Abstract
Objectives
This study compares the infectivity of SARS-CoV-2 in respiratory samples from patients with mild COVID-19 with those from hospitalized patients with severe bilateral pneumonia. In severe COVID-19, we also analysed the presence of neutralizing activity in paired sera.
Methods
We performed cell cultures on 193 real-time reverse transcription polymerase chain reaction respiratory samples, positive for SARS-CoV-2, obtained from 189 patients at various times, from clinical diagnosis to follow-up. Eleven samples were obtained from asymptomatic individuals, 91 samples from 91 outpatients with mild forms of COVID-19 and 91 samples from 87 inpatients with severe pneumonia. In these patients, neutralizing activity was analysed in 30 paired sera collected after symptom onset >10 days.
Results
We detected a cytopathic effect (CPE) in 91/193 (47%) samples. Viral viability was maintained for up to 10 days in patients with mild COVID-19. In patients with severe COVID-19, the virus remained viable for up to 32 days after the onset of symptoms. Patients with severe COVID-19 presented infectious virus at a significantly higher rate in the samples with moderate to low viral load (cycle threshold value ≥ 26): 32/75 (43%) versus 14/63 (22%) for mild cases (p < 0.01). We observed a positive CPE despite the presence of clear neutralizing activity (NT50 > 1:1024 in 10% (3/30) of samples.
Discussion
Patients with severe COVID-19 might shed viable virus during prolonged periods of up to 4 weeks after symptom onset, even when presenting high cycle threshold values in their respiratory samples and despite having developed high neutralizing antibody titres.
Keywords: Cell culture, COVID-19, Infection control, Neutralizing antibodies, SARS-CoV-2
Introduction
SARS-CoV-2, a novel human coronavirus that emerged in Wuhan (China) in late 2019 [1,2] has been responsible for the largest pandemic in a century.
The use of real-time reverse transcription polymerase chain reaction (rRT-PCR) [3] as a diagnostic and follow-up tool for SARS-CoV-2 infection has led to hypotheses regarding infectivity duration, the possibility of reactivation and even re-infection [4]. Although rRT-PCR is the reference standard diagnostic method, it is less useful as a follow-up technique, because samples from patients who have overcome either mild or severe SARS-CoV-2 infection still have detectable viral RNA for variable periods of time [[5], [6], [7]]. In the absence of diagnostic methods with reliable quantification, the cycle threshold (Ct) value obtained in amplification has been employed as a semiquantitative measure and has been proposed as a parameter for elaborating approaches to removing patients from isolation [8]. Establishing a reliable cut-off Ct value is difficult, given the large number of available rRT-PCR-based diagnostic tests; the need to use more than one molecular test in most clinical laboratories to meet growing demand; and the use of different types of samples during patient follow-up. Hence, the importance of establishing the duration of virus viability in various clinical situations. The assessment of SARS-CoV-2 viability will help establish criteria for isolating patients.
The role of anti-SARS-CoV-2 neutralizing antibodies in controlling viral excretion has recently been evaluated [9,10], finding differences in the titres achieved and antibody persistence depending on illness severity. It has also been suggested that the presence of neutralizing antibodies is correlated with the lack of viral viability in respiratory samples [7].
This study compared viral detection by rRT-PCR and the infectivity of SARS-CoV-2 in respiratory samples from patients with mild COVID-19 with those from hospitalized patients with severe bilateral pneumonia. In those patients with severe COVID-19, we also analysed the presence of anti-SARS-CoV-2 immunoglobulin G (IgG) and the neutralizing activity in paired sera with respiratory samples, as well as the correlation between its presence and viral viability.
Materials and methods
Design, setting and ethics
This retrospective study focused on respiratory samples obtained during a 2-month period that met the following requirements: (a) clinical record is available; (b) collection on viral transport medium that ensures virus viability; (c) sufficient residual volume after routine diagnostic assays; (d) samples processed with the same rRT-PCR assay; (e) when a reduction in Ct values was detected during follow-up. The study was approved by our institutional review board (Reference CEIm: 20/232).
Samples and patients
A total of 193 respiratory samples (186 nasopharyngeal exudates and seven bronchial aspirates) were processed by rRT-PCR and cell culture. All the samples were from adult patients. Ninety-one samples were obtained from 91 patients with COVID-19-compatible symptoms who did not require hospital admission and who were mostly healthcare workers (HCWs (n = 76) attending the Occupational Health and Safety Service for a first consultation or follow-up after a first positive rRT-PCR sample. Eleven samples were collected from a different group of 11 asymptomatic individuals in whom the virus was detected during presurgical or delivery screening for hospital admission or during contact studies. Ninety-one samples were obtained from 87 hospitalized patients with severe COVID-19 pneumonia. The diagnosis of severe COVID-19 was established by respiratory, laboratory and radiographic findings. Samples were obtained at various time points covering the time from clinical diagnosis to follow-up during hospital care. Bronchial aspirates were collected during the follow-up of patients admitted to the intensive care units (ICUs).
Microbiological methods
Nasopharyngeal samples were collected with flocked swabs in universal transport medium (Copan Diagnostics, Brescia, Italy). A previously published rRT-PCR protocol for detecting the E gene [3] was adapted for processing on the Panther Fusion Hologic (San Diego, CA, USA) automated molecular diagnostic platform, using its open access functionality [11]. The Ct value obtained in this assay was employed as a measure of relative quantification throughout the study.
For the cell culture, an aliquot (250 μL) of the residual sample was decontaminated using gentamicin and amphotericin B, inoculated into 24-well plates on Vero E6 cells (ATCC CCL-81) and cultured in Medium 199 supplemented with L-glutamine and 10% foetal bovine serum. The plates were incubated in a 5% carbon dioxide atmosphere for 5 days. The development of a cytopathic effect (CPE) was examined daily. SARS-CoV-2 CPE specificity was confirmed by immunofluorescence (shell-vial technique) by using a commercial anti-SARS-CoV-2 N protein (Rockland Immunochemicals, Inc., Limerick, PA, USA) as the primary antibody and a goat anti-rabbit IgG labelled with Alexafluor 488 (Abcam, Cambridge, UK) as the secondary antibody. Upon CPE observation and at the end of the cell culture incubation period, culture supernatants were collected from each well and an rRT-PCR was performed, which was confirmed positive if it was at least 3 Ct lower than the original sample. All cell culture-related procedures were performed at a biosafety level 3 facility.
Specific anti-SARS-CoV-2 antibody detection
For 27 patients with severe COVID-19, we study a serum sample collected at least 10 days after symptom onset, paired with the analysed respiratory sample. In total, we analysed the presence of IgG and neutralizing antibodies [6] in 30 serum samples through an IgG anti-SARS-CoV-2 chemiluminescent immunoassay (Abbott Laboratories) and neutralization assays. We employed the SARS-CoV-2-pseudotyped recombinant vesicular stomatitis virus-expressing luciferase system to test the neutralizing activity. Virus-containing transfection supernatants were normalized for infectivity to a 0.5–1 multiplicity of infection and incubated with the serum sample dilutions at 37°C for 1 hr in 96-well plates. After the incubation, 2 × 104 Vero E6 cells were seeded onto the virus–plasma mixture and incubated at 37°C for 24 hr. Cells were then lysed and assayed for luciferase expression. We calculated the 50% neutralization titre (NT50) using a nonlinear regression model fit with settings for log (inhibitor) versus normalized response curves.
Data analysis
We recorded and analysed the demographic data, COVID-19 severity, symptom onset to test time (STT), whether the patient was undergoing immunosuppressive therapy at the time of infection, Ct values and CPE detection. NT50 neutralizing activity was correlated with viral viability in the paired respiratory samples.
Quantitative variables are described using median and interquartile range (IQR) and were compared using the Mann–Whitney U test. Categorical variables are expressed as relative frequencies and were compared using Fisher's exact test. A p value < 0.05 was considered statistically significant. The statistical analysis was performed using GraphPad Prism v8 software.
Results
Patient and sample descriptions
The mean age of the asymptomatic patients was 52.9 years (range 22–76), and 45% (5/11) were women. The mean age of the patients with mild COVID-19 was 40.7 years (range 20–81) and 75% (68/91) were women. This mean age and sex distribution are because most of the individuals included in this group are HCWs. Inpatients with severe COVID-19 had a mean age of 65.2 years (IQR 17–94), and 34% were women (30/87).
The patients with mild COVID-19 consulted for their symptoms earlier (mean 3.2 days (range 1–10), median 3 days (IQR 2–3)) than those with severe COVID-19 (mean 7.5 days (range 3–27), median 6 days (IQR, 4–10); p < 0.001).
Seven (7/87, 8%) patients with severe COVID-19 were admitted to the ICUs and underwent mechanical ventilation.
In total, 7/87 (8%) patients with bilateral pneumonia died, presenting a higher median age than the patients with bilateral pneumonia who recovered (80.0 vs. 64.5 years, p < 0.01).
Eighteen (18/87, 21%) patients with severe COVID-19 were undergoing immunosuppressive therapy when they acquired the infection (12 had malignancies, three were solid transplant recipients and three had autoimmune diseases).
For the entire patient group, 109 samples were obtained at clinical diagnosis, and 73 were collected during patient follow-up. The median Ct value was 29.2 (IQR 26.0–32.3) for the inpatients' first samples (n = 63) and 25.2 (IQR 21.5–29.1) for the outpatients (n = 46) (p 0.007). The seven patients who died presented higher viral loads in the diagnostic sample than the other patients with pneumonia (median Ct values 21.0 vs. 29.5, p 0.009). In contrast, first samples from the immunocompromised patients did not presented significantly lower Ct values (27.0 vs. 29.5, p 0.2).
Cell culture
A CPE was detected in the cell culture in 91/193 (47%) samples and was detectable in most cases in 72 hr (Fig. 1 ). Initial samples presented viral replication at a higher proportion than the follow-up samples: 69% (75/109) vs. 15% (11/73) (p < 0.001). The mean collection time for the initial samples was 5 days (range 1–20, median 3, IQR 2–7), whereas for the follow-up samples it was 18.8 days (range 10–32, median 20, IQR 10–25).
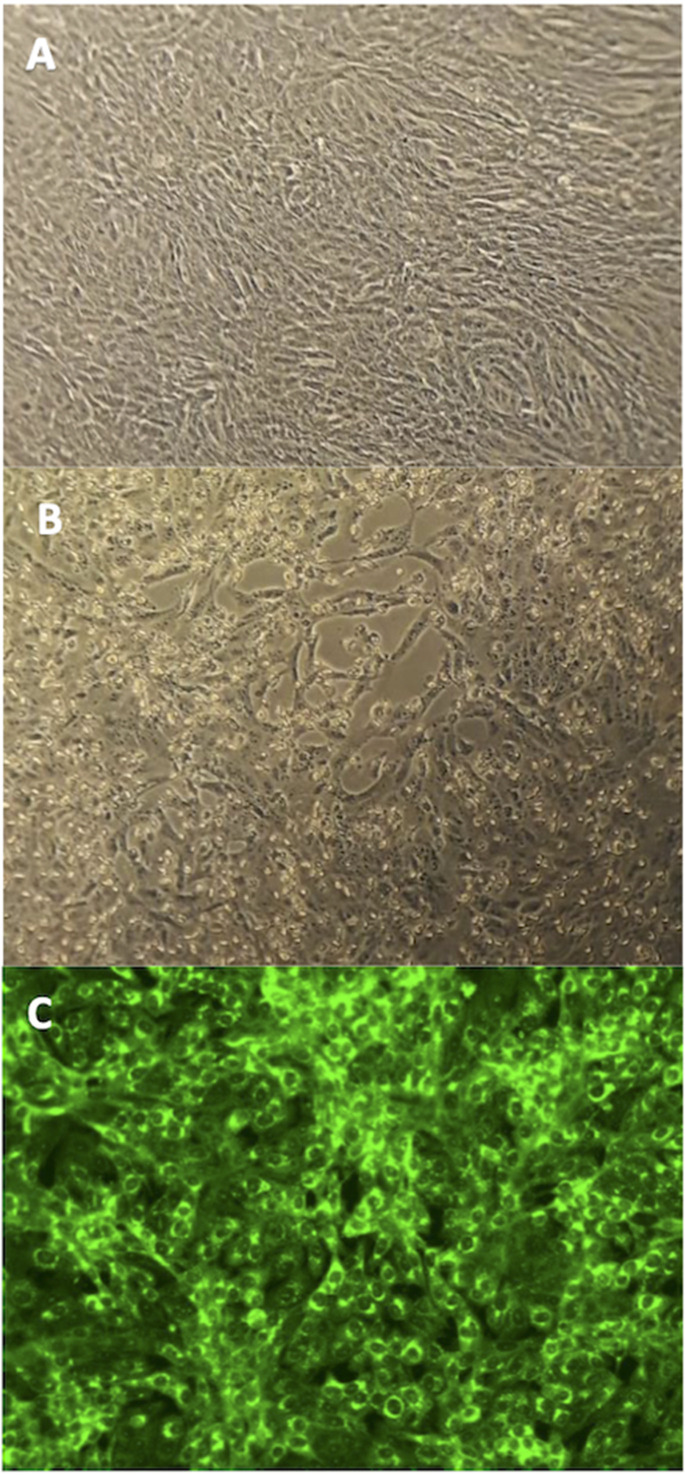
Fig. 1.
Cytopathic effect (CPE) produced by SARS-CoV-2 on Vero E6 cell line. (A) Normal appearance of the cell line. (B) CPE development after 48 hours' incubation. (C) Confirmation of the specificity of the CPE observed by shell-vial technique. Microscopic visualization at 20× magnification.
The percentage of samples that presented viral replication for each of the patient groups is shown in Table 1 , along with other sample data and patient demographics.
Table 1.
Main patient and sample data for all patient groups
| Asymptomatic | Mild COVID-19 HCW | Mild COVID-19 non-HCW | Severe COVID-19 immunocompromised | Severe COVID-19 exitus | Severe COVID-19 other pneumonia | |
|---|---|---|---|---|---|---|
| Number of patients (total n = 189) | 11 | 76 | 15 | 18 | 7 | 62 |
| Age, mean (range) | 52.9 (22–76) | 40.2 (20–62) | 43.1 (26–81) | 59.1 (42–77) | 79.28 (70–91) | 65.7 (17–94) |
| Female sex number (%) | 5 (45) | 59 (78) | 5 (33) | 6 (30) | 3 (43) | 24 (39) |
| Number of samples (total n = 193) | 11 | 76 | 15 | 18 | 7 | 66 |
| rRT-PCR Ct value median (IQR) | 34.9 (21.3–39.5) | 32.1 (26.0–37.6) | 25.3 (24.0–35.8) | 28.5 (22.6–35.9) | 21.1 (19.9–26.4) | 31.5 (28.2–34.9) |
| STT mean (range) | NA | 9.5 (2.0–16.0) | 7 (3.0–10.0) | 8.5 (5.0–20.2) | 5 (4.0–10.0) | 9.5 (5.0–15.2) |
| CPE positive samples number (%) | 5 (45) | 31 (41) | 8 (53) | 11 (61) | 6 (86) | 30 (48) |
HCW = healthcare worker; IQR = interquartile range; STT = symptom onset to test time; CPE = cytopathic effect; rRT-PCR = real-time reverse transcription polymerase chain reaction; Ct = cycle threshold.
Correlation between virus viability and time from symptom onset
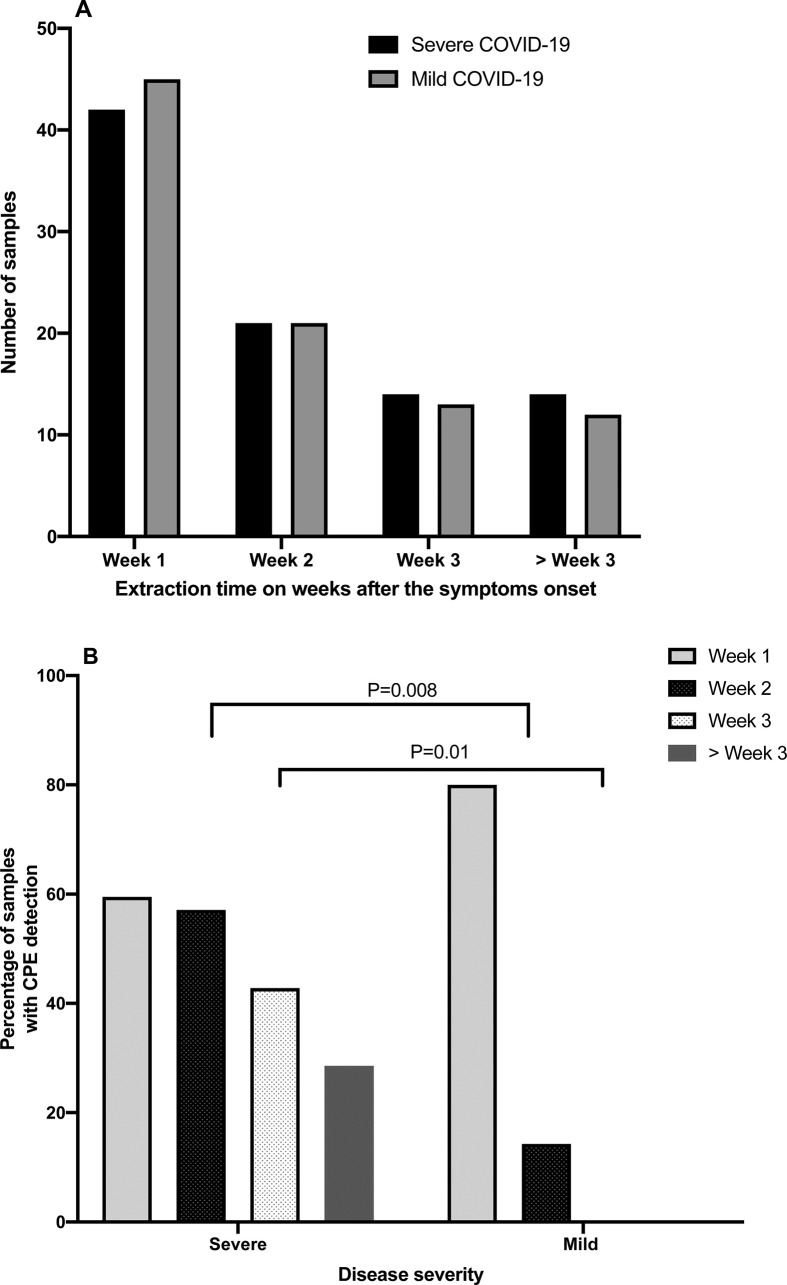
For the outpatients, a CPE was detected in 71% (17/24) of the samples obtained in the first week after symptom onset. In this group of patients with mild COVID-19, the maximum STT of a CPE-positive sample during follow-up was 10 days.
In the hospitalized patients with severe COVID-19, the virus was viable in 59% (16/27), 56% (9/16) and 64% (7/11) of the samples obtained in the first, second and third week, respectively, and in 25% (2/8) of the samples obtained beyond the third week STT. The maximum STT of a CPE-positive sample in the severe COVID-19 group was 32 days.
Figure 2 shows the distribution of samples analysed by the collection week after symptom onset, the percentage of samples with CPE in cell cultures in each week for both patient groups, and their statistical significance.
Fig. 2.
Distribution of samples according to symptom time to test (STT) in weeks (A) and percentage of samples with cytopathic effect (CPE) according to STT (B) in groups of patients with severe and mild COVID-19.
Correlation between virus viability and viral load
In both the mild and severe COVID-19 groups, the samples that showed viral replication had significantly (p < 0.001) lower Ct values than the samples without viable virus (23.3 (IQR 20.5–28.0) vs. 36.4 (IQR 31.8–39.1), respectively, for mild COVID-19 and 27.7 (IQR 23.2–30.0) vs. 33.0 (IQR 30.4–38.0), respectively, for severe COVID-19) (Fig. 3 ).
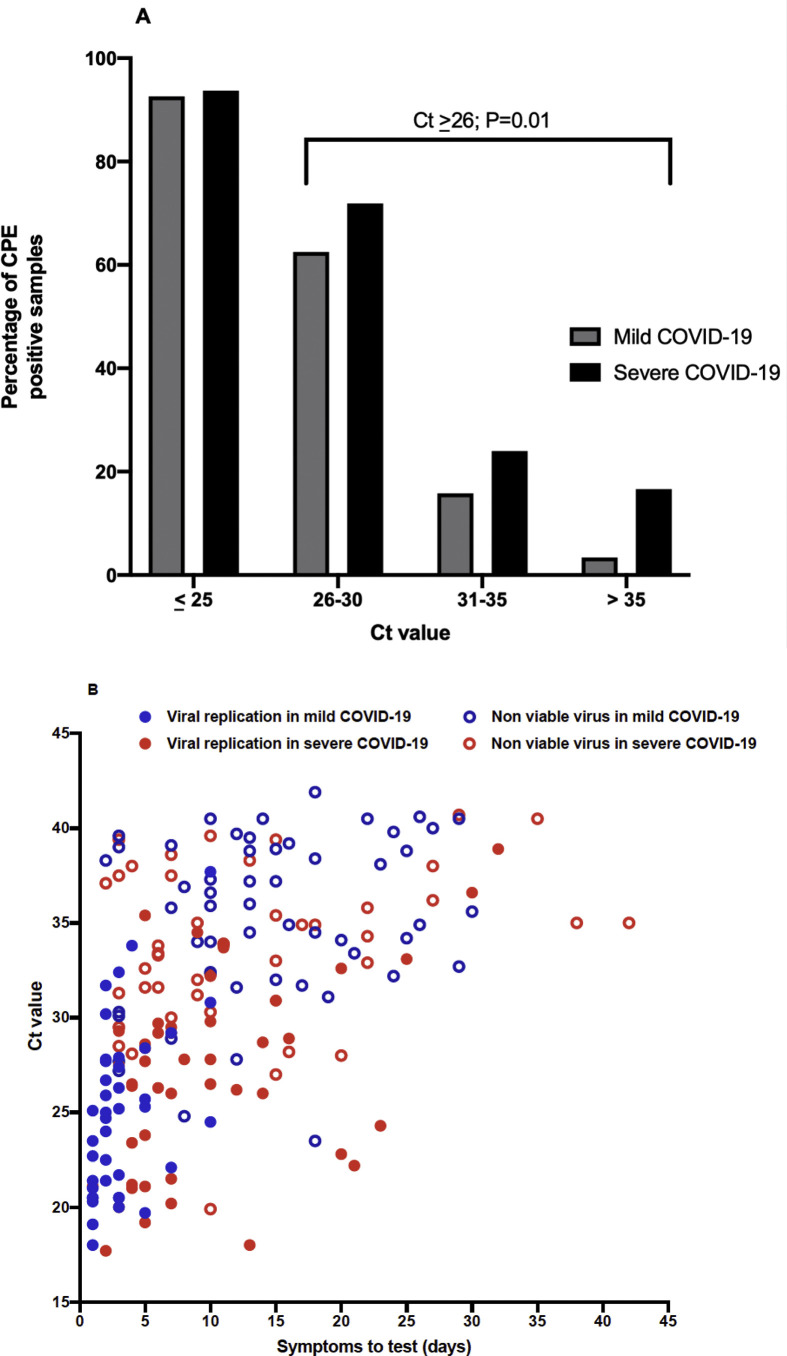
Fig. 3.
Percentage of samples with cytopathic effect (CPE) according to the Ct amplification value (A) and the correlation of viral replication with Ct value and symptom onset (B) in groups of patients with severe and mild COVID-19. Two samples collected during the follow-up of the same patient at days 42 and 61 after symptom onset are not represented in the graph. There are statistically significant differences (p < 0.001) between the Ct values found in the samples with viral replication in both mild and severe COVID-19 compared with the samples that did not present viral replication, regardless of the severity of the disease.
The samples with higher viral loads (Ct ≤ 25) in both patient groups showed viable virus at a rate >90%. However, even the samples with low viral loads (Ct ≥ 35) could harbour viable virus, although at a much lower proportion (5% for mild COVID-19 and 15% for severe illness). Differences in viral viability between the outpatients and hospitalized patients were dramatic in the samples with moderate or low viral loads (Ct ≥ 26). Patients with severe COVID-19 presented infective virus at a significantly higher rate (47%, 24/51) than outpatients (18%, 7/38) (p < 0.01).
In this regard, it is noteworthy that two of seven bronchial aspirates presented CPE despite the fact that the median Ct value for this type of sample was 35.0 (IQR 32.6–38.9).
Correlation between viral replication and presence of anti-SARS-CoV-2 antibodies
Of the 30 sera collected with STT >10 days, 12 were paired with a CPE-positive respiratory sample, and 18 were paired with a CPE-negative respiratory sample.
In seven samples, the presence of IgG and neutralizing activity was not detected, five of which paired with CPE-positive respiratory samples. In the remaining samples, both assays were positive.
There was a significant difference between the NT50 geometric mean titre between the samples with and without CPE (107.2 vs. 699.69, p 0.04). Most of the sera paired with CPE-negative respiratory samples (16/18, 89%) had an NT50 > 1:80, whereas only 5/12 (42%) sera paired with CPE-positive respiratory samples had an NT50 > 1:80, p 0.032). This difference was not due to a greater proportion of samples from
immunocompromised patients in the group of sera being paired with respiratory samples presenting CPE (25%, 3/12 vs. 11%, 2/18; p 0.32).
Production of high neutralizing antibody titres >1:1024 was present in almost half (14/30, 46.7%) of the samples. Despite this neutralizing activity, viral replication was detected in 21% (3/14) of the paired respiratory samples.
Discussion
A systematic review and meta-analysis of the duration of viral shedding and infectivity [12] have shown that although the shedding of RNA in respiratory samples can be prolonged, the detection of viable viruses does not occur after more than 9 days of illness. Previous studies [5,7] have shown prolonged viral shedding in patients with severe COVID-19 and its relation to high viral loads. Although we observed a significant positive correlation between low Ct values and the presence of viable virus, this viral load estimate appears insufficient for discriminating samples harbouring infective virus. It is important to highlight that Ct values obtained for the same sample in different rRT-PCR assays can vary remarkably [13]; thus, the correlation between Ct value and viral viability should be determined for each assay.
Prolonged detection of viral replication has been demonstrated in immunosuppressed patients [14]; however, our results show that viral replication can also be detected in immunocompetent patients, even with moderate or low viral loads, for longer periods of time than those previously described [7,12,15]. It remains to be seen whether this finding is related to our higher cell culture positivity rate (51.6%) in patients with severe COVID-19 than with that reported previously (9%) [7] due to technical factors such as cell line permissiveness to SARS-CoV-2 [16]. Ideally, viral viability should be measured in human nasopharyngeal epithelium cell culture.
The use of different types of samples from the upper respiratory tract has been proposed for diagnosing SARS-CoV-2 [4]. The demonstration that the nasal epithelium has the highest expression of the angiotensin-converting enzyme 2 virus cell receptor [17] indicates that nasopharyngeal exudate is the more suitable respiratory sample to investigate virus viability, which was the upper respiratory tract sample type analysed in our study.
We have found a positive correlation between serum neutralization activity and SARS-CoV-2 non-viability in cell cultures. Nevertheless, we observed a positive CPE in patients with severe COVID-19, despite the presence of clear neutralizing activity (NT50 > 1:80). It remains to be seen whether this high level of neutralizing antibodies plays some pathogenic role [18,19]. In our series, two patients who presented very high (>1:1024) NT50 titres required ICU admission and mechanical ventilation. Interestingly, this fact has been reported for patients with SARS-CoV-1 infection, in whom rapid production of high neutralizing titres was associated with poor prognoses [20,21], and recently for SARS-CoV-2 infection [22,23]. These apparently contradictory results can only be explained by performing longitudinal studies to assess the kinetics of viral replication and of antibodies, as well as virus-specific T cell response, in patients with varying disease severity.
In summary, we detected a completely different pattern of SARS-CoV-2 viability in upper respiratory tract samples from mild cases, in which viral replication in the upper respiratory tract occurs for a short period (maximum STT, 10 days), compared with hospitalized patients with severe COVID-19, in whom viable virus can frequently be demonstrated during prolonged periods of up to 4 weeks, both in their upper and in their lower respiratory tract samples, even in the presence of high levels of neutralizing activity. These results have important implications to discontinue isolation precautions, given we have demonstrated that immunocompetent patients with severe disease can shed viable virus for long periods of time. For mild COVID-19, quarantine should be extended to at least 10 days.
Transparency declaration
We declare that we have no conflicts of interest. This study was supported by the Research Institute Carlos III (grants FIS PI 1801007 and FIS PI1800740), by the European Union Commission Horizon 2020 Framework Program (Project VIRUSCAN FETPROACT-2016: 731868) and by Fundacion Caixa-Health Research (Project StopEbola).
Author contributions
M.D.F. and R.D. were involved in the design and supervision of this study; J.L., F.L. and M.D.F performed experiments; A.P.R. collected data; and M.D.F., J.L., A.P.R. and R.D. performed the data analysis. All the authors were involved in writing the paper and have approved the final version.
Acknowledgements
We would like to express our gratitude to Dra. Estela Paz and the Immunology Department for their help in providing residual serum samples. The professional editing service ServingMed.com provided technical editing of the manuscript prior to submission.
Editor: L. Kaiser
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chun D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 5.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020;ciaa638 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Kampen JJA, van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. 2020 doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tom M.R., Mina M.J. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71:2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 11.Cordes A.K., Heim A. Rapid random access detection of the novel SARS-coronavirus-2 (SARS-CoV-2, previously 2019-nCoV) using an open access protocol for the Panther Fusion. J Clin Virol. 2020;125:104305. doi: 10.1016/j.jcv.2020.104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SAES-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirden M., Feghoul L., Bertine M., Nere M.L., Le Hingrat Q., Abdi B. Multicenter comparison of the Cobas 6800 system with the RealStar RT-PCR kit for the detection of SARS-CoV-2. J Clin Virol. 2020;130:104573. doi: 10.1016/j.jcv.2020.104573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L. Case study: prolonged Infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh K.A., Spillane S., Comber L., Cardwell K., Harrington P., Connell J. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect. 2020;81:847–856. doi: 10.1016/j.jinf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vabret N., Britton G.J., Gruber C., Hedge S., Kim J., Kuksin M. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho M.-S., Chen W.-J., Chen H.-Y., Lin S.-F., Wang M.-C., Di J. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11:1730–1737. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]