Abstract
Objectives
Institutionalized older adults have a high prevalence of frailty and disability, which may make them more vulnerable to the negative consequences of coronavirus disease 2019 (COVID-19). We investigated the impact of COVID-19 on the level of frailty, physical, and cognitive performance in nursing home residents.
Design
Nested case-control study.
Setting and Participants
The study included nursing home residents who were infected with COVID-19 (case group, n = 76), matched by age to a control group (n = 76).
Methods
Participants’ sociodemographic and medical data were collected, and they were also assessed for physical function (handgrip and walking speed), cognitive performance (Mini-Mental State Examination) and frailty (Frail-NH scale) before the first wave of the COVID-19 pandemic (October to December 2019, pre-COVID-19) and after (June to July 2020, post-COVID-19). COVID-19 symptoms and clinical course were recorded for the cases.
Results
Between the pre- and post-COVID-19 assessments, we found a 19% greater deterioration in handgrip, a 22% greater decrease in walking speed, and a 21% greater increase in Frail-NH scores in cases compared with controls. In both cases and controls, on the other hand, there was a significant 10% decrease in Mini-Mental State Examination scores over the study period. Multivariable logistic regression showed that COVID-19 survivors had a 4-fold increased chance of developing frailty compared with controls (odds ratio 4.95, 95% confidence interval 1.13–21.6, P = .03), but not cognitive decline.
Conclusions and Implications
COVID-19 can accelerate the aging process of institutionalized older adults in terms of physical performance and frailty by around 20%. However, we found similar levels of decline in cognitive performance in both cases and controls, likely because of the burden of social isolation and containment measures on neuropsychological health.
Keywords: COVID-19, aged, frailty, physical functional performance, nursing homes
The older population has been shown to be highly vulnerable to the negative consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 , 2 In Italy, the mean age of individuals who have died from coronavirus disease 2019 (COVID-19) is 80 years, and the oldest age groups have the highest lethality rate.3 Moreover, the presence of frailty, a common condition in older age,4 has been associated with atypical presentation and a more severe course of SARS-CoV-2 infection,5 including increased mortality.6 Frailty is especially frequent in nursing homes, where its prevalence can be as much as 75%.7 These are, therefore, settings where there is not only a high risk of contagion due to logistical characteristics (eg, shared living environments), but also a high risk of complications that can affect their residents.8
In recent months, several studies have investigated SARS-CoV-2 infection in institutionalized individuals, focusing on contagion, clinical presentation, and mortality.1 , 9, 10, 11 However, there is still a lack of evidence regarding the extent to which the pandemic has affected the cognitive and physical performance of such a vulnerable population, in particular those having survived COVID-19. The negative impact of the COVID-19 pandemic on the health of nursing home residents could be a direct result of the disease, but may also be an indirect result of the implementation of preventive measures, such as physical distancing, the restriction of informal visits, and lack of cognitively and physically stimulating activities. In these ways, the COVID-19 pandemic might overall have accelerated the aging process of institutionalized individuals. The degree of this acceleration with regard to different health parameters and the actual impact of COVID-19 per se could be estimated by evaluating the changes in individual performances before and after the disease, and comparing them with the changes in people not affected by COVID-19.
Although in most cases the rapid spread of the pandemic did not allow individuals to be evaluated before the disease, for the present study, we were able to use the evaluations of residents involved in a pilot study on influenza burden in the nursing home setting carried out pre-COVID-19 during fall 2019. Using these data, we were able to test the hypothesis that institutionalized people affected by COVID-19 present a greater deterioration in cognitive and physical function than their nonaffected counterparts.
The aim of this study was, therefore, to evaluate the changes in frailty status and in physical and cognitive performance in relation to SARS-CoV-2 infection in nursing home residents.
Methods
Study Design and Study Population
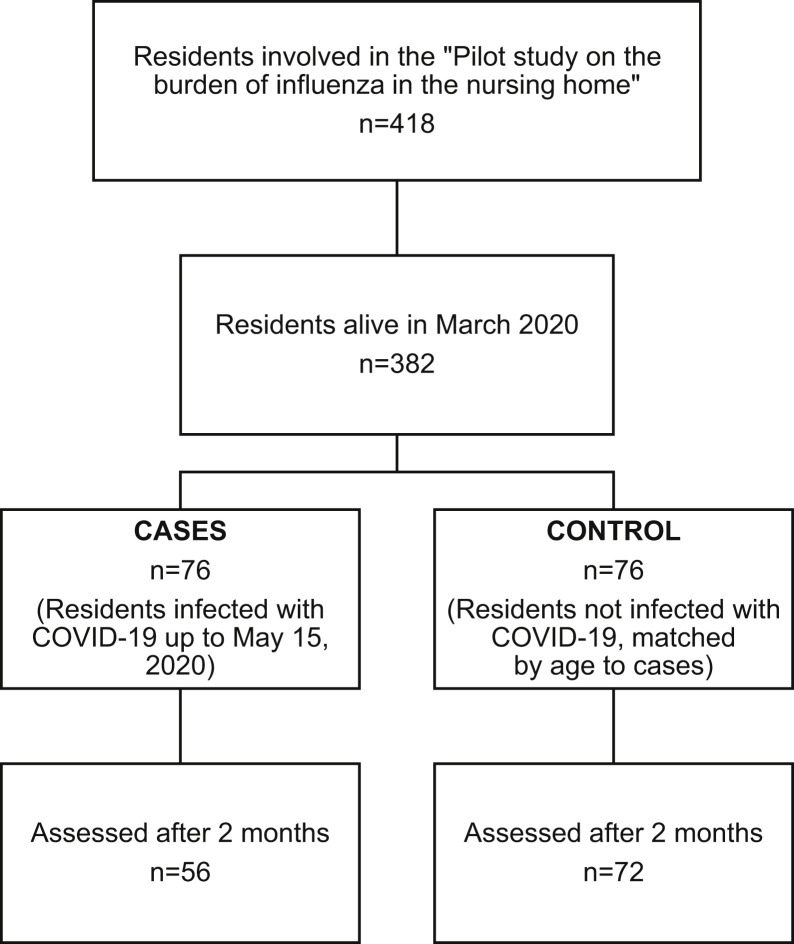
We designed a nested case-control study based on the cohort involved in an ongoing study (Pilot Study on the Burden of Influenza in the Nursing Home) being conducted in the Istituto AltaVita-IRA nursing home in the city of Padua, Italy. For this pilot study, 418 residents underwent a multidimensional assessment by experienced geriatric physicians between October and December 2019. Of that sample, 382 individuals were still residents in the nursing home in March 2020, 76 of whom became infected with SARS-CoV-2 up to May 15, 2020, and were, therefore, recruited as cases in the present study. We selected 76 controls matched to the cases by age ±3 years from those residents who were not infected by SARS-CoV-2 and were still alive up to June 2020. Among the cases, 20 residents (26.3%) deceased from March to July 2020, and 56 underwent the post-COVID-19 assessment (the study flow-chart is shown in Supplementary Figure 1). The study was approved by the local Ethics Committee of the Istituto AltaVita-IRA nursing home, and participants (or their next of kin) gave their written consent to participate in the study.
Supplementary Fig. 1.
Flow chart of the study.
Data Collection
Data pertinent to the pre-COVID-19 phase were collected between October and December 2019 and included sociodemographic information, medical history, use of drugs, and vaccinations in the last year. Patients’ comorbidity levels were evaluated through the cumulative illness rating scale comorbidity index and severity index. The comorbidity index indicates the number of disease categories out of the first 13 (excluding psychiatric/behavioral illnesses) rated as moderate to severe to obtain a score ranging from 0 to 13. The severity index is the average score of the ratings of the first 13 disease categories evaluated in the cumulative illness rating scale.12 Frailty status was assessed through the Frail-NH scale, a validated tool for nursing homes that evaluates seven domains (ie, fatigue, resistance, ambulation, incontinence, weight loss, nutritional approach, and help with dressing) to obtain a total score ranging from 0 to 14 points.13 In the present study, total Frail-NH scores were used to classify residents as nonfrail (≤7) or frail (>7), according to Kaehr et al13; and as nonfrail (0–1 points), mild-to-moderate frail (2–5), and most frail (6–14), according to Theou et al.14
For physical performance, we considered the following examinations. (1) Handgrip strength (kg) was measured using a DynEX hand-held dynamometer (Akern). Three tests were carried out for each hand, and grip strength was calculated as the mean of the maximum performance at the dominant and no-dominant hand. (2) Walking speed (m/s) was measured through the 4-meter usual-pace walking test. The test was performed 2 times consecutively. The best time necessary to cover the established distance was used for our assessment of walking speed.15
As regards cognitive performance, we considered the Mini-Mental State Examination (MMSE), a validated scale ranging from 0 (worse) to 30 (best cognitive performance),16 estimating global cognitive function.
Diagnostic testing for SARS-CoV-2 infection in the nursing home has been performed through nasopharyngeal swab since March 2020, when the first COVID-19 case was registered in a resident. Surveillance testing of all residents by the same method was carried out every 15––20 days in accordance with local health authority policies, in addition to testing of residents who presented with COVID-19 symptoms (fever, respiratory symptoms) or who had been in contact with infected individuals. The clinical presentation and mortality of COVID-19 cases was also recorded.
In the post-COVID-19 phase of the study, between June and July 2020, participants were assessed for frailty status, handgrip strength, walking speed, and cognitive function using the same methods described for the pre-COVID-19 phase.
Statistical Analyses
Controls were matched to cases by age ±3 years using the SAS GMATCH macro (Department of Quantitative Health Sciences, Mayo Clinic Research, US).17
The sample characteristics are expressed as counts and percentages for the categorical variables, and as means ± standard deviations or medians (interquartile range for the continuous variables. Differences between the characteristics of cases and controls were evaluated using the paired Student t-test or the Wilcoxon signed-rank test for the continuous variables, and conditional logistic regression for categorical variables, as appropriate. The characteristics associated with the incidence of frailty evaluated with the Frail-NH scale were assessed by logistic regression models.
Changes in MMSE scores, handgrip, walking speed, and the Frail-NH scale pre- vs post-COVID-19 in the case and control groups were analyzed using mixed models for repeated measures, adjusting for the within-block (case-control) correlation of observations. The characteristics associated with cognitive decline (defined as a drop in MMSE score ≥2 points) were assessed through logistic regression.
Statistical analyses were performed using the SAS 9.4 software (SAS Institute Inc.,Cary, NC).
Results
The baseline characteristics of cases (only survivors) and controls are reported in Table 1 (Supplementary Table 1 shows the baseline characteristics of all COVID-19 cases and controls). As shown, at the pre-COVID-19 assessment, cases and controls differed only in the prevalence of upper gastrointestinal, and musculoskeletal and skin pathologies, which were higher in the control group. Although the 2 groups had a similar prevalence of frailty at baseline, single-item frailty measures showed that COVID-19 survivors were more likely to have better mobility levels than controls, with 34% vs 19%, respectively, able to stand up independently (P = .011), and 38% vs 23%, respectively, able to move in and out of the facility autonomously (P = .057).
Table 1.
Characteristics of Cases and Controls at the Pre-COVID-19 Assessment (Only Survivors)
| Cases (n = 56) | Controls (n = 74) | P Value | |
|---|---|---|---|
| Sex, female, n (%) | 41 (73.2) | 55 (74.3) | .82 |
| Age, y, mean ± SD | 84.4 ± 7.3 | 85.1 ± 7.4 | .75 |
| Educational level, primary school or less, n (%) | 28 (52.8) | 44 (62.8) | .26 |
| Number of drugs, median (IQR) | 7 (5, 10) | 8 (6, 11) | .43 |
| Chronic diseases (moderate severity according to CIRS), n (%) | |||
| Cardiac | 28 (50.0) | 30 (40.5) | .21 |
| Hypertension | 25 (44.6) | 35 (46.1) | .72 |
| Vascular/hematological | 26 (46.4) | 32 (43.2) | .56 |
| Respiratory | 13 (23.2) | 18 (24.3) | .82 |
| Ophthalmologic and otorhinolaryngologic | 20 (35.7) | 17 (23.0) | .08 |
| Upper gastrointestinal | 19 (33.9) | 46 (62.2) | .01 |
| Lower gastrointestinal | 18 (32.1) | 26 (35.1) | 1.00 |
| Hepatic and pancreatic | 4 (7.1) | 5 (6.8) | .71 |
| Renal | 4 (7.1) | 4 (5.4) | 1.00 |
| Genitourinary | 39 (69.6) | 58 (78.4) | .53 |
| Musculoskeletal and dermatologic | 32 (57.1) | 58 (78.4) | .04 |
| Neurologic (excluding dementia) | 21 (37.5) | 36 (48.7) | .09 |
| Endocrine - metabolic | 23 (41.1) | 31 (41.9) | .49 |
| Psychiatric/behavioral | 51 (91.1) | 68 (91.9) | .74 |
| CIRS-comorbidity index, median (IQR) | 5 (4, 6) | 5 (4, 6) | .27 |
| CIRS-severity index, median (IQR) | 2.1 (1.9, 2.3) | 2.2 (2, 2.4) | .12 |
| MMSE, mean ± SD | 16.3 ± 7.4 | 16.8 ± 7.8 | .42 |
| No. residents with available ratings | n = 48 | n = 61 | |
| Handgrip (kg), mean ± SD | 8.6 ± 4.2 | 8.6 ± 5.7 | .07 |
| No. residents with available ratings | n = 24 | n = 45 | |
| 4-m walking test (m/s), mean ± SD | 0.8 ± 0.2 | 0.5 ± 0.2 | .10 |
| No. residents with available ratings | n = 15 | n = 23 | |
| Frail-NH score (range 0–13) | 7.4 ± 3.4 | 7.8 ± 3.1 | .79 |
| Frailty (Frail-NH score >7), n (%) | 30 (53.6) | 50 (67.5) | .10 |
| Frailty, n (%) | .16 | ||
| Nonfrail (Frail-NH score 0–1) | 8 (14.3) | 7 (9.5) | |
| Mild or moderate frailty (Frail-NH score 2–5) | 12 (21.4) | 9 (12.2) | |
| Most frail (Frail-NH score 6–14) | 36 (64.3) | 58 (78.3) |
CIRS, cumulative illness rating scale; IQR, interquartile range; SD, standard deviation.
Considering both COVID-19 cases who survived and those who did not (n = 76), we found that almost one-half (49%) presented with at least 1 symptom, the most frequent being fever (61.5%), low-grade fever (23.1%), dyspnea (43.6%), cough (28.2%), fatigue (7.7%), cold (5.1%), myalgia and arthralgia (2.6%), and diarrhea (2.6%). There were marginally significant differences in the prevalence of frailty at baseline between symptomatic and asymptomatic COVID-19 cases (Supplementary Table 2).
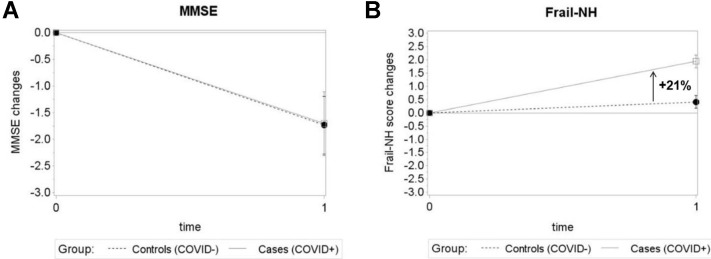
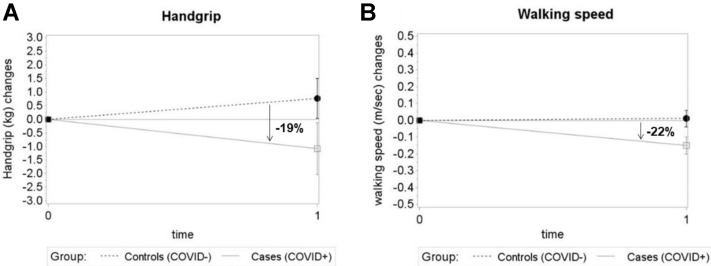
Table 2 shows the estimated within- and between-group differences in MMSE, Frail-NH, handgrip strength, and walking speed obtained from repeated measures models. In the group of COVID-19 survivors (average time interval between infection and post-COVID-19 assessment: 92 ± 10 days), we found significant worsening in MMSE, Frail-NH, and walking speed from the pre- to the post-COVID-19 phases, and borderline significant changes in handgrip strength. Among the controls, only MMSE worsened significantly from the pre- to the post-COVID-19 phases. As Supplementary Figures 2 and 3 show, there were significant differences between cases and controls in the extent of the changes in Frailty-NH, handgrip strength, and walking speed during the observation period, with a steeper worsening in the former group. In particular, we found a 21% greater increase in frailty scores in cases compared with controls, and 19% and 22% greater reductions in handgrip strength and walking speed, respectively. Instead, no significant differences between cases and controls were found in the frequency of residents who became unable to perform the physical performance test at the post-COVID-19 phases (Appendix 1), as well as in the MMSE variations.
Table 2.
Estimated Within-Group and Post-between-Group Differences in Cognitive Function, Frailty and Physical Performance (Survived Cases vs Controls)
| n | Average Differences Within-Group |
Mean Between-Group Differences |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (COVID-19 +) |
n | Controls (COVID-19 −) |
|||||||||||
| Pre-COVID | Post-COVID | Δ Post-Pre | P Value | Pre-COVID | Post-COVID | Δ Post-Pre | P Value | Δ Cases-Controls | P Value | Effect Size, d | |||
| MMSE | 40 | 17.0 (0.4) | 15.3 (0.5) | −1.7 (0.6) | .005 | 60 | 17.1 (0.4) | 15.4 (0.5) | −1.7 (0.5) | .002 | −0.02 (0.6) | .97 | 0.37 |
| Frail-NH score | 56 | 7.2 (0.2) | 9.1 (0.2) | 1.9 (0.3) | <.001 | 72 | 7.5 (0.2) | 7.9 (0.2) | 0.4 (0.2) | .32 | 1.3 (0.3) | <.001 | 0.33 |
| Handgrip (kg) | 43 | 9.1 (0.6) | 8.1 (0.7) | −1.1 (0.9) | .05 | 57 | 9.1 (0.5) | 9.8 (0.5) | 0.8 (0.7) | .28 | 1.8 (0.9) | .05 | 0.10 |
| Walking speed (m/s) | 17 | 0.72 (0.03) | 0.57 (0.04) | −0.15 (0.05) | .003 | 22 | 0.64 (0.03) | 0.65 (0.03) | 0.01 (0.04) | .80 | 0.08 (0.06) | .01 | 0.53 |
Numbers are means (standard error).
Supplementary Fig. 2.
Changes in cognitive status (A) and frailty (B) in cases and controls from the pre- to the post-COVID-19 assessments. Figures show the estimated mean changes from the pre-COVID-19 (time 0) to the post-COVID-19 phases (time 1) for MMSE and the Frail-NH scale. Error bars are SE. The percentage shown in (B)indicates the difference between cases and controls in the mean Frail-NH score changes. SE, standard error.
Supplementary Fig. 3.
Changes in handgrip strength (A) and walking speed (B) in cases and controls from the pre- to the post-COVID-19 assessments. Figures illustrate the estimated mean changes from the pre-COVID-19 (time 0) to the post-COVID-19 phase (time 1) for handgrip and walking speed. Error bars are SE. Percentages indicate the differences between cases and controls in the mean handgrip and walking speed changes. SE, standard error.
Moreover, considering the study cases, we found that the changes in MMSE, physical performance, and frailty did not significantly correlate with the number of days between the COVID-19 episode and the post-COVID-19 assessment (data not shown).
The cumulative incidence of frailty over the study period was 38% (46.2% among cases, 29.2% among controls) using the Kaehr et al cut-off13; and 53.4% (75% among cases, 28.6% among controls) using the Theou et al cut-off.14 As reported in Table 3 , logistic regression showed that having been infected with COVID-19 was associated with a 4-fold higher chance of developing frailty (odds ratio 4.95, 95% confidence interval 1.13–21.6, P = .03). Single-item Frail-NH measures (data not shown) showed that COVID-19 increased the chance of experiencing a loss in weight ≥10% of normal weight (odds ratio 5.47, 95% confidence interval 1.66–18.0, P = .005).
Table 3.
Characteristics Associated With Incident Frailty and Cognitive Decline
| Frailty |
Cognitive Decline |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| COVID-19 (yes vs no) | 4.95 (1.13–21.6) | .03 | 1.89 (0.69–5.21) | .22 |
| Age ≥80 y (vs <80) | 2.84 (0.73–11.0) | .13 | 2.17 (0.53–8.93) | .28 |
| Sex (female vs male) | 4.44 (0.75–26.3) | .10 | 0.63 (0.23–1.69) | .36 |
| CIRS-comorbidity index ≥5 (vs <5) | 3.31 (0.93–11.8) | .06 | 1.72 (1.03–7.17) | .04 |
| Frailty (vs nonfrailty) | – | – | 2.76 (1.07–7.12) | .03 |
CI, confidence Interval; CIRS, Cumulative Illness Rating Scale; OR, odds ratio.
Characteristics associated with incident frailty and cognitive decline (defined as a reduction in MMSE score >2 points from pre- to post-COVID assessments) were evaluated through logistic regression models. Independent variables included COVID-19, age, sex, CIRS-comorbidity index and, only for cognitive decline, frailty at the pre-COVID assessment.
Cognitive decline, defined as a reduction in MMSE score ≥2 points from the pre- to the post-COVID-19 phases, was observed in 14 cases (36.8%) and 17 controls (32.7%), with no differences between groups (P = .41). Multivariable logistic regression (Table 3) showed that the factors significantly associated with cognitive decline were frailty and comorbidity, but not COVID-19.
Discussion
Our study shows that COVID-19 may accelerate age-related deterioration in the physical performance and frailty status of nursing home residents by around 20%. Furthermore, the preventive measures associated with the COVID-19 pandemic that limited social interaction and cognitively/physically stimulating activities seemed to have influenced cognitive function similarly in individuals who were infected with SARS-CoV-2 and those who were not.
Regarding mortality, 26.3% of our COVID-19 cases died over the observation period (80% of these because of COVID-19, 20% for other diseases), in line with previous studies.6 , 9 , 18 However, the disease also placed a substantial burden on the health of COVID-19 survivors. Indeed, the latter showed a greater reduction in walking speed and muscle strength, and a higher chance of frailty developing and worsening than controls, with negative effects especially on nutritional status and psychological well-being. With respect to the changes in physical performance and frailty from the pre- to the post-COVID-19 phases, we found that COVID-19 determined a 20% steeper worsening in frailty, walking speed, and handgrip strength in cases compared with controls.
Several mechanisms may underlie the impact of COVID-19 on these health domains.
First, it is well-known that both acute and chronic inflammation can negatively affect muscle strength and performance. SARS-CoV-2 infection, in particular, induces a severe inflammatory response characterized by cytokine storm that drives systemic inflammation.19 Previous studies have demonstrated that acute inflammatory status can cause a reduction in muscle mass and strength, mainly because of protein catabolism upregulation and concurrent anabolism downregulation, which overall lead to decreased muscle protein synthesis.20 , 21 These effects may be more marked in older individuals who already present with chronic inflammation (inflammaging), which in the long term can negatively affect multiple organs and systems, including the musculoskeletal system.22 , 23
Second, reduced mobility or immobilization plays an important role in the loss of muscular mass and strength.21 , 24 In this regard, it has been shown that 2 days in bed reduces muscle mass by around 1.7%, and by 5.5% after only 7 days.25 As a consequence of the ongoing acute disease and to prevent contagion, individuals with a positive SARS-Cov-2 swab test moved around less and were unable to benefit from individual and group physical rehabilitation programs, as these had been suspended.
Third, preventive measures to limit contagion in the nursing home also entailed a reduction in psychological counseling, which, on top of the ban on visits from informal caregivers and physical distancing, led to increased feelings of loneliness and depression. The erosion of psychological well-being is a further factor affecting physical performance, as already demonstrated for depression.26
Concerning cognitive function, our study did not reveal any differences between COVID-19 survivors and controls in terms of loss of cognitive performance. Both groups exhibited an average reduction in MMSE scores of 1.7 points over the observation period, and the chance of undergoing cognitive decline was not significantly associated with COVID-19. In this regard, comparing our results with data on people with cognitive disorders,27 , 28 we cannot rule out that a similar cognitive decline would have also been experienced irrespective of the COVID-19 pandemic. However, the fact that we found no differences in the cognitive decline between cases and controls suggests that the possible burden attributable to preventive measures and social restriction could have involved all the nursing home residents. Nonetheless, we cannot exclude the possibility that SARS-CoV-2 infection leads to a worsening of cognitive status in the longer term and, therefore, support the need to closely monitor the cognitive status of patients with COVID-19 over time to identify possible subsequent decline.29
The main strength of this work lies in the availability of data from a pre-COVID-19 assessment carried out in the context of another ongoing study. This makes our study the first to investigate the impact of COVID-19 on frailty and on physical and cognitive performance in older residents who survive SARS-CoV-2 infection. Limitations, however, are the small sample size, which affects the statistical power of our analyses (study power 0.75 for incident frailty and <0.10 for cognitive decline), and the unavailability of details on the single chronic diseases presented by the study participants. Moreover, the use of age and not of a propensity score to match cases and controls may represent a further limitation of our work. Nonetheless, we considered a relatively homogeneous population of nursing home residents, who, differently from the community-dwelling, present a high prevalence of chronic conditions, such as multimorbidity, polypharmacy, and disability. The high prevalence of such conditions likely made the matching process based on age similar to what potentially would have obtained with a propensity score, as confirmed by comparing the baseline characteristics of cases and selected controls.
Conclusions and Implications
COVID-19 may place a considerable burden on older people's health not only in terms of mortality, but also in terms of worsening frailty and physical function among survivors of the disease. This effect may translate into an acceleration of around 20% in the ongoing aging process. Moreover, our study revealed the detrimental effect of the preventive measures put in place in the nursing home, such as physical distancing and the cessation of routine leisure activities, on the cognitive status of older residents, regardless of whether they were infected with SARS-CoV-2 or not. These findings highlight the importance of maintaining daily activities, even in alternative forms, to ensure that institutionalized individuals receive adequate physical and cognitive stimulation. Unlike the first wave of the pandemic, which found nursing homes and healthcare systems generally unprepared for such an emergency and resulted in restrictive preventive measures being put in place, the following waves of SARS-CoV-2 cases pose a challenge to maintaining the best standards of medical and nursing care, in terms of both clinical health and psychological and physical well-being.
Acknowledgments
We thank all the nursing home residents who participated in the study and the scientific editor, Tessa Say, for her help in the manuscript preparation.
Footnotes
This study was made possible by an educational grant from Sanofi for the project: Pilot Study on the Burden of Influenza in the Nursing Home.
The authors declare no conflicts of interest.
Appendix 1. Frequency of participants who were unable or refused to undergo physical performance tests
Considering only survivors, at the pre-COVID-19 assessment, the frequency of controls unable to perform the handgrip test was 23 (60.8%), while 6 (8.1%) refused; the correspondent frequencies among cases were 23 (41.1%) and 9 (16.1%). At the post-COVID-19 assessment, 6 controls and 3 cases (9.4%) became unable to perform the test (11.8%) (P = 1.00).
For walking speed, at the pre-COVID-19 assessment, the frequency of controls unable to perform the test was 49 (66.2%), while 2 (2.7%) refused; the correspondent frequencies among cases were 39 (69.6%) and 2 (3.6%). At the post-COVID-19 assessment, 3 controls (12%), and 4 cases (25%) became unable to perform the walking speed test (P = .40).
When evaluating whether transitions from able to unable to undergo physical performance tests were at least partly captured by frailty assessment, we found that 3 (2 cases, 1 controls) and 4 (3 cases, 1 controls) residents who did not perform the post-COVID-19 assessment of handgrip and walking speed, respectively, had incident frailty.
Supplementary Table 1.
Characteristics of Cases and Controls at the Pre-COVID-19 Assessment
| Cases (n = 76) | Controls (n = 76) | P Value | |
|---|---|---|---|
| Sex, female, n (%) | 55 (72.4) | 56 (73.7) | .84 |
| Age, y, mean ± SD | 85.3 ± 7.4 | 85.3 ± 7.4 | .70 |
| Educational level, primary school or less, n (%) | 36 (51.4) | 45 (62.5) | .17 |
| Number of drugs, median (IQR) | 7 (5, 10) | 8 (6, 11) | .47 |
| Chronic diseases (moderate severity according to CIRS), n (%) | |||
| Cardiac | 41 (54.0) | 31 (40.8) | .13 |
| Hypertension | 32 (42.1) | 35 (46.1) | .65 |
| Vascular/hematologic | 36 (47.4) | 34 (44.7) | .75 |
| Respiratory | 15 (19.7) | 18 (23.7) | .55 |
| Ophthalmologic and otorhinolaryngologic | 19 (38.2) | 17 (22.4) | .03 |
| Upper gastrointestinal | 30 (39.5) | 47 (61.8) | .01 |
| Lower gastrointestinal | 26 (34.2) | 26 (34.2) | 1.00 |
| Hepatic and pancreatic | 4 (5.3) | 5 (6.6) | .74 |
| Renal | 6 (7.9) | 4 (5.3) | .53 |
| Genitourinary | 53 (69.7) | 60 (79.0) | .21 |
| Musculoskeletal and dermatologic | 48 (63.2) | 60 (79.0) | .04 |
| Neurologic (excluding dementia) | 36 (47.4) | 37 (48.7) | .87 |
| Endocrine – metabolic | 30 (39.5) | 31 (40.8) | .85 |
| Psychiatric/behavioral | 71 (93.4) | 70 (92.1) | .76 |
| CIRS-comorbidity index, median (IQR) | 5 (4, 6) | 5 (4, 7) | .42 |
| CIRS-severity index, median (IQR) | 2.1 (1.9, 2.3) | 2.2 (2, 2.3) | .16 |
| MMSE, mean ± SD | 15.8 ± 7.2 | 17 ± 7.8 | .23 |
| No. residents with available ratings | n = 60 | n = 61 | |
| Handgrip (kg), mean ± SD | 8.7 ± 4.2 | 8.6 ± 5.7 | .07 |
| No. residents with available ratings | n = 31 | n = 45 | |
| 4-m walking test (m/s), mean ± SD | 0.8 ± 0.2 | 0.5 ± 0.2 | .10 |
| No. residents with available ratings | n = 17 | n = 23 | |
| Frail-NH score (range 0-13) | 7.4 ± 3.4 | 7.8 ± 3.1 | .79 |
| Frailty (Frail-NH score >7), n (%) | 47 (61.8) | 52 (68.4) | .34 |
| Frailty, n (%) | .43 | ||
| Nonfrail (Frail-NH score 0–1) | 8 (10.5) | 7 (9.2) | |
| Mild or moderate frailty (Frail-NH score 2–5) | 14 (18.4) | 9 (11.8) | |
| Most frail (Frail-NH score 6–14) | 54 (71.1) | 60 (79.0) |
CIRS, cumulative illness rating scale; IQR, interquartile range; SD, standard deviation.
Supplementary Table 2.
Characteristics of COVID-19 Cases Presenting With and Without Symptoms (n = 76)
| COVID+ |
P Value | ||
|---|---|---|---|
| Symptomatic (n = 37) | Asymptomatic (n = 39) | ||
| Sex, male, n (%) | 9 (24.3) | 12 (30.8) | .53 |
| Age, y, mean ± SD | 84.9 ± 7.8 | 85.7 ± 6.9 | .67 |
| CIRS-comorbidity index, median (IQR) | 5 (4, 7) | 5 (4, 6) | .27 |
| CIRS-severity index, median (IQR) | 2.1 (1.9, 2.4) | 2.1 (1.9, 2.3) | .42 |
| Number of drugs, median (IQR) | 8 (5, 10) | 7 (5, 9) | .32 |
| MMSE, mean ± SD | 15.8 ± 7.0 | 15.3 ± 7.7 | .76 |
| Vaccinated for influenza, n (%) | 26 (70.3) | 33 (84.6) | .13 |
| Chronic diseases (moderate severity according to CIRS), n (%) | |||
| Cardiac | 41 (54.0) | 31 (40.8) | .13 |
| Hypertension | 32 (42.1) | 35 (46.1) | .65 |
| Vascular/hematologic | 36 (47.4) | 34 (44.7) | .75 |
| Respiratory | 15 (19.7) | 18 (23.7) | .55 |
| Ophthalmologic and otorhinolaryngologic | 19 (38.2) | 17 (22.4) | .03 |
| Upper gastrointestinal | 30 (39.5) | 47 (61.8) | .01 |
| Lower gastrointestinal | 26 (34.2) | 26 (34.2) | 1.00 |
| Hepatic and pancreatic | 4 (5.3) | 5 (6.6) | .74 |
| Renal | 6 (7.9) | 4 (5.3) | .53 |
| Genitourinary | 53 (69.7) | 60 (79.0) | .21 |
| Musculoskeletal and dermatologic | 48 (63.2) | 60 (79.0) | .04 |
| Neurologic (excluding dementia) | 36 (47.4) | 37 (48.7) | .87 |
| Endocrine – metabolic | 30 (39.5) | 31 (40.8) | .85 |
| Psychiatric/behavioral | 71 (93.4) | 70 (92.1) | .76 |
| Frailty (Frail-NH score >7), n (%) | 27 (73.0) | 20 (51.3) | .05 |
| Frailty, n (%) | .06 | ||
| Non-frail (Frail-NH score 0–1) | 2 (5.4) | 6 (15.4) | |
| Mild or moderate frailty (Frail-NH score 2–5) | 4 (10.8) | 10 (25.6) | |
| Most frail (Frail-NH score 6–14) | 31 (83.8) | 23 (59.0) | |
CIRS, cumulative illness rating scale; IQR, interquartile range; SD, standard deviation.
References
- 1.Cesari M., Montero-Odasso M. COVID-19 and older adults. lessons learned from the Italian epicenter. Can Geriatr J. 2020;23:155–159. doi: 10.5770/cgj.23.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahid Z., Kalayanamitra R., McClafferty B. COVID-19 and older adults: What we know. J Am Geriatr Soc. 2020;48:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Characteristics of COVID-19 patients dying in Italy. Available at: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths. Accessed October 29, 2020.
- 4.Fried L.P., Tangen C.M., Walston J. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Tay H.S., Harwood R. Atypical presentation of COVID-19 in a frail older person. Age Ageing. 2020;49:523–524. doi: 10.1093/ageing/afaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aw D., Woodrow L., Ogliari G., Harwood R. Association of frailty with mortality in older inpatients with Covid-19: A cohort study. Age Ageing. 2020;49:915–922. doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima G. Prevalence of frailty in nursing homes: A systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Gardner W., States D., Bagley N. The coronavirus and the risks to the elderly in long-term care. J Aging Soc Policy. 2020;32:310–315. doi: 10.1080/08959420.2020.1750543. [DOI] [PubMed] [Google Scholar]
- 9.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortality associated with COVID-19 outbreaks in care homes: Early international evidence –Resources to support community and institutional long-term care responses to COVID-19. https://ltccovid.org/2020/04/12/mortality-associated-with-covid-19-outbreaks-in-care-homes-early-international-evidence/ Available at:
- 12.Parmelee P.A., Thuras P.D., Katz I.R., Lawton M.P. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaehr E., Visvanathan R., Malmstrom T.K., Morley J.E. Frailty in nursing homes: The FRAIL-NH scale. J Am Med Dir Assoc. 2015;16:87–89. doi: 10.1016/j.jamda.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Theou O., Sluggett J.K., Bell J.S. Frailty, hospitalization, and mortality in residential aged care. J Gerontol Ser A Biol Sci Med Sci. 2018;73:1090–1096. doi: 10.1093/gerona/glx185. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:2. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Kosanke J., Rochester EB, of MMCC . 2004. GMATCH Macro: Match 1 or more controls to cases using the GREEDY algorithm. Available at http://bioinformaticstools.mayo.edu/downloads/sas/gmatch.sas. Accessed May 7, 2020. [Google Scholar]
- 18.Hewitt J., Carter B., Vilches-Moraga A. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meftahi G.H., Jangravi Z., Sahraei H., Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflammation Res. 2020;69:825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J.Y.J., Reijnierse E.M., Van Ancum J.M. Acute inflammation is associated with lower muscle strength, muscle mass and functional dependency in male hospitalised older patients. PLoS One. 2019;14:4. doi: 10.1371/journal.pone.0215097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirwan R., McCullough D., Butler T. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bano G., Trevisan C., Carraro S. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morley J.E., Kalantar-Zadeh K., Anker S.D. COVID-19: A major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilroe S.P., Fulford J., Jackman S.R. Temporal muscle-specific disuse atrophy during one week of leg immobilization. Med Sci Sports Exerc. 2020;52:944–954. doi: 10.1249/MSS.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T., Umegaki H., Makino T. Association between sarcopenia and depressive mood in urban-dwelling older adults: A cross-sectional study. Geriatr Gerontol Int. 2019;19:508–512. doi: 10.1111/ggi.13650. [DOI] [PubMed] [Google Scholar]
- 27.Stanley K., Whitfield T., Kuchenbaecker K. Rate of cognitive decline in Alzheimer’s disease stratified by age. J Alzheimer’s Dis. 2019;69:1153–1160. doi: 10.3233/JAD-181047. [DOI] [PubMed] [Google Scholar]
- 28.Mungas D., Reed B.R., Ellis W.G., Jagust W.J. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 29.Serrano-Castro P.J., Estivill-Torrús G., Cabezudo-García P. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurol (English Ed) 2020;35:245–251. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]