Abstract
Objective
Coronavirus Disease 2019 (COVID-19) caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is still spreading worldwide, which may progress to pulmonary fibrosis (PF), leading to the worsen outcome. As the markers of lung injury, the correlation of Krebs von den Lungen-6 (KL-6) and fibronectin (Fn) with pulmonary fibrosis in COVID-19 was still unclear.
Methods
113 patients diagnosed as COVID-19 were enrolled in this retrospective study, and divided into three categories as mild, moderate and severe cases. The concentrations of serum KL-6 and Fn at hospital admission were tested using the method of latex agglutination assay and immunoturbidimetic assay, respectively.
Results
Compared with that in the non-severe COVID-19 cases and normal control subjects, serum KL-6 concentration on admission was significantly higher in the severe group, which was positively correlated with C-reactive protein, and negatively correlated with lymphocytes count. Whereas, no obvious elevation in serum Fn concentration was investigated in COVID-19 patients with the different phenotypes. The severe cases displayed the higher incident rate of pulmonary fibrosis at hospital discharge. Compared with non-PF patients, the COVID-19 cases with PF had the higher serum KL-6 values.
Conclusion
Serum KL-6 concentration was significantly elevated in severe COVID-19 patients, which may be useful for evaluating the disease severity. For early prevention of the development of pulmonary fibrosis, high concentrations of serum KL-6 in the early stage of COVID-19 should be paid close attention.
Keywords: Coronavirus Disease 2019, Krebs von den Lungen-6, Pulmonary fibrosis, Fibronectin, SARS-CoV-2
1. Background
Coronavirus Disease 2019 (COVID-19) is a serious pandemic disease affecting more than 2.8 million people worldwide, which is caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], [2], [3]. The targeting organs of SARS-CoV-2 are believed to be the lungs and bronchus at all levels. One of the most important pathological changes was mechanization of alveolar exudate and interstitial lung fibrosis [4], [5]. Pulmonary fibrosis (PF) was closely related to tissue inflammation and damage repair, which has been characterized as a risk factor for a more severe evolution and higher mortality of COVID-19 [6], [7], [8], [9]. Also, patients recovering from severe and critical COVID-19 disease are at serious risk of developing postinflammatory PF through the follow-up computed tomography (CT) scan [4], [10]. Thus, it is urgent to identify the laboratory indicators for predicting the development of PF in the early stage of COVID-19 patients, which is useful for taking timely intervention measures to prevent patients turning into further PF.
It has been currently accepted that Krebs von den Lungen-6 (KL-6), a glycoprotein secreted by type II alveolar pneumocytes and bronchiolar epithelial cells, is a biomarker of several interstitial lung diseases (ILD), including idiopathic pulmonary fibrosis [11], [12], [13]. A recent study reported that there is a complexity of interactions between coexisting idiopathic pulmonary fibrosis/ILD and COVID-19 disease [6]. Serum KL-6 has been suggested to be a useful biomarker for evaluating the severity of COVID-19, however, the results from another research did not demonstrate that KL-6 can discriminate different ventilatory phenotypes in COVID-19 patients [14], [15], [16]. Moreover, pulmonary fibrosis is identified by excessive deposition of extracellular matrix (ECM) proteins, such as fibronectin (Fn) [17]. Previous studies showed that increased Fn deposition was present in lung tissues from lung fibrosis patients [18], [19]. Recently, it has been reported that SARS-CoV-2 infection may induce Fn gene expression in alveolar epithelial cells, indicating the fibrotic processes could be induced in COVID-19 disease [20]. Herein, we tested fibrosis markers KL-6 and Fn in sera samples from COVID-19 patients with the different phenotypes to investigate whether serum KL-6 and Fn may be used as the sensitive marker for assessing the development of SARS-CoV-2-induced pulmonary fibrosis.
2. Patients and methods
2.1. Study population
Our study retrospectively analyzed 113 cases of confirmed COVID-19 (diagnosed according to positive viral nucleic acid test results in throat swab samples), and 36 suspected cases (no positive viral nucleic acid results but diagnosis was made based on presence of exposure, symptoms or lung imaging features consistent with virus pneumonia). These patients were admitted to Zhongnan Hospital of Wuhan University from February 12 to March 15, 2020. Normal healthy subjects (n = 65) who had routine laboratory tests between April 10 to April 30, 2020 were included as the control group, with exclusion of SARS-CoV-2 infection. This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2020058 K).
2.2. Data collection
Electronic data regarding age, sex, medical history, symptoms and signs, diagnosis and severity assessment, standard laboratory tests and chest computed tomography (CT) findings. According to the 7th Version of the Diagnosis and Treatment Guidelines of COVID-19 by the National Health Commission of China (NHCC) and a previous literature [21], all confirmed COVID-19 patients (n = 113) were classified into the following subtypes: mild cases (n = 49; having mild clinical symptoms without CT imaging features of pneumonia), moderate cases (n = 28; having clinical symptoms, such as fever and cough, as well as CT imaging features of pneumonia) and severe cases (n = 36; having dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates > 50% within 24 to 48 h). Chest CT imaging results for at least 2 times including on admission and discharge were collected to evaluate the features of coronavirus pneumonia or lung fibrosis development.
2.3. Laboratory tests
All laboratory tests were carried out at the department of Laboratory Medicine of Zhongnan Hospital of Wuhan University. All enrolled cases were detected for SARS-CoV-2 nucleic acid on samples from the respiratory tract using a Coronavirus PCR Fluorescence Diagnostic Kit (Daan Gene Co., Ltd. Of Sun Yat-Sen University, Guangzhou, China). The primers and probes for quantitative real-time polymerase chain reaction (qRT-PCR) were reported previously [22].
Blood samples were collected from each of the 113 fasting confirmed COVID-19 patients and 36 clinically diagnosed cases on admission, as well as from the 65 fasting control subjects. The blood samples were centrifuged for 10 min at 2700g after standing for 30 min, then the serum samples were separated and preserved at −80 ℃ for further analysis. Serum concentrations of KL-6 and Fn were measured on an Olympus 5800 analyzer (Beckman Coulter, CA, USA) using the latex agglutination assay (Nanopia KL-6, Sekisui Medical Co., Ltd., Tokyo, Japan) and immunoturbidimetic assay (Fibronectin, Beijia Biochemical Co., Ltd., Shanghai, China), respectively.
2.4. Statistical analysis
GraphPad Prism 7.0 (GraphPad Software) were used for statistical analysis. Descriptive data were presented as mean ± standard error of mean (SEM), median (interquartile range, IQR) or proportions. Between-group and across-group differences were compared by Student’s t-test, Mann-Whitney U test, one-way analysis of variance (ANOVA), Kruskal-Wallis test or χ2 test, where appropriate. The receiver operating characteristic (ROC) curve was done for measuring the sensitivity and specificity of fibrosis markers at different cut-off values. p < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of enrolled COVID-19 patients
The basic clinical characteristics of 113 COVID-19 patients (divided into 49 mild, 28 moderate, and 36 severe cases), 36 suspected cases and 65 healthy control subjects are shown in Table 1 . The gender and age distribution did not differ significantly among the control group, the COVID-19 subgroups with different severity, and the suspected group. Compared to the control subjects and suspected cases, hypertension was the more prevalent morbidity in severe COVID-19 patients. Consistent with the previous reports, the decreased white blood cells (WBCs) and lymphocytes (LC) count, and the elevated C-reactive protein (CRP) could also be detected in COVID-19 patients in our study [23], [24], [25].
Table 1.
Clinical characteristics of enrolled COVID-19, suspected cases and control subjects.
| Characteristic | Normal range | Control | COVID-19 (n = 113) |
Suspected Cases | P | ||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||||
| Number of patients | 65 | 49 | 28 | 36 | 36 | ||
| Male, n (%) | 28 (43.1) | 25 (51.0) | 12(42.9) | 24 (66.7) | 20 (55.6) | NS | |
| Age, y, median (IQR) | 50 (22–69) | 45 (16–72) | 51 (27–75) | 56 (28–86) | 55 (20–70) | NS | |
| Morbidities, n (%) | |||||||
| Hypertension | 7 (10.8) | 8 (16.3) | 8 (28.6) | 17 (47.2) | 6 (16.7) | &, a | |
| Diabetes | 5 (7.7) | 8 (16.3) | 6 (21.4) | 7 (19.4) | 7 (19.4) | NS | |
| Heart diseases | 3 (4.6) | 3 (6.1) | 2 (7.1) | 3 (8.3) | 1 (2.8) | NS | |
| COPD | 4 (6.2) | 3 (6.1) | 3 (10.7) | 3 (8.3) | 4 (11.1) | NS | |
| WBCs count, ×109/L median (IQR) |
3.5–9.5 | 7.52 (3.37–10.22) |
6.61 (3.30–9.34) |
4.25 (2.50–9.77) |
2.91 (1.09–5.23) |
6.77 (3.55–10.30) |
#, &, a |
| LC count, ×109/L median (IQR) |
1.15–6.00 | 4.18 (1.20–6.01) | 2.05 (0.65–3.09) |
1.15 (0.44–2.07) |
0.65 (0.24–2.16) |
2.19 (1.01–5.76) |
*, #, &, a, b |
| CRP, mg/L median (IQR) |
0.0–10.0 | 5.0 (1.0–8.5) |
9.5 (1.0–155.8) |
47.9 (2.0–350.0) | 105.8 (21.2–369.0) |
11.9 (0.6–88.1) |
#, &, a, b |
Note: Data reported as number (%) or median (IQR). Abbreviations: COVID-19, coronavirus disease 2019; IQR: interquartile range; COPD:
chronic obstructive pulmonary disease; WBCs: white blood cells; LC: lymphocytes; CRP: C-reactive protein; NS: not significant (p > 0.05).
*control vs mild COVID-19 and suspected cases, p < 0.05; #control vs moderate COVID-19 cases, p < 0.05; &control vs severe COVID-19
cases, p < 0.05; a severe vs mild COVID-19 and suspected cases, p < 0.05; b severe vs moderate COVID-19 cases, p < 0.05.
3.2. Serum concentrations of KL-6 and Fn in different COVID-19 phenotypes
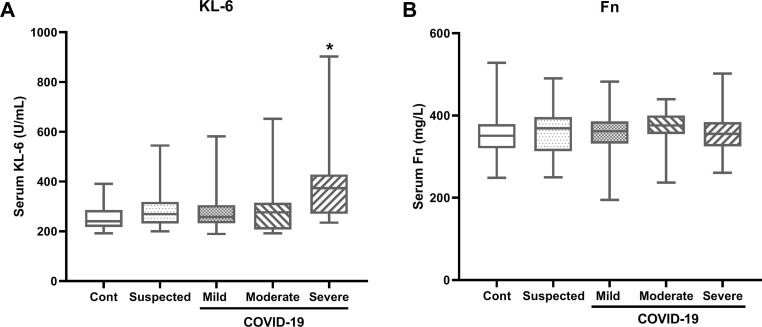
First we tested the serum concentrations of KL-6 and Fn in COVID-19 patients with different phenotypes. Our results showed that the median values of serum KL-6 and Fn in control subjects were 240.5 U/mL (IQR, 217.5–285.5) and 351.0 mg/L (IQR, 320.6–379.4), respectively. Compared to the control subjects, the median value of serum KL-6 was significantly higher in the severe COVID-19 cases (373.7 U/mL, IQR 269.9–428.1; p < 0.001), but not in the suspected cases, mild and moderate COVID-19 subjects (Fig. 1 A). For the serum Fn level, no obvious difference was detected among the control group, the suspected cases and the COVID-19 patients with different phenotypes (Fig. 1B).
Fig. 1.
Serum Krebs von den Lungen-6 (KL-6) and fibronectin (Fn)concentrationsin COVID-19 patients. Serum concentrations of KL-6 (A) and Fn (B) in the control subjects (n = 65), suspected cases (n = 36), mild COVID-19 cases (n = 49), moderate COVID-19 cases (n = 28), and severe COVID-19 cases (n = 36). Data are shown as median (interquartile range), *p < 0.05, compared with the other groups.
3.3. Serum KL-6 positively correlated with CRP concentration and negatively correlated with LC count in severe COVID-19 patients
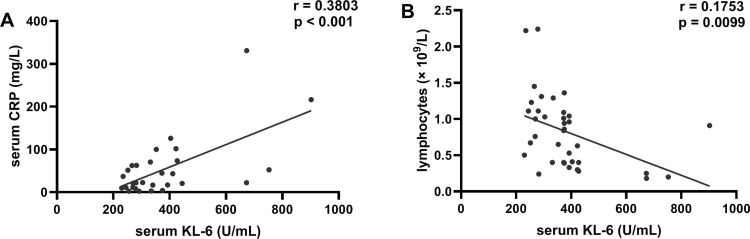
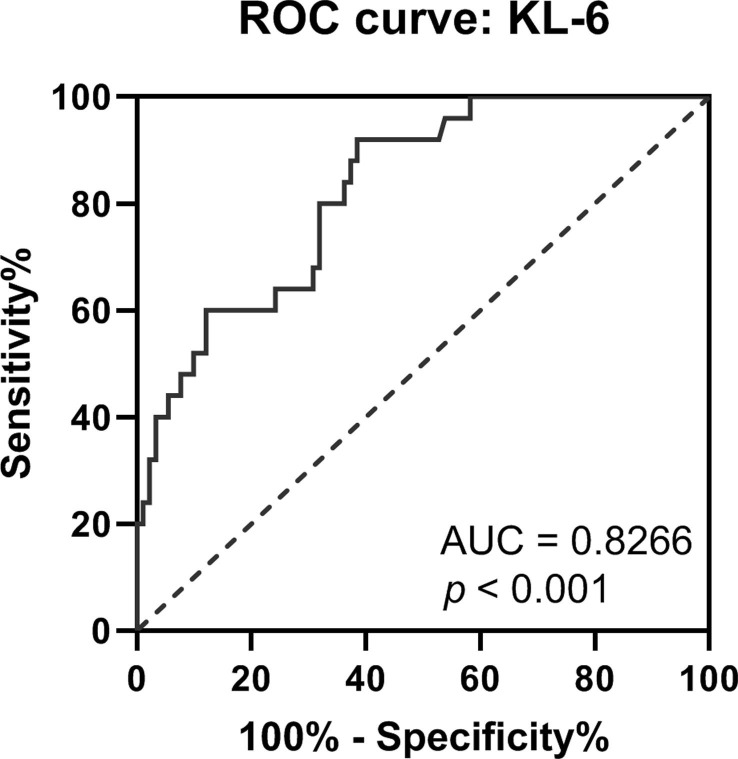
Correlation coefficients were calculated with the purpose to access the association between the disease severity and the serum KL-6 concentrations in severe COVID-19 cases. Previous studies have reported that the increased serum CRP and the depleted LC count could reflect disease severity [24], [25], [26]. We found that there was a significant positive correlation between the serum KL-6 and CRP concentration in severe COVID-19 patients (Fig. 2 A), whereas the elevated serum KL-6 was correlated negatively with LC count in the severe cases (Fig. 2B). ROC curve showed that the area under curve (AUC) of KL-6 in severe cases was 0.8266, and the optimal cut-off value of 278.3 U/mL yield sensitivity at 80.00% and specificity at 68.13% (Fig. 3 ). Therefore, higher concentrations of serum KL-6 may indicate the more severe status of COVID-19 disease, and these patients should be paid close attention.
Fig. 2.
Serum KL-6 positively correlated with CRP concentration and negatively correlated with LC count in severe COVID-19 patients. (A) Correlation between serum Krebs von den Lungen-6 (KL-6) and C-reactive protein (CRP) (n = 36). (B) Correlation between between serum KL-6 and lymphocytes (LC) count in severe COVID-19 cases (n = 36).
Fig. 3.
Receiver operating characteristic (ROC) curve analysis of serum KL-6 in severe COVID-19 patients.
3.4. Serum KL-6 concentration was significantly higher in COVID-19 patients with signs of pulmonary fibrosis
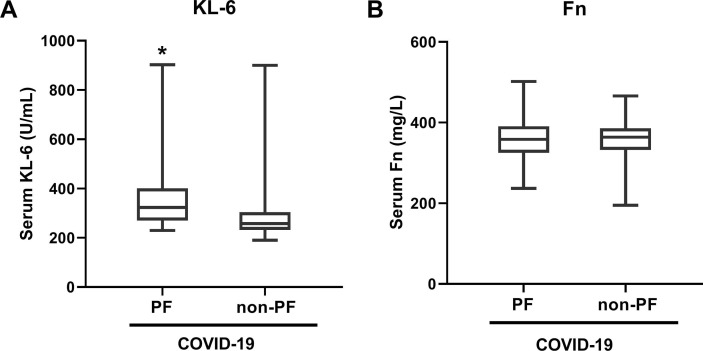
Among the enrolled 113 patients with SARS-CoV-2 infection, a total of 19 cases (16.81%) displayed the signs of pulmonary fibrosis on lung CT images when hospital discharge, including 2 in 49 (4.08%) mild cases, 4 in 28 (14.29%) moderate cases, and 13 in 36 (36.11%) severe cases. The incident rate of pulmonary fibrosis in severe COVID-19 patients was obviously higher than that in mild or moderate cases (Table 2 ). We further analyzed the serum concentrations of KL-6 and Fn on admission in COVID-19 with and without lung fibrosis, and the result showed that serum KL-6 value in COVID-19 patients developing pulmonary fibrosis was higher than that in COVID-19 cases without pulmonary fibrosis (Fig. 4 A), whereas no significant difference was detected in serum Fn concentration (Fig. 4B). High concentration of serum KL-6 may be used as a predictive marker for assessing the development of SARS-CoV-2-induced pulmonary fibrosis.
Table 2.
Incident rate of pulmonary fibrosis in the different phenotypes of COVID-19.
| COVID-19 |
||||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Total cases (n) | 49 | 28 | 36 | 113 |
| Pulmonary fibrosis cases (n) | 2 | 4 | 13 | 19 |
| Ratio (%) | 4.08 | 14.29 | 36.11* | 16.81 |
*p < 0.05, compared with mild or moderate cases.
Fig. 4.
Comparison of serum KL-6 and Fn concentrations between COVID-19 patients with and without pulmonary fibrosis (PF). Serum concentrations of KL-6 (A) and Fn (B) in the PF-developed (n = 19) and non-PF COVID-19 (n = 94) patients. Data are shown as median (interquartile range), *p < 0.05, compared with the non-PF COVID-19 group.
4. Discussion
Severe COVID-19 pneumonia may progress to pulmonary fibrosis, leading to impair outcome significantly, and antifibrotic therapies may have a role in preventing fibrosis after SARS-CoV-2 infection [7], [10]. How to classify the severity of COVID-19 has been reported in several reports, nevertheless, no criteria for pulmonary fibrosis evaluation has yet been adopted into the severity classification in COVID-19 [27], [28]. In the present study, we investigated the usefulness of serum KL-6 and Fn, which are known as the biomarkers of lung fibrosis [11], [12], [13], [17], [18], [19]. Compared with the non-severe groups including mild and moderate cases, the serum KL-6 value on admission was significantly higher in severe COVID-19 patients. Whereas, no difference in the concentration of serum KL-6 was found in non-severe groups and normal control subjects. SARS-CoV-2 is known to have specific tropism for alveolar epithelial cells, which may cause interstitial lung injury and epithelial lung alterations in the acute phase [15]. As a secreted glycoprotein, KL-6 is mainly produced by damaged or regenerating type II alveolar pneumocytes and bronchiolar epithelial cells [29]. Thus, the elevated serum KL-6 in severe COVID-19 patients may due to the induction of SARS-CoV-2 infection, and could be activated to secret into circulation. On the other hand, the elevated serum KL-6 may be also related to the more severe lung injury occurring in the severe COVID-19 patients. The previous studies have reported that serum KL-6 may be used to evaluate the severity of COVID-19 [14], [30]. A very recent study also reported that serum KL-6 may be a novel biomarker for accessing COVID-19 severity and predicting the prognosis of lung injury [31]. We also found that serum KL-6 concentration in severe COVID-19 patients correlated positively with serum CRP concentration, and negatively with peripheral blood lymphocytes count. The cut-off value of 278.3 U/mL with a sensitivity at 80.00% and specificity at 68.13%, may be an indicator to evaluate the severity of lung injury and inflammation. On the contrary, the increased serum Fn was not significant in the different phenotypes of COVID-19 patients. As a component of the ECM, whether the circulated Fn concentration could be parallel to its change in ECM in the lung tissues need to be further elucidated.
Furthermore, whether high serum KL-6 could reflect the high risk of pulmonary fibrosis development in COVID-19 patients is still unknown. Our results showed that the incident rate of pulmonary fibrosis in severe COVID-19 group was obviously higher than that in non-severe groups, suggesting that high serum KL-6 in severe COVID-19 patients may be useful for predicting the fibrotic lung involvement. The further analysis indicated that the serum KL-6 concentration was higher in the COVID-19 patients displaying the signs of pulmonary fibrosis at discharge, consistent with the finding reported in another study [32]. In the patients with idiopathic pulmonary fibrosis (IPF), serum KL-6 changes tend to increase with IPF progression, and serial serum KL-6 test may reflect the clinical status of fibrotic lung abnormalities [29], [33], [34]. Thus, in the early stage of patients diagnosed with SARS-CoV-2 infection, high serum KL-6 may indicate the higher risk for the development of pulmonary fibrosis.
There are still some limitations in our study. First, it was a retrospective, single-center study with a small number of COVID-19 patients admitted to hospital. Second, the serum KL-6 concentrations before SARS-CoV-2 infection in several COVID-19 cases with the morbidity of chronic obstructive pulmonary disease (COPD) were unknown, as the high KL-6 concentrations in serum and sputum samples have been reported in Japanese COPD patients [35], [36]. Moreover, collecting enough cases of critical COVID-19 patients seems to be difficult. Additional larger sizes of COVID-19 patients from multiple centers undergoing follow-up evaluations are needed to validate our findings.
5. Conclusion
In conclusion, this study demonstrated that serum KL-6 concentrations but not Fn were high in severe COVID-19 patients, which were associated with the severity of lung damage, positively correlated with CRP and negatively correlated with lymphocytes count. Serum KL-6 may be a candidate marker for pulmonary fibrosis evaluation, which may be adopted into the severity classification in COVID-19. Patients with high serum KL-6 on admission may have the high risk for the fibrotic lung involvement, deserving the careful concern. Given a potential role of antifibrotic therapy in preventing fibrosis after SARS-CoV-2 infection, maybe antifibrotic therapies could be performed to further evaluate the treatment effectiveness according to the value of serum KL-6 in the early stage of COVID-19 disease.
Funding
This study was supported by Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund, Project znpy2019053.
Ethical approval and consent to participate
This study was approved by the ethics committee of Zhongnan Hospital of Wuhan University (No. 2020058K).
CRediT authorship contribution statement
Ding-Hui Peng: Data curation, Formal analysis, Investigation, Methodology, Project administration. Yi Luo: Data curation, Formal analysis, Project administration, Writing - original draft, Funding acquisition. Li-Jun Huang: Conceptualization, Methodology, Writing - review & editing. Fan-Lu Liao: Resources, Visualization. Yan-Yuan Liu: Data curation, Software. Peng Tang: Investigation, Validation. Han-Ning Hu: Conceptualization, Supervision, Visualization, Writing - review & editing. Wei Chen: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The author declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Professor Yirong Li, Dr. Wei Xiao, who helped with the topic development and with improving the article.
References
- 1.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat. Med. 2020;26(3):317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B., Li X., Wang Y., Han Y., Wang Y., Wang C., Zhang G., Jin J., Jia H., Fan F., Ma W., Liu H., Zhou Y. Diagnostic value and key features of computed tomography in Coronavirus Disease 2019. Emerging Microbes Infect. 2020;9(1):787–793. doi: 10.1080/22221751.2020.1750307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damiani S., Fiorentino M., De Palma A., et al. Pathological post mortem findings in lungs infected with SARS-CoV-2. J. Pathol. 2021;253(1):31–40. doi: 10.1002/path.5549. [DOI] [PubMed] [Google Scholar]
- 6.Crisan-Dabija R., Pavel C.A., Popa I.V., Tarus A., Burlacu A. “A chain only as strong as its weakest link”: an up-to-date literature review on the bidirectional interaction of pulmonary fibrosis and COVID-19. J. Proteome Res. 2020;19(11):4327–4338. doi: 10.1021/acs.jproteome.0c00387. [DOI] [PubMed] [Google Scholar]
- 7.Letellier A., Gibelin A., Voiriot G., Fartoukh M., Djibré M. Destructive pulmonary fibrosis after severe COVID-19 pneumonia. Int. J. Infect. Diseases. 2020;100:377–378. doi: 10.1016/j.ijid.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y., Gu H., Weng D., Zhou Y., Li Q., Zhang F., Zhang Y., Shen L.i., Hu Y., Li H. Association of serum levels of laminin, type IV collagen, procollagen III N-terminal peptide, and hyaluronic acid with the progression of interstitial lung disease. Medicine. 2017;96(18):e6617. doi: 10.1097/MD.0000000000006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding M., Zhang Q., Li Q., Wu T., Huang Y.-z. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir. Med. 2020;167:105981. doi: 10.1016/j.rmed.2020.105981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory Medicine. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.S., Lee E.Y., Ha Y.-J., Kang E.H., Lee Y.J., Song Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019;21(1) doi: 10.1186/s13075-019-1835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa N., Hattori N., Yokoyama A., Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respiratory Investigation. 2012;50(1):3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Kuwana M., Shirai Y., Takeuchi T. Elevated serum krebs von den lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J. Rheumatol. 2016;43(10):1825–1831. doi: 10.3899/jrheum.160339. [DOI] [PubMed] [Google Scholar]
- 14.Awano N., Inomata M., Kuse N., Tone M., Takada K., Muto Y., Fujimoto K., Akagi Y.u., Mawatari M., Ueda A., Izumo T. Serum KL-6 level is a useful biomarker for evaluating the severity of coronavirus disease 2019. Respirat. Investigat. 2020;58(6):440–447. doi: 10.1016/j.resinv.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d'Alessandro M., Cameli P., Refini R.M., Bergantini L., Alonzi V., Lanzarone N., Bennett D., Rana G.D., Montagnani F., Scolletta S., Franchi F., Frediani B., Valente S., Mazzei M.A., Bonella F., Bargagli E. Serum KL‐6 concentrations as a novel biomarker of severe COVID‐19. J. Med. Virol. 2020;92(10):2216–2220. doi: 10.1002/jmv.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d'Alessandro M., Cameli P., Bergantini L., Franchi F., Scolletta S., Bargagli E. Serum concentrations of Krebs von den Lungen‐6 in different COVID‐19 phenotypes. J. Med. Virol. 2021;93(2):657. doi: 10.1002/jmv.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G., Cooley M.A., Nair P.M., Donovan C., Hsu A.C., Jarnicki A.G., Haw T.J., Hansbro N.G., Ge Q.i., Brown A.C., Tay H., Foster P.S., Wark P.A., Horvat J.C., Bourke J.E., Grainge C.L., Argraves W.S., Oliver B.G., Knight D.A., Burgess J.K., Hansbro P.M. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c: Fbln1c regulates the pathogenesis of chronic asthma. J. Pathol. 2017;243(4):510–523. doi: 10.1002/path.4979. [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Cooley M.A., Jarnicki A.G., et al. Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis. JCI Insight. 2019;5(16) doi: 10.1172/jci.insight.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., Baralle F.E., Toews G.B., White E.S. An essential role for fibronectin extra type III domain a in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177(6):638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020;21(1) doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 22.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet. Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.B. Diao, C. Wang, Y. Tan, et al., Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19), Front Immunol. 11 (2020) 827. [DOI] [PMC free article] [PubMed]
- 24.F. Wang, J. Nie, H. Wang, et al., Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia, J. Infect. Dis. 221(11) (2020) 1762–1769. [DOI] [PMC free article] [PubMed]
- 25.Wang L. C-reactive protein levels in the early stage of COVID-19. Médecine et Maladies Infectieuses. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 – a systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health. Clinical spectrum of SARS-CoV-2 infection. http://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ [Accessed 17 December, 2020].
- 28.General Office of National Health Committee, Office of State Administration of Traditional Chinese Medicine. 2019-nCoV. Notice on the issuance of a programme for the diagnosis and treatment of novel coronavirus pneumonia. 2020. trial seventh edition, http://yzs.satcm.gov.cn/zhengcewenjian/2020-05-28/15455.html [Accessed 4 March, 2020].
- 29.d’Alessandro M., Bergantini L., Cameli P., et al. Krebs von den Lungen-6 as biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomark. Med. 2020;68(6):414–421. doi: 10.2217/bmm-2019-0545. [DOI] [PubMed] [Google Scholar]
- 30.Xue M., Zheng P., Bian X., Huang Z., Huang H., Zeng Y., Hu H., Liu X., Zhou L., Sun B., Wu J.-L., Zhong N. Exploration and correlation analysis of changes in Krebs von den Lungen-6 levels in COVID-19 patients with different types in China. BST. 2020;14(4):290–296. doi: 10.5582/bst.2020.03197. [DOI] [PubMed] [Google Scholar]
- 31.K. Deng, Q. Fan, Y. Yang, et al., Prognostic roles of KL-6 in disease severity and lung injury in COVID-19 patients: a longitudinal retrospective analysis, J. Med. Virol. 2021, doi: 10.1002/jmv.26793. [DOI] [PMC free article] [PubMed]
- 32.d’Alessandro M., Bergantini L., Cameli P., et al. Serial KL-6 measurement in COVID-19 patients. Intern. Emerg. Med. 2021 doi: 10.1007/s11739-020-02614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakamatsu K., Nagata N., Kumazoe H., Oda K., Ishimoto H., Yoshimi M., Takata S., Hamada M., Koreeda Y., Takakura K., Ishizu M., Hara M., Ise S., Izumi M., Akasaki T., Maki S., Kawabata M., Mukae H., Kawasaki M. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respirat. Investigat. 2017;55(1):16–23. doi: 10.1016/j.resinv.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Bergantini L., Bargagli E., Cameli P., Cekorja B., Lanzarone N., Pianigiani L., Vietri L., Bennett D., Sestini P., Rottoli P. Serial KL-6 analysis in patients with idiopathic pulmonary fibrosis treated with nintedanib. Respirat. Invest. 2019;57(3):290–291. doi: 10.1016/j.resinv.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa N., Hattori N., Tanaka S., Horimasu Y., Haruta Y., Yokoyama A., Kohno N., Kinnula V.L. Levels of surfactant proteins A and D and KL-6 are elevated in the induced sputum of chronic obstructive pulmonary disease patients: a sequential sputum analysis. Respiration. 2011;82(1):10–18. doi: 10.1159/000324539. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa N., Hattori N., Kohno N., et al. Airway inflammation in Japanese COPD patients compared with smoking and nonsmoking controls. Int. J. Chron. Obstruct. Pulmon. Dis. 2015;10:185–192. doi: 10.2147/COPD.S74557. [DOI] [PMC free article] [PubMed] [Google Scholar]