Abstract
Patients with chronic heart failure (HF) are among the most vulnerable populations in the COVID era. HF patients infected with COVID-19 are at a significant risk of severe illness and death. They usually present with shortness of breath and radiologic signs of an acute decompensation, which can mask the manifestations of COVID-19. Delay in the diagnosis increases the risk of individual poor outcomes and jeopardizes healthcare workers if protective and isolation measures are not established promptly. Furthermore, the COVID-19 pandemic is forcing health-care systems to modify the delivery of care to patients. Outpatient services are being done virtually, and elective procedures postponed. These may have an impact on the quality of life and survival of chronic HF patients. We present two cases of patients with the previous history of HF who developed an acute exacerbation secondary to COVID-19 infection. In this review, we focused on the main challenges physicians face when dealing with COVID-19 in chronic HF patients at the individual and system levels.
Keywords: Cardiovascular, COVID-19, hear failure, SARS-CoV-2
INTRODUCTION
COVID-19, an ongoing pandemic, has had significant socioeconomic, clinical, and mental health repercussions.[1] This viral disease typically involves various organ systems, most commonly the pulmonary system[2] Commonly reported cardiac manifestations of COVID-19 are myopericarditis, heart failure (HF), and myocardial infarction.[3,4] Like other viruses, SARS-CoV-2 can induce, prolong, or complicate the clinical course of HF.[5] We present two cases of acute exacerbation of HF. They were diagnosed with COVID-19 infection on workup. The first patient succumbed to the SARS-CoV-2 which led to acute HF and death, while the second patient recovered.
We discuss the challenges of COVID-19 diagnosis in patients with HF, the possible pathophysiologic mechanisms involved, and its management. Finally, we have explored the unfavorable effects of the modification of the outpatient services in the HF population from physical to telehealth clinics and possible solutions to overcome the resulting problems.
CASE DESCRIPTION OF PATIENT 1
A 42-year-old Bangali male presented to the emergency room with a 1-day history of fever, associated with a dry cough, shortness of breath (SOB), and chest pain. Two months previously, he was admitted with an extensive anterior wall myocardial infarction. He had a primary percutaneous coronary intervention to a totally occluded left anterior descending artery. His hospital course was complicated by pulmonary edema secondary to severe left ventricular dysfunction (Ejection fraction [EF] 28%) with akinesia of the anterior wall from base to apex.
After his initial discharge, he had four consecutive re-admissions to the hospital with acute decompensated HF [Table 1].
Table 1.
Heart failure workup for the first patient
| Parameter | Admission 1 | Admission 2 | Admission 3 | Admission 4 | Normal range |
|---|---|---|---|---|---|
| LOS (days) | 6 | 27 | 7 | 1 | NA |
| WBC count | 12.2 | 13.5 | 6.9 | 5.3 | 4-10×10^3/uL |
| HB | 9.2 | 9.8 | 9.3 | 7.7 | 13-17 g/dL |
| Lymphocyte count | 1.5 | 2.3 | 2.1 | 1.2 | 1-3×10^3/uL |
| Pro-BNP | 4714 | 3763 | 4428 | 8213 | <300 pg/mL |
| Creatinine | 124 | 130 | 115 | 62-106 umol/L | |
| Troponin T level | 1554 | 681.5 | 35 | 49 | 0-10 ng/L |
| Pro-BNP | 4714 | 3763 | 4428 | 8213 | <300 pg/mL |
| CRP | 100.4 | 52.9 | 26 | 26.7 | 0-5 mg/L |
| Procalcitonin | 0.24 | 0.1 | 0.9 | - | <0.5 ng/ml |
| D-dimer | - | - | 3.21 | 3.18 | 0-4.9 FEU |
| Blood cultures | - | No growth | - | Negative | - |
| Urine culture | - | No growth | - | - | - |
| Common Viral panel | - | Negative | - | - | - |
| SARS-CoV-2 PCR | - | Negative | Negative | Positive | - |
| Sick contact | Negative | Negative | Negative | Negative | - |
| ECG | NNC | NNC | NNC | NNC | - |
LOS: Length of stay, WBC: White blood cell, HB: Hemoglobin, BNP: Blood natriuretic peptide, CRP: C reactive protein, ECG: Electrocardiogram, NNC: No new changes, Polymerase chain reaction
In his first re-admission (re-admission 1), he was treated in the emergency department with an adequate response to intravenous (IV) diuretics [Figure 1]. Chest X-ray revealed bilateral pulmonary infiltrates [Figure 1a]. After discharge, he was re-admitted the following day (re-admission 2) with worsening dyspnea and cough associated with chest pain. Physical examination revealed basal inspiratory crackles in both lungs with decreased breath sounds at the bases and mild pitting edema in both lower limbs. There were no new electrocardiographic (ECG) changes and no new rise in cardiac enzymes. His chest X-ray (CXR) revealed bilateral perihilar haziness associated with interstitial lung parenchymal congestion and bilateral pleural effusions [Figure 1b]. His condition necessitated admission to the cardiac intensive care unit (ICU), where he was treated along standard lines for acute decompensated HF. He required inotropic support for associated hypotension and received IV antibiotics for suspicion of superadded pneumonia.
Figure 1.
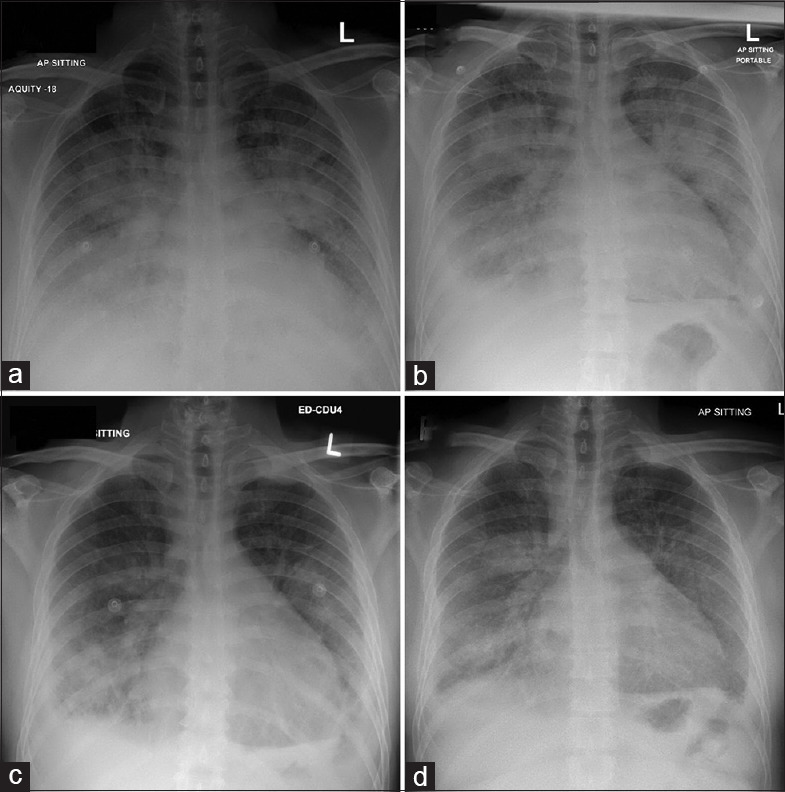
Chest X-rays for the first patient (1a: 1st admission, 1b: 2nd admission, 1c: 3rd admission, 1d: 4th admission)
He had a prolonged hospital stay (4 weeks) due to intractable HF and a transient acute kidney injury. The hypotension and kidney injury prevented initiation of disease-modifying HF medications (such as beta-blockers, angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARBs), or angiotensin receptor-neprilysin inhibitors). On discharge, he was given a follow-up appointment in the HF outpatient clinic four days later. However, due to the lockdown of his neighborhood (in the setting of the COVID-19 pandemic), he was unable to attend the clinic, and the consultation was done virtually.
One week later, he was again re-admitted (re-admission 3) with acute decompensated HF. There was no fever. CxR showed cardiomegaly, bilateral predominately basal air space shadowing, and bilateral pleural effusion [Figure 1c]. He improved with IV diuretics, but again due to associated hypotension and acute kidney injury, he could not tolerate meaningful doses of beta-blocker or ACE-I/ARBs.
Despite an initially negative SARS-CoV-2 nasopharyngeal real-time reverse-transcription polymerase chain reaction (RT-PCR) test, there was still a clinical suspicion of COVID-19 as he had elevated inflammatory markers with bilateral infiltrates and pleural effusions on the CXR, lived in the industrial area (pandemic zone) and shared a room with multiple people. A computed tomography (CT) scan of the thorax was performed, which showed diffuse pleural thickening and faint pleural calcification spots. Enlarged mediastinal lymph nodes and bilateral lower lobe atelectatic bands were noted, but he had no significant pulmonary infiltrates to suggest COVID-19 infection. Standard cultures were also negative. He was discharged after a 1-week stay in the emergency department.
Due to the continued pandemic restrictions, an outpatient follow-up visit for his HF was done virtually over the telephone a few days later.
He was re-admitted 10 days later (final re-admission) with a one-day history of fever, dry cough, SOB, and chest pain. At presentation, he was pyrexial (38.2°C), blood pressure (BP) was 88/56 (subsequently dropped to 76/57 mm Hg), pulse 127 beats/min (BPM), and oxygen saturation (SpO2) 97% on room air. He had bilateral basal crepitations in his chest. ECG showed sinus tachycardia, poor R-wave progression in V1-V4, ST/T changes inferolaterally, but there were no new/significant changes as compared to baseline [Table 2]. He was anemic with an Hb of 7.7 g/dL, NT Pro-brain natriuretic peptide (BNP) was significantly elevated at 8213 pg/mL (normal value <300 pg/mL).
Table 2.
Heart failure workup for the second patient
| Investigation | Result | Normal range |
|---|---|---|
| WBC count | 8.4 | 4-10×10^3/uL |
| HB | 8.2 (on baseline) | 13-17 g/dL |
| Lymphocyte count | 0.8 | 1-3×10^3/uL |
| Creatinine | 203 | 62-106 umol/L |
| Pro-BNP | 9926 | <300 pg/mL |
| Troponin T level | 71 | 0-10 ng/L |
| TSH | 8.8 | 0.3-4.2 mIU/L |
| FT4 | 16.7 | 11.6-21.9 pmol/ |
| D-Dimer | 1.4 | 0-4.9 FEU |
| CRP | 42.4 | 0-5 mg/L |
| Procalcitonin | 0.14 | <0.5 ng/ml |
| Lactic acid | 1.1 | 0.5-2.2 mmol/L |
| Blood cultures | No growth | - |
| Urine culture | No growth | - |
| Common viruses panel | Negative | - |
| Medication noncompliance | Negative | - |
| Sick contact | Negative | - |
| New drop in EF | Negative | - |
| ECG | Normal | - |
ECG: Electrocardiogram, EF: Ejection fraction, WBC: White blood cell, HB: Hemoglobin, BNP: Blood natriuretic peptide, TSH: Thyroid-stimulating hormone, FT4: Free thyroxine, CRP: C reactive protein
CXR was reported as showing bilateral veiling opacities with fluffy confluent patchy opacities, suspected underlying consolidations, and bilateral blunting of both costophrenic angles likely due to underlying mild reaction/effusion [Figure 1d]. He was thus diagnosed with acute decompensated HF with a high suspicion of COVID-19 related bilateral pneumonia and associated shock. He was started on vasopressors and admitted to the ICU. A few hours later, he had an episode of supraventricular tachycardia (reverted with IV adenosine), followed by high-grade ventricular arrhythmias and subsequently ventricular fibrillation and cardiac arrest. He did not survive despite extended cardiopulmonary resuscitation. His COVID-19 PCR result came back positive (previously negative on two separate occasions). His cause of death was related to shock, acute decompensated HF, and COVID-19 infection.
CASE DESCRIPTION OF PATIENT 2
A 59-year-old Qatari female presented with a 2-day history of gradually progressive SOB. She was a known case of HF with reduced EF of 42%, type-2 diabetes mellitus, hypertension, chronic renal failure with anemia, coronary artery disease, paroxysmal atrial fibrillation, and hypothyroidism. There were no associated symptoms of chest pain, cough, fever, or upper respiratory tract infection. She had two recent hospital admissions, with decompensated HF and hypoglycemia, respectively.
BP was 130/63 and pulse rate was 78 BPM. Her SPO2 on room air was 85%. Her physical examination revealed decreased breath sounds in the bases of the lungs bilaterally, with inspiratory crackles. The rest of the examination was unremarkable. A CXR showed new bilateral mid and lower zone opacities with bilateral pleural effusions [Figure 2]. Blood works revealed a raised NT pro-BNP level 9926 (normal value <300 pg/mL). A diagnosis of acute HF was made based on raised BNP, and a detailed workup to identify the cause of HF exacerbation was carried out [Table 2]. RT-PCR test for a SARS-CoV-2 (via the GeneXpert system) was negative.
Figure 2.
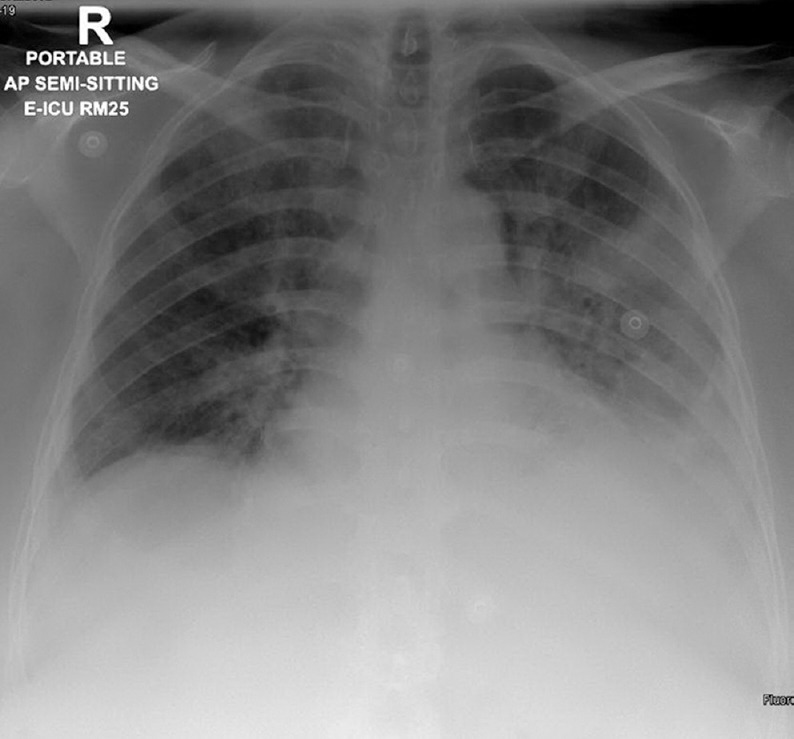
Chest X-ray for the second patient at presentation
The patient was started empirically on ceftriaxone 1 g IV, azithromycin 500 mg IV daily, and oseltamivir 75 mg oral twice daily for suspicion of underlying infective pathology (both bacterial and viral). Her HF was treated with IV infusions of loop diuretics and nitrates. However, despite optimal initial management, her condition deteriorated with increasing oxygen requirements. By day 3, she required noninvasive ventilation with continuous positive airway pressure. RT-PCR test was repeated due to the unexpected deterioration and high clinical suspicion, and it came back positive. A diagnosis of severe COVID-19-related pneumonia leading to acute decompensated HF was made, and the patient was transferred to the ICU. She was intubated and treated as per the local guidelines at that time with hydroxychloroquine 400 mg daily, azithromycin 500 mg daily, oseltamivir 75 mg twice daily, methylprednisolone 40 mg twice daily for 5 days, and tocilizumab 600 mg IV once. The patient's interleukin-6 (IL-6) was 674, (normal range was ≤ 7 pg/mL). The patient was extubated after 4 days. SARS-CoV-2 PCR was negative by day 7, and she was discharged home after 15 days of her initial presentation. She was asymptomatic, with an SpO2 of 98% on room air.
DISCUSSION
COVID-19 infection, caused by the SARS-CoV-2 virus, most commonly involves the respiratory system but can have many different manifestations.[2,6] Cardiovascular involvement has been extensively reported and includes myopericarditis, tamponade, HF, and myocardial infarction.[3,4] Zhou et al. studied the clinical course of COVID-19 in 191 patients and found HF to be present in around 23% of patients infected by the SARS-CoV-2. Furthermore, 52% of the patients who died had HF as an outcome.[7]
Mechanisms of heart failure in COVID-19
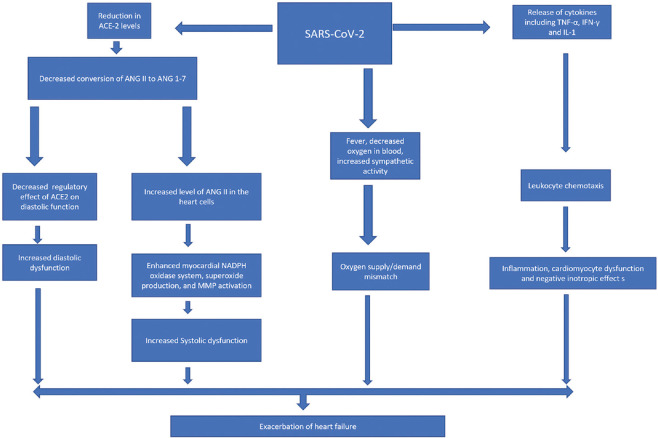
Several pathophysiologic mechanisms have been proposed to explain the relation between COVID-19 infection and HF [Figure 3].
Figure 3.
Proposed mechanisms of heart failure in the setting of COVID-19 (ANG II angiotensin 2, ANG 1-7 angiotensin 1-7, NADPH Reduced nicotinamide)
Downregulation of angiotensin-converting enzyme 2 receptors
The SARS-CoV-2 virus has a high affinity for ACE 2 receptors. Although these receptors are mainly present in the lungs, they are also found in other organs, including the heart.[8] Recent investigations have demonstrated that ACE-2 receptor internalization induced by SARS-CoV-2 in the myocardium can induce HF.[9] This internalization leading to a reduction in ACE-2 receptors increases the angiotensin 2 levels in the body, causing an increased chance of the development of both diastolic and systolic HF by different mechanisms. Systolic dysfunction is secondary to “worsened cardiac remodeling in response to pressure-overload-induced biomechanical stress,” as a result of the increase in angiotensin 2 levels, leading to HF with reduced EF.[10]
The increase in the angiotensin-2 levels also promotes the release of various enzyme complexes, i.e., nicotinamide adenine dinucleotide phosphate oxidase system, superoxides, and matrix metalloproteinases. These complexes consequently lead to ventricular dilatation and systolic dysfunction.[10]
HF with preserved EF can also occur secondary to the downregulation of ACE-2, which itself is a negative regulator of diastolic dysfunction.[10] Recombinant human ACE-2 has been used to demonstrate reversal of ACE-2 led cardiac hypertrophy and fibrosis with the ultimate correction of diastolic dysfunction, validating the pivotal role of ACE-2 in HF.[10]
Hyperinflammatory response to SARS-CoV-2
Another proposed mechanism of SARS-CoV-2 led HF is consequent to the accompanying inflammation in COVID-19-related infection. The role of inflammation leading to HF is well-studied and is secondary to the release of various cytokines, including tumor necrosis factor-alpha, interferon-gamma, and IL-1.[11]
The increased levels of angiotensin-2 are also associated with cardiac cell hypoxia, wall shear stress, and cell programmed death and necrosis, with the release of different types of cytokines that further results in augmentation of the reactive inflammatory process.
Increased sympathetic activity
Finally, fever and hypoxemia can lead to increased sympathetic activity in the body, leading to an oxygen supply and demand mismatch, resulting in myocardial damage and HF.[5]
DIAGNOSING COVID-19 INFECTION IN PATIENTS WITH HEART FAILURE
The diagnosis of COVID-19 in a patient presenting with acute HF can be challenging. Both conditions can have a similar presentation, i.e., SOB and cough, and comparable abnormalities on CXRs. Moreover, the sensitivity of the viral RT-PCR, the most widely used diagnostic test, is only 79%.[12] In one study from China, the sensitivity was reported even lower, around 71%.[13]
Both our cases had negative RT-PCR results before the final positive one. A false-negative result can be attributed to factors such as improper sampling techniques, testing when the patient is having a low viral load, and suboptimal quality control of the collected specimen. The delay in COVID-19 diagnosis associated with false-negative PCR can worsen patient outcomes and increase the risk of health-care workers' exposure. To prevent this, it is highly recommended that protective and isolation measures are kept in place despite the RT-PCR result, when there is a high suspicion. In addition, a CT of the thorax (showing ground-glass opacities) can aid in the diagnosis of patients with a false-negative PCR.[13]
ACUTE HEART FAILURE MANAGEMENT IN COVID-19
In-hospital acute HF therapy is generally the same as for the patients without COVID-19, as per the European society of cardiology guidelines.[14] The key to effective treatment is the judicious use of diuretics, vasodilators, vasopressors, and a careful assessment of the need for dialysis and assisted ventilation.[15]
Dexamethasone has recently emerged as a life-saving drug in SARS-CoV-2-infected patients on ventilation or in need of supplemental oxygen.[16] Nevertheless, the use of systemic steroids in HF patients can be challenging as they induce sodium and water retention and therefore, can precipitate or worsen lung congestion. Thus, in those COVID-19 patients complicated with acute HF, dexamethasone should be used cautiously, with close volume status monitoring and adjustment of diuretic doses when needed.
Considerable controversy has arisen concerning the potential harm of ACE-I and ARBs in COVID-19. These drugs theoretically increase the exposure of SARS-CoV-2 to ACE2 receptors that could lead to more severe presentations. However, ACE-I and ARBs have well-demonstrated efficacy in HF by counteracting the effect of the renin-angiotensin-aldosterone system, which has a vital role in the development of congestion and inflammation. A recent retrospective study observed that the use of ACE1 and ARBs was associated with better outcomes in patients infected with COVID-19.[17] Therefore, international societies strongly recommend the continuation of these life-saving drugs in the absence of clear evidence about possible harmful effects.
HEART FAILURE OUTPATIENT CARE DURING COVID-19 PANDEMIC CHALLENGES AND FUTURE DIRECTIONS
Outpatient management of HF is being affected by the restrictions imposed in almost every country hit by the pandemic. The optimization of diuretic therapy and guideline-directed HF therapy (such as adding or maximizing doses of ACE-I, ARBs, sacubitril/valsartan, spironolactone, and beta-blockers) necessitate knowledge of vital signs, details of physical examination and serial biochemical blood tests. This monitoring has become difficult during the pandemic as most outpatient clinics have adopted telehealth mechanisms (due to infection control preventions in place to mitigate the spread of SARS-CoV-2). In the first case described, the patient could not physically attend his early follow-up appointments due to the lockdown, which may have been a factor in his readmissions over a short period. Similarly, elective procedures such as implantable cardioverter-defibrillator and cardiac resynchronization device implantations have also been postponed.[18]
The direct and indirect effects of the COVID-19 pandemic may have an impact on the long-term management of HF patients. To counter these effects, there is a dire need to explore further possibilities of enhancing telecommunication with the patients without compromising the best possible care. In this regard, the development and implementation of transitional care programs for patients at high risk of rehospitalization are now needed more than ever.
Homecare is known to be one of the most effective interventions to reduce hospitalization and mortality in chronic HF patients.[19] Home visits by HF nurse specialists or physicians allow assessment of vital signs and clinical findings and the extraction of regular blood samples. These data are crucial for optimizing anti-failure treatments and early management of decompensations of HF. This, in turn, may have a significant impact on the prevention of admissions with acute decompensation of HF.[20] In this COVID era, such home-based interventions have the added benefit of potentially reducing the risk of exposure to COVID-19 patients in the health-care facility while, at the same time, ensuring the best care for the patients.
Thus, we postulate that the combination of telemedicine with homecare visits and case-management should be the cornerstone of the outpatient management of HF patients during the COVID-19 pandemic. Incorporating these interventions into preexisting HF services will ensure optimal management of high-risk HF patients after discharge and have a favorable impact on the short, medium, and long-term outcomes.
Furthermore, such mechanisms can ensure that the chronic HF population, which is at high risk of readmissions, is minimally affected by the challenges that COVID-19 pandemic imposes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dubey S, Biswas P, Ghosh R, Chatterjee S, Dubey MJ, Chatterjee S, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14:779–88. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-segment elevation in patients with COVID-19-A case series? N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. doi: 101056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac Tamponade Secondary to COVID-19. JACC Case Rep 2020;? doi: 10.1016/j.jaccas.2020.04.009. doi: 101016/jjaccas202004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, et al. COVID 19 and heart failure: From infection to inflammation and angiotensin II stimulation.Searching for evidence from a new disease? Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1871. doi: 101002/ejhf1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ata F, Almasri H, Sajid J, Yousaf Z. COVID-19 presenting with diarrhoea and hyponatraemia. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-235456. doi: 101136/bcr-2020-235456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–90. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–26. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017;19:27. doi: 10.1007/s11883-017-0660-3. [DOI] [PubMed] [Google Scholar]
- 12.He JL, Luo L, Luo ZD, Lyu JX, Ng MY, Shen XP, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. doi: 10.1016/j.rmed.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115–7. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreini D. ESC guidance for the diagnosis and management of CV disease during The COVID-19 Pandemic: European Society of Cardiology. 2020. [[Last acessed on 2020 Jun 10]]. Available from: https://www Escardio Org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance#p09 .
- 15.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 16.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19: Preliminary report. medRxiv. 2020 doi: 101101/2020062220137273. [Google Scholar]
- 17.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Reza N, DeFilippis EM, Jessup M. Secondary Impact of the COVID-19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13:e007219. doi: 10.1161/CIRCHEARTFAILURE.120.007219. [DOI] [PubMed] [Google Scholar]
- 19.Van Spall HG, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427–43. doi: 10.1002/ejhf.765. [DOI] [PubMed] [Google Scholar]
- 20.Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating study evaluating outcomes of advising and counseling in heart failure (COACH) Arch Intern Med. 2008;168:316–24. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]