Abstract
Uncovering the moment-to-moment dynamics of functional connectivity (FC) in the human brain during early development is crucial for understanding emerging complex cognitive functions and behaviors. To this end, this paper leveraged a longitudinal resting-state functional magnetic resonance imaging dataset from 51 typically developing infants and, for the first time, thoroughly investigated how the temporal variability of the FC architecture develops at the “global” (entire brain), “mesoscale” (functional system), and “local” (brain region) levels in the first 2 years of age. Our results revealed that, in such a pivotal stage, 1) the whole-brain FC dynamic is linearly increased; 2) the high-order functional systems tend to display increased FC dynamics for both within- and between-network connections, while the primary systems show the opposite trajectories; and 3) many frontal regions have increasing FC dynamics despite large heterogeneity in developmental trajectories and velocities. All these findings indicate that the brain is gradually reconfigured toward a more flexible, dynamic, and adaptive system with globally increasing but locally heterogeneous trajectories in the first 2 postnatal years, explaining why infants have rapidly developing high-order cognitive functions and complex behaviors.
Keywords: brain development, connectome, dynamic functional connectivity, infant, temporal variability
Introduction
The first 2 postnatal years are a pivotal period of life with rapid development in the human brain structure and function. During this period, the overall brain size of the infant reaches 80% of adult’s volume (Knickmeyer et al. 2008) concurrent with dramatic increases in cortical thickness (Lyall et al. 2015) and surface area (Li et al. 2013). Brain functions also achieve remarkable development, including the improved vision (Courage and Adams 1990) and body manipulation, and acquisition of several higher-order cognitive functions, such as self-awareness (Amsterdam 1972), spatial attention (Haith et al. 1988), and working memory (Reznick 2008). Compared with the anatomical organization, functional neural circuits represent more direct mediators of the brain’s diverse functional capabilities (Gao et al. 2017). Delineating their development in the first 2 years of life would provide unprecedented insights into the neuro-mechanism of infants’ fast-growing behavioral repertoire in early life.
In recent years, resting-state functional magnetic resonance imaging (rs-fMRI) has been emerging as a useful tool for probing functional brain development (Smyser et al. 2010, 2011; Gao et al. 2011, 2012, 2015a, 2015b; Alcauter et al. 2014; Damaraju et al. 2014; De Asis-Cruz et al. 2015; Wen et al. 2019). The most popular approach for this type of study is to represent the infant brain as a complex network that consists of brain regions as nodes linked by edges, estimated by functional connectivity (FC), and then delineate developmental trajectories of various network topological properties across age (Smyser et al. 2011; Gao et al. 2011; Gao et al. 2015a; Damaraju et al. 2014; De Asis-Cruz et al. 2015; Wen et al. 2019). Based on this method, existing studies have revealed the brain functional network development at multiple levels, including global (i.e., whole brain) (Gao et al. 2011; Wen et al. 2019), mesoscale (i.e., functional subnetworks) (Gao and Lin 2012; Gao et al. 2015a; Gao et al. 2015b), and local levels (i.e., brain regions) (Wen et al. 2019). Generally, the brain network is globally reconfigured to be a more efficient balance between local and global information communications from neonates to 2 years of age (Gao et al. 2011; Wen et al. 2019). Different functional subnetworks are developed with diverse developmental trajectories, with primary systems mature earlier than high-order function-related systems (Gao et al. 2015a; Gao et al. 2015b). From the local level, our recent findings suggest that more association regions gradually emerge as connector hubs facilitating information exchange and integration among functional subnetworks, while many primary regions become less centralized (Gao et al. 2011; Wen et al. 2019). Although providing valuable insights, these studies all investigate FC from a “static” point of view, ignoring its essential “temporal dynamics.”
Mountainous evidence has been indicating that the brain functional network dynamically reconfigures itself from connectivity patterns of subnetworks to functional architectures of brain regions over seconds to minutes both at rest (Zalesky et al. 2014) and during task performance (Braun et al. 2015), supporting the adaptive integration between different neural systems in response to rapidly changing internal and external stimulus (Hutchison et al. 2013; Calhoun et al. 2014). Distinct functional subnetworks or brain regions establish different degrees of variability across time relevant to their roles/functions in neural coordination for the implementation of diverse cognitions and behaviors (Cole et al. 2013; Braun et al. 2015; Betzel et al. 2016; Zhang et al. 2016). For instance, Cole et al. (2013) found that, among all between-network functional connections, the couplings between the frontal–parietal system and other systems display the highest temporal variability suggesting it may be served as a mediator for task switching. Zhang et al. (2016) found that the association cortices and the limbic regions display higher temporal variability in FC architecture than the primary areas, providing evidence that the transmodal areas tend to participate in more complex and integrated cognitive activities. Hence, shifting the investigation of brain network from “static” to “dynamic” views provides new insights to build the relationship between time-varying FC and neural and behavioral adaptability. More importantly, the analysis of temporal variation has more potential to capture the sensitive and subtle changes than the static FC analysis method (Rashid et al. 2016).
Characterizing FC dynamics during normative development could help to deepen our understandings of functional flexibility and behavioral changes. Previous studies have revealed age-related development in the frequency and dwelling time (how long the brain spends on a given mode) of specific FC “modes” (Allen et al. 2014; Hutchison and Morton 2015; Qin et al. 2015; Marusak et al. 2017; Medaglia et al. 2018) and between-state transitions from late childhood to young adult (Allen et al. 2014; Qin et al. 2015). Such developmental changes in the dynamic FC are related to the reduced behavioral variability and more accurate performance (McIntosh et al. 2008). However, the understanding of the early brain developmental process in FC dynamics remains in the dark, and the similar delineation of time-varying FC during this pivotal period is warranted. Given that the infant brain regulates information flow from whole-brain (Gao et al. 2011; Wen et al. 2019), mesoscale (Gao and Lin 2012; Gao et al. 2015a; Gao et al. 2015b), and local levels (Wen et al. 2019) during the development, we characterized the development of FC dynamics at these three levels to provide a comprehensive and thorough insight into how brain dynamics change in early life.
In this paper, leveraging longitudinal rs-fMRI from 51 typically developing infants, we used recently developed novel dynamic FC approaches to characterize the development of the temporal variation of FC profiles at the regional, subnetwork, and whole-brain levels during the first 2 years of life. Specifically, we first calculated the temporal variabilities of 217 predefined functional regions of interest (ROIs) (Zhang et al. 2016) and 7 functional subnetworks (including within- and between-network variabilities) (Dong et al. 2018; Sun et al. 2018). We then averaged the variability across 217 ROIs as a measure of the whole-brain FC temporal dynamic. After that, we, respectively, charted developmental trajectories of temporal variability at three different levels by using the linear mixed-effect regression model (Verbeke 1997). Given an increasingly efficient brain network during early development (Gao et al. 2011; Wen et al. 2019), we assumed that the whole-brain functional connectome would become more and more flexible and adaptive from neonate to 2 years old. At the subnetwork level, previous studies have revealed that most high-order functional systems in adults display time-varying FC patterns to meet the requirement of diverse high-level cognitive functions at different temporal periods (Liu, Nie, et al. 2013; Liu and Duyn 2013; Li et al. 2015). With gradually maturing functional subnetworks (Gilmore et al. 2018) and remarkable achievement of brain functions in the first 2 years of life (Amsterdam 1972; Haith et al. 1988; Reznick 2008), we hypothesized that the higher-order function-related subnetworks tend to display increased FC temporal variability within the network to meet the increasing demand of cognitive flexibility for conducting high-level functions. At the regional level, based on the finding that the functional connectome of association areas is more variable than that of the primary areas in the adult population (Zhang et al. 2016), we assumed that the brain regions with significantly increased FC variability are more likely located at high-order function-related areas.
Materials and Method
Subjects
Images were obtained from the subjects enrolled in the “Multi-visit Advanced Pediatric brain imaging study for characterizing structural and functional development (MAP Study).” Study procedures were approved by the University of North Carolina at Chapel Hill Institutional Review Board, and informed written consent was obtained from the parents of participants. After quality control, data of 51 normal developing infants with 200 longitudinal rs-fMRI scans, that is, at 0 month (29 scans), 3 months (26 scans), 6 months (32 scans), 9 months (31 scans), 12 months (31 scans), 18 months (31 scans), and 24 months (20 scans), entered the analysis. The distribution of the ages of all included subjects was presented in Figure 1. Detailed inclusion and exclusion criteria were described in (Gao et al. 2015a).
Figure 1.
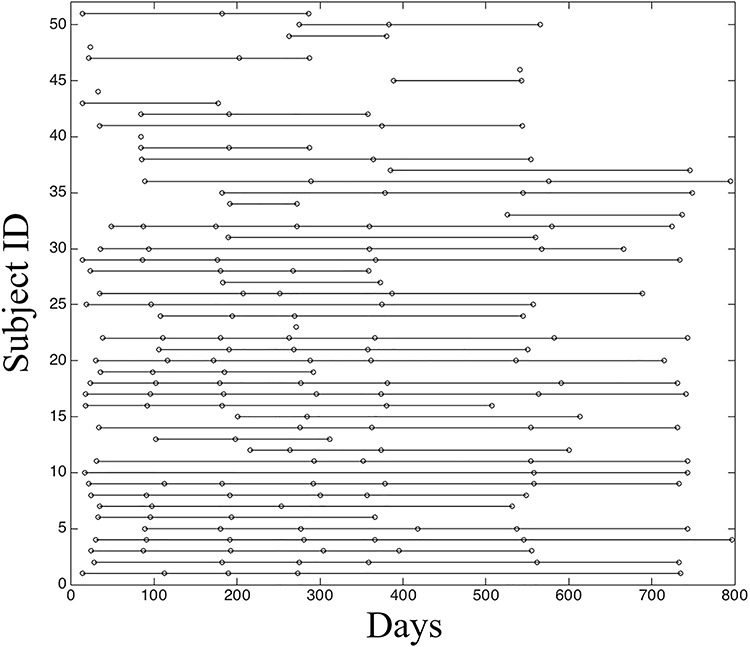
The distribution of rs-fMRI scans at different ages (i.e., days) for all included subjects. Each dot represents one successful scan from a subject at a certain age, and the dots along each line represent all the available longitudinal scans of a subject.
Data Acquisition
During data acquisition, all subjects were in a natural sleeping state. No sedation was conducted. All subject data were acquired with a Siemens 3-T MR scanner. Rs-fMRI was acquired using T2-weighted EPI sequence with the following parameters: TR = 2 s, TE = 32 ms, 33 slices, voxel size = 4 × 4 × 4 mm3, and total volumes = 150 (5 min). Structural images were acquired with 3D MP-RAGE sequence with the following imaging parameters: TR = 1820 ms, TE = 4.38 ms, inversion time = 1100 ms, and voxel size = 1×1×1 mm3.
Data Preprocessing
Functional data were preprocessed using the tools from FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl). It includes the following steps: discarding the first 10 volumes, slice-timing correction, motion correction, and band-pass filtering (0.01 ~ 0.08 Hz). The mean signals from the white matter (WM), cerebrospinal fluid (CSF), and six motion parameters were removed using a linear regression model. To further reduce the head motion effects, wavelet despiking was used to remove both prolonged motion artifacts and higher-frequency events (Patel et al. 2014). The subjects with the percentage of the detected spikes larger than 5% were excluded from further analysis.
Considering dramatic changes in imaging appearance and brain geometry during early infancy, we used a stepwise, infant-dedicated registration strategy to align each rs-fMRI data to the Montreal Neurological Institute (MNI) space, as described elsewhere (Zhang et al. 2018; Wen et al. 2019; Yin et al. 2019). Specifically, for each subject of each age, we first registered the first volume of the rs-fMRI data to its corresponding structural MRI with linear registration. Second, for each subject, we conducted within-subject across-age longitudinal registration that aligned all the structural images from a certain subject at different ages to a “group-mean” image in a common space by using a groupwise longitudinal registration toolbox (GLIRT, Wu et al. 2012). Third, we registered the group-mean image of each subject to the standard symmetric “MNI-152” template by using Demons, a nonparametric nonrigid registration algorithm based on ITK (Ibanez et al. 2005). To alleviate the effect of weak contrast in infant T1-weighted structural MRI, we adopted tissue-segmented label (i.e., gray matter, WM, and CSF) images derived from infant-dedicated segmentation with LINKS (Wang et al. 2015) instead of the original intensity (gray scale) T1-weighted MRI to conduct the registration. Finally, by combining all the linear transformation matrices and deformation fields from the above steps, we registered each infant’s longitudinal rs-fMRI data to the common, standard MNI space. We visually inspected all the registration results to guarantee good registration quality.
Temporal Variability in FC Profile of Brain Regions and the Whole Brain
To evaluate regional-wise FC variability, we first parcellated the whole brain into 268 ROIs by using a volumetric groupwise parcellation atlas provided by (Shen et al. 2013). This is an FC-based brain parcellation generated with a rs-fMRI dataset from 79 healthy adults based on the groupwise clustering of voxel-wise FCs, which has been widely used in many functional brain studies (Bertolero et al. 2015; Rosenberg et al. 2016; Beaty et al. 2018). Compared with other anatomical information-based parcellations, this functional atlas is more suitable for rs-fMRI studies as it provides highly homogeneous and functionally coherent brain parcellations and well-established region-to-network associations (Arslan et al. 2018). As the infant cerebellum registration is much more difficult than the cerebral area registration due to its smaller size and weaker contrast, we excluded 51 cerebellar regions from further analysis. This resulted in 217 ROIs in the cortical and subcortical areas. For each ROI, we extracted its rs-fMRI time series by averaging the blood-oxygen-level–dependent (BOLD) signals of all voxels within it and segmented the time course into N nonoverlapping windows with an equal length of L. In each window, we computed pairwise Pearson’s correlation among each pair of ROIs using the windowed time series, generating a 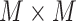 FC matrix (denoted by Fi, i = 1, 2, 3, …, N; M is the total number of ROIs; here, M = 217). The kth row (or kth column) in Fi characterizes the FC architecture for the ROI k at the ith time window, represented as
FC matrix (denoted by Fi, i = 1, 2, 3, …, N; M is the total number of ROIs; here, M = 217). The kth row (or kth column) in Fi characterizes the FC architecture for the ROI k at the ith time window, represented as 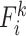 . Then, the regional temporal variability of the ROI k (
. Then, the regional temporal variability of the ROI k (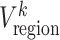 ) was defined as
) was defined as
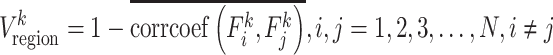 |
(1) |
In equation 1, 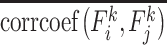 measures the averaged similarity of the FC architecture of the region k between any two different time windows and corrcoef denotes Pearson’s correlation coefficient. By deducting from 1,
measures the averaged similarity of the FC architecture of the region k between any two different time windows and corrcoef denotes Pearson’s correlation coefficient. By deducting from 1, 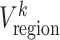 indicates the temporal variability of FC architecture of the region k (Zhang et al. 2016). To avoid the arbitrary choice of the window length and starting point of the window, we computed Vregion with different window lengths (L = 10, 11, …, 20 time points) using different starting points (S = 1, 2,…, L
indicates the temporal variability of FC architecture of the region k (Zhang et al. 2016). To avoid the arbitrary choice of the window length and starting point of the window, we computed Vregion with different window lengths (L = 10, 11, …, 20 time points) using different starting points (S = 1, 2,…, L 1) and then averaged across all window length and starting point parameters as the final regional dynamic FC variability.
1) and then averaged across all window length and starting point parameters as the final regional dynamic FC variability.
After obtaining all regional-wise FC variabilities, we calculated the whole-brain FC dynamic, Vwhole-brain, for each scan of each subject by averaging Vregion across all the 217 ROIs of the corresponding brain.
Temporal Variability of FC Architecture within and between Functional Subnetworks
Besides calculating ROI-wise and whole-brain FC dynamics, we also measured the temporal variation of FC architecture from the mesoscale level. To this end, we first parcellated all infant brains into several functional subnetworks, each of which contains a set of ROIs of a certain functional system. Considering the dramatic reorganization of brain functional networks during early infancy, we adopted a unified atlas as the template of brain parcellation and applied it to all age groups to ensure the comparability of functional networks across age. This atlas was generated on adults, which parcellated the brain into seven functional subnetworks, including visual network (VN), sensorimotor network (SMN), dorsal attention network (DAN), ventral attention network (VAN), limbic/striatum network (LN/SN), frontal–parietal network (FPN), and default mode network (DMN) (Yeo et al. 2014). Because there are two major types of FC in terms of subnetwork affiliations of two nodes that form a link for each functional subnetwork, we computed the within-network temporal variability of FC (i.e., Vwithin-net) and between-network temporal variability of FC (i.e., Vbetween-net) according to a modified algorithm of the regional temporal variability method of FC architecture (Dong et al. 2018). Specifically, 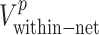 is defined as the time-varying dynamic of the FC architecture within functional subnetwork p according to equation 2 and
is defined as the time-varying dynamic of the FC architecture within functional subnetwork p according to equation 2 and  as the time-varying dynamic of the FC architecture between functional subnetworks p and q according to equation 3
as the time-varying dynamic of the FC architecture between functional subnetworks p and q according to equation 3
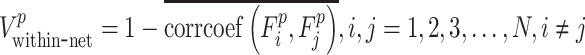 |
(2) |
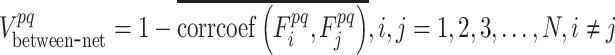 |
(3) |
In equations 2 and 3, p and q are indexes of seven functional subnetworks. 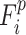 is the FC architecture consisting of all FCs in functional subnetwork p at the ith time window.
is the FC architecture consisting of all FCs in functional subnetwork p at the ith time window. 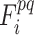 represents the FC architecture including all FCs between subnetworks p and q at the ith time window. Similar to ROI-wise temporal variability calculation, we computed Vwithin-net and Vbetween-net with multiple window lengths (L = 10, 11, …, 20 time points) using multiple starting points (S = 1, 2,…, L
represents the FC architecture including all FCs between subnetworks p and q at the ith time window. Similar to ROI-wise temporal variability calculation, we computed Vwithin-net and Vbetween-net with multiple window lengths (L = 10, 11, …, 20 time points) using multiple starting points (S = 1, 2,…, L 1) and took an average to generate the final version of the Vwithin-net and Vbetween-net to improve the robustness of the result.
1) and took an average to generate the final version of the Vwithin-net and Vbetween-net to improve the robustness of the result.
Characterization of Developmental Trajectories of Temporal Variability
The developmental trajectories of the temporal variability of FC architecture at the whole-brain, subnetwork, and regional levels were delineated by using the linear mixed-effect regression (LMER) model. The LMER was used in this study due to its ability to handle missing data in longitudinal studies (Verbeke 1997). For each measurement (Vwhole-brain, Vwithin-net, Vbetween-net, and Vregion), both a linear model (with age as a fixed effect variable) and a log-linear/nonlinear model [with log(age) as a fixed effect variable] were built. The log-linear model was used to delineate the uneven developmental speed during the first 2 years of life, which has been identified to follow the exponential function (Gao et al. 2015b). In each LMER model, Vwhole-brain, Vwithin-net, Vbetween-net, or Vregion is the dependent variable, and age or log(age) in days and gender information are independent variables (Jha et al. 2019). Random intercept and subject effects were included as the random effects to characterize the temporal correlation. Akaike information criterion (AIC) was used to gauge the selection between linear and nonlinear models. Significant developmental changes were determined by P < 0.05 after multiple comparison correction by false discovery rate (FDR).
Results
Temporal Variability of Whole-Brain FC Architecture
The temporal variability of the whole-brain FC architecture was found significantly increased linearly during the first 2 years of life (P < 0.05), suggesting that the brain becomes more and more flexible along with the development in early life (Fig. 2a). To clearly show spatial pattern changes at different ages, especially the regions with high FC variability, we further visualized the top 20% of the regions in terms of age-averaged regional FC temporal variability for the ages of 0, 3, 6, 9, 12, 18, and 24 months (Fig. 2b). In general, we found that the spatial pattern of the high FC temporal variability regions is shifted from the posterior to the anterior of the brain, especially from the occipital and temporal lobes to the frontal lobe. Specifically, for neonates, brain regions with highly variable FC profiles are mainly located at the visual cortex, middle and inferior temporal cortices, and thalamus. After 3 months of the development, those in the visual cortex, temporal cortex, and thalamus shrink largely, while bilateral sensorimotor areas and angular areas emerge. From 6 to 9 months old, the sensorimotor areas have continuously increased FC variability, while those in other regions are gradually weakened. Since 12 months of age, the spatial distribution of the top variable brain regions tends to be stable with a continuous pattern in bilateral sensorimotor areas but with prominent extension to the premotor area and superior frontal areas.
Figure 2.
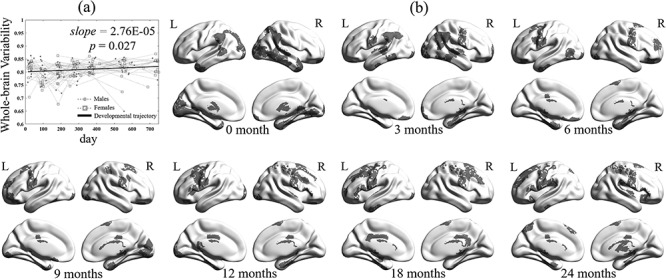
(a) Developmental trajectory of whole-brain FC temporal variability in the first 2 years of life. In the figure, dots represent the males and squares represent females. (b) Spatial patterns of 20% brain regions with the highest variability across seven age groups (i.e., 0, 3, 6, 9, 12, 18, and 24 months of age).
Temporal Variability of Subnetwork’s FC Architecture
Figure 3 plotted the developmental patterns of within- and between-network FC temporal variabilities for seven functional subnetworks, with Supplementary Table 1 summarizing the fitted developmental trajectories from the LMER model. For within-network FC variability, we observed nonlinearly decreasing trajectories for two primary subnetworks, VN and SMN, and linearly increasing trajectories for two high-order functional subnetworks, VAN and DMN (Fig. 3a). Among them, VN displays a larger decreasing speed than that of SMN, while VAN increases slightly faster than that of DMN (Fig. 3b).
Figure 3.
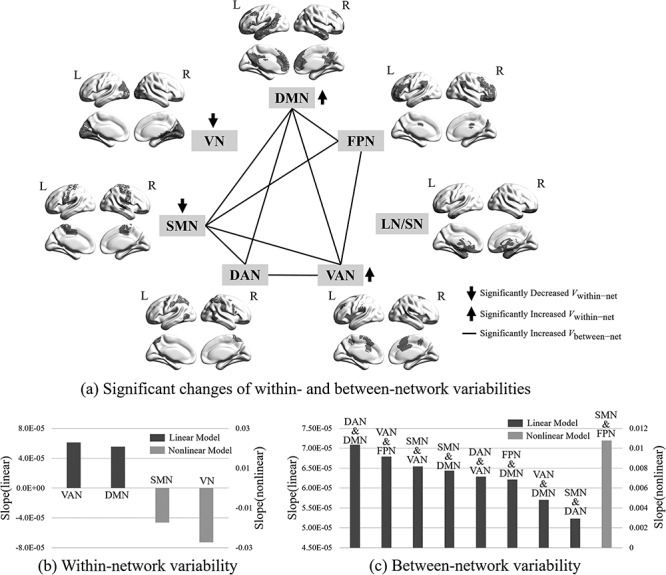
Illustration of developmental patterns of within-network (Vwithin-net) and between-network FC temporal variabilities (Vbetween-net) on seven functional subnetworks, including visual network (VN), sensorimotor network (SMN), dorsal attention network (DAN), ventral attention network (VAN), limbic/striatum network (LN/SN), frontal–parietal network (FPN), and default mode network (DMN). The network parcellation is based on the template provided by (Yeo et al. 2014). The significance level is set to P < 0.05 after FDR correction. (a) Significantly changed Vwithin-net and Vbetween-net. Down and up arrows indicate significantly decreased and increased Vwithin-net, respectively. The black lines between two subnetworks indicate significantly increased Vbetween-net between them. (b) Different developing speeds of Vwithin-net for different functional subnetworks. (c) Different developing speeds of Vbetween-net for different subnetwork pairs. Black bars represent linear developmental trajectories and gray ones represent nonlinear developmental trajectories.
For between-network FC temporal variability, we found all significantly developmental changes (P < 0.05 after FDR correction) are increased with age (Fig. 3a). Eight of them showed the linear increase and one showed the exponential (i.e., nonlinear/log-linear) increase. Interestingly, there is no Vbetween-net increase between VN (and LN/SN) and any other functional subnetworks. For the other five functional subnetworks, all their Vbetween-net are found to be increasing across age. Specifically, for the SMN, VAN, and DMN, more than half of their connections (four out of six) have the increased Vbetween-net; and for the DAN and FPN, half of their connections (three out of six) have the increased Vbetween-net. Notably, except SMN, all the other networks with significantly increased Vbetween-net are the high-level functional networks (i.e., DAN, VAN, FPN, and DMN). Regarding developing speed, we observed that the Vbetween-net between DAN and DMN had the fastest linear increase, followed by that of VAN and FPN, SMN and VAN, SMN and DMN, DAN and VAN, FPN and DMN, VAN and DMN, and SMN and DAN (Fig. 3c). Only the between-network FC variability between SMN and FPN showed a nonlinear increasing trajectory (Fig. 3c).
Temporal Variability of Regional FC Architecture
In addition to the above results at whole-brain and subnetwork levels, we also delineated developmental trajectories of FC dynamics for all brain regions. To clarify different developmental patterns, we classified the ROIs with significant changes in FC variability into four categories based on their fitted developmental trajectories, including linear increase (Type 1), nonlinear increase (Type 2), linear decrease (Type 3), and nonlinear decrease (Type 4). Figure 4a shows the spatial distributions of brain regions with significant developmental changes in Vregion, separately plotted for each developmental type, where the grayscale values coded different developmental velocities (i.e., the slope of the age effect in the LMER model). The detailed information for all fitted parameters was summarized in Supplementary Table 2. We found that the number of brain regions with increasing FC variability (57 ROIs) is much more than that with a decrease (12 ROIs) and most of them are linear increases. Different types of brain regions tend to sit at distinct brain areas. Specifically, increasing developmental trajectories are mainly located at high-order function-related areas, including inferior and superior frontal areas, medial prefrontal areas, cingulate cortices, some subcortical regions (including putamen, caudate, and insula) and ventral precentral gyrus in Type 1 (i.e., linear increase), and dorsolateral prefrontal areas and ventral precentral areas in Type 2 (i.e., nonlinear increase). Among these brain regions, the left anterior and middle cingulate areas (referred to as dorsal anterior cingulate cortex, dACC), as well as the left prefrontal gyrus (orbital and triangular parts) and the right prefrontal gyrus (orbital part), showed steeper developmental slopes than the other regions. These regions are particularly included in the executive control network and default mode network. For those brain regions with decreasing developmental trajectories, they mainly sit at primary areas, including the middle temporal area in Type 3 (i.e., linear decrease) and visual areas and temporal lobe in Type 4 (i.e., nonlinear decrease).
Figure 4.
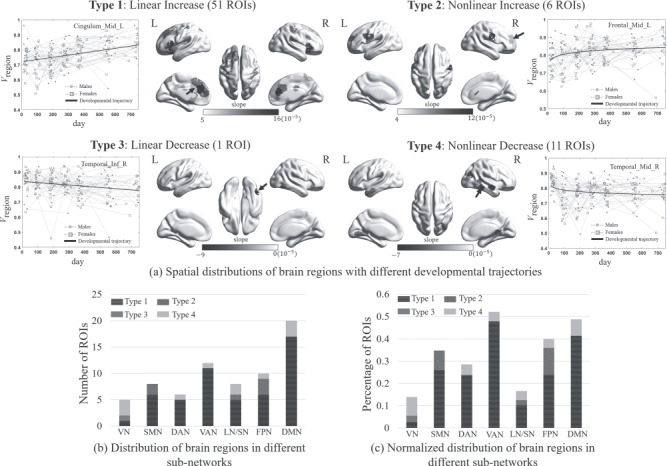
Illustration of developmental patterns of regional-wise FC temporal variability (Vregion) and their spatial distributions, where the brain regions with significant developmental trends (P < 0.05 after FDR correction) were divided into four different categories (Types 1–4) based on their fitted developmental trajectories, including linear increase (Type 1), nonlinear increase (Type 2), linear decrease (Type 3), and nonlinear decrease (Type 4). (a) Spatial distribution of brain regions in each category, where different grayscale values coded different developing velocities (i.e., the slopes of the fitted curves) of Vregion. An exemplary trajectory of Vregion was plotted beside each surface rendering map, with males and females separately plotted by dots and squares. (b) Distributions of four categories of brain regions in different functional brain subnetworks measured by the number of brain regions. (c) Distributions of four categories of brain regions in different functional brain subnetworks measured by the percentage of brain regions. The subnetworks in Figures. (b) and (c) were defined based on the template provided by (Yeo et al. 2014), including visual network (VN), sensorimotor network (SMN), dorsal attention network (DAN), ventral attention network (VAN), limbic/striatum network (LN/SN), frontal–parietal network (FPN), and default mode network (DMN).
Additionally, we also characterized how four types of brain regions are distributed in seven predefined functional subnetworks based on two indexes, one of which counts the number of brain regions of each type located at each subnetwork and the other calculates the percentage of ROIs, that is, the number of brain regions of each type at one network divided by the total number of brain regions in that network. The results were displayed with cumulative histograms as shown in Figure 4b,c. We found that measured by either of two indexes, the brain regions with linearly increasing FC flexibility were dominant to all subnetworks but VN (to which nonlinear decrease is dominant) and FPN (for which significant more nonlinear increased Type 2 regions were found).
Finally, to investigate the relationship between “static FC” and “dynamic FC,” we further compared the static FC strength (FCS) between the brain regions with increasing FC variability (i.e., ROIs in Types 1 and 2) and those with decreasing or nonsignificantly changed FC variability based on two-sample t-test. For each brain region, the static FCS was calculated by summing all the FC weights from the other regions positively connected to this region at 2 years old. The result showed that the static FCS of the regions with increasing FC variability is significantly lower than that of the other regions (t = 3.42, P = 0.0004).
Discussions
New Findings of Developmental Patterns of FC Dynamics
To the authors’ knowledge, this paper provides the first-ever early development report of brain FC dynamics in the first two postnatal life. A recently proposed, robust metric measuring temporal variability of the FC profiles was adopted to evaluate such FC dynamics (Zhang et al. 2016; Dong et al. 2018). To comprehensively and accurately investigate how brain dynamics develop in early life, we measured the FC temporal variability from multiple levels and utilized a longitudinal infant natural sleeping fMRI data with longitudinal data regression model (LMER), instead of cross-sectional data, to capture their longitudinal developmental trajectories. Similar to previous studies on static FC development (Gao et al. 2011; Wen et al. 2019), we also found age-dependent changes in FC dynamics at different levels, including 1) linearly increased global FC dynamic (Fig. 2a), 2) reduced intranetwork FC dynamics in two primary systems and increased FC dynamics in two high-order functional systems (Fig. 3b), 3) increased internetwork FC dynamics among five functional subnetworks (Fig. 3c), and 4) a major linearly increasing pattern of the regional FC dynamics in frontal areas (Fig. 4a). These new findings in our study provide a new view angle of the rapidly developing brain functions in early life and could be a good supplement to the previous static FC development studies.
Gradually Increased Global Functional Flexibility in Early Development
Although the FC temporal variability was first calculated for each region, the whole-brain averaging process still revealed a significantly increased pattern from neonates to 2 years of age. Such a global dominance indicates most of the regions have increased FC dynamics, as both found at a coarse scale (internetwork FC dynamics, Fig. 3) and a fine scale (regional FC dynamics, Fig. 4). This finding suggests that the infant’s brain is globally evolved to be more and more flexible in early life providing new evidence for the previous theory obtained with task-related EEG and fMRI, that is, the early FC reorganization drives the brain from a deterministic system to a more stochastic one (McIntosh et al. 2010). Specifically, the human brain could be considered as a nonlinear dynamic system functioning at the “edge of criticality” between diverse brain states or functional network architectures (Ghosh et al. 2008; Deco et al. 2009, 2011; McIntosh et al. 2010; Garrett et al. 2011). As the brain matures during early infancy, the number of possible functional network configurations (corresponding to resting-state networks, RSNs) around the stable anatomical framework is increased to respond various new emerging high-order cognitive functions, such as self-awareness (Amsterdam 1972), spatial attention (Haith et al. 1988), and working memory (Reznick 2008), and thus the human brain is bound to be optimized as a more variable system to ensure the information processing capacity among the increasing number of brain states (Deco et al. 2009; McIntosh et al. 2010). Despite the absence of external stimuli, the resting-state brain functional connectome is also dynamically changed because the internally driven system visits different network configurations spontaneously (Deco et al. 2011). Thus, with resting-state fMRI, we found a similar result with those from task-related studies.
The more flexible global FC with age found in our study also provides additional evidence for previous long-standing hypothetical models, that is, there exists continuously increased brain functional organization efficiency at the global level from birth to early childhood. In Cao et al. (2017), from a connectome (topology of the entire brain static FC) perspective, they proposed that the brain networks are changing toward an “organized and optimized” configuration, with a “strengthening balance between local and global information processing.” That is, the local (reflecting functional segregation) and global efficiency (reflecting functional integration) are increasing at an equal rate. Similar hypothetic models of the local and global efficiency development are also reported in another review paper of early FC development (Zhang et al. 2019) and another study based on a longitudinal infant fMRI data from a modular perspective (Wen et al. 2019). Studies on the whole-brain structural connectome revealed increasing integration but decreasing functional segregation, possibly due to different definitions between FC and structural connectivity (Yap et al. 2011). Nevertheless, all these previous studies on the efficiency development are based on regional-wise paired relationship and topological analysis of static FC, mostly stronger ones, which generally measures the “backbone” of the connectome. To better characterize “efficiency,” we proposed a temporal dynamics perspective of efficiency, which further defines regional flexibility (the FC profile between each region to all other regions changes along time). Such temporal flexibility may create a transient “shortcut” from one functional system to another, despite their weak static FC, to facilitate information flow and exchanges. This speculation is supported by our finding that the brain regions with increasing FC variability tend to have weaker static FC than those with decreasing or unchanging FC variability. Thus, from the dynamic perspective, we again validate the hypothesis that there is a globally increasing trajectory of the connectivity efficiency (in terms of flexibility), but we also reported a new finding that such a flexibility change has a linearly increasing pattern, indicating that the FC flexibility is still under a fast increasing track and does not reach its peak at 2 years of age.
Different Developmental Trajectories of Intranetwork FC Flexibility between Primary and High-Order Subnetworks
The decreased Vwithin-net for VN and SMN but increased Vwithin-net for DMN and VAN provides additional evidence for different developmental patterns between primary and high-order functional systems. Such differences among networks maybe because the primary function-related brain regions mature earlier than the high-order function-related regions, similarly revealed by many conventional static FC studies (Gao et al. 2015a; Gao et al. 2015b; Cao et al. 2017; Gilmore et al. 2018). Specifically, they found that primary functional networks show adult-like topologies in preterm and term infants, while higher-order networks have not been established before the second year of the postnatal life or even later. This result is also supported by protracted structural development in the association regions compared with early matured primary regions. Postmortem histologic studies of early infancy cortical development revealed that the period of synaptogenesis in the auditory and visual sensory areas is completed much earlier than that in high-order function-related areas (Huttenlocher & Dabholkar 1997). Due to the formation of synapse occurring concurrently with dendritic and axonal growth and with myelination of the cortical WM, previous studies also found much earlier maturation of short cortico-cortical connections (Kostović et al. 2014), dendritic arborizations (Petanjek et al. 2011), and myelination of cortical afferents and efferents (Gao et al. 2009; Huang et al. 2013) in the primary regions than high-order regions. Such developmental order in both brain function and structure allows the resources of the infant brain be focused on primary networks first as essential for early survival while enabling enriched development of higher-order function-related networks through the prolonged postnatal gene-by-environment interactions.
Rather than measuring the properties of the static FCs in each subnetwork, Vwithin-net evaluates how within-network FC fluctuates across time, where a larger Vwithin-net value indicates richer FC patterns possibly underlying more frequent information communications among different components in the same functional systems (Dong et al. 2018). Previous studies on the adult population have revealed that most of the maturing high-order functional systems often constitute several subsystems to mediate different complex functions. For example, the DMN was found to have multiple functional subnetworks, each of which may be responsible for distinctive functions (Buckner et al. 2008; Assaf et al. 2010; Li, Liu, et al. 2013). A meta-analysis study with task-based activities found that the DMN is functionally heterogeneous, corresponding to different behavioral metadata and indicating that the DMN is differentially specialized (Laird et al. 2009). With temporal lag-respected task fMRI source separation, four different DMN subnetworks were revealed, each with different spatiotemporal relationships to different cognitive components (Van De Ville et al. 2012). A recent dynamic FC study conducted a clustering analysis on transient coactivations of rs-fMRI signals and revealed multiple DMN-related coactivation patterns, each of which indicates a transient connection between the DMN and other regions from other networks (Liu and Duyn 2013). Such spatiotemporal flexibility of the DMN was even observed in a sustained attention task (Li et al. 2015). Collectively, the coordination and interaction among different DMN subnetworks are important for the infant to accomplish more complex tasks, which could be reflected by the increasing Vwithin-net of the DMN. In the same vein, the VAN (or, equivalently, salience network) has also been suggested to have different subsystems (Chand et al. 2017) and could be heavily involved in mediating the switch between DMN and FPN (Sridharan et al. 2008; Menon 2011; Nekovarova et al. 2014). Thus, we speculated that the intranetwork FC within DMN and VAN are becoming more flexible as their respective subsystem structures become much clearer along the development to facilitate high-level cognitive functions.
The reversed developmental trajectories of Vwithin-net of the VN and SMN (the only two primary functional subnetworks) may, on the other hand, indicate increasingly homogeneous FC architecture of each network along with the development. It is reasonable because previous studies have found that the brain functional subnetworks are generally defined according to the anatomical closeness at birth, but with long-term FC becoming stronger, they are gradually developed to be spatially distributed (Yap et al. 2011; Li et al. 2014; Cao et al. 2017; Wig 2017; Gilmore et al. 2018). As the VN and SMN atlases are defined according to an adult-based parcellation, they may not well suit for neonates (also include some regions from other functional subnetworks), leading to greater variability in their respective within-network FC. With the increase of age, the VN and SMN become more and more similar to the adult pattern with more focal spatial distributions and thus generate a smaller FC variability, reflecting the highly specialized function of each subnetwork. Another possibility is that the increasing myelination within the primary networks makes the intranetwork FC within the VN and SMN much stronger, leading to less freedom for these FCs to be fluctuating. Such stability of their FC could be due to the strong within-network FC backbone, which has the ceiling effect, making the FC fluctuation less possible (Bassett et al. 2013). On the other hand, the decreasing flexibility could indicate that the primary sensory functions require more stable FC to maintain a stable and robust bottom-up information feeding to high-level functional subnetworks. Yet, such decreasing Vwithin-net of the VN and SMN seems to follow nonlinear (exponential) trajectories, meaning their Vwithin-net turns out to be smaller, but such changes are becoming less and less until the Vwithin-net are stable in later ages. Such earlier stabilized Vwithin-net changes in the primary function-related subnetworks versus the possibility of prolonged changes in the high-order functional systems again show fundamental differences between the two types of functional systems.
Increased Internetwork FC Flexibility
We found that nearly all the significantly changed Vbetween-net are linearly increasing (more flexible and diverse) with age. If not counting the LN/SN and VN, all the Vbetween-net among the rest of the five subnetworks (SMN, DAN, DMN, FPN, and VAN) are increasing with age. This indicates that internetwork information communication becomes more frequent and more active in the first 2 years of age. For example, the VAN, FPN, and DMN that are involved in a “triple network” hypothetic model (Menon 2011) showed linear increases in their pairwise Vbetween-net. It has been proposed that such three networks are the “core” neurocognitive networks and their functional interactions are important for complex, high-order cognitive functions (Menon 2011) and could be responsible for psychiatric and neurological disorders, such as depression (Berman et al. 2011), schizophrenia (Palaniyappan et al. 2011), and dementia (Zhou et al. 2010; Yu et al. 2017). Recently, researchers have found that the dynamic network switch supports flexible attention (mainly via DAN and VAN) and cognitive control (mainly via FPN) to meet time-varying changes in cognitive demand, and the loss of such optimized dynamics may lead to poor task performance in a decision-making task (Taghia et al. 2018). Such flexibility among the three networks could be associated with attention-deficit/hyperactivity disorder (ADHD) (Cai et al. 2018) and schizophrenia (Supekar et al. 2019). Similarly, another study from the “modular” perspective also found the relationships between the three core networks and the learning ability (Bassett et al. 2011) in healthy subjects and with the pathophysiology of schizophrenia (Braun et al. 2016). It is worth noting that a previous static FC-based study has identified significant changes in the averaged internetwork FC strength between the VAN and DMN and between the FPN and DMN, but not between the VAN and DMN (Gao et al. 2015b). Our finding of all increasing Vbetween-net among the three core networks gives the first evidence that interactions among these three networks are also very important for supporting the remarkable development of complex cognitive abilities throughout development during early infancy. The use of dynamic FC further increases the sensitivity in the detection of developing internetwork functional interactions. As such, all high-order function-related subnetworks have increased Vbetween-net in the first 2 years of age.
Another interesting finding is that the SMN has increasing Vbetween-net with all the other four high-order cognitive function-related subnetworks (DAN, VAN, DMN, and FPN) but had decreasing Vwithin-net itself. We tentatively interpreted such a result as differently developed functional integration and segregation from a dynamic viewpoint. For within-SMN FC, the connectivity becomes stable while gaining its strength to make sensory inputs and motor outputs interact and coordinate better (more stable) and less affected by other factors. However, the input sensory information needs to be spread and further processed in high-order functional subnetworks such as DAN, VAN, DMN, and FPN, and the motor performance could be highly modulated and adaptively controlled by these high-order functional subnetworks (Hutchison et al. 2013; Calhoun et al. 2014). During early development, the increasingly adaptive ability of the brain is to better fit for or response to the moment-to-moment changes in the environment, as well as time-varying attention and task control demands during the conduct of complex cognitive functions, all of which requires timely response and continuous preparedness at different attention levels. The increasing Vbetween-net of the SMN suggests richer FC patterns underlying feedback and feed-forward interactions between it and other high-order functional subnetworks (Dong et al. 2018), which may allow infants to carry out more and more complex tasks (Reddy et al. 2018). Combing with the heightened stability (decreased Vwithin-net) of FC within the SMN, we speculated that the increased dynamic reconfigurations between SMN and high-order networks may due to the enhanced ability of infants for integrating internal and external sensory and perception input to achieve the rapid development of complex functions.
Heterogeneous Developmental Trajectories of Brain Regions’ FC Dynamics
The measurement of regional FC variability at the finest scale provides us a more detailed picture of the rapidly developing brain dynamics. As shown in Figure 2b (cross-sectional) and Figure 4a (longitudinal), convergent evidence show complex spatiotemporal changes in the flexibility of FC architecture across ages. Generally, those with decreasing FC flexibility are located at the inferior part of the brain, while those with increasing FC flexibility were widely distributed at the anterior and superior part of the brain. This is consistent with the previous finding of the development order of brain functions, that is, from posterior to anterior, from inferior to superior, and from medial to the lateral areas (Gao et al. 2015a).
The diverse developmental trajectories of brain regions’ temporal FC variability indicate there are regional differences in the development of functional connections. Specifically, many brain regions in Type 1 (i.e., regions with linearly increased FC variability) and Type 2 (i.e., regions with nonlinearly increased FC variability) are located at the frontal association areas, suggesting an unestablished reorganization of FC architecture in these areas at 2 years old. Many of these regions are proposed to serve as a bridge for information exchange and integration among different functional subsystems (Wen et al. 2019). They receive inputs from all other cortical regions for various higher-order functions, such as planning and directing motor, cognitive, affective, and social behavior across time (Kolb et al. 2012). Their prolonged development allows for acquiring these complex cognitive abilities through experience. The structure backbone underlying such a function-related finding of the frontal associative areas is their late-delayed neuronal maturation, including synaptogenesis (Huttenlocher and Dabholkar 1997), synaptic pruning (Huttenlocher and Dabholkar 1997), and dendritic growth (Petanjek et al. 2011). All these protracted microscopic structural development may altogether manifest as increasing functional flexibility. For example, the difference in the timing of overproduction and elimination of synapses could affect the timing of the plasticity or flexibility of these regions. The later a region’s synaptic reorganization completes, the longer the region remains plastic and the more flexible its FC organization will be. For the primary regions, the pruning effect could happen earlier, making their FC shape to a more efficient, more matured, and less flexible status.
Additionally, we found that the number of brain regions with linearly increased FC variability (51 ROIs) is much more than those with nonlinearly increased variability (6 ROIs), suggesting that most of the complex cognitive functions have protracted development at later ages. The frontal areas are generally split into two categories; the Type 1 category (linearly increase) mainly covered the inferior and the superior frontal areas with functions like self-awareness (Goldberg et al. 2006) and motor planning (Grier 2005), while the Type 2 category (nonlinearly increase with earlier saturation) mainly sits at the ventral precentral areas (mouth, tongue, and laryngeal movements). These results suggest that the language-related motor functions may have been largely in place by the end of 2 years old, while other more complex functions such as language production, movement preparation, initialization, and control could continue to be developing at later ages.
Techniques Considerations, Limitations, and Future Directions
Several technical considerations, limitations, and future directions deserve further discussion. First, large voxels in the rs-fMRI data may worsen the partial volume effect in infant studies due to relatively small infant brains. Although the ROI-based strategy could largely alleviate such an effect, the final solution will be high-resolution fMRI. For instance, an ongoing Baby Connectome Project (BCP) has been collecting infant fMRI data with a high spatial resolution (voxel size = 2 × 2 × 2 mm3) by implementing a multiband echo-planar imaging sequence (Howell et al. 2019). A future study can investigate the effect of spatial resolution based on this dataset. Second, our study adopted an adult-based template to define functional subnetworks for infants rather than constructing infant-dedicated templates for different age groups. While the functional subsystems are basically in place by the 2 year of life, the brain is continually developing by fine-tuning and those subsystems are yet to mature (Cao et al. 2017; Gilmore et al. 2018). In the future, it is necessary to construct a set of age-specific infant-dedicated network parcellation templates. Third, mounting evidence has been indicating that human brain dynamics are important for predicting cognitive functions (Bassett et al. 2011; Bassett and Mattar 2017). Due to the limited sample size, we could not perform such an association or prediction study with sufficient statistical power. It warrants further investigations with a better-designed data acquisition protocol, such as that in the BCP (Howell et al. 2019). Finally, another urgent study in the human brain is to uncover the structural substrates of the FC dynamics and the development of such neural substrates, such as how dendritic and axonal growth, myelination, and reorganization of cortical layers could shape FC flexibility. This topic requires further dedicated study on animal models with combined imaging techniques focusing on different scales.
Conclusions
In this study, we provided a first-ever comprehensive report of how brain functional dynamics develop in the first 2 years after birth at different spatial scales. Our results indicate that the brain is becoming increasingly flexible, dynamic, and adaptive with complex spatiotemporal developmental patterns. The unraveled detailed changes in the brain functional dynamics pave a road for the future works for a better understanding of infant brain development in the chronnectome perspective.
Notes
This work uses the data from “Multi-visit Advanced Pediatric brain imaging study for characterizing structural and functional development (MAP Study).”
Conflict of Interest: None declared.
Funding
National Institutes of Health (EB022880, AG041721, MH110274, MH116225, and MH117943).
Supplementary Material
References
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 34(27):9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 24(3):663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam B 1972. Mirror self-image reactions before age two. Dev Psychobiol. 5(4):297–305. [DOI] [PubMed] [Google Scholar]
- Arslan S, Ktena SI, Makropoulos A, Robinson EC, Rueckert D, Parisot S. 2018. Human brain mapping: a systematic comparison of parcellation methods for the human cerebral cortex. Neuroimage. 170:5–30. [DOI] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 53(1):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. 2011. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 108(18):7641–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST. 2013. Task-based core-periphery organization of human brain dynamics. PLoS Comput Biol. 9(9):e1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Mattar MG. 2017. A network neuroscience of human learning: potential to inform quantitative theories of brain and behavior. Trends Cogn Sci. 21(4):250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Kenett YN, Christensen AP, Rosenberg MD, Benedek M, Chen Q, Fink A, Qiu J, Kwapil TR, Kane MJ. 2018. Robust prediction of individual creative ability from brain functional connectivity. Proc Natl Acad Sci U S A. 115(5):1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, Gonzalez R, Demiralp E, Gotlib IH, Hamilton P et al. 2011. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 11(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D'Esposito M. 2015. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A. 112(49):E6798–E6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Satterthwaite TD, Gold JI, Bassett DS. 2016. A positive mood, a flexible brain. arXiv preprint arXiv. 1601:07881. [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H et al. 2015. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 112(37):11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Bassett DS, Rausch F, Schweiger JI, Bilek E, Erk S, Romanczuk-Seiferth N, Grimm O, Geiger LS et al. 2016. Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proc Natl Acad Sci U S A. 113(44):12568–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 1124(1):1–38. [DOI] [PubMed] [Google Scholar]
- Cai W, Chen T, Szegletes L, Supekar K, Menon V. 2018. Aberrant time-varying cross-network interactions in children with attention-deficit/hyperactivity disorder and the relation to attention deficits. Biol Psychiatry Cogn Neurosci Neuroimaging. 3(3):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adalı T. 2014. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 84(2):262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Huang H, He Y. 2017. Developmental connectomics from infancy through early childhood. Trends Cogn Sci. 40(8):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand GB, Wu J, Hajjar I, Qiu D. 2017. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 7(7):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 16(9):1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage ML, Adams RJ. 1990. Visual acuity assessment from birth to three years using the acuity card procedure: cross-sectional and longitudinal samples. Optom Vis Sci. 67(9):713–718. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, Phillips JP. 2014. Functional connectivity in the developing brain: a longitudinal study from 4 to 9 months of age. Neuroimage. 84:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Asis-Cruz J, Bouyssi-Kobar M, Evangelou I, Vezina G, Limperopoulos C. 2015. Functional properties of resting state networks in healthy full-term newborns. Sci Rep. 5:17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh AR, Sporns O, Kötter R. 2009. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci U S A. 106(25):10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. 2011. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 12(1):43–56. [DOI] [PubMed] [Google Scholar]
- Dong D, Duan M, Wang Y, Zhang X, Jia X, Li Y, Xin F, Yao D, Luo C. 2018. Reconfiguration of dynamic functional connectivity in sensory and perceptual system in schizophrenia. Cereb Cortex. 29(8):3577–3589. [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. 2009. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol. 30(2):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, Lin W. 2011. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One. 6(9):e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. 2012. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 23(3):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. 2012. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 33(1):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. 2015a. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex. 25(9):2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. 2015b. Development of human brain cortical network architecture during infancy. Brain Struct Funct. 220(2):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, Gilmore JH. 2017. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 23(2):169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. 2011. The importance of being variable. J Neurosci. 31(12):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Rho Y, McIntosh AR, Kötter R, Jirsa VK. 2008. Noise during rest enables the exploration of the brain's dynamic repertoire. PLoS Comput Biol. 4(10):e1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. 2018. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 19(3):123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. 2006. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 50(2):329–339. [DOI] [PubMed] [Google Scholar]
- Grier EC 2005. School neuropsychology: a practitioner's handbook. Psychol Sch. 42(4):452–453. [Google Scholar]
- Haith MM, Hazan C, Goodman GS. 1988. Expectation and anticipation of dynamic visual events by 3.5-month-old babies. Child Dev. 1:467–479. [PubMed] [Google Scholar]
- Howell BR, Styner MA, Gao W, Yap PT, Wang L, Baluyot K, Yacoub E, Chen G, Potts T, Salzwedel A et al. 2019. The UNC/UMN baby connectome project (BCP): an overview of the study design and protocol development. Neuroimage. 185:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y. 2013. Development of human brain structural networks through infancy and childhood. Cereb Cortex. 25(5):1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J. 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 80:360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. 2015. Tracking the brain's functional coupling dynamics over development. J Neurosci. 35(17):6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Schroeder W, Ng L, Cates J. 2005. The ITK software guide: updated for ITK version 2.4. New York: Kitware. [Google Scholar]
- Jha SC, Xia K, Ahn M, Girault JB, Li G, Wang L, Shen D, Zou F, Zhu H, Styner M et al. 2019. Environmental influences on infant cortical thickness and surface area. Cereb Cortex. 29(3):1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28(47):12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. 2012. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 109:17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N, Radoš M, Sedmak G, Benjak V, Kostović-Srzentić M, Vasung L, Čuljat M, Radoš M, Hüppi P. 2014. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct. 219(1):231–253. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. 2009. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 29(46):14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng L, Hu D. 2013. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 74(1):48–54. [DOI] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH, Shen D. 2013. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb Cortex. 23(11):2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. 2014. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 24(5):1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zang Y, Zhang H. 2015. Exploring dynamic brain functional networks using continuous “state-related” functional MRI. Biomed Res Int. 2015:824710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chang C, Duyn JH. 2013. Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns. Front Syst Neurosci. 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. 2013. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A. 110(11):4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D, Gilmore JH. 2015. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 25(8):2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala Hamrick K, Gotlib IH, Thomason ME. 2017. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp. 38(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Itier RJ. 2008. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput Biol. 4(7):e1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. 2010. The development of a noisy brain. Arch Ital Biol. 148(3):323–337. [PubMed] [Google Scholar]
- Medaglia JD, Satterthwaite TD, Kelkar A, Ciric R, Moore TM, Ruparel K, Gur RC, Gur RE, Bassett DS. 2018. Brain state expression and transitions are related to complex executive cognition in normative neurodevelopment. Neuroimage. 166:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15(10):483–506. [DOI] [PubMed] [Google Scholar]
- Nekovarova T, Fajnerova I, Horacek J, Spaniel F. 2014. Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory. Front Behav Neurosci. 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. 2011. Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr Res. 129(2–3):163–168. [DOI] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD, Suckling J, Bullmore ET. 2014. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, Kostović I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108(32):13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Chen S, Hu D, Zeng L, Fan Y, Chen X, Shen H. 2015. Predicting individual brain maturity using dynamic functional connectivity. Front Hum Neurosci. 9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, Calhoun VD. 2016. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 134:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PG, Mattar MG, Murphy AC, Wymbs NF, Grafton ST, Satterthwaite TD, Bassett DS. 2018. Brain state flexibility accompanies motor-skill acquisition. Neuroimage. 171:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS 2008. Working memory in infants and toddlers In: Courage M, Cowan N, editors. The development of memory in infancy and childhood. 2nd ed. London: Psychology Press, pp. 355–378. [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. 2016. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 19:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, Constable RT. 2013. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 82:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 20(12):2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. 2011. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 56:1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 105(34):12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu Z, Rolls ET, Chen Q, Yao Y, Yang W, Wei D, Zhang Q, Zhang J, Feng J et al. 2018. Verbal creativity correlates with the temporal variability of brain networks during the resting state. Cereb Cortex. 29(3):1047–1058. [DOI] [PubMed] [Google Scholar]
- Supekar K, Cai W, Krishnadas R, Palaniyappan L, Menon V. 2019. Dysregulated brain dynamics in a triple-network saliency model of schizophrenia and its relation to psychosis. Biol Psychiatry. 85(1):60–69. [DOI] [PubMed] [Google Scholar]
- Taghia J, Cai W, Ryali S, Kochalka J, Nicholas J, Chen T, Menon V. 2018. Uncovering hidden brain state dynamics that regulate performance and decision-making during cognition. Nat Commun. 9(1):2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Ville D, Jhooti P, Haas T, Kopel R, Lovblad K, Scheffler K, Haller S. 2012. Recovery of the default mode network after demanding neurofeedback training occurs in spatio-temporally segregated subnetworks. Neuroimage. 63:1775–1781. [DOI] [PubMed] [Google Scholar]
- Verbeke G 1997. Linear mixed models for longitudinal data In: Molenberghs G, editor. Linear mixed models in practice. Vol 126 New York: Springer, pp. 63–153. [Google Scholar]
- Wang L, Gao Y, Shi F, Li G, Gilmore JH, Lin W, Shen D. 2015. LINKS: learning-based multi-source IntegratioN frameworK for segmentation of infant brain images. Neuroimage. 108:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang H, Li G, Liu M, Yin W, Lin W, Zhang J, Shen D. 2019. First-year development of modules and hubs in infant brain functional networks. Neuroimage. 185:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS 2017. Segregated systems of human brain networks. Trends Cogn Sci. 21(12):981–996. [DOI] [PubMed] [Google Scholar]
- Wu G, Wang Q, Shen D, Alzheimer'S DNI. 2012. Registration of longitudinal brain image sequences with implicit template and spatial–temporal heuristics. Neuroimage. 59:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap P, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D. 2011. Development trends of white matter connectivity in the first years of life. PLoS One. 6(9):e24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Chee MW, Buckner RL. 2014. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 88:212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Chen M, Hung S, Baluyot KR, Li T, Lin W. 2019. Brain functional development separates into three distinct time periods in the first two years of life. Neuroimage. 189:715–726. [DOI] [PubMed] [Google Scholar]
- Yu E, Liao Z, Tan Y, Qiu Y, Zhu J, Han Z, Wang J, Wang X, Wang H, Chen Y. 2017. High-sensitivity neuroimaging biomarkers for the identification of amnestic mild cognitive impairment based on resting-state fMRI and a triple network model. Brain Imaging Behav. 13(1):1–14. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. 2014. Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A. 111(28):10341–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Stanley N, Mucha PJ, Yin W, Lin W, Shen D. 2018. Multi-layer large-scale functional connectome reveals infant brain developmental patterns In: Frangi A, Schnabel J, Davatzikos C, Alberola-López C, Fichtinger G, editors. Medical image computing and computer assisted intervention– MICCAI 2018. Vol 11072 Granada (Spain): Springer, pp. 136–144. [Google Scholar]
- Zhang H, Shen D, Lin W. 2019. Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage. 185:664–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cheng W, Liu Z, Zhang K, Lei X, Yao Y, Becker B, Liu Y, Kendrick KM, Lu G. 2016. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain. 139(8):2307–2321. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 133(5):1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


