Abstract
Aims
Our recent study demonstrated that increased Ca2+ sparks and spontaneous transient outward currents (STOCs) played an important role in uterine vascular tone and haemodynamic adaptation to pregnancy. The present study examined the role of ryanodine receptor (RyR) subtypes in regulating Ca2+ sparks/STOCs and myogenic tone in uterine arterial adaptation to pregnancy.
Methods and results
Uterine arteries isolated from non-pregnant and near-term pregnant sheep were used in the present study. Pregnancy increased the association of α and β1 subunits of large-conductance Ca2+-activated K+ (BKCa) channels and enhanced the co-localization of RyR1 and RyR2 with the β1 subunit in the uterine artery. In contrast, RyR3 was not co-localized with BKCa β1 subunit. Knockdown of RyR1 or RyR2 in uterine arteries of pregnant sheep downregulated the β1 but not α subunit of the BKCa channel and decreased the association of α and β1 subunits. Unlike RyR1 and RyR2, knockdown of RyR3 had no significant effect on either expression or association of BKCa subunits. In addition, knockdown of RyR1 or RyR2 significantly decreased Ca2+ spark frequency, suppressed STOCs frequency and amplitude, and increased pressure-dependent myogenic tone in uterine arteries of pregnant animals. RyR3 knockdown did not affect Ca2+ sparks/STOCs and myogenic tone in the uterine artery.
Conclusion
Together, the present study demonstrates a novel mechanistic paradigm of RyR subtypes in the regulation of Ca2+ sparks/STOCs and uterine vascular tone, providing new insights into the mechanisms underlying uterine vascular adaptation to pregnancy.
Keywords: Pregnancy, Uterine arteries, Ryanodine receptor, Large-conductance Ca2+-activated K+, channel, Ca2+ sparks, Spontaneous transient outward currents, Myogenic tone
Graphical Abstract
1. Introduction
To accommodate the demand of foetal growth during pregnancy, uterine vascular tone reduces substantially and uterine blood flow increases dramatically.1 Uterine vascular adaptation has been extensively studied, and yet the mechanisms underlying this phenomenon remain not fully understood. Previous studies have demonstrated that upregulation of the β1 subunit (BKCa β1) of the large-conductance Ca2+-activated K+ channel and enhanced channel function in uterine arteries are essential for reduced uterine vascular tone and increased uterine blood flow.2–7 Under physiological conditions, the activity of BKCa channels in vascular smooth muscle cells is stimulated by ryanodine receptor (RyR)-mediated Ca2+ sparks and exists in the form of spontaneous transient outward currents (STOCs). The K+ efflux carried by STOCs promotes vascular smooth muscle cell membrane hyperpolarization and subsequent closure of L-type voltage-gated Ca2+ (CaV1.2) channels, leading to vasorelaxation.8 The functional coupling between RyRs and BKCa channels is highly efficient that virtually a one-to-one relationship exists between Ca2+ sparks and STOCs.9 The regulation of vascular tone by the Ca2+ spark-STOC coupling has been frequently observed in vascular beds of cerebral and mesenteric circulations.10–14 We have recently revealed that pregnancy attenuates myogenic tone in uterine arteries by promoting Ca2+ spark-STOC coupling in uterine arteries, providing a novel mechanism underlying the uterine vascular adaptation.15 RyRs consist of three subtypes: RyR1, RyR2, and RyR3. All of them are expressed in smooth muscle cells.16 Consistently, uterine arteries also express all RyR subtypes.15 Both RyR1 and RyR2 have been shown to contribute to the generation of Ca2+ sparks.17–19 However, RyR3 was found either not to initiate Ca2+ sparks or to negatively regulate Ca2+ spark generation.17,20 The sarcoplasmic reticulum runs parallel with the plasma membrane and they stay close to each other. This structural arrangement permits a close interaction between RyRs in the sarcoplasmic reticulum membrane and BKCa channels in the plasma membrane. It is estimated that activation of the BKCa channel by Ca2+ requires the channel being located within 10–30 nm of RyRs.21 Interestingly, a Ca2+ microdomain created by Ca2+ sparks may cover a radius of ∼200 nm.22 BKCa β1 residing within the spatial boundaries of the Ca2+ microdomain could then function as a Ca2+ sensor and transmit the Ca2+ signal to the BKCa channel, leading to increased BKCa channel activity.23,24 Importantly, the spatial organization of RyRs and BKCa channels is critical for the Ca2+ spark-STOC coupling.25,26
Our recent study revealed that all three subtypes of RyRs were upregulated in uterine arteries during pregnancy, and the upregulation of RyRs conferred pregnancy-induced increases in Ca2+ spark activity and STOCs and reduction in uterine arterial myogenic tone.15 A question of great importance that needs to be addressed is which subtype(s) of RyRs and its spatial organization with the BKCa channel contribute to the enhanced Ca2+ spark-STOC coupling in uterine arteries in pregnancy. Herein, we present evidence that pregnancy promotes co-localization of RyR1 and RyR2 with BKCa β1, leading to the upregulation of Ca2+ spark activity and STOCs and attenuation of myogenic tone in uterine arteries. Moreover, RyR1 and RyR2 also play a role in regulating the expression of BKCa β1and its association with the BKCa channel α subunit (BKCa α) in uterine arteries. These findings provide novel insights into the mechanisms underlying the physiological adaptation of uterine vasculature during pregnancy.
2. Methods
2.1 Tissue preparation and treatment
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Tissue collection was carried out under anaesthesia. After tissue collection, animals were killed via intravenous injection of 15 mL T-61 solution (Hoechst-Rousel, Somervile, NJ, USA), according to American Veterinary Medical Association guidelines.
Uterine arteries were harvested from non-pregnant or near-term (∼142–145 days of gestation) pregnant sheep.27 Animals were anaesthetized with intravenous injection of propofol (2 mg/kg) followed by intubation, and anaesthesia was maintained on 1.5–3.0% isoflurane balanced in O2 throughout the surgery. An incision was made in the abdomen, and the uterus was exposed. Resistance-sized uterine arteries (∼150–200 µm in diameter) were used in all experiments. Arteries were isolated and removed without stretching and placed into a physiological salt solution (PSS) containing (in mmol/L) 130.0 NaCl, 10.0 HEPES, 6.0 glucose, 4.0 KCl, 4.0 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.18 KH2PO4, and 0.025 EDTA (pH 7.4). To knockdown RyRs, resistance-sized uterine arteries of pregnant sheep were transfected with RyR siRNAs (Dharmacon Inc., Lafayette, CO, USA) as described previously.6 Target or scrambled siRNAs were mixed with HiPerfect Transfection Reagent (Qiagen) and Opti-MEM I (ThermoFisher) for 30–45 min at room temperature and subsequently added into DMEM/F12 supplemented with 1% charcoal-stripped foetal bovine serum. Tissues were incubated with the medium containing target or scrambled siRNAs (control siRNA, the final concentration of 200 nmol/L) in an incubator at 37°C for 48 h.
2.2 Real-time RT-PCR
Total RNA was isolated using TRIzol reagent (Thermo Fisher) and subjected to reverse transcription with iScript cDNA Synthesis system (Bio-Rad, Hercules, CA, USA). The mRNA abundance of RyRs was measured with real-time polymerase chain reaction (PCR) using iQ SYBR Green Supermix (Bio-Rad), as described previously.28 Primers used were 5′-CAGAGGGGGAAAAAGAGGAC-3′ (forward) and 5′-ACGGTGCTGTAGCTCTTGGT-3′ (reverse) for RyR1, 5′-TGAGGCTCACAGGCTTTTCT-3′ (forward) and 5′-ATGCAGGGGATACAGGTTTG-3′ (reverse) for RyR2, and 5′-TAAAGTATGGGCCCGAAGTG-3′ (forward) and 5′-TTTCATTTCTGCTGCCTGTG-3′ (reverse) for RyR3. Ribosomal protein L4 (RPL4) content in uterine arteries was not altered by pregnancy15 and was used for normalization of the abundance of mRNAs. PCR was performed in triplicate, and threshold cycle numbers (CT), generated by CFX connect Real-time System (Bio-Rad), were averaged for each sample. The RNA quality was assessed by a Nanodrop Spectrophotometer (ThermoFisher) by determining A260/A280, A260/A230 values. The relative gene expression of the gene of interest (GOI) was calculated by the modified method.29
2.3 Immunoblotting
Protein abundance of RyR1, RyR2, and RyR3 in uterine arteries was measured as described previously.5 Briefly, tissues were homogenized in a lysis buffer followed by centrifugation at 4°C for 10 min at 10 000 g, and the supernatants were collected. Samples with equal proteins were loaded onto NuPAGE 3–8% Tris-Acetate Protein Gels (Thermo Fisher Scientific, Waltham, MA, USA) and were separated by electrophoresis at 150 V for 1 H. Proteins were then transferred onto nitrocellulose membranes. After blocking non-specific binding sites by dry milk, membranes were incubated with primary antibodies (1:1000 dilution) against rabbit polyclonal RyR1 (8153, Cell Signaling, Danvers, MA, USA), rabbit polyclonal RyR2 (ARR-002, Alomone Labs, Israel), rabbit polyclonal RyR3 (AB9082, EMD Millipore), mouse monoclonal BKCa channel α, or mouse monoclonal BKCa channel β1 (Santa Cruz Biotechnology, Dallas, TX, USA). After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. Results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software (Kodak, Rochester, NY, USA). The target protein abundance was normalized to the abundance of β-actin as a protein loading control, whose abundance in uterine arteries was not altered by pregnancy.15
2.4 Ca2+ spark measurements
Ca2+ sparks were measured in endothelium-denuded uterine arteries loaded with the Ca2+ sensitive dye Fluo-4 AM and using a Zeiss LSM 710 NLO laser scanning confocal imaging workstation on an inverted microscope platform (Zeiss Axio Observer Z1).30 The endothelium was mechanically disrupted by gently pulling a silver wire across the intimal surface of the uterine arterial segments five times, with confirmation by visual analysis of the preparations on the confocal microscope after loading the tissue with Fluo-4. Arterial segments were incubated with 10 μmol/L Fluo-4 AM (Thermo Fisher, Waltham, MA, USA) dissolved in DMSO along with 0.1% pluronic F127 (Thermo Fisher) for 1–1.5 h at room temperature. Tissues were then washed for 30 min to allow dye esterification and then cut into linear strips. The arterial segments were pinned to Sylgard blocks and placed in an open bath imaging chamber mounted on the confocal imaging stage. Cells were illuminated at 488 nm with a krypton argon laser, and the emitted light was collected using a photomultiplier tube. Line scans were imaged at 529 frames/s with the emission signal recorded at 493–622 nm. The acquisition period for Ca2+ spark recordings was 18.9 s. The resultant pixel size ranged from 0.021 to 0.1 μm per pixel. To ensure that sparks within the cell were imaged, the pinhole was adjusted to provide an imaging depth of 2.5 μm. This depth is roughly equivalent to the width of 50% of the cell based on the morphological examination of live preparations. Line scans were analysed using Sparklab 4.2.1 to characterize Ca2+ spark parameters such as frequency (sparks/μm/s), amplitude (F/F0), spatial size [the full width at half maximum (FWHM)], and duration [the full duration at half maximum (FDHM)]. The fractional fluorescence intensity was calculated as F/F0 = F-baseline/F0-baseline, where baseline is the intensity from a region of interest with no cells, F is the fluorescence intensity for the region of interest, and F0 is the fluorescence intensity during a period from the beginning of the recording when there was no Ca2+ activity.
2.5 Measurement of STOCs
Vascular smooth muscle cells were enzymatically dissociated from resistance-sized uterine arteries as described previously.15 Briefly, uterine arteries were minced and incubated (37°C, 10 min) in low-Ca2+ HEPES-buffered physiological salt (PSS) solution containing (in mmol/L) 140.0 NaCl, 5.0 KCl, 0.1 CaCl2, 1.2 MgCl2, 10.0 HEPES, and 10.0 glucose (pH 7.4). Vessels were then exposed to a two-step digestion process that involved: (i) a 60-min incubation in low-Ca2+ HEPES-buffered PSS (37°C) containing 1.5 mg/mL papain (Worthington Biochemical; Lakewood, NJ, USA), 1.5 mg/mL dithiothreitol (MilliporeSigma, St. Louis, MO, USA), and 1.5 mg/mL bovine serum albumin (MilliporeSigma); and (ii) a 60-min incubation in low-Ca2+ HEPES-buffered PSS (37°C) containing 1.5 mg/mL collagenase IV (Worthington), and 1.5 mg/mL bovine serum albumin (MilliporeSigma). Following the enzyme treatment, tissues were washed with low Ca2+ HEPES-buffered PSS. Single smooth muscle cells were released by gently inverting the tube(s) containing low Ca2+ HEPES-buffered PSS and digested tissues several times. The cells were kept at 4°C, and experiments were conducted within 6 h of cell isolation. STOCs were recorded in the whole-cell configuration of the perforated patch-clamp technique using an EPC 10 patch-clamp amplifier with Patchmaster software (HEKA, Lambrecht/Pfalz, Germany) at room temperature as described previously.15 Briefly, cell suspension drops were placed in a recording chamber, and adherent cells were continuously superfused with HEPES-buffered PSS containing (in mmol/L) 140.0 NaCl, 5.0 KCl, 1.8 CaCl2, 1.2 MgCl2, 10.0 HEPES, and 10.0 glucose (pH 7.4). Only relaxed and spindle-shaped vascular smooth muscle cells were used for recording. Micropipettes were pulled from borosilicate glass and had resistances of 2–5 MΩ when filled with the pipette solution containing (in mmol/L) 140.0 KCl, 1.0 MgCl2, 5.0 Na2ATP, 5.0 EGTA, 10.0 HEPES (pH 7.2) with 250 μg/mL amphotericin B. CaCl2 was added to bring free Ca2+ concentrations to 100 nmol/L as determined using WinMAXC software (Chris Patton, Stanford University). Membrane currents were recorded while the cells were held at steady membrane potentials between −50 and 10 mV in 10 mV-increments. STOCs were analysed with Mini Analysis program (Synaptosoft, Leonia, NJ, USA) with a threshold for detection set at 10 pA. The currents were normalized to cell capacitance and expressed as picoampere per picofarad (pA/pF).
2.6 Measurement of pressure-dependent myogenic tone
The pressure-dependent myogenic tone of resistance-sized uterine arteries was measured as described previously.5 Briefly, the arterial segments (∼150 μm diameter) were mounted and pressurized in an organ chamber (Living Systems Instruments, Burlington, VT, USA). The intraluminal pressure was controlled by a servo-system to set transmural pressures, and arterial diameter was recorded using the SoftEdge Acquisition Subsystem (IonOptix LLC, Milton, MA, USA). After the equilibration period, the intraluminal pressure was increased in a stepwise manner from 10 to 100 mmHg in 10-mmHg increments, and each pressure was maintained for 5 min to allow vessel diameter to stabilize before the measurement. Ca2+-free PSS contains zero Ca2+ and 3 mM EGTA. PSS was allowed to pass through the lumen of the pressurized vessels before the detection of myogenic tone, and the myogenic tone was measured under the static flow. The passive pressure–diameter relationship was conducted in Ca2+-free PSS to determine the maximum passive diameter. The following formula was used to calculate the percentage of pressure-dependent tone at each pressure step: % tone = (D1 − D2)/D1 × 100, where D1 is the passive diameter in Ca2+-free PSS, and D2 is the active diameter with normal PSS in the presence of extracellular Ca2+.
2.7 Immunofluorescence staining and Duolink proximity ligation assay
The co-localization of RyRs and BKCa channel was examined using immunofluorescent staining as described previously with modifications.31 After immersion in optimal cutting temperature compound, uterine arteries were sectioned at a thickness of 8 µm using a Leica cryostat (Leica, Buffalo Grove, IL, USA). Uterine arterial slices were blocked in 5% donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) at room temperature (RT) for 1 h, and then incubated with primary antibodies, rabbit polyclonal MaxiKβ antibody (1:200; sc-33608, Santa Cruz Biotechnology), rabbit polyclonal RyR1 antibody (1:100; 8153, Cell Signaling, Danvers, MA, USA), rabbit polyclonal RyR2 (1:100; ARR-002, Alomone Labs, Israel), rabbit polyclonal RyR3 (1:100; AB9082, EMD Millipore), mouse monoclonal BKCa channel α (1:200; sc-374142), or mouse monoclonal BKCa channel β1 (1:200; sc-377023) (Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight. After washing with phosphate-buffered saline (PBS), sections were incubated with corresponding fluorescent secondary antibodies (1:1000, Thermo Fisher Scientific) at RT for 1 h, and then mounted and coverslipped using fluorescent mounting media with a classic nuclear counterstain DAPI (diamidino-2-phenylindole) (VECTOR LABORATORIES, INC., Burlingame, CA, USA). All slices were scanned with a Zeiss LSM 710 confocal microscopy (Zeiss, Oberkochen, Germany). Images were acquired using a z-stack of 1.0 µm intervals. Image stacks were analysed using NIH Image J software.
Proximity ligation assay (PLA) was performed using the Duolink in situ kit (MilliporeSigma, St. Louis, MO, USA) according to the manufacturer’s instructions. After pre-incubation with a blocking agent for 60 min, cryo-fixed samples were incubated overnight with the primary antibodies against RyR1 or RyR2 (1:300) and BKCa β1 (1:300). The PLUS and MINUS PLA probes were diluted (1:5 in the Duolink® Antibody Diluent) and applied to the slides, followed by incubation for 2 h in a pre-heated humidity chamber at 37°C. Unbound PLA probes were removed by washing. The samples were incubated in the ligation solution consisting of Duolink Ligation stock (1:5) and Duolink Ligase (1:40) for 30 min at 37°C. Detection of the amplified probe was done with the Duolink Detection Kit. Duolink Detection stock was diluted at 1:5 and applied for 100 min at 37°C. After final washing, coverslips were mounted onto the slides using a minimal volume of Duolink® in situ mounting medium containing DAPI nuclear stain. Image acquisition was carried out on a Zeiss LSM 710 confocal microscopy. Maximum projection images were analysed using ImageJ (National Institutes of Health, Bethesda, MD, USA) to quantify PLA punctate signals. Images were smoothed, and a threshold to distinguish signal from background fluorescence was applied equally to all images and the number of puncta quantified using the ‘Analyze Particles’ macro with the exclusion criteria of the size of objects being higher than 5 µm.32
2.8 Co-immunoprecipitation
The co-immunoprecipitation experiments were performed with Pierce™ Co-Immunoprecipitation Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Briefly, 20 µg of the monoclonal BKCa α antibody (Santa Cruz Biotechnology, Dallas, TX, USA) were incubated with the delivered resin and covalently coupled. The antibody-coupled resin was incubated with 200 µL of the whole sheep uterine artery protein lysates overnight at 4°C. The resin was washed, and the protein complexes bound to the antibody were eluted. Subsequently, western blot analyses were performed. BKCa α and BKCa β1 protein levels in the immunoprecipitates were analysed by monoclonal mouse antibodies against BKCa α and BKCa β1 (Santa Cruz Biotechnology) and immunoblotting.
2.9 Statistical analysis
Data were expressed as means ± SEM obtained from the number of experimental animals. Data were analysed with GraphPad Prism (GraphPad Software, San Diego, CA, USA). Differences were evaluated for statistical significance (P < 0.05) by one-way analysis of variance (ANOVA) with the post hoc Bonferroni/Dunn test or independent-samples t-test where appropriate.
3. Results
3.1 Pregnancy increased the co-localization of RyR1 and RyR2 with BKCa β1 in uterine arteries
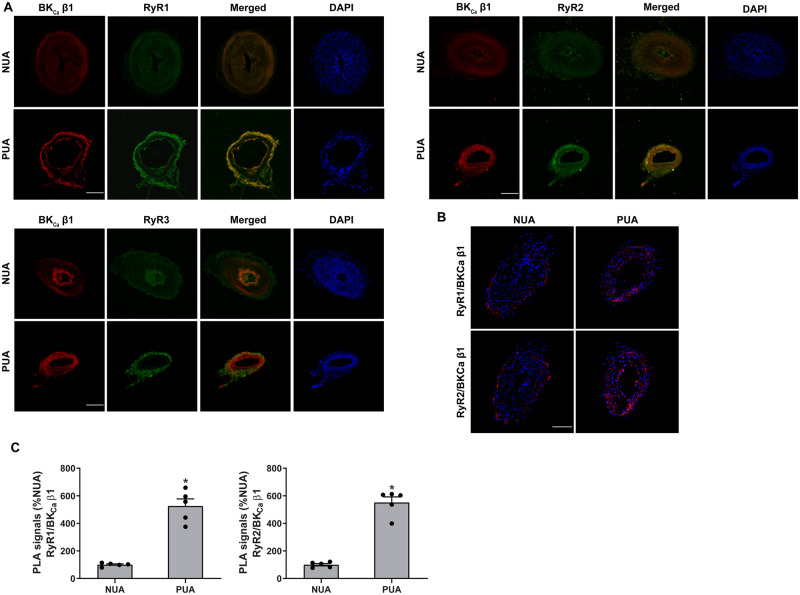
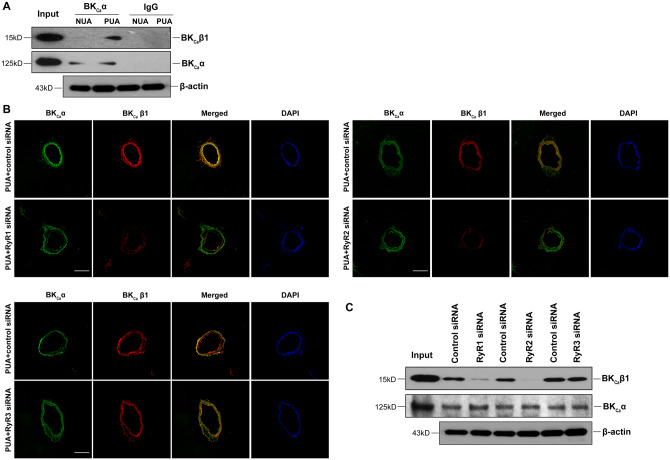
BKCa β1 is the primary sensor of the BKCa channel to Ca2+ sparks mediated by RyRs.33 Hence, we first determined the spatial organization of BKCa β1 and RyRs in uterine arteries using immunofluorescence and confocal microscopy. Figure 1 shows immunofluorescence confocal images of BKCa β1 and RyRs expression in uterine arteries of non-pregnant and pregnant sheep. Apparently, pregnancy increased the expression of BKCa β1 and all three subtypes of RyRs in the tunica media of uterine arteries (Figure 1A), consistent with western blot findings in our previous studies.5,15 Moreover, pregnancy also promoted immunofluorescence co-localization of BKCa β1 with RyR1 and RyR2, respectively. In contrast, the co-localization of BKCa β1 with RyR3 was negligible and not affected by pregnancy. The interactions between BKCa β1 and RyR1/RyR2 were further examined using the PLA assay. As shown in Figures1B andC, pregnancy significantly increased the close co-localization between BKCa β1 and RyR1/RyR2.
Figure 1.
Pregnancy increased co-localization of RyR1/RyR2 with BKCa channel in uterine arteries. (A) Representative confocal immunofluorescence images from five replicates show the co-localization of RyRs and BKCa channels in uterine arteries. Uterine arteries of non-pregnant (NUA) and pregnant (PUA) animals were stained with antibodies against the β1 subunit of BKCa channel (BKCa β1, red) and RyR1, RyR2, or RyR3 (green). Merged images show the co-localization of RyR1 or RyR2 with BKCa β1 (in yellow). The nuclear region was stained with DAPI and shows in blue. Scale bar: 100 µm. (B) PLA assay to confirm the co-localization of RyR1/RyR2 and BKCa β1 in uterine arteries. (C) Quantification of the PLA signals. Images from five independent replicates were analysed. The nuclear region was stained with DAPI and shown in blue. Scale bar: 50 µm. Data are means ± SEM from five animals of each group; independent-samples t-test; *P < 0.05, PUA vs. NUA.
3.2 Knockdown of RyRs functionally impaired Ca2+ sparks and STOCs in uterine arteries
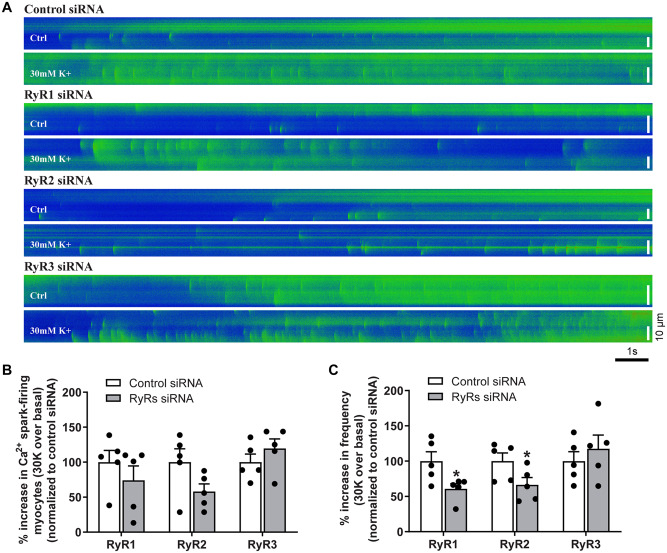
Our previous study revealed that pan-inhibition of RyRs with ryanodine inhibited the Ca2+ spark-STOC coupling in uterine arteries of pregnant sheep.15 To determine the functional importance of individual RyR subtypes, we examined Ca2+ sparks and STOCs in uterine artery vascular smooth muscle cells following knockdown of each RyR subtype using siRNAs. As shown in Supplementary material online, Figure S1, the expression levels of mRNA and protein of each RyR1, RyR2, and RyR3 in uterine arteries were significantly reduced by corresponding RyR siRNAs. Figure 2A illustrates representative line-scan confocal images of Ca2+ sparks in control siRNA and RyR siRNA-treated uterine arteries from pregnant sheep loaded with the Ca2+ indicator Fluo-4. The percentage of Ca2+ spark firing smooth muscle cells in uterine arteries was not significantly altered by the knockdown of RyRs (Figure 2B). However, the ability of membrane depolarization with 30 mmol/L K+ to stimulate Ca2+ spark frequency was significantly decreased by the knockdown of RyR1 and RyR2, respectively, but not significantly affected by RyR3 knockdown (Figure 2C). The other Ca2+ spark parameters such as amplitude (F/F0), width (FWHM), and duration (FDHM) were not or slightly altered by knocking down RyRs in uterine arterial smooth muscle cells (Supplementary material online, Figure S2).
Figure 2.
Knockdown of RyR1/RyR2 decreased Ca2+ sparks in uterine arteries of pregnant sheep. Uterine arteries of pregnant animals were treated with scramble control siRNA or siRNAs for RyR1, RyR2, and RyR3, respectively. Ca2+ sparks in uterine arteries were measured 48 h later. (A) Representative line-scan images of Fluo-4AM loaded uterine arteries showing Ca2+ sparks recorded before and after the sequential application of 30 mmol/L K+ (30 K) following siRNA treatments. (B) Percentage of Ca2+ spark-firing vascular smooth muscle cells. (C) Ca2+ spark frequency. Data are means ± SEM from five animals of each group; independent-samples t-test; *P < 0.05, RyR siRNAs vs. control siRNA.
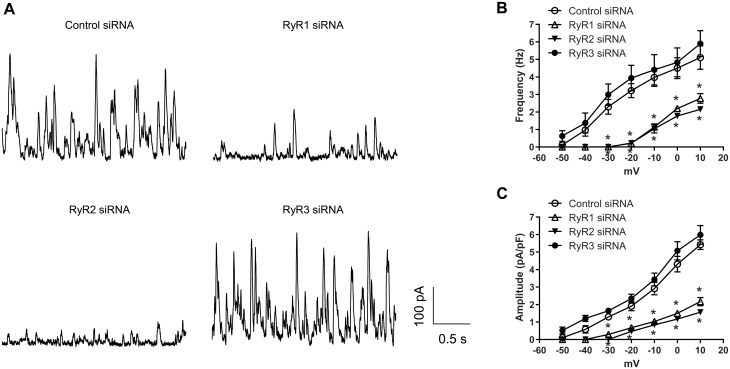
We then examined the contribution of individual RyRs in the regulation of STOCs in uterine arteries of pregnant sheep. Figure 3A shows representative tracing of STOCs in vascular smooth muscle cells isolated from uterine arteries treated with either scramble control siRNA or RyR siRNAs at a holding potential of 10 mV. In agreement with the actions of RyR siRNAs on Ca2+ sparks, RyR1 and RyR2, but not RyR3, knockdown suppressed STOCs. The occurrence of STOCs in RyR1/RyR2-knockdown uterine artery vascular smooth muscle cells was shifted to a much more positive membrane potential (from −40 mV to −20 mV), approximating the phenotype of non-pregnant animals, as demonstrated previously.15 Moreover, knockdown of RyR1 or RyR2 significantly reduced both STOC frequency (Figure 3B) and amplitude (Figure 3C) at membrane potentials between −30 and 10 mV. For example, STOC frequency and amplitude at 10 mV were decreased by 44.7 ± 5.5% and 60.1 ± 4.4% in RyR1-knockdown uterine artery vascular smooth muscle cells, and by 57.2 ± 1.7% and 71.2 ± 2.3% in RyR2-knockdown uterine artery vascular smooth muscle cells, respectively. In contrast, RyR3 knockdown did not significantly alter STOC frequency and amplitude (Figure3B andC). Treatments with control siRNA or RyR siRNAs apparently did not alter the inhibitory effect of BKCa channel inhibitor iberiotoxin on STOCs. Similar to the previous findings in vascular smooth muscle cells from freshly harvested uterine arteries,15 iberiotoxin produced a progressive inhibition of STOCs in smooth muscle cells of siRNA-treated uterine arteries (Supplementary material online, Figure S3).
Figure 3.
Knockdown of RyR1/RyR2 suppressed STOCs in uterine arteries of pregnant sheep. Uterine arteries of pregnant animals were treated with scramble control siRNAs or siRNAs for RyR1, RyR2, and RyR3, respectively. STOCs were measured in uterine artery vascular smooth muscle cells 48 hours later. (A) Representative traces showing STOCs at holding potentials of 10 mV in uterine artery vascular smooth muscle cells. (B) STOC frequency. (C) STOC amplitude. Data are means ± SEM from five animals of each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, RyR siRNAs vs. control siRNA.
3.3 Ryr1 and RyR2 knockdown increased pressure-dependent myogenic tone in uterine arteries
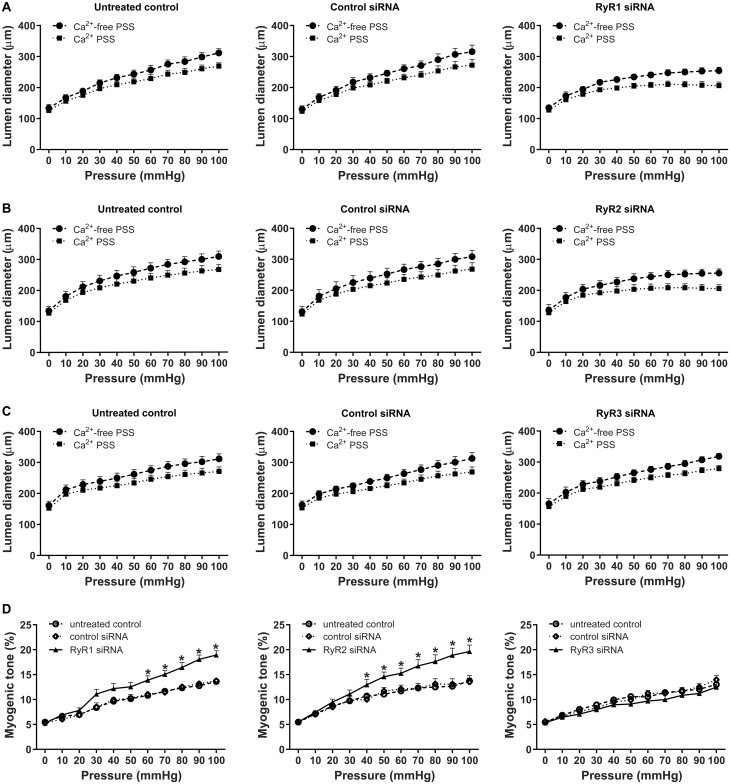
Increased Ca2+ sparks and STOCs are of critical importance in pregnancy-induced attenuation in uterine arterial myogenic tone.15 We thus investigated the functional importance of individual RyR subtypes in regulating pressure-dependent myogenic tone in uterine arteries of pregnant animals by knocking down individual RyR subtypes. Representative traces and averaged data of the myogenic response were shown in Supplementary material online, Figure S4 and Figure 4A–C, respectively. As shown in Figure 4, knockdown of RyR1 or RyR2 significantly increased pressure-dependent myogenic tone (Figure 4D). Consistent with the lack of effect on Ca2+ sparks and STOCs, knockdown of RyR3 had no significant effect on uterine arterial myogenic tone (Figure 4D). Control siRNA had no effect on myogenic tone compared to the untreated control (Figure 4D). Furthermore, treatment with control siRNA did not alter the effect of iberiotoxin on increasing uterine arterial myogenic tone (Supplementary material online, Figure S5). Our previous study demonstrated that iberiotoxin or RyR blockade with ryanodine increased myogenic tone of uterine arteries from pregnant sheep, and combined action of iberiotoxin and ryanodine did not produce additive effects on myogenic tone, as compared to the response induced by each alone.15 Similar findings were obtained in the present study. Iberiotoxin had no additive effect on increased myogenic tone induced by knockdown of RyR1 or RyR2, respectively (Supplementary material online, Figure S5). In contrast to pregnant animals, treatments of uterine arteries of non-pregnant animals with RyR siRNAs had no significant effect on myogenic tone (Supplementary material online, Figure S6).
Figure 4.
Knockdown of RyR1/RyR2 increased myogenic tone in uterine arteries of pregnant sheep. Uterine arteries of pregnant animals were treated with scramble control siRNAs or siRNAs for RyR1, RyR2, and RyR3, respectively. Pressure-dependent myogenic tone was measured in uterine arteries 48 h later. (A–C) Changes of lumen diameters of uterine arteries in response to increases in intravascular pressure. (D) Data summary showing the percentage myogenic tone in RyR siRNA-treated uterine arteries. Data are means ± SEM from five animals of each group; repeated measures ANOVA with the post hoc Bonferroni/Dunn test; *P < 0.05, RyR siRNAs vs. untreated control or control siRNA.
3.4 Ryrs regulated BKCa β1 expression and its association to BKCa α subunit in uterine arteries
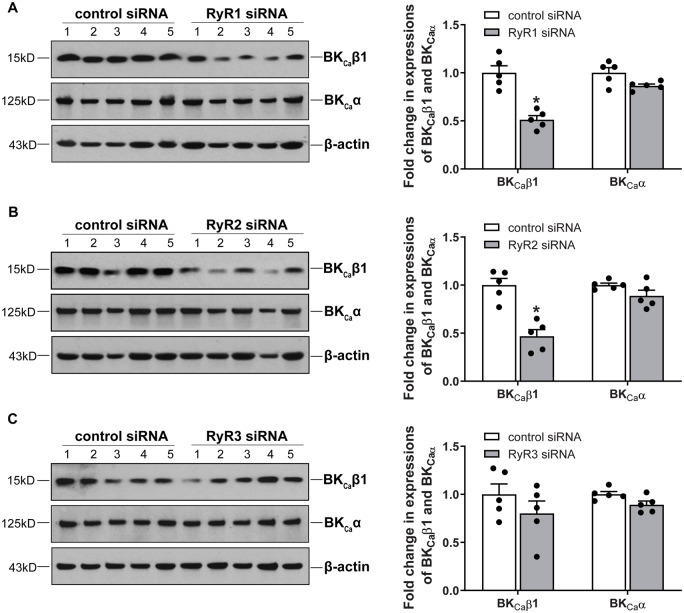
The BKCa channel in smooth muscle cells is heteromeric of the pore-forming α subunit and auxiliary β1 subunit.34,35 The β1:α subunit stoichiometry is dynamically regulated under various physiological and pathophysiological conditions and plays a vital role in regulating the BKCa channel activity in vascular smooth muscle. As shown in Figure 5A, co-immunoprecipitation and western blot demonstrated that pregnancy increased the association of BKCa α and BKCa β1 in uterine arteries. Co-localization of BKCa α and BKCa β1 examined by immunofluorescence confocal microscopy revealed that knockdown of RyR1 or RyR2, but not RyR3, decreased the co-localization of BKCa α and BKCa β1 (Figure 5B). This was further confirmed by co-immunoprecipitation and western blot, as illustrated in Figure 5C. Of interest, knockdown of either RyR1 or RyR2 in uterine arteries of pregnant sheep markedly reduced protein abundance of BKCa β1 when compared to the scramble control siRNA treatment (Figure6A andB). In contrast, the protein expression of BKCa α was not altered by RyR1/RyR2 knockdown (Figure6A andB). Unlike RyR1 or RyR2, RyR3 knockdown altered neither BKCa α nor BKCa β1 protein expression (Figure 6C).
Figure 5.
Knockdown of RyR1/RyR2 reduced the interaction of BKCa channel α and β1 subunits in uterine arteries. (A) Representative immunoblots from five replicates show co-immunoprecipitation of BKCa channel α and β1 subunits in uterine arteries of non-pregnant (NUA) and pregnant (PUA) sheep. Uterine arteries were treated with scramble control siRNA or siRNAs for RyR1, RyR2, and RyR3, respectively, for 48 h. IgG was used as a control to show antibody specificity. (B) Representative confocal immunofluorescence images from five replicates show the co-localization of BKCa channel α and β1 subunits in uterine arteries of pregnant sheep after control siRNAs or RyR siRNAs treatments. The arteries were stained with antibodies against α (green) and β1 (red) subunits. Merged images show in yellow. The nuclear region was stained with DAPI and shows in blue. Scale bar: 100 µm. (C) Representative immunoblots from five replicates show co-immunoprecipitation of BKCa channel α and β1 subunits in uterine arteries of pregnant sheep after control siRNA or RyR siRNAs treatments. β-Actin blots showing equal total protein lysates (input).
Figure 6.
Knockdown of RyR1/RyR2 downregulated the expression of BKCa channel β1 subunit in uterine arteries of pregnant animals. Uterine arteries of pregnant sheep were treated with scramble control siRNA or siRNAs for RyR1, RyR2, and RyR3, respectively. Protein abundance of BKCa channel α and β1 subunits was measured by western blot in uterine arteries 48 h after the treatment. (A) RyR1 siRNAs treatment. (B) RyR2 siRNAs treatment. (C) RyR3 siRNAs treatment. Data are means ± SEM from five animals of each group; independent-samples t-test; *P < 0.05, RyR siRNAs vs. control siRNA.
4. Discussion
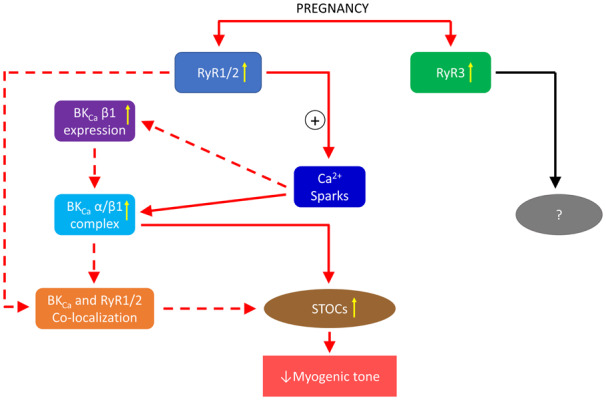
In the present study, we examined the contribution of individual RyR subtypes to pregnancy-induced increase in the Ca2+ spark-STOC coupling and uterine vascular adaptation. The major findings are: (i) pregnancy increased the co-localization of BKCa β1 and RyR1/RyR2 in uterine arteries; (ii) RyR3 was not co-localized with BKCa β1; (iii) RyR1 or RyR2, but not RyR3, knockdown impaired Ca2+ sparks/STOCs and increased myogenic tone in uterine arteries; (iv) pregnancy augmented the association of BKCa α and BKCa β1 in uterine arteries, which was suppressed by RyR1/RyR2, but not RyR3, knockdown; (v) knockdown of RyR1/RyR2 deceased BKCa β1 but not BKCa α expression in uterine arteries.
Owing to its large conductance and high density, the BKCa channel plays a critical role in regulating vascular smooth muscle cell membrane potential and vascular tone.36 Although the BKCa channel can be activated by either voltage or Ca2+, its activity in smooth muscle cells is primarily regulated by Ca2+ sparks mediated by RyRs under physiological conditions.37 The binding affinity (Kd) of Ca2+ for the BKCa channel in smooth muscle cells is ∼20 μmol/L at the physiological membrane potential around −40 mV. A single Ca2+ sparks can rise [Ca2+]i to 10–30 μmol/L, and this Ca2+ microdomain covers a radius of ∼200 nm.22,38 It should be noted that [Ca2+]i decreases sharply when it travels away from the Ca2+ source.21,25 This requires proper spatial organization of the BKCa channel and RyRs for the BKCa channel to sense the Ca2+ signal within the Ca2+ microdomain. The BKCa channel is a heteromeric assembly of the pore-forming BKCa α and auxiliary BKCa β1 in smooth muscle cells and the association of BKCa β1 to BKCa α significantly increases the channel activity by enhancing channel’s Ca2+ sensitivity.33,39 BKCa α co-localized with RyR1/RyR2 in airway and cerebral arterial vascular smooth muscle cells.25,40 To our knowledge, the present study is the first to show the co-localization of BKCa β1 and RyR1/RyR2 in uterine artery vascular smooth muscle cells using immunofluorescence and confocal microscopy and proximity ligation assay that detects protein–protein interaction within 40 nm. It remains to be determined whether the co-localization of RyRs with BKCa β1 may alter the association between BKCaα and BKCaβ1 subunits. Similar to the dynamic association of BKCa β1 and BKCa α subunits in vascular smooth muscle cells,41 it is likely that the co-localization of BKCa β1 with RyR1/RyR2 in uterine artery vascular smooth muscle cells also undergoes a dynamic process. It is currently not known whether the co-localization of RyR1/RyR2 with BKCa β1 may alter the half-life of BKCa β1. Such a spatial proximity keeps BKCa β1 in close contact with RyRs and enables BKCa channel to be activated by Ca2+ sparks, facilitating the functional coupling between Ca2+ sparks and STOCs. A disruption of this spatial organization may impair the coupling. For example, an increase in the distance between sarcoplasmic reticulum and plasma membranes by microtubule depolymerization using nocodazole reduced the number of close contacts between BKCa α and RyR2 in rat cerebral arteries, leading to almost abolition of STOCs.26 Whether and to what extent SR distance from the plasma membrane is altered during pregnancy remain to be determined. Only a small fraction of BKCa channels and RyRs are co-localized in smooth muscle cells.25,40 Interestingly, pregnancy significantly increased the co-localization of BKCa β1 and RyR1/RyR2, suggesting potentially increased contact sites between these proteins, which may account for the enhanced Ca2+ spark-STOC coupling in uterine arteries of pregnant animals, as demonstrated in our previous study.15 Among other mechanisms, the increased co-localization of BKCa β1 and RyR1/RyR2 is probably due to their upregulation in uterine arteries.5,15 Previous studies demonstrated that BKCaβ1 and RyR1/RyR2 mRNA and protein levels in uterine arteries increased in a parallel manner during pregnancy,5,15 suggesting an increase in transcription. It also remains possible that pregnancy may alter half-lives of these proteins, which may contribute to the increase in their abundance in uterine arteries.
The close co-localization of RyRs and BKCa channels allows BKCa β1 to function as a vital relay that functionally couples the Ca2+ sparks to STOCs. The integrity of the coupling machinery is essential for signal transduction. Knockdown of RyR1/RyR2, which could interrupt the coupling of RyRs and BKCa channels, impaired the Ca2+ spark-STOC coupling as evidenced by blunted 30 mmol/L K+-stimulated Ca2+ spark activity and diminished STOCs in uterine artery vascular smooth muscle cells. These findings are in agreement with previous observations.17–19,42 The finding that knockdown of RyR1/2 had no significant effect on the proportion of Ca2+ spark-firing smooth muscle cells, but reduced Ca2+ spark frequency is intriguing and suggests that the primary action of RyR knockdown is to reduce Ca2+ spark frequency within the cells (which hence decreases the Ca2+ spark-STOC coupling), but not the number of firing cells. This is consistent with the notion that gene knockdown only reduces, but not silences, the gene of interest in the cells. In contrast to RyR1/RyR2, RyR3 was not co-localized with BKCa β1 in uterine arteries. Similarly, RyR3 and BKCa α were not co-localized in airway smooth muscle cells.25 Functionally, RyR3 knockdown did not alter either Ca2+ sparks or STOCs. This is in line with observations reported by Mironneau et al.,17 but not by Löhn et al. and Matsuki et al.20,43 The finding that RyR1/RyR2, but not RyR3 was co-localized with BKCa β1 is intriguing. Several previous studies demonstrated a distinct spatial distribution of RyRs in smooth muscle cells.25,44–46 In general, RyR2 is predominantly located in the subplasmalemmal region and RyR3 is in the perinuclear region, whereas RyR1 is located in both subplasmalemmal and perinuclear regions. This distinct spatial distribution of RyRs is likely to contribute to the differential interaction of RyRs and BKCa channels. The aberrant expression of BKCa β1 could also impact the Ca2+ spark-STOC coupling in vascular smooth muscle cells. The coupling was attenuated and boosted by BKCa β1 deficiency and BKCa β1 overexpression, respectively.23,33,47
We previously demonstrated that enhanced Ca2+ spark-STOC coupling in the uterine arteries during pregnancy was associated with upregulation of all three subtypes of RyRs.15 Physiologically, the enhanced Ca2+ spark-STOC coupling during pregnancy was found to promote uterine vascular adaptation by attenuating uterine arterial myogenic tone.15 However, the roles of individual RyRs in the pregnancy-induced enhancement of Ca2+ spark-STOC coupling remain elusive. Interestingly, in the present study, we demonstrated that knockdown of RyR1/RyR2, but not RyR3, in uterine arteries of pregnant animals repressed the Ca2+ spark-STOC coupling and increased uterine arterial myogenic tone. The finding that knockdown of RyR1/2 has little effect on myogenic tone in uterine arteries of non-pregnant animals is not surprising, and indeed is consistent with the previous finding that the pan-inhibition of ryanodine receptors with ryanodine had no significant effect on myogenic tone in uterine artery of non-pregnant sheep.15 Apparently, repressing RyR1/RyR2 in uterine arteries of pregnant animals using the RNA interference tool produced a phenotype resembles uterine arteries of non-pregnant animals. The finding that siRNAs knockdown RyR1 and RyR2 about 40% at the mRNA level but about 60% of protein abundance is not surprising. Indeed, it is not uncommon that changes in mRNA abundance may not always precisely correlate to changes in protein abundance due to post-transcriptional, translational, and protein degradation regulations.48–50 Although downregulation of RyRs had no significant effect on Ca2+ spark firing smooth muscle cells, knockdown of RyR1 and RyR2 significantly decreased Ca2+ spark frequency, STOC frequency and amplitude, and increased myogenic tone in uterine arteries. Thus, the present study established a cause-and-effect relationship between RyR1/RyR2 and the Ca2+ spark-STOC coupling in the regulation of uterine arterial myogenic tone during pregnancy. Although RyR3 is upregulated in uterine arteries during pregnancy,15 its physiological relevance is currently unclear. Both RyR1 and RyR2 participate in generating Ca2+ sparks in smooth muscle cells.18,19,51 Arterial stiffness is affected by vascular smooth muscle cell tone.52 It is not surprising that knockdown of RyR1 or RyR2 affected arterial stiffness via altering myogenic tone in uterine arteries. The apparent redundancy of increasing both RyR1 and RyR2 in uterine arteries during pregnancy is likely to provide a protective mechanism to ensure the adaptation of Ca2+ dynamics and myogenic tone in uterine arteries and the optimization of uterine blood flow during pregnancy. Whether and to what extent RyR1 and RyR2 may produce additive or synergistic effect in the regulation of Ca2+ sparks remains to be determined. Together, this study revealed an explicit contribution of RyR1/RyR2 in mediating enhanced Ca2+-release events that resulted in reduced vascular tone in the uterine circulation during pregnancy.
The association of BKCa β1 to BKCa α is essential for the channel to regulate arterial vascular smooth muscle cell membrane potential, contractility, and blood pressure.23,33,35 It is not surprising that pregnancy increased the association of BKCa β1 to BKCa α in the uterine artery in the present study. However, it is somewhat surprising that RyR1/RyR2 knockdown promoted BKCa β1 downregulation and decreased the association of BKCa α and BKCa β1 in uterine arteries of pregnant animals. These observations are puzzling at first glance. However, digging into Ca2+ sparks’ action could help unfold this mysterious issue. RyR-mediated Ca2+ sparks in vascular smooth muscle cells have been shown to counter vasoconstriction via BKCa channel-mediated hyperpolarization of the vascular smooth muscle cell membrane and closing CaV1.2.10,11 It is conceivable that RyR1/RyR2 knockdown would diminish the inhibitory effect of Ca2+ sparks on CaV1.2 in uterine artery vascular smooth muscle cells, thus leading to increased Ca2+ influx through CaV1.2. The regulation of gene expression by CaV1.2 activity is a well-established phenomenon and frequently involves transcription factors such as cAMP-response element binding protein and nuclear factor of activated T-cells (NFAT).53 Intriguingly, in an animal model of hypertension, in vivo administration of angiotensin II activated NFATc3 via stimulating CaV1.2-mediated Ca2+ influx.54 The activation of NFATc3 consequently led to downregulation of BKCa β1 in vascular smooth muscle cells. Significantly, RyR inhibition with ryanodine enhanced transcriptional activities of NFAT in skeletal muscle fibres and potentiated UTP-induced NFATc3 nuclear accumulation in cerebral arteries.55,56 It is possible that RyR1/RyR2 knockdown induces the activation of NFATc3, which confers the downregulation of BKCa β1 in uterine arteries. The diminished BKCaβ1 abundance in uterine arterial smooth muscle probably then contributed to the reduced association of BKCa α and BKCa β1. To our knowledge, this study is the first to provide evidence that RyR1/RyR2 participate in the regulation of BKCa β1 expression. These findings suggest that RyRs play an important role in maintaining BKCa β1 homeostasis and the dynamic interaction of BKCa α and BKCa β1 in the uterine artery in pregnancy.
Adequate uterine vascular adaptation is essential for a successful pregnancy. We recently demonstrated an important role of RyR-mediated Ca2+ sparks and BKCa channel-mediated STOCs in this adaptation.15 In the present study, we revealed (i) enhanced close co-localization between RyR1/RyR2 and BKCa β1 in uterine arteries during pregnancy and (ii) a cause-and-effect relationship of RyR1/RyR2 in the enhanced Ca2+ spark-STOC coupling and attenuated uterine arterial myogenic tone in uterine arteries of pregnant animals. Moreover, our findings also suggest the regulatory divergence of RyRs on the Ca2+ spark-STOC cascade at two levels: direct regulation of Ca2+ sparks and indirect regulation of BKCa β1 expression and the dynamic interaction of BKCa α and BKCa β1 as a consequence of altered Ca2+ sparks. Together, these novel findings provide new mechanistic insights into pregnancy-induced uterine vascular adaptation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health Grants (HD083132, HL128209, and HL137649 to L.Z.).
Time for primary review: 32 days
Translational perspective
A successful pregnancy requires adequate uterine vascular adaptation. The present study demonstrates that pregnancy-induced RyR1 and RyR2 upregulation in uterine arteries exerts their regulatory role on uterine arterial myogenic tone via impacting the Ca2+ spark-STOC coupling, BKCa β1 expression, and association of BKCa β1 and BKCa α. Our findings reveal a crucial role of the RyRs-BKCa channel partnership in mediating pregnancy-induced uterine vascular adaptation. Thus, our findings provide valuable insights into understanding the mechanisms underlying uterine vascular adaptation in pregnancy.
References
- 1. Ducsay CA, Goyal R, Pearce WJ, Wilson S, Hu XQ, Zhang L.. Gestational hypoxia and developmental plasticity. Physiol Rev 2018;98:1241–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenfeld CR, White RE, Roy T, Cox BE.. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol 2000;279:H319–H328. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld CR, Roy T, DeSpain K, Cox BE.. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig 2005;12:402–408. [DOI] [PubMed] [Google Scholar]
- 4. Rosenfeld CR, Liu XT, DeSpain K.. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol 2009;296:H1878–H1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L.. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension 2011;58:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu XQ, Dasgupta C, Chen M, Xiao D, Huang X, Han L, Yang S, Xu Z, Zhang L.. Pregnancy reprograms large-conductance Ca(2+)-activated K(+) channel in uterine arteries: roles of ten-eleven translocation methylcytosine dioxygenase 1-mediated active demethylation. Hypertension 2017;69:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L.. MicroRNA-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance Ca(2+)-activated K(+) channel expression and function in ovine uterine arteries. Hypertension 2017;70:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brayden JE, Nelson MT.. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992;256:532–535. [DOI] [PubMed] [Google Scholar]
- 9. Perez GJ, Bonev AD, Patlak JB, Nelson MT.. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 1999;113:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ.. Relaxation of arterial smooth muscle by calcium sparks. Science 1995;270:633–637. [DOI] [PubMed] [Google Scholar]
- 11. Knot HJ, Standen NB, Nelson MT.. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 1998;508: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang GH, Xi Q, Leffler CW, Jaggar JH.. Hydrogen sulfide activates Ca(2)(+) sparks to induce cerebral arteriole dilatation. J Physiol 2012;590:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson-Weaver O, Osmond JM, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL.. Intermittent hypoxia in rats reduces activation of Ca2+ sparks in mesenteric arteries. Am J Physiol Heart Circ Physiol 2015;309:H1915–H1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khavandi K, Baylie RA, Sugden SA, Ahmed M, Csato V, Eaton P, Hill-Eubanks DC, Bonev AD, Nelson MT, Greenstein AS.. Pressure-induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels. Sci Signal 2016;9:ra100–ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu XQ, Song R, Romero M, Dasgupta C, Huang X, Holguin MA, Williams V, Xiao D, Wilson SM, Zhang L.. Pregnancy increases Ca(2+) sparks/spontaneous transient outward currents and reduces uterine arterial myogenic tone. Hypertension 2019;73:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Essin K, Gollasch M.. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol 2009;2009:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mironneau J, Coussin F, Jeyakumar LH, Fleischer S, Mironneau C, Macrez N.. Contribution of ryanodine receptor subtype 3 to Ca2+ responses in Ca2+-overloaded cultured rat portal vein myocytes. J Biol Chem 2001;76:11257–11264. [DOI] [PubMed] [Google Scholar]
- 18. Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI.. RYR2 proteins contribute to the formation of Ca(2+) sparks in smooth muscle. J Gen Physiol 2004;123:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fritz N, Morel JL, Jeyakumar LH, Fleischer S, Allen PD, Mironneau J, Macrez N.. RyR1-specific requirement for depolarization-induced Ca2+ sparks in urinary bladder smooth muscle. J Cell Sci 2007;120:3784–3791. [DOI] [PubMed] [Google Scholar]
- 20. Matsuki K, Kato D, Takemoto M, Suzuki Y, Yamamura H, Ohya S, Takeshima H, Imaizumi Y.. Negative regulation of cellular Ca(2+) mobilization by ryanodine receptor type 3 in mouse mesenteric artery smooth muscle. Am J Physiol Cell Physiol 2018;315:C1–C9. [DOI] [PubMed] [Google Scholar]
- 21. Fakler B, Adelman JP.. Control of K(Ca) channels by calcium nano/microdomains. Neuron 2008;59:873–881. [DOI] [PubMed] [Google Scholar]
- 22. McCarron JG, Chalmers S, Bradley KN, MacMillan D, Muir TC.. Ca2+ microdomains in smooth muscle. Cell Calcium 2006;40:461–493. [DOI] [PubMed] [Google Scholar]
- 23. Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O.. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 2000;87:E53–E60. [DOI] [PubMed] [Google Scholar]
- 24. Lohn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M.. beta(1)-Subunit of BK channels regulates arterial wall[Ca(2+)] and diameter in mouse cerebral arteries. J Appl Physiol (1985) 2001;91:1350–1354. [DOI] [PubMed] [Google Scholar]
- 25. Lifshitz LM, Carmichael JD, Lai FA, Sorrentino V, Bellve K, Fogarty KE, ZhuGe R.. Spatial organization of RYRs and BK channels underlying the activation of STOCs by Ca(2+) sparks in airway myocytes. J Gen Physiol 2011;138:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pritchard HAT, Gonzales AL, Pires PW, Drumm BT, Ko EA, Sanders KM, Hennig GW, Earley S.. Microtubule structures underlying the sarcoplasmic reticulum support peripheral coupling sites to regulate smooth muscle contractility. Sci Signal 2017;10: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L.. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension 2010;56:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen M, Dasgupta C, Xiong F, Zhang L.. Epigenetic upregulation of large-conductance Ca2+-activated K+ channel expression in uterine vascular adaptation to pregnancy. Hypertension 2014;64:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, Menon BK, Mufti RE, Watson T, Starreveld Y, Furstenhaupt T, Muellerleile PR, Kurjiaka DT, Kyle BD, Braun AP, Welsh DG.. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res 2014;115:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma Q, Dasgupta C, Li Y, Huang L, Zhang L.. MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int J Mol Sci 2017;18:1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cahill E, Pascoli V, Trifilieff P, Savoldi D, Kappes V, Luscher C, Caboche J, Vanhoutte P.. D1R/GluN1 complexes in the striatum integrate dopamine and glutamate signalling to control synaptic plasticity and cocaine-induced responses. Mol Psychiatry 2014;19:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW.. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 2000;407:870–876. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka Y, Koike K, Alioua A, Shigenobu K, Stefani E, Toro L.. Beta1-subunit of MaxiK channel in smooth muscle: a key molecule which tunes muscle mechanical activity. J Pharmacol Sci 2004;94:339–347. [DOI] [PubMed] [Google Scholar]
- 35. Hu XQ, Zhang L.. Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells. Drug Discov Today 2012;17:974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ledoux J, Werner ME, Brayden JE, Nelson MT.. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. [DOI] [PubMed] [Google Scholar]
- 37. Jaggar JH, Porter VA, Lederer WJ, Nelson MT.. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 2000;278:C235–C256. [DOI] [PubMed] [Google Scholar]
- 38. Perez GJ, Bonev AD, Nelson MT.. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 2001;281:C1769–C1775. [DOI] [PubMed] [Google Scholar]
- 39. Cox DH, Aldrich RW.. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol 2000;116:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pritchard HAT, Pires PW, Yamasaki E, Thakore P, Earley S.. Nanoscale remodeling of ryanodine receptor cluster size underlies cerebral microvascular dysfunction in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2018;115:E9745–E9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leo MD, Zhai X, Muralidharan P, Kuruvilla KP, Bulley S, Boop FA, Jaggar JH.. Membrane depolarization activates BK channels through ROCK-mediated beta1 subunit surface trafficking to limit vasoconstriction. Sci Signal 2017;10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaßmann M, Szijártó IA, García-Prieto CF, Fan G, Schleifenbaum J, Anistan Y-M, Tabeling C, Shi Y, Le Noble F, Witzenrath M, Huang Y, Markó L, Nelson MT, Gollasch M.. Role of ryanodine type 2 receptors in elementary Ca(2+) signaling in arteries and vascular adaptive responses. J Am Heart Assoc 2019;8:e010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LöHn M, Jessner W, FüRstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M, Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res 2001;89:1051–1057. [DOI] [PubMed] [Google Scholar]
- 44. Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS.. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L338–L348. [DOI] [PubMed] [Google Scholar]
- 45. Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM.. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 2008;44:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM.. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am J Physiol Cell Physiol 2010;299:C264–C278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H.. Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res 2007;101:493–502. [DOI] [PubMed] [Google Scholar]
- 48. Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M.. Global quantification of mammalian gene expression control. Nature 2011;473:337–342. [DOI] [PubMed] [Google Scholar]
- 49. Vogel C, Marcotte EM.. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012;13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Beyer A, Aebersold R.. On the dependency of cellular protein levels on mRNA abundance. Cell 2016;165:535–550. [DOI] [PubMed] [Google Scholar]
- 51. Coussin F, Macrez N, Morel JL, Mironneau J.. Requirement of ryanodine receptor subtypes 1 and 2 for Ca(2+)-induced Ca(2+) release in vascular myocytes. J Biol Chem 2000;275:9596–9603. [DOI] [PubMed] [Google Scholar]
- 52. Zieman SJ, Melenovsky V, Kass DA.. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriscler Thromb Vasc Biol 2005;25:932–943. [DOI] [PubMed] [Google Scholar]
- 53. Barbado M, Fablet K, Ronjat M, De Waard M.. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta 2009;1793:1096–1104. [DOI] [PubMed] [Google Scholar]
- 54. Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF.. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 2007;282:3231–3240. [DOI] [PubMed] [Google Scholar]
- 55. Jordan T, Jiang H, Li H, DiMario JX.. Inhibition of ryanodine receptor 1 in fast skeletal muscle fibers induces a fast-to-slow muscle fiber type transition. J Cell Sci 2004;117:6175–6183. [DOI] [PubMed] [Google Scholar]
- 56. Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT.. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem 2002;277:37756–37764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.