. (A) Quantification of CD68 expression and representative microphotographs of aortic root plaques in Apoe−/− mice treated with vehicle (PBS) or Bcl-6 inhibitor under NCD and HCD (n = 6–7 mice per group/5 slides per mice). (B) Quantification of CD68 expression and representative microphotographs of aortic root plaques in Apoe−/− mice treated with vehicle (PBS) or TFR cells (TFR cell transfer) under NCD and HCD (n = 6–7 mice per group/5 slides per mice) Original magnification, ×10. Scale bars, 400 μm. (C) Effect of Bcl-6 inhibitor on mRNA expression of lymphangiogenic regulators in the spleen under NCD and HCD fed mice (prox1: n = 6–15 mice/group; lyve1: n = 7–10 mice/group; ftl4: n = 7–11 mice/group; il4: n = 8–11 mice/group; ifng: n = 8–15 mice/group). (D) Effect of TFR cells transfer on mRNA expression of lymphangiogenic regulators in the spleen of NCD and HCD fed mice (prox1: n = 5–8 mice/group; lyve1: n = 6–7 mice/group; ftl4: n = 4–8 mice/group; il4: n = 7–8 mice/group; ifng: n = 6–7 mice/group). (E) Quantification and representative microphotographs of the number of LYVE-1+ lymphatic vessels in Apoe−/− mice treated with vehicle (PBS) or Bcl-6 inhibitor under NCD and HCD. Red dotted line surround the plaques (n = 8 mice per group/5 slides per group). (F) Quantification and representative microphotographs of the number of LYVE-1+ lymphatic vessels in Apoe−/− mice treated with vehicle (PBS) or TFR cells (TFR cell transfer) under NCD and HCD. Red dotted line surround the plaques (n = 8 mice per group/5 slides per mice). (G) Effect of Bcl-6 inhibitor on secondary lymphoid organs CD4+IL-4+-producing cells in NCD and HCD fed mice (PLN IL-4 n = 5–8 mice/group; spleen IL-4: n = 5–7 mice/group). (H) Effect of TFR cell transfer to secondary lymphoid organs CD4+IL-4+-producing cells in NCD and HCD feed mice (PLN IL-4 n = 5–6 mice/group; spleen IL-4: n = 4–8 mice/group). (I) Effect of Bcl-6 inhibitor on mRNA expression of reverse cholesterol efflux regulators (abcg1 and abca1) on the spleen of NCD and HCD fed mice (n = 5–15 mice/group). (J) Effect of TFR cell transfer on mRNA expression of reverse cholesterol efflux regulators (abcg1 and abca1) in the spleen of NCD and HCD fed mice (n = 6–8 mice/group). (K) Effect of Bcl-6 inhibitor on serum level of total cholesterol and LDL-c (Friedewald) in NCD and HCD fed mice (n = 5–8 mice/group). (L) Effect of TFR cell transfer on serum level of total cholesterol and LDL-c (Friedewald) in NCD and HCD fed mice (n = 7–8 mice/group). The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005. All data were represented as mean ± SEM.
The lymphatic system is involved in cholesterol homoeostasis. Martel et al.11 have specifically shown that the lymphatic vessel route is crucial for RCT, independently of the efflux capacity of cholesterol from macrophages. We, however, examined whether TFH and TFR cell depletion, as well as TFR cell adoptive transfer, could modulate other steps of the RCT process, an observed their effect on ATP-binding cassette transporters ABCA1 and ABCG1, which in turn are involved in cholesterol efflux. Our data indicate that abca1- and abcg1-mRNA expression are increased in the spleen in advance atherosclerosis, although neither Bcl-6 inhibitors nor transferred TFR cells modulate the ABCA1 gene (Figure 5I and J). On the other hand, in both NCD- and HCD-fed mice, abca1 mRNA expression is up-regulated when Bcl-6 inhibitors deplete TFH and TFR cells, whereas it is restrained when TFR cells are transferred (Figure 5I and J). These effects could be also observed with the liver X receptors-α (LXRα) gene (see Supplementary material online, Figure S6C and D). In Apoe−/− mice fed an NCD or HCD, levels of plasmatic LDL-C, calculated using the Friedewald equation, and total cholesterol follow the same profiles as abca1 gene expression (Figure 5K and L). These data suggest that TFH cells regulate lymphangiogenesis through IL-4 secretion. The results further demonstrate that reduction of both TFH and TFR cell populations leads to up-regulation of RCT-involved genes, whereas TFR cells decrease RCT genes. Results also indicated that TFR cell populations regulate plasma cholesterol levels.
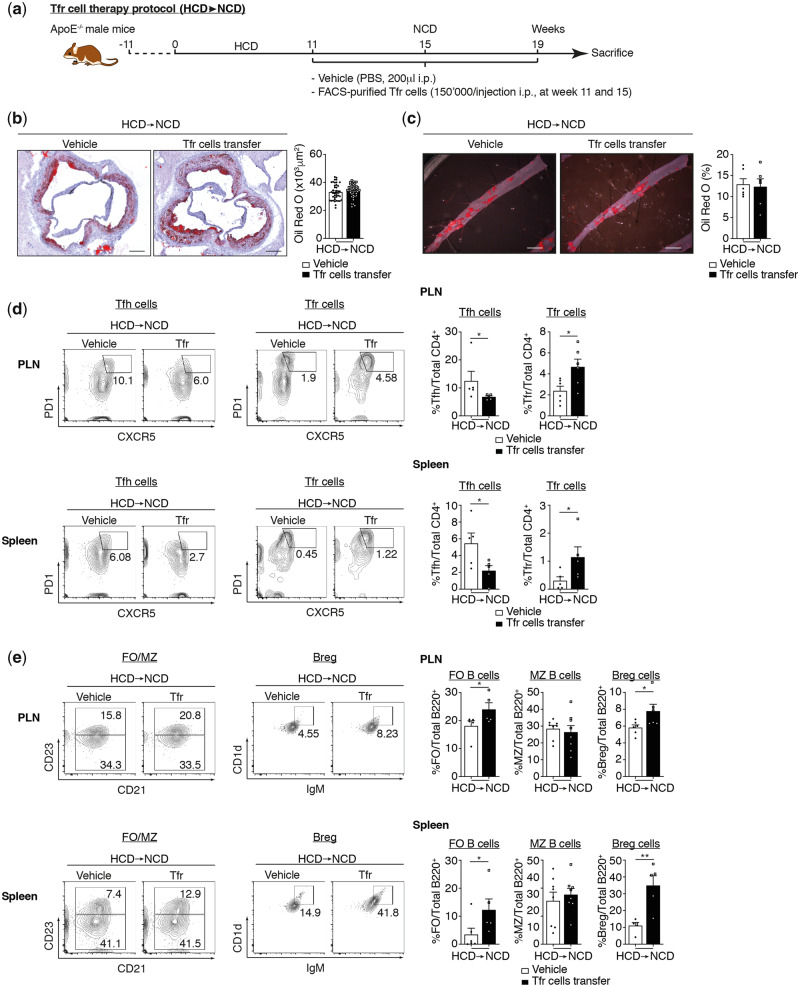
3.4 TFR cell population is not able to repair established atherosclerosis lesions
TFR cell populations carry atheroprotective functions as demonstrated by adoptive transfer (Figure 2C and D). We, therefore, examined the potential atheroregression properties of TFR cells. We used the same protocol as that described previously but this time Apoe−/− mice were fed an HCD for 11 weeks and then switched to an NCD before twice-adoptive transferring TFR cells (Figure 6A). TFR cells transferred to Apoe−/− mice with established atherosclerotic lesions had no significant impact on the size of lesions present in root and abdominal aorta (Figure 6B and C). However, the frequency of TFR and TFH cells in secondary lymphoid organs was increased and decreased by the adoptive transfer of TFR cells, respectively (Figure 6D). As regards the B cell population, we observed that TFR cell transfer increased both BREG and FOB cell populations while MZ B cells were not affected. TFR cell transfer triggers a significant augmentation of IgM and a diminution of IgE in the serum of mice (see Supplementary material online, Figure S6E). These data indicate that TFR cell populations are involved in the regulation of atherogenesis through a dampening of the progression of atherosclerotic lesions. Similarly, reducing TFH cell populations following the establishment of atherosclerotic lesions has no effect on lipid deposition. Altogether, these results show that TFR cell populations cannot be considered as a means of treating established atherosclerosis. Instead, it may be worth evaluating the use of TFR cells in the primary setting for patients with familial hypercholesterolaemia.
Figure 6.
TFR cell population is not able to repair established atherosclerosis lesions. (A) Mouse atherosclerosis protocols and adoptive transfer strategy. (B) Quantification of atherosclerotic lipid content and representative microphotographs of aortic root plaques (Oil Red O staining) in Apoe−/− mice treated with vehicle (PBS) or Bcl-6 inhibitor under NCD and HCD (n = 6 mice/group). Original magnification, ×10. Scale bars, 400 μm. (C) Quantification of atherosclerotic lipid content and representative microphotographs from Oil Red O-stained abdominal aortas (n = 6 mice/group). Scale bars, 500 μm. (D) Effect of TFR cell transfer on secondary lymphoid organs TFH and TFR populations on established atherosclerosis mice (n = 5–6 mice/group). (E) Effect of TFR cell transfer on secondary lymphoid organs in B cell populations on established atherosclerosis mice (n = 5–8 mice/group). The non-parametric Mann–Whitney U test was used for statistical analysis: *P ≤ 0.05. All data were represented as mean ± SEM.
4 Discussion
The important role played by specific B and T cell populations with regard to the adaptive immune response in atherosclerosis appears to be markedly greater than previously established. In fact, it was previously shown that MZB cells, TFH cells, as well as CD8 regulatory T cell populations, can modulate atherosclerosis.5,6 To our knowledge, the function of TFR cells within this context has not been previously investigated. Our results suggest that TFR cell populations play a crucial role in the establishment of atherosclerotic lesions in response to an HCD.
TFH cells clearly constitute a crucial cell population involved in atherogenesis. It has in fact recently been shown that TFH cells carry atherogenic properties and that their defective regulation characterizes atherogenesis.5,6 Consistently, our results have shown a high TFH/TFR cell ratio in young and adult Apoe−/− mice fed on an NCD, while an increase in TFH cell regulation by adoptive transferred TFR cells strongly limits plaque development in an HCD. Nus et al.5 have also demonstrated that TFH cells have proatherogenic functions and that their frequency is regulated by MZB cells when subjected to an HCD. These finding raise the question of mutual regulation of T and B cells in the TFH–GC B cell axis. Our data suggest that BREG cell populations are strongly increased in advanced atherosclerosis. Adoptive transfer of TFR cells reinforces this growth and, in vitro, a differentiation assay of B cells confirmed that TFR cells control BREG differentiation. However, TFR-dependent differentiation of BREG cells required direct contact between both of them, as demonstrated by the disability/inability of a supernatant from in vitro differentiation assay to trigger BREG differentiation. Both B cell populations (MZB and BREG cell populations) possess CD1d molecules on their cell surface.31,32 CD1 proteins belong to a family of major histocompatibility complexes that present lipid molecules or hydrophobic peptide antigens to T cells.33–35 It appears that the uptake of antigenic lipids by CD1-positive DCs might facilitate cell activation, while macrophages take up lipid antigen and degrade it.36 This is consistent with the antigen-presenting cell properties of B cells especially since these cells have regulatory functions in atherogenesis.
While the regulatory mechanisms operating within the follicles prevent autoreactivities they do enable the selection of high-affinity antibody-producing cells. In this context, regulatory B cells correspond to B lymphocytes that control the proportion and function of T and B effector cells. It has been revealed that BREG cells are increased in hypercholesterolaemic mice and protect from lesion development.7 Achour et al.37 have further demonstrated that human BREG cells control TFH cell maturation, expand TFR cells, and inhibit TFH cell-mediated antibody secretion. Our findings indicate that TFR cells control BREG cell populations. It thereby seems that BREG and TFR cell populations are mutually self-regulated, leading to an amplification loop of regulation dampening TFH cell frequency and, in turn, atherogenesis. Consistently, while TFH cell populations are the main producers of IL-4 in GC, IL-4 is able to indirectly inhibit BREG cell expansion.38 IL-4 thus seems to play a crucial function in atherogenesis. It has been clearly demonstrated that double knockout mice, Apoe−/−/IL-4−/−, show a 58% decrease in plaque size in the aortic arch compared to Apoe−/− mice.39 In this context, the anti-lymphangiogenic effects of IL-4 have been established and the functions of lymphangiogenesis in atherosclerosis suggest that the lymphatic system possesses anti-atherogenic properties through the modulation of cholesterol homoeostasis,8,14 at least in the late stages of atherosclerosis.12 It has also been demonstrated that CD4+ T cells transferred to anti-CD3-treated mice decrease the development of lymph node lymphatic vessels.9 Additionally, Gousopoulos et al.40 have shown that Foxp3+ cells transferred into mice which have undergone surgical lymphedema induction promotes lymphatic vessel function in association with a reduced level of expression of IL-4. These findings are consistent with our present data showing that TFR cells stimulate lymphatic vessel expansion through the inhibition of TFH cell-produced IL-4 in a HDC. Altogether, these data demonstrate that TFR cell populations drive pro-lymphangiogenesis and carry anti-atherogenic functions.
The lymphatic system is intimately related to cholesterol homoeostasis, and it has been suggested that Bcl-6 inhibitors are also able to modulate lipid metabolism.11,12,41 As a result, Bcl-6-deficient mice typically have higher serum levels of cholesterol.41 These findings are consistent with our data showing that the Bcl-6 inhibitor induces an increase in the total level of cholesterol. Inversely, it was recently shown that increasing the level of cholesterol leads to CD4+ T cell differentiation in Foxp3+ T cells.42 Hypercholesterolaemia thus induces Foxp3 expression and promotes T cell antigen receptor signalling in CD4+ T cells.43 On the other hand, TREG cell depletion increases atherosclerosis by modulating lipoprotein metabolism.3 Although the exact mechanism underlining the regulation of lipoprotein metabolism by regulatory immunity is not fully understood, our data indicate that the RCT-related gene abca1, (but not abcg1) is increased by Bcl6 inhibitors and decreased by adoptive transfer of TFR cells through LXRα in Apoe−/− mice on an HCD. While inverse variations should be expected because abca1 and abcg1 promote cholesterol efflux from the cells, a deficiency of abca1 promotes foam cell accumulation and accelerates atherosclerosis in mice.44 Similarly, enhanced abcg1 expression increases atherosclerosis in mice with advanced atherosclerosis.45,46 As TFR cell population is able to modulate lipid metabolism, this ability could be a part of to the anti-atherogenic properties of TFR cells. Overall, studies suggest that TREG cell, TFR cell, and lipoprotein metabolism are mutually affected, although the exact mechanisms underlying these interactions remain to be unravelled.
We further established that TFR cells exert atheroprotective effects in at least three different ways. The first is by controlling expansion of the pro-atherogenic TFH cell population in Apoe−/− mice in advanced atherosclerosis. The second is by controlling expansion of BREG cell populations in Apoe−/− mice in advanced atherosclerosis and in vitro. The third is by controlling lymphangiogenesis through the inhibition of IL-4-dependent production by TFH cell populations and decreasing lipoprotein atherogenic profiles. All of which have proved to be mostly anti-atherogenic processes.7,47 Finally, our results indicate that TFR cell populations are unable to repair established atherosclerotic lesions. Consequently, ex vivo expansion of TFR cell populations could be considered for cellular therapy in the context of primary, but not secondary, care treatment of atherosclerosis patients.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
K.J.B. conceived, designed, and supervised the study. F.B., K.M., A.R., D.B., R.F.-S., and K.J.B. performed the experiments. K.J.B., F.B., K.M., C.M., and F.M. analysed the data. K.J.B. and F.M. wrote the article.
Supplementary Material
Acknowledgements
The authors thank also Prof. Mauri and Prof. Monaco for helpful discussions and, especially Aliki Buhayer (Prism Scientific Sàrl; www.prismscientific.ch) for critical review of the present article.
Conflict of interest: none declared.
Funding
This study was supported by European Commission (FP7-INNOVATION I HEALTH-F2-2013-602114; Athero-B-cell: targeting and exploiting B cell function for treatment in CVD). This work was also supported by Swiss National Science Foundation Grants to Prof. François Mach (No. 310030_152912/1) and a Geneva Private Foundation. The funders had no role in study design, data collection, or analysis, nor influenced the decision to publish or preparation of the article.
Time for primary review: 37 days
Translational perspective
This research demonstrates that TFR cells have atheroprotective functions through modulation of different important biological processes present during atherogenesis. Among these biological processes, lymphangiogenesis and BREG proliferation appear to have particular importance across their ability to modulate immune response, inflammation, and cholesterol levels. It may be worth evaluating the use of TFR cells in the primary setting for patients with familial hypercholesterolaemia, although TFR cell populations cannot be considered as a means of treating established atherosclerosis.
References
- 1. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z.. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006;12:178–180. [DOI] [PubMed] [Google Scholar]
- 2. Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH.. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 2011;124:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK.. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 2013;123:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aloulou M, Carr EJ, Gador M, Bignon A, Liblau RS, Fazilleau N, Linterman MA.. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun 2016;7: 10579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, Finigan A, Kitt L, Figg N, Schirmbeck R, Kneilling M, Yeo GSH, Binder CJ, de la Pompa JL, Mallat Z.. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med 2017;23:601–610. [DOI] [PubMed] [Google Scholar]
- 6. Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston AT, Delbosc S, Alsac JM, Bruneval P, Deschildre C, Le Borgne M, Castier Y, Kim HJ, Cantor H, Michel JB, Caligiuri G, Nicoletti A.. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 2015;131:560–570. [DOI] [PubMed] [Google Scholar]
- 7. Strom AC, Cross AJ, Cole JE, Blair PA, Leib C, Goddard ME, Rosser EC, Park I, Hultgardh Nilsson A, Nilsson J, Mauri C, Monaco C.. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via IL-10. Thromb Haemost 2015;114:835–847. [DOI] [PubMed] [Google Scholar]
- 8. Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, Garcia Nores GD, Hespe GE, Nitti MD, Kataru RP, Mehrara BJ.. Th2 cytokines inhibit lymphangiogenesis. PLoS One 2015;10:e0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY.. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011;34:96–107. [DOI] [PubMed] [Google Scholar]
- 10. Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, Swartz MA, Randolph GJ.. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol 2013;190:4608–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ.. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 2013;123:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milasan A, Dallaire F, Mayer G, Martel C.. Effects of LDL receptor modulation on lymphatic function. Sci Rep 2016;6:27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milasan A, Jean G, Dallaire F, Tardif JC, Merhi Y, Sorci-Thomas M, Martel C.. Apolipoprotein A-I modulates atherosclerosis through lymphatic vessel-dependent mechanisms in mice. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kutkut I, Meens MJ, McKee TA, Bochaton-Piallat ML, Kwak BR.. Lymphatic vessels: an emerging actor in atherosclerotic plaque development. Eur J Clin Invest 2015;45:100–108. [DOI] [PubMed] [Google Scholar]
- 15. Carbone F, Crowe LA, Roth A, Burger F, Lenglet S, Braunersreuther V, Brandt KJ, Quercioli A, Mach F, Vallee JP, Montecucco F.. Treatment with anti-RANKL antibody reduces infarct size and attenuates dysfunction impacting on neutrophil-mediated injury. J Mol Cell Cardiol 2016;94:82–94. [DOI] [PubMed] [Google Scholar]
- 16. Montecucco F, Bondarenko AI, Lenglet S, Burger F, Piscitelli F, Carbone F, Roth A, Liberale L, Dallegri F, Brandt KJ, Fraga-Silva RA, Stergiopulos N, Di Marzo V, Mach F.. Treatment with the GPR55 antagonist CID16020046 increases neutrophil activation in mouse atherogenesis. Thromb Haemost 2016;116:987–997. [DOI] [PubMed] [Google Scholar]
- 17. Brandt KJ, Fickentscher C, Kruithof EK, de Moerloose P.. TLR2 ligands induce NF-kappaB activation from endosomal compartments of human monocytes. PLoS One 2013;8:e80743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burger D, Fickentscher C, de Moerloose P, Brandt KJ.. F-actin dampens NLRP3 inflammasome activity via Flightless-I and LRRFIP2. Sci Rep 2016;6:29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH.. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol 2010;185:4410–4419. [DOI] [PubMed] [Google Scholar]
- 20. Sage PT, Sharpe AH, In vitro assay to sensitively measure Tfr suppressive capacity and Tfh stimulation of B cell responses In Espéli M, Linterman M (eds). T Follicular Helper Cells: Methods and Protocols. New York, NY: Springer New York, 2015. pp. 151–160. [Google Scholar]
- 21. Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C.. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2007;27:373–379. [DOI] [PubMed] [Google Scholar]
- 22. Montecucco F, Vuilleumier N, Pagano S, Lenglet S, Bertolotto M, Braunersreuther V, Pelli G, Kovari E, Pane B, Spinella G, Pende A, Palombo D, Dallegri F, Mach F, Roux-Lombard P.. Anti-apolipoprotein A-1 auto-antibodies are active mediators of atherosclerotic plaque vulnerability. Eur Heart J 2011;32:412–421. [DOI] [PubMed] [Google Scholar]
- 23. Sage PT, Sharpe AH.. T follicular regulatory cells. Immunol Rev 2016;271:246–259. [DOI] [PubMed] [Google Scholar]
- 24. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 25. Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H, Owens GK.. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med 2018;24:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ.. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A 2004;101:11779–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ.. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006;24:203–215. [DOI] [PubMed] [Google Scholar]
- 28. Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, Craft J.. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016;17:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG.. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011;17:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zampell JC, Avraham T, Yoder N, Fort N, Yan A, Weitman ES, Mehrara BJ.. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am J Physiol Cell Physiol 2012;302:C392–C404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A.. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol 1998;160:3121–3127. [PubMed] [Google Scholar]
- 32. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK.. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002;16:219–230. [DOI] [PubMed] [Google Scholar]
- 33. Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA.. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol 1999;155:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porcelli SA, Modlin RL.. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 1999;17:297–329. [DOI] [PubMed] [Google Scholar]
- 35. Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM.. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 2000;12:211–221. [DOI] [PubMed] [Google Scholar]
- 36. Major AS, Joyce S, Van Kaer L.. Lipid metabolism, atherogenesis and CD1-restricted antigen presentation. Trends Mol Med 2006;12:270–278. [DOI] [PubMed] [Google Scholar]
- 37. Achour A, Simon Q, Mohr A, Seite JF, Youinou P, Bendaoud B, Ghedira I, Pers JO, Jamin C.. Human regulatory B cells control the TFH cell response. J Allergy Clin Immunol 2017;140:215–222. [DOI] [PubMed] [Google Scholar]
- 38. Taitano SH, Lundy SK.. Regulation of regulatory B cells by Th2 cytokines. J Immunol 2016;196:204–224-204.224. [Google Scholar]
- 39. Davenport P, Tipping PG.. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol 2003;163:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gousopoulos E, Proulx ST, Bachmann SB, Scholl J, Dionyssiou D, Demiri E, Halin C, Dieterich LC, Detmar M.. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 2016;1:e89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LaPensee CR, Lin G, Dent AL, Schwartz J.. Deficiency of the transcriptional repressor B cell lymphoma 6 (Bcl6) is accompanied by dysregulated lipid metabolism. PLoS One 2014;9:e97090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng HY, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, McNamara CA, Sorci-Thomas M, Hedrick CC.. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J Clin Invest 2016;126:3236–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mailer RKW, Gistera A, Polyzos KA, Ketelhuth DFJ, Hansson GK.. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep 2017;7:15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR.. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 2007;117:3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basso F, Amar MJ, Wagner EM, Vaisman B, Paigen B, Santamarina-Fojo S, Remaley AT.. Enhanced ABCG1 expression increases atherosclerosis in LDLr-KO mice on a western diet. Biochem Biophys Res Commun 2006;351:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aiello RJ, Brees D, Francone OL.. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler Thromb Vasc Biol 2003;23:972–980. [DOI] [PubMed] [Google Scholar]
- 47. Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, Yla-Herttuala S.. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.