Abstract
Background
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Up to 20%–30% of patients hospitalized with COVID-19 have evidence of cardiac dysfunction. Xuebijing injection is a compound injection containing five traditional Chinese medicine ingredients, which can protect cells from SARS-CoV-2-induced cell death and improve cardiac function. However, the specific protective mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction remains unclear.
Methods
The therapeutic effect of Xuebijing injection on COVID-19 was validated by the TCM Anti COVID-19 (TCMATCOV) platform. RNA-sequencing (RNA-seq) data from GSE150392 was used to find differentially expressed genes (DEGs) from human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) infected with SARS-CoV-2. Data from GSE151879 was used to verify the expression of Angiotensin I Converting Enzyme 2 (ACE2) and central hub genes in both human embryonic-stem-cell-derived cardiomyocytes (hESC-CMs) and adult human CMs with SARS-CoV-2 infection.
Results
A total of 97 proteins were identified as the therapeutic targets of Xuebijing injection for COVID-19. There were 22 DEGs in SARS-CoV-2 infected hiPSC-CMs overlapped with the 97 therapeutic targets, which might be the therapeutic targets of Xuebijing injection on COVID-19-induced cardiac dysfunction. Based on the bioinformatics analysis, 7 genes (CCL2, CXCL8, FOS, IFNB1, IL-1A, IL-1B, SERPINE1) were identified as central hub genes and enriched in pathways including cytokines, inflammation, cell senescence and oxidative stress. ACE2, the receptor of SARS-CoV-2, and the 7 central hub genes were differentially expressed in at least two kinds of SARS-CoV-2 infected CMs. Besides, FOS and quercetin exhibited the tightest binding by molecular docking analysis.
Conclusion
Our study indicated the underlying protective effect of Xuebijing injection on COVID-19, especially on COVID19-induced cardiac dysfunction, which provided the theoretical basis for exploring the potential protective mechanism of Xuebijing injection on COVID19-induced cardiac dysfunction.
Keywords: Network pharmacology, RNA-sequencing, Molecular docking, Xuebijing injection, COVID-19, Cardiac dysfunction
1. Introduction
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has rapidly turned into a pandemic [1]. Angiotensin I Converting Enzyme 2 (ACE2), the receptor of SARS-CoV-2, mainly distributes among heart, kidney and testis [2]. Although the clinical manifestations of COVID-19 are dominated by typical respiratory symptoms, up to 20%–30% of patients hospitalized with COVID-19 were diagnosed with elevated myocardial enzymes and abnormal echocardiography and suffered from cardiac events, including acute myocardial injury, myocarditis, heart failure and arrhythmia [[3], [4], [5]]. In a study of 18 ST-segment elevation cases with COVID-19, the average peak value of cTnI was 44.4 ng/ml, of which 9 cases (50.0%) were diagnosed with ejection fraction reduction by echocardiography. Further, 6 cases (66.7%) were diagnosed with coronary heart disease in 9 cases who underwent coronary angiography [6]. In another retrospective observational study, 11% of COVID-19 patients had increased hs-cTnI levels (>40 ng/L) on admission and the serum hs-cTnI levels were positively associated with the severity of medical conditions [7]. Those studies suggested that SARS-CoV-2 infection might cause cardiac dysfunction or aggravate the original underlying heart disease.
In addition, patients with COVID-19 complicated with cardiac dysfunction have a significantly higher risk of poor prognosis [8]. A multi-center retrospective study showed that 13.7% of 657 COVID-19 patients had elevated creatine kinase (CK) levels, and 41% of patients were with increased lactate dehydrogenase (LDH). It's worth noting that the abnormally elevated CK and LDH were closely related to the poor prognosis of COVID-19 patients [9]. Han et al. showed that the higher concentration in venous blood of CK-MB, MYO, ultra‐TnI, and NT‐proBNP was associated with the severity and case fatality rate (CFR) of COVID‐19 [10]. Shi et al. found that nearly 19.7% of patients had myocardial injury among 416 hospitalized patients with severe COVID-19, and 51.2% of them eventually departed. In contrast, the mortality rate of patients without myocardial injury was only 4.5% [11]. This indicated that the occurrence of myocardial injury might increase the risk of death for COVID-19 patients by 3–4 times. A study involving 191 COVID-19 patients reported a higher proportion of heart failure patients in the death group compared with the cured group, suggesting that heart failure in patients infected with SARS-CoV-2 was a sign of poor prognosis [12]. A meta-analysis enrolled 43 studies containing 3600 patients also came to a similar conclusion that the intensive group has a higher prevalence of heart failure compared with the non-intensive group [13]. These published studies manifest that in the treatment of severe and critically ill COVID-19 patients, in addition to the treatment of pneumonia, attention should also be paid to myocardial protection and the follow-up of myocardial markers.
Xuebijing injection is a compound injection containing five traditional Chinese medicine ingredients, including Hong Hua (Carthami Flos), Chi Shao (Paeoniae Radix Rubra), Chuan Xiong (Chuanxiong Rhizoma), Dan Shen (Salviae Miltiorrhizae Radix Et Rhizoma), and Dang Gui (Angelicae Sinensis Radix). In critically ill patients with severe community-acquired pneumonia, Xuebijing injection led to a statistically significant improvement in the primary endpoint of the pneumonia severity index as well a significant improvement in the secondary clinical outcomes of mortality, duration of mechanical ventilation and duration of Intensive Care Unit (ICU) stay [14]. Xuebijing injection could significantly protect cells from SARS-CoV-2-induced cell death and could attribute to the blocking of the proliferation of virus, and inhibit the expression of pro-inflammatory cytokines induced by SARS-CoV-2 [15]. Current studies have shown that Xuebijing injection could reduce the levels of cTnI, NT-proBNP and PCT in patients with septic myocardial injury and had a protective effect on myocardial injury [16]. Xuebijing injection can effectively improve cardiac function in rats with myocardial hypoxia/reoxygenation injury [17]. However, the specific protective mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction remains unclear.
In this study, traditional Chinese medicine anti COVID-19 (TCMATCOV) platform was used to verify the therapeutic effects of Xuebijing injection and its components on COVID-19. We combinedly used network pharmacology, RNA-sequencing (RNA-seq), bioinformatics and molecular docking technology to further recognize genes and pathways involved in the treatment of COVID-19-induced cardiac insufficiency through Xuebijing injection in order to construct a theoretical basis for exploring the therapeutic mechanism of Xuebijing injection.
2. Methods
2.1. Anti−COVID-19 Chinese medicine validation
TCMATCOV (http://47.92.232.154:90/) is a platform to predict the potential therapeutic efficacy of traditional Chinese medicine against COVID-19 [18]. The curative effects of Xuebijing injection and its main components against COVID-19 were evaluated by interference scores, which were predicted and calculated based on the TCMATCOV platform and were positively correlated with the curative efficacy of corresponding drugs. Ban Xia Baizhu Tianma decoction (BXTM) was selected as the negative control, while the Huashi Baidu formula (HSBD) was taken as the positive control.
2.2. Identification of COVID-19 related therapeutic target genes
Therapeutic target genes related with COVID-19 were obtained from five databases, including DrugBank (https://www.drugbank.ca/), GeneCard (https://www.genecards.org/), Online Mendelian Inheritance in Man (OMIM, https://omim.org/), Therapeutic Target Database (TTD, http://db.idrblab.net/ttd/) and TCMATCOV. The keywords “COVID-19” and “SARS-CoV-2” were used in the retrieval process.
2.3. Recognition of effective compounds of Xuebijing injection
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://tcmspw.com/tcmsp.php) was used to identify the effective compounds of Xuebijing injection. Oral bioavailability (OB) ≥ 30 and drug-likeness (DL) ≥ 0.18 were set as the criteria for screening bioactive constituents of each component from Xuebijing injection [19].
2.4. Prediction of effective compounds related target genes
The compounds related target proteins were predicted depending on chemical similarities and pharmacophore models through TCMSP. Perl software was used to convert all protein names to gene names based on UniProt Knowledgebase (UniprotKB, https://www.uniprot.org/uniprot/).
2.5. Effective compound-target network construction
The target genes that were commonly related with both COVID-19 and Xuebijing injection were obtained by taking the intersection of COVID-19 related therapeutic genes and the effective compounds related genes of Xuebijing injection. Cytoscape (3.7.2) software was used to construct the effective compounds-target genes network [20].
2.6. RNA-seq data analysis
RNA-seq data of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) infected with or without SARS-CoV-2 were obtained from GSE150392, which contained three controls and three cases [21]. For hiPSC-CMs with SARS-CoV-2 infection, viral inoculum (USA-WA1/2020, MOI = 0.1) prepared using serum-free media was added. For hiPSC-CMs with mock infection, serum-free media alone was added. The inoculated plates were incubated for 1 h at 37 °C with 5% CO2. At the end of incubation, the inoculum was replaced with fresh hiPSC-CM culture medium. Cells remained at 37 °C with 5% CO2 for 72 h before analysis. The R package named “limma” was adopted in the normalization of RNA-seq data and the identification of differentially expressed genes (DEGs) [22]. |log2 (foldchange)| > 1 and adjusted p value < 0.05 were taken as the screening criteria.
2.7. Gene set enrichment analysis (GSEA)
GSEA was performed by WebGestalt (http://www.webgestalt.org) based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the Reactome Pathway Knowledgebase, respectively [23,24].
2.8. GO and KEGG enrichment analyses
The R package “clusterProfiler” was utilized to implement Gene ontology (GO) enrichment analysis and KEGG pathway analysis [23,25,26]. More precisely, GO enrichment analysis was carried out within three classical subschemas: biological process (BP), cellular component (CC), and molecular function (MF). The cutoff value of statistical significance was set as p < 0.05.
2.9. PPI network establishment and central hub genes identification
Analysis of Protein-Protein Interaction (PPI) network is beneficial to explore the interactions of various proteins in complex diseases and it is helpful in systematically studying the molecular mechanism of diseases and distinguishing novel drug targets. In this study, STRING (v11.0) (https://string-db.org/) was utilized to establish a PPI network, 0.4 was set as the threshold of confidence score, and disconnected nodes in the network were hidden [27]. The PPI network was further visualized by Cytoscape 3.7.2. Subsequently, the central hub genes were screened based on the medians of six important topological parameters, including betweenness centrality (BC), closeness centrality (CC), degree centrality (DC), eigenvector centrality (EC), network centrality (NC), and local average connectivity (LAC).
2.10. Verification of ACE2 and central hub genes
GSE151879 contained RNA-seq data of hESC-derived cardiomyocytes (hESC-CMs) and adult human CMs with or without SARS-CoV-2 infection (USA-WA1/2020, MOI = 0.1) [28]. Each subgroup included three samples. Cells were incubated at 37 °C with 5% CO2 for 24 h before analysis. GSE151879 was used to analyze the expression of ACE2 and central hub genes to validate the results of GSE150392.
2.11. Molecular docking
The 2D structure of effective compounds was downloaded through PubChem (https://pubchem.ncbi.nlm.nih.gov/) and converted into 3D models by ChemOffice software. The X-ray crystal structures of the therapeutic target proteins were obtained from the RCSB PDB database (http://www.rcsb.org/). Molecular dockings of molecule ligands and protein receptors were performed by AutoDock Vina, and the binding free energy was utilized as the basis for evaluating the docking results.
3. Results
3.1. Predict the effect of Xuebijing injection on COVID-19 by TCMATCOV
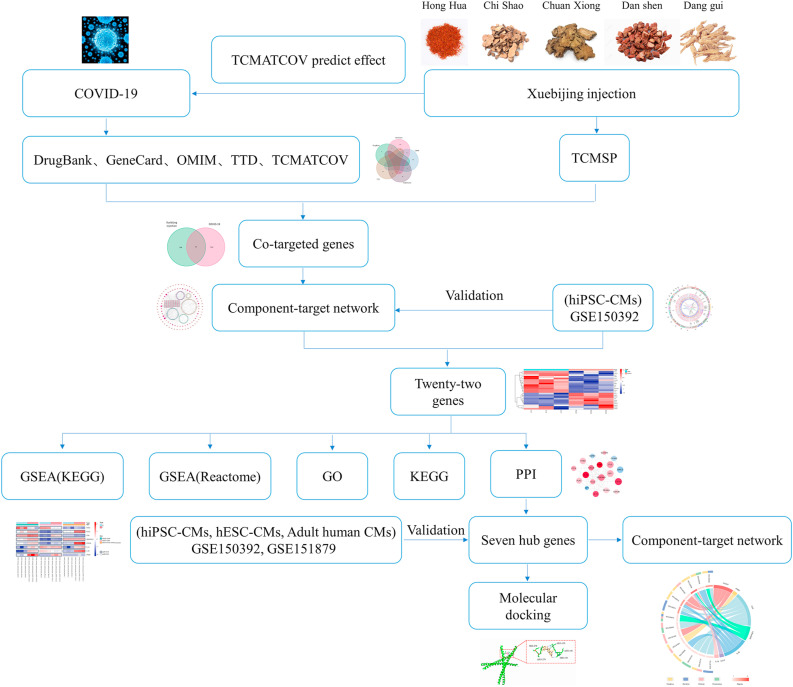
The research flow diagram of this study was shown in Fig. 1 . It can be noticed from the results exhibited in Table 1 that the therapeutic effect of Xuebijing injection on COVID-19 was almost equal to the positive control (HSBD), indicating the therapeutic ability of Xuebijing injection was quite compelling. The curative potential of the 5 traditional Chinese medicine herbs in Xuebijing injection was also evaluated through TCMATCOV. Hong Hua, Chuan Xiong and Dang Gui were all identified with satisfactory therapeutic effects on COVID-19, while Dan Shen showed rather mediocre curative ability. Chi Shao was not enrolled in the analysis due to the deficiency of corresponding pharmacological data in TCMATCOV.
Fig. 1.
Flow diagram of the analysis process.
Table 1.
Xuebijing injection and TCM prescriptions validation results by TCMATCOV platform.
| TCM herbs | Sum score | Average Degree | Average shortest path | Degree centrality | Closeness centrality |
|---|---|---|---|---|---|
| Negative Control (BXTM) | 14.52 | −2.32 | 4.59 | −1.15 | −6.46 |
| Positive Control (HSBD) | 22.71 | −3.78 | 11.17 | −1.25 | −6.52 |
| Xuebijing injection | 21.78 | −4.38 | 9.88 | −1.3 | −6.21 |
| Chishao (herb) | – | – | – | – | – |
| Chuanxiong (herb) | 22.65 | −5.37 | 10.46 | −0.98 | −5.84 |
| Danggui (herb) | 22.55 | −5.18 | 10.25 | −1.12 | −6 |
| Danshen (herb) | 17.06 | −3.95 | 5.63 | −1.42 | −6.07 |
| Honghua (herb) | 23.09 | −5.34 | 11.07 | −1.18 | −5.51 |
BXTM: Banxia Baizhu Tianma decoction; HSBD: Huashi Baidu formula.
3.2. COVID-19 related target genes
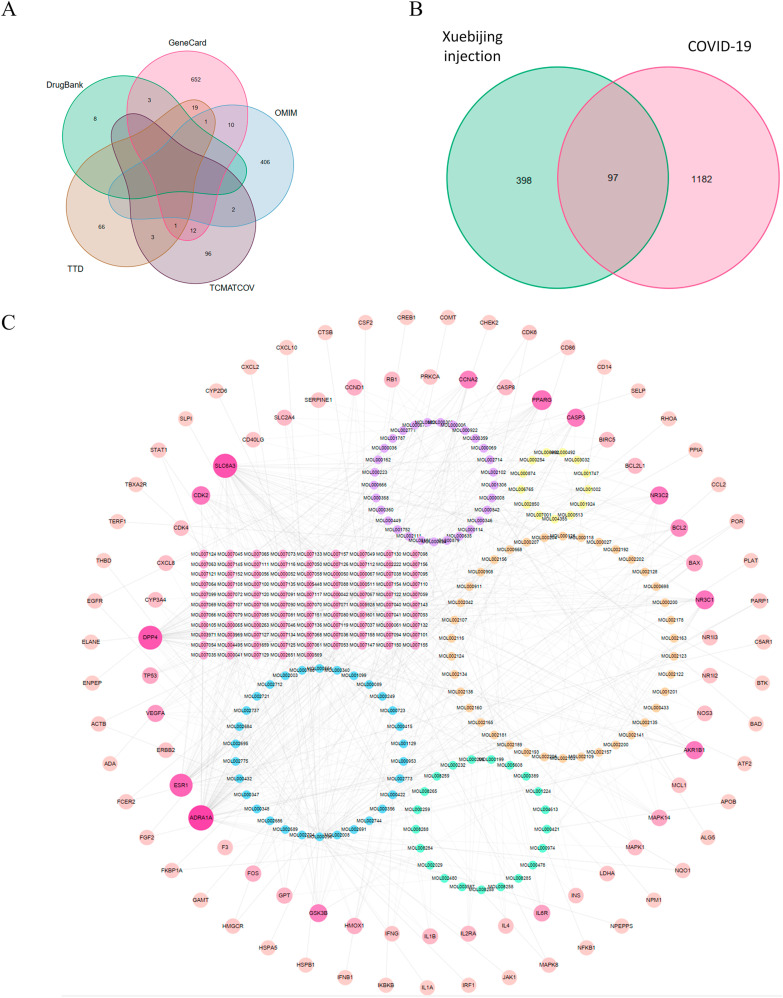
COVID-19 related therapeutic target genes were retrieved in five databases, including 11 from DrugBank, 698 from GeneCard, 419 from OMIM, 90 from TTD, and 114 from TCMATCOV (Fig. 2 A, Table S1). After removing duplicate targets, a total of 1279 COVID-19 related therapeutic targets were confirmed.
Fig. 2.
The network of effective compound-target. (A) Venn diagram of COVID-19 related therapeutic target genes from 5 databases. (B) Venn diagram for candidate targets in Xuebijing injection and COVID-19. (C) The effective compound-target network of Xuebijing injection on COVID-19. The two outer rings were composed of 97 therapeutic target proteins. In the inner circle, the effective ingredients in Chi Shao, Chuan Xiong, Dang Gui, Hong Hua, Dan Shen were shown in circle colored yellow, orange, green, blue, pink, respectively. Besides, the purple circle represented effective ingredients in multiple herbs.
3.3. Effective components and targets of Xuebijing injection
A total of 459 effective compounds and 495 target proteins from five traditional Chinese herbs in Xuebijing injection were distinguished through the TCMSP database (Table S2). Among them, 97 proteins were identified as the therapeutic targets of Xuebijing injection for COVID-19 (Fig. 2B).
3.4. Effective compound-target network of Xuebijing injection on COVID-19
The effective compound-target network of Xuebijing injection on COVID-19 was constructed to elaborate the multiplex interplay that contained 323 nodes (226 effective compounds and 97 target proteins) and 630 edges (Fig. 2C). The 2 peripheral rings were composed of 97 target proteins. The deeper color and the larger radius represented the greater connectivity among target proteins and effective compounds. In the inner layer, the yellow circle represented effective ingredients in Chi Shao, the orange circle represented effective ingredients in Chuan Xiong, the green circle represented effective ingredients in Dang Gui, the blue circle represented effective ingredients in Hong Hua, the pink circle represented effective ingredients in Dan Shen, and the effective ingredients that commonly existed in multiple herbs were exhibited by the purple circle.
3.5. Identification of DEGs in hiPSC-CMs infected with SARS-CoV-2
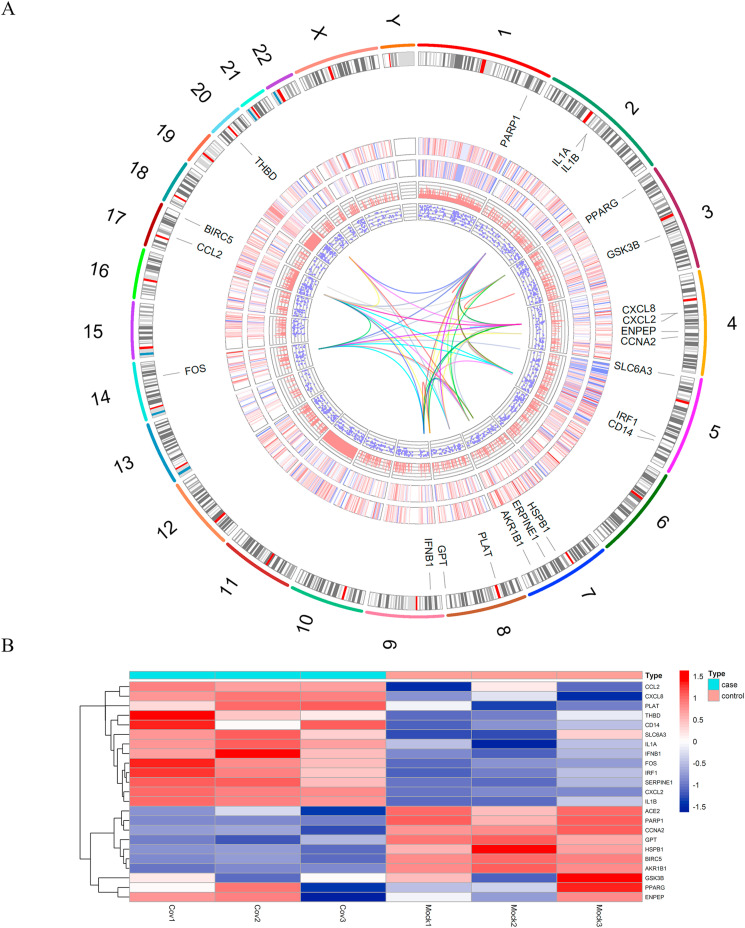
To further explore the underlying curative targets of Xuebijing injection on COVID-19-induced cardiac insufficiency, GSE150392 was enrolled in analysis and 2029 DEGs were identified, among which 1054 were up-regulated, 975 were down-regulated in hiPSC-CMs infected with SARS-CoV-2. It was noteworthy that ACE2, the major receptor of SARS-CoV-2, was significantly decreased in the SARS-CoV-2 infected hiPSC-CMs. There were 22 DEGs overlapped with the previously mentioned 97 therapeutic targets of Xuebijing injection on COVID-19. The 22 DEGs might be the hub therapeutic targets of Xuebijing injection on COVID-19-induced cardiac dysfunction. As Fig. 3 A showed, the chromosomal locations of the 22 hub DEGs were exhibited on the outermost track. The outer heatmap and the inner heatmap indicated the expression status of the 2029 DEGs of one representative sample from the control and case subgroup, respectively. The log2 (foldchange) and corresponding adjust p value of the 2029 DEGs were shown in the inner bar plot and scatter plot. The innermost track represented the PPI connection among the 22 hub DEGs. To visualize the expression status of the 22 hub DEGs and ACE2 in the control and case subgroup more clearly, a heatmap was drawn as Fig. 3B.
Fig. 3.
Data analysis of GSE150392. (A) Circos plot of DEGs in GSE150392. The outer track represented each chromosome, the second track was gene names of 22 overlapped genes between DEGs of SARS-CoV-2 infected hiPSC-CMs and the 97 therapeutic targets, the third track was a heatmap of 2029 DEGs (a sample of a control group), the fourth track was a heatmap of 2029 DEGs (a sample of a case group), the fifth track was the bar plot for log2 (foldchange) of 2029 DEGs, the sixth track was the scatter plot for log2 (adjust p value) of 2029 DEGs, the innermost track was the PPI connection of the 22 hub DEGs. (B) Expression heatmap of ACE2 and 22 hub DEGs in GSE150392.
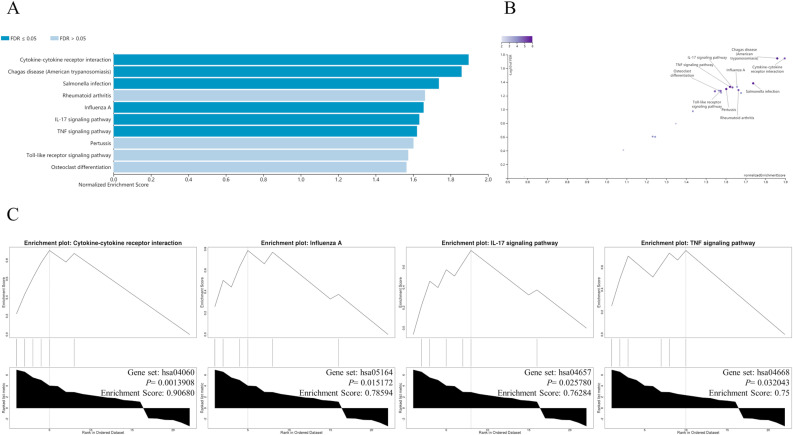
3.6. The GSEA of 22 hub DEGs
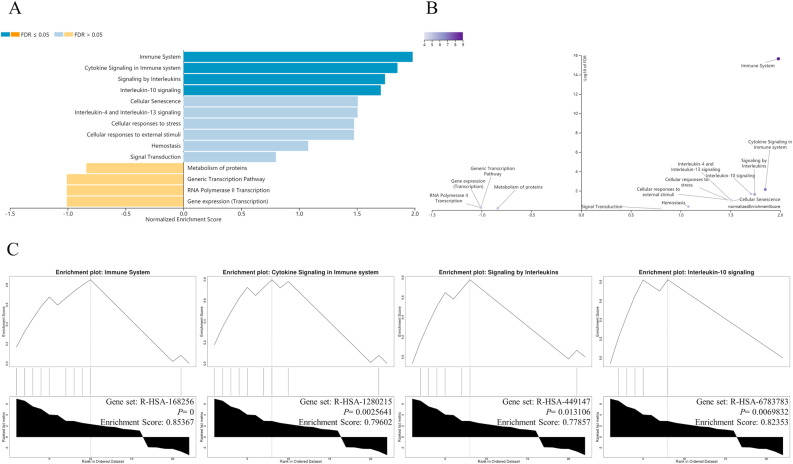
GSEA based on the KEGG gene set showed that the 22 hub DEGs were enriched in cytokine-cytokine receptor interaction, influenza A, IL-17 signaling pathway, TNF signaling pathway, pertussis, Toll-like receptor signaling Pathway and so on (Fig. 4 ). GSEA executed on the grounds of Reactome Pathway Knowledgebase indicated the 22 hub DEGs were principally enriched in immune system, cytokine signaling in immune system, signaling by interleukins, interleukin-10 signaling, cellular senescence, interleukin-4 and interleukin-13 signaling, cell responses to stress, cell responses to external stimuli and signal transduction (Fig. 5 ). These results hinted that the therapeutic mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction might mainly lie in inhibiting inflammatory storm, delaying cell senescence, and inhibiting oxidative stress.
Fig. 4.
The GSEA enrichment results for 22 hub DEGs by WebGestalt based on the KEGG gene set. (A) The GSEA enrichment results shown in a bar chart. (B) The GSEA enrichment results exhibited in a volcano plot. (C) The GESA enrichment plots of typical pathways.
Fig. 5.
The GSEA enrichment results for 22 hub DEGs by WebGestalt on the grounds of Reactome Pathway Knowledgebase. (A) The GSEA enrichment results shown in a bar chart. (B) The GSEA enrichment results exhibited in a volcano plot. (C) The GESA enrichment plots of typical pathways.
3.7. GO and KEGG enrichment analysis of 22 hub DEGs
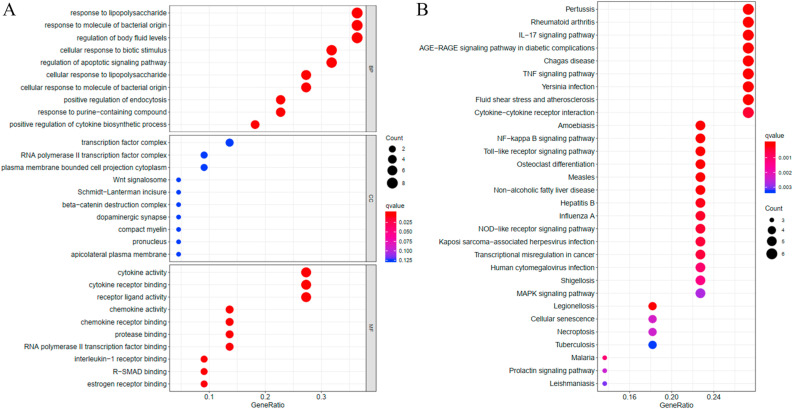
The GO functional enrichment analysis revealed that the 22 hub DEGs were enriched in multiple BP related terms, including response to lipopolysaccharide, regulation of apoptotic signaling pathway, cellular response to lipopolysaccharide, and positive regulation of cytokine biosynthetic process (Fig. 6 A). The hub DEGs were also observed to be enriched in Wnt signalosome and beta-catenin destruction complex, which belonged to the CC group. Cytokine related MF terms were enriched by the majority of the hub DEGs, such as cytokine activity, cytokine receptor binding, receptor ligand activity, chemokine activity, chemokine receptor binding and interleukin-1 receptor binding.
Fig. 6.
Functional analysis of 22 hub DEGs. (A) GO functional enrichment analysis of 22 hub DEGs. BP: biological process, CC: cellular component, MF: molecular function. GeneRatio: The ratio of genes enriched in each term. (B) KEGG pathway enrichment analysis of 22 hub DEGs. GeneRatio: The ratio of genes enriched in each pathway.
The results of KEGG pathway analysis revealed that the 22 hub DEGs mainly participated in pathways associated with inflammatory injury and cardiac dysfunction, including IL-17 signaling pathway, TNF signaling pathway, fluid shear stress and atherosclerosis, Cytokine-cytokine receptor interaction, NF-kappa B signaling pathway, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway, MAPK Signaling pathway, cellular senescence, necroptosis and so on. In addition, the pathological mechanism of COVID-19-induced cardiac dysfunction might be similar to that in Pertussis and Influenza A (Fig. 6B).
3.8. PPI network and central hub genes
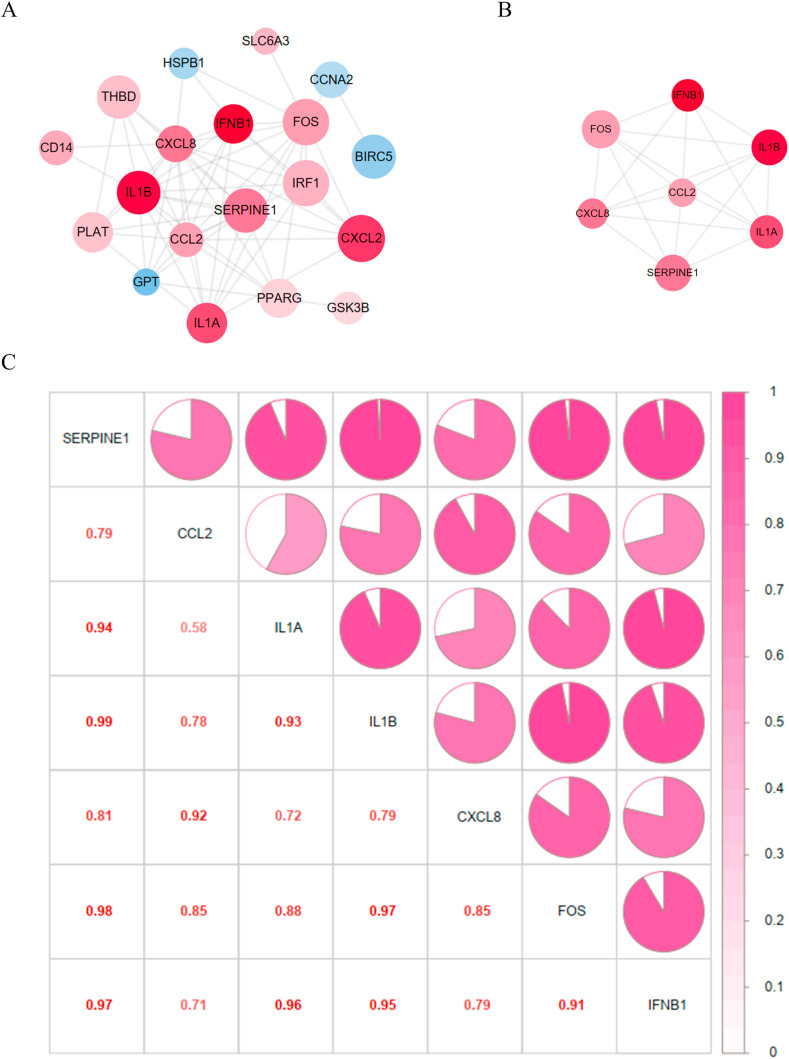
After hiding disconnected nodes, the 22 hub DEGs formed a PPI network with 19 nodes and 63 edges (Fig. 7 A). The up-regulated genes in SARS-CoV-2 infected hiPSC-CMs were shown in red, while the down-regulated ones were shown in blue. The deeper color of the genes indicated increased log2 (foldchange), and the larger diameter stood for a more significant statistical difference. According to the screening criteria “BC ≥ 1.13, CC ≥ 0.58, DC ≥ 7, EC ≥ 0.21, NC ≥ 5.80, LAC ≥4.67″, a sub-network with 7 central hub genes and 20 edges was constructed (Fig. 7B). The 7 central hub genes (CCL2, CXCL8, FOS, IFNB1, IL-1A, IL-1B, SERPINE1) were all up-regulated in SARS-CoV-2 infected hiPSC-CMs and were all positively correlated with each other (Fig. 7C).
Fig. 7.
PPI network and the correlation among 7 central hub genes. (A) PPI network of the 22 hub DEGs. The up-regulated genes were shown in red, while the down-regulated ones were shown in blue. The deeper color of the genes indicated increased log2 (foldchange), and the larger diameter stood for a more significant statistical difference. (B) PPI network of the 7 central hub genes. (C) Correlation heatmap of the 7 central hub genes. The area of the colored pie and the depth of color were positively associated with the correlation coefficient. The specific correlation coefficients were also marked as numbers in the corresponding sites.
3.9. Expression of ACE2 and central hub genes in three kinds of CMs infected with SARS-CoV-2
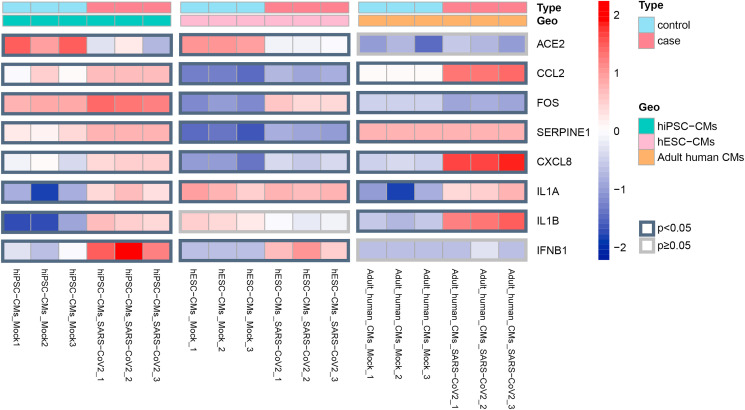
To enhance the reliability of our research, we added another dataset (GSE151879) to validate the expression status of ACE2 and 7 central hub genes. Combining the data from GSE150392 and GSE151879, we found ACE2 and 7 central hub genes were all differentially expressed in at least two kinds of SARS-CoV-2 infected CMs (hiPSC-CMs, hESC-CMs, adult human CMs) (Fig. 8 , Table S3). Besides, ACE2 was down-regulated and 7 hub genes were up-regulated.
Fig. 8.
Heatmap showed the detailed expression patterns of ACE2 and 7 central hub genes in three kinds of SARS-CoV-2 infected CMs (hiPSC-CMs, hESC-CMs, adult human CMs). The borders of DEGs with p value < 0.05 were marked in black. While the borders of DEGs with p value > 0.05 were marked in gray.
3.10. Molecular docking
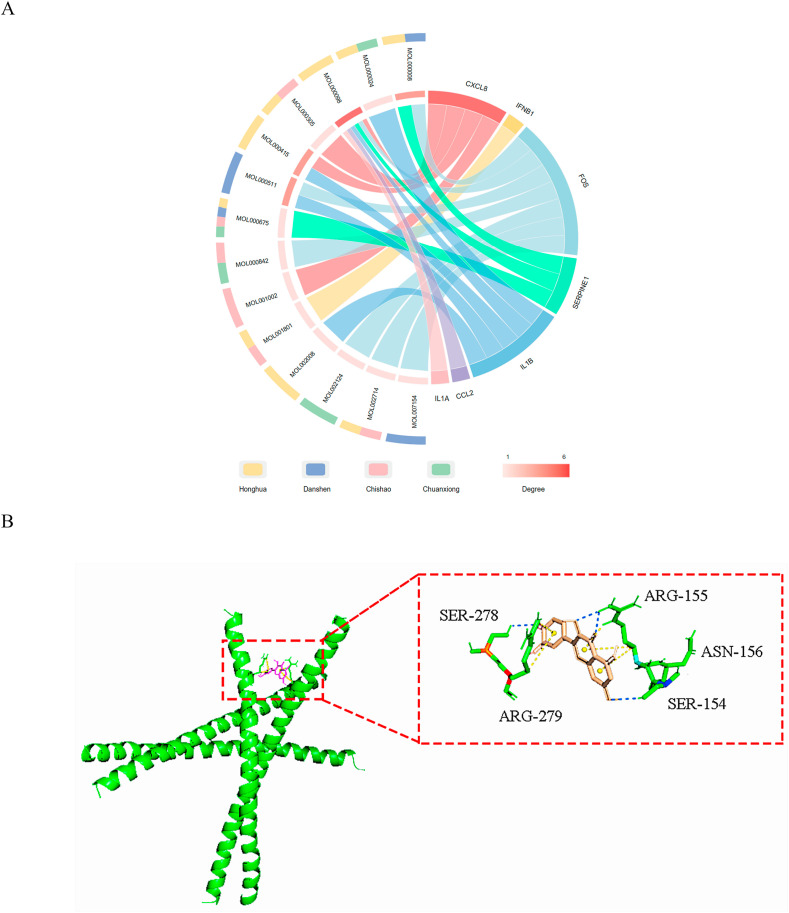
The 7 central hub genes and their corresponding effective compounds from the components of Xuebijing injection were displayed in Fig. 9 A. In order to further verify the binding ability of the effective compounds with hub targets, molecular docking was performed. The results of molecular docking were shown in Table 2 , where FOS and quercetin exhibited the tightest binding (Fig. 9B).
Fig. 9.
Relationship between 7 central hub genes and their corresponding effective compounds. (A) Chord plot of the 7 central hub genes and their corresponding effective compounds from the components of Xuebijing injection. The outter semicircle showed the components source of corresponding compounds. The inner chord plot showed the interreaction among the 7 central hub genes and the effective compounds. (B) Molecular docking diagram of FOS and quercetin. The green helix represented the molecular structure of FOS. The predicted bond between FOS and quercetin were exhibited in the dashed box.
Table 2.
Molecular docking between Xuebijing injection ingredients and seven hub genes.
| Target | PDB ID | Mol ID | Mol Name | 2D Structure | Affinity (kcal/mol) |
|---|---|---|---|---|---|
| CCL2 | 4DN4 | MOL000098 | quercetin | 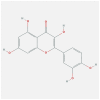 |
−8.3 |
| CXCL8 | 5D14 | MOL001002 | ellagic acid | 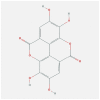 |
−7 |
| CXCL8 | 5D14 | MOL000305 | lauric acid | 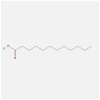 |
−4.3 |
| CXCL8 | 5D14 | MOL000098 | quercetin | 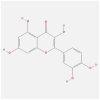 |
−7.9 |
| CXCL8 | 5D14 | MOL000415 | rutin | 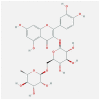 |
−8.9 |
| FOS | 1FOS | MOL000008 | apigenin | 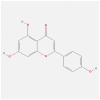 |
−9.4 |
| FOS | 1FOS | MOL002714 | baicalein | 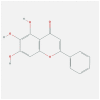 |
−9.7 |
| FOS | 1FOS | MOL002124 | beta-asarone | 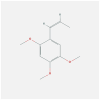 |
−6.2 |
| FOS | 1FOS | MOL000098 | quercetin | 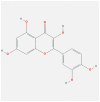 |
−10.4 |
| FOS | 1FOS | MOL000842 | sucrose | 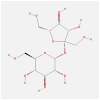 |
−8.6 |
| FOS | 1FOS | MOL007154 | tanshinone iia | 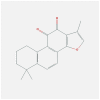 |
−10.4 |
| FOS | 1FOS | MOL000511 | ursolic acid | 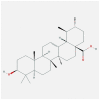 |
−9.4 |
| IFNB1 | 1AU1 | MOL001801 | salicylic acid | 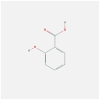 |
−6.9 |
| IL1A | 2KKI | MOL000098 | quercetin | 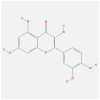 |
−7 |
| IL1B | 4 × 3A | MOL000024 | alpha-humulene | 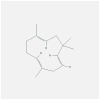 |
−5.7 |
| IL1B | 4 × 3A | MOL002008 | myricetin | 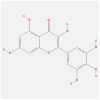 |
−6.7 |
| IL1B | 4 × 3A | MOL000098 | quercetin | 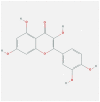 |
−6.7 |
| IL1B | 4 × 3A | MOL000415 | rutin | 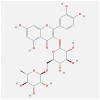 |
−7.7 |
| IL1B | 4 × 3A | MOL000511 | ursolic acid |  |
−7.4 |
| SERPINE1 | 9PAI | MOL000008 | apigenin |  |
−7.3 |
| SERPINE1 | 9PAI | MOL000675 | oleic acid |  |
−3.8 |
| SERPINE1 | 9PAI | MOL000098 | quercetin |  |
−7.3 |
4. Discussion
COVID-19 has developed into a global pandemic, which is caused by SARS-CoV-2. COVID-19 mainly manifests as respiratory symptoms, but cardiac complications such as arrhythmia, heart failure, and viral myocarditis are also prevalent [29,30]. Xuebijing injection has been adopted in the adjuvant treatment of severe and critical COVID-19 patients, and has been proved to have a protective effect on myocardial injury induced by infection and inflammation [[15], [16], [17]]. Therefore, we speculated that Xuebijing injection might also have a therapeutic effect on COVID-19-induced cardiac dysfunction.
Network pharmacology and RNA-seq were jointly used to identify potential therapeutic target genes of Xuebijing injection on COVID-19-induced cardiac dysfunction. After a series of bioinformatics analyses, 22 hub genes were distinguished and were found to be mainly enriched in cytokine signaling related terms and pathways, including interleukin signaling, TNF signaling pathway, NF-kappa B signaling pathway, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway, MAPK signaling pathway. The hub genes might also participate in cellular senescence, cell responses to stress, regulation of apoptotic signaling pathway, fluid shear stress and atherosclerosis.
More stringent criteria were used to screen out 7 central hub genes (CCL2, CXCL8, FOS, IL-1A, IL-1B, SERPINE1, IFNB1). A number of published studies have demonstrated the 7 central hub genes were up-regulated in COVID-19 patients, which were consistent with the results of our analysis. For example, Taus et al. found cytokines (IL-1A, IL-1B) and chemokines (CCL2) were significantly elevated in plasma and platelet releasate of COVID-19 patients compared with healthy subjects [31]. Kang et al. demonstrated the plasma from severe COVID-19 patients exhibited increased CCL2, CXCL8 and SERPINE1 levels in comparison with healthy controls. Meanwhile, the increased SERPINE1 could cause endothelial cell injury [32]. Another study stated that the CCL2+ inflammatory macrophage amount was abundant in peripheral blood of severe COVID-19 patients and inflammatory genes such as IL-1B were highly expressed in the CCL2+ inflammatory macrophages [33]. CXCL8, IL-1B, FOS and IFNB1 were identified to be up-regulated in COVID-19 patients and it was suggested that IFN-β might play a pivotal role in exacerbating inflammation in severe COVID-19 patients by enhancing TNF/IL-1B-driven inflammation [34]. Moreover, increased IFNB1 could upregulate the SARS-CoV-2 receptor ACE2, which promoted the course of disease in patients with COVID-19 [35].
The published investigations suggested the mechanisms of myocardial injury secondary to COVID-19 infection mainly included direct damage to the CMs, systemic inflammation, myocardial interstitial fibrosis, interferon mediated immune response and hypoxia [36]. Previous studies have confirmed the biological role of the 7 central hub genes in cardiac dysfunction. For example, the increased inflammatory cytokines and chemokines such as IL-1A, IL-1B and CCL2 led to oxidative stress, endothelial dysfunction and hypercoagulation, thus increasing the risk of cardiovascular events [37,38]. As a nonselective IL-1 antagonist acting on both IL-1A and IL-1B receptors, anakinra has been verified to be beneficial for treating severe COVID-19 patients [39]. Considering anakinra is also effective in improving cardiac function [40], it could be assumed that COVID-19-induced cardiac dysfunction might be improved by restraining the function of IL1-A and IL-1B. In addition, Frangogiannis et al. revealed that CCL2 and CXCL8 were markedly increased in the infarcted myocardium and might regulate leukocyte infiltration and granulation tissue formation [41]. Besides, the upregulation of CXCL8 in CMs could lead to damage of actomyosin cytoskeleton distribution and mitochondrial network organization [42]. AP-1 (c-JUN/c-FOS) was also reported to be involved in cardiac remodeling, myocardial dysfunction and progression of heart failure [43].
From the results of the molecular docking simulation, 6 out of the 7 central hub genes (CCL2, CXCL8, FOS, IL-1A, IL-1B, SERPINE1) could be molecularly docked with quercetin, hinting the crucial role of quercetin in the pharmacology network. It has been reported that quercetin could inhibit angiotensin-induced cardiac fibrosis [44]. In addition, quercetin was found to against myocardial ischemia reperfusion injury through alleviating oxidative stress injury, exerting anti-inflammatory and anti-apoptotic effects [45]. Apigenin was the molecular ligand that could be bound by both FOS and SERPINE1. Apigenin could attenuate the differentiation of cardiac fibroblasts, reduce the mitochondrial/lysosome damage and oxidative stress of CMs [46,47]. The common ligand shared by CXCL8 and IL-1B was rutin, which was demonstrated to improve myocardial damage via miR-22-5p-regulated RAP1/ERK signaling pathway [48]. FOS and IL-1B might attenuate the mitochondrial and lysosomal dysfunction through docking with ursolic acid [49]. Combining the published studies and our bioinformatics analysis, we hypothesized that Xuebijing injection might play a protective effect on COVID-19-induced cardiac dysfunction by targeting the 7 central hub genes.
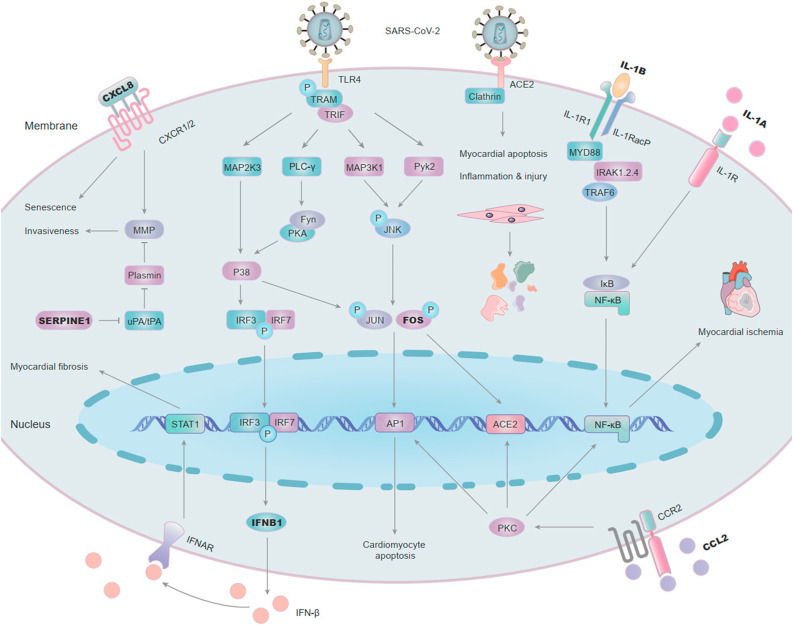
Finally, a pathway diagram of the underlying mechanism of the 7 central hub genes in CMs infected with SARS-CoV-2 was established (Fig. 10 ). Our bioinformatics analysis found ACE2 was significantly down-regulated in SARS-CoV-2 infected CMs, which was consistent with the prior reports of examining SARS-CoV-2 infected myocardium [50]. SARS-CoV-2 mediated myocardial apoptosis, cardiogenic shock, myocardial ischemia and inflammatory injury were related to the down-regulation of ACE2 in the myocardium [51,52]. The spike protein of SARS-CoV-2 might bind to TLR4 to trigger transmembrane stimulation [53,54]. Then, TLR4 activated interferon (IFN)-regulatory factors (IRF3 and IRF7), leading to increased expression of one of the hub genes IFNB1 and further enhancive the secretion of IFN-β [55,56]. It has been proved the increased IFN-β could stimulate STAT1 and finally resulting in myocardial fibrosis [57,58]. Besides, the TLR4-induced c-JUN/c-FOS activation could enhance the expression of ACE2, so that more SARS-CoV-2 could entry into the host cells via ACE2 as a receptor [59,60]. At the same time, the activited c-JUN/c-FOS eventually accelerated the apoptosis of CMs [61]. Herman et al. and Lavrentyev et al. demonstrated the hub gene CCL2 bound to its receptor CCR2 and activated the protein kinase C (PKC) signaling pathway, inducing the increase of AP-1 (c-JUN/c-FOS), NF-κB and ACE2, and ultimately leading to CM apoptosis and myocardial ischemia [62,63]. Likewise, the NF-κB signaling pathway could also be activated by IL-1A and IL-1B, two hub genes of our analysis, and subsequently contributed to myocardial ischemia [64,65]. The combination of CXCL8 and its receptor CXCR1/2 promoted cell senescence, and enhanced cell invasion in conjunction with another hub gene SERPINE1 through matrix metalloprotein (MMP) pathway [66,67]. Hence Xuebijing injection might play a protective role on COVID-19-induced cardiac dysfunction by inhibiting the above pathways correlated with the 7 central hub genes.
Fig. 10.
Potential cell signal transduction pathways of 7 central hub genes in CMs infected with SARS-CoV-2. SARS-CoV-2 might bind to TLR4, then activated IRF3 and IRF7, leading to increased expression of IFNB1 and IFN-β. IFN-β could stimulate STAT1 and finally resulting in myocardial fibrosis. Besides, the TLR4-induced c-JUN/c-FOS activation could enhance the expression of ACE2 to allow more SARS-CoV-2 to entry into the host cells. At the same time, the activited c-JUN/c-FOS might eventually accelerate the apoptosis of CMs. CCL2 bound to its receptor CCR2 and activated the PKC signaling pathway, inducing the increase of AP-1 (c-JUN/c-FOS), NF-κB and ACE2, and ultimately leading to CM apoptosis and myocardial ischemia. NF-κB signaling pathway could also be activated by IL-1A/IL-1B and subsequently contributed to myocardial ischemia. The combination of CXCL8 and its receptor CXCR1/2 promoted cell senescence, and enhanced cell invasion in conjunction with SERPINE1 through MMP pathway.
Although we conducted a series of analysis to illustrate the potential protective mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction, there were still some limitations in this research: (1) The expression levels of the 7 central hub genes were verified in two published datasets and showed a high degree of consistency in three kinds of CMs (hiPSC-CMs, hESC-CMs, adult human CMs) infected with SARS-CoV-2, but the total sample size was still limited. (2) The two datasets (the type and MOI of virus added in the two models were the same while the processing time was different) contained RNA-seq data of only one single time point after SARS-CoV-2 infection, which also marked the limitation of the current study. (3) Since the development of COVID-19 involves complex pathological processes, the mechanism predicted above of Xuebijing injection in treating COVID-19-induced cardiac dysfunction still needs to be further verified by in vivo and in vitro experiments. (4) Though the effectiveness and safety of Xuebijing injection have been clinically demonstrated in large numbers of Chinese COVID-19 patients [68,69], the efficacy and security of Xuebijing injection in treating patients of other ethnicities still require careful evaluation.
5. Conclusion
In conclusion, our bioinformatics analysis indicated the underlying protective effect of Xuebijing injection on COVID-19, especially on COVID19-induced cardiac dysfunction. Xuebijing injection might treat COVID-19-induced cardiac dysfunction through inhibiting oxidative stress, preventing atherosclerotic plaque formation, restraining inflammatory and apoptosis by targeting 7 hub genes (CCL2, CXCL8, FOS, IFNB1, IL-1A, IL-1B, SERPINE1). Our research provided a theoretical basis for exploring the potential protective mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction.
Funding information
This research was funded by the Cooperation Project of Wuhan Science and Technology Bureau and Ningbo MedicalSystem Biotechnology Co., Ltd (250000469), Joint Fund of Health Commission of Hubei Province (WJ2019H034) and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (ZNPY2017054).
Author contribution
DDH and XKZ designed the project, drafted the manuscript and visualized the data. XYZ, FFH, ZW were involved in data analysis and interpretation. JCT revised the manuscript. All authors approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Declaration of competing interest
The authors declare that this study received funding from Ningbo MedicalSystem Biotechnology Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.compbiomed.2021.104293.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230–232. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., Ibrahim H., Friedman G.H., Thompson C., Alviar C.L., Chadow H.L., Fishman G.I., Reynolds H.R., Keller N., Hochman J.S. ST-segment elevation in patients with covid-19 - a case series. N. Engl. J. Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J., Zheng Y., Luo Z., Mei Z., Yao Y., Liu Z., Liang C., Yang H., Song Y., Yu K., Gao Y., Zhu C., Huang Z., Qian J., Ge J. Myocardial injury and COVID-19: serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease. Theranostics. 2020;10:9663–9673. doi: 10.7150/thno.47980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C., Xu C., Li S.S., Zeng H.S. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients] Zhonghua Xinxueguanbing Zazhi. 2020;48:456–460. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 9.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., Chen L., Hou W., Feng Y., Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.-F., Duan Q., Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K., Liu L., Xu Y., Liu Z., Zhou Q., Wang Y., Ma Z., Zheng Y., Wu D., Tang Z., Zhang M., Pan S., Chai Y., Song Y., Zhang J., Pan L., Liu Y., Yu H., Yu X., Zhang H., Wang X., Du Z., Wan X., Tang Y., Tian Y., Zhu Y., Wang H., Yan X., Liu Z., Zhang B., Zhong N., Shang H., Bai C. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2019;47 doi: 10.1097/CCM.0000000000003842. e735–e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., Chen L., Zhou J., Liao Y., Chen Q., Zheng Q., Cai L., Shen L., Yang Z. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol. Res. 2020;160:105073. doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Wei L., Zhao G., Liu S., Zhang Z., Zhang J., Yang Y. Protective effect of Xuebijing injection on myocardial injury in patients with sepsis: a randomized clinical trial. J. Tradit. Chinese Med. = Chung i Tsa Chih Ying Wen Pan. 2016;36:706–710. doi: 10.1016/s0254-6272(17)30003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J.-B., Yang X., Luo Z.-Y., Yuan P.-G., Song D., Xiang B.-Q., Chen X.-W., Wang W.-T. [Effects of xuebijing injection on cardiac function and structure in rats with myocardial hypoxia/reoxygenation injury] J. Appl. Physiol. 2016;32:173–176. doi: 10.13459/j.cnki.cjap.2016.02.021. Zhongguo ying yong sheng li xue za zhi = Zhongguo yingyong shenglixue zazhi = Chinese. [DOI] [PubMed] [Google Scholar]
- 18.Guo F.-F., Zhang Y.-Q., Tang S.-H., Tang X., Xu H., Liu Z.-Y., Huo R.-L., Li D., Yang H.-J. [TCMATCOV--a bioinformatics platform to predict efficacy of TCM against COVID-19] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chinese Mater. medica. 2020;45:2257–2264. doi: 10.19540/j.cnki.cjcmm.20200312.401. [DOI] [PubMed] [Google Scholar]
- 19.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A., Garcia G.J., Wang Y., Plummer J.T., Morizono K., Arumugaswami V., Svendsen C.N. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Reports. Med. 2020;1:100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jassal B., Matthews L., Viteri G., Gong C., Lorente P., Fabregat A., Sidiropoulos K., Cook J., Gillespie M., Haw R., Loney F., May B., Milacic M., Rothfels K., Sevilla C., Shamovsky V., Shorser S., Varusai T., Weiser J., Wu G., Stein L., Hermjakob H., D'Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gene Ontology Consortium Going forward. Nucleic Acids Res. 2015;43:D1049–D1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., Jensen L.J. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Yang L., Nilsson-Payant B., Han Y., Jaffré F., Zhu J., Wang P., Zhang T., Redmond D., Houghton S., Møller R., Hoagland D., Horiuchi S., Acklin J., Lim J., Bram Y., Richardson C., Chandar V., Borczuk A., Huang Y., Xiang J., Ho D., Schwartz R., tenOever B., Evans T. SARS-CoV-2 infected cardiomyocytes recruit monocytes by secreting CCL2. Res. Sq. 2020 doi: 10.21203/rs.3.rs-94634/v1. [DOI] [Google Scholar]
- 29.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., Rabbani L., Brodie D., Jain S.S., Kirtane A.J., Masoumi A., Takeda K., Kumaraiah D., Burkhoff D., Leon M., Schwartz A., Uriel N., Sayer G. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 31.Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., Petrova V., Barouni R.M., Dima F., Dalbeni A., Romano S., Poli G., Benati M., De Nitto S., Mansueto G., Iezzi M., Tacconelli E., Lippi G., Bronte V., Minuz P. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40:2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K., Ogura H., Matsuura Y., Kishimoto T. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F., Mears J.R., Shakib L., Beynor J.I., Shanaj S., Korsunsky I., Nathan A., Donlin L.T., Raychaudhuri S. IFN- γ and TNF- α drive a CXCL10 + CCL2 + macrophage phenotype expanded in severe COVID-19 and other diseases with tissue inflammation. BioRxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.08.05.238360. [DOI] [Google Scholar]
- 34.Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H., Choi B., Nam S.K., Sa M., Kwon J.-S., Jeong S.J., Lee H.K., Park S.H., Park S.-H., Choi J.Y., Kim S.-H., Jung I., Shin E.-C. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finney L.J., Glanville N., Farne H., Aniscenko J., Fenwick P., V Kemp S., Trujillo-Torralbo M.-B., Loo S.L., Calderazzo M.A., Wedzicha J.A., Mallia P., Bartlett N.W., Johnston S.L., Singanayagam A. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korakas E., Ikonomidis I., Kousathana F., Balampanis K., Kountouri A., Raptis A., Palaiodimou L., Kokkinos A., Lambadiari V. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020;319 doi: 10.1152/ajpendo.00198.2020. E105–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreakos E., Papadaki M., Serhan C.N. 2020. Dexamethasone, Pro-resolving Lipid Mediators and Resolution of Inflammation in COVID-19. Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M.G., Spyridopoulos T., Verheggen R.J., Hoogerwerf J., Lachana A., van de Veerdonk F.L., Giamarellos-Bourboulis E.J. Favorable anakinra responses in severe covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123. doi: 10.1016/j.chom.2020.05.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikonomidis I., Pavlidis G., Katsimbri P., Andreadou I., Triantafyllidi H., Tsoumani M., Varoudi M., Vlastos D., Makavos G., Kostelli G., Βenas D., Lekakis J., Parissis J., Boumpas D., Alexopoulos D., Iliodromitis E. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin. Res. Cardiol. 2019;108:1093–1101. doi: 10.1007/s00392-019-01443-9. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis N.G., Entman M.L. Chemokines in myocardial ischemia. Trends Cardiovasc. Med. 2005;15:163–169. doi: 10.1016/j.tcm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Buoncervello M., Maccari S., Ascione B., Gambardella L., Marconi M., Spada M., Macchia D., Stati T., Patrizio M., Malorni W., Matarrese P., Marano G., Gabriele L. Inflammatory cytokines associated with cancer growth induce mitochondria and cytoskeleton alterations in cardiomyocytes. J. Cell. Physiol. 2019;234:20453–20468. doi: 10.1002/jcp.28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palomer X., Capdevila-Busquets E., Botteri G., Davidson M.M., Rodríguez C., Martínez-González J., Vidal F., Barroso E., Chan T.O., Feldman A.M., Vázquez-Carrera M. miR-146a targets Fos expression in human cardiac cells. Dis. Model. Mech. 2015;8:1081–1091. doi: 10.1242/dmm.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Tan A., An X., Xia Y., Xie Y. Quercetin Dihydrate inhibition of cardiac fibrosis induced by angiotensin II in vivo and in vitro. Biomed. Pharmacother. 2020;127:110205. doi: 10.1016/j.biopha.2020.110205. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y.-M., Zhang Z.-Y., Wang R.-X. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 2020;11:956. doi: 10.3389/fphys.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F., Fan K., Zhao Y., Xie M.-L. Apigenin attenuates TGF-β1-stimulated cardiac fibroblast differentiation and extracellular matrix production by targeting miR-155-5p/c-Ski/Smad pathway. J. Ethnopharmacol. 2020;265:113195. doi: 10.1016/j.jep.2020.113195. [DOI] [PubMed] [Google Scholar]
- 47.Jahedsani A., Khezri S., Ahangari M., Bakhshii S., Salimi A. Apigenin attenuates Aluminum phosphide-induced cytotoxicity via reducing mitochondrial/Lysosomal damages and oxidative stress in rat Cardiomyocytes. Pestic. Biochem. Physiol. 2020;167:104585. doi: 10.1016/j.pestbp.2020.104585. [DOI] [PubMed] [Google Scholar]
- 48.Qin M., Li Q., Wang Y., Li T., Gu Z., Huang P., Ren L. Rutin treats myocardial damage caused by pirarubicin via regulating miR-22-5p-regulated RAP1/ERK signaling pathway. J. Biochem. Mol. Toxicol. 2020 doi: 10.1002/jbt.22615. [DOI] [PubMed] [Google Scholar]
- 49.Radhiga T., Senthil S., Sundaresan A., V Pugalendi K. Ursolic acid modulates MMPs, collagen-I, α-SMA, and TGF-β expression in isoproterenol-induced myocardial infarction in rats. Hum. Exp. Toxicol. 2019;38:785–793. doi: 10.1177/0960327119842620. [DOI] [PubMed] [Google Scholar]
- 50.Wong C.-K., Luk H.K.-H., Lai W.-H., Lau Y.-M., Zhang R.R., Wong A.C.-P., Lo G.C.-S., Chan K.-H., Hung I.F.-N., Tse H.-F., Woo P.C.-Y., Lau S.K.-P., Siu C.-W. Human-induced pluripotent stem cell-derived cardiomyocytes platform to study SARS-CoV-2 related myocardial injury. Circ. J. 2020;84:2027–2031. doi: 10.1253/circj.CJ-20-0881. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y.-M., Li W.-W., Wu J., Han M., Li B.-H. The diagnostic value of circulating microRNAs in heart failure. Exp. Ther. Med. 2019;17:1985–2003. doi: 10.3892/etm.2019.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loganathan S., Kuppusamy M., Wankhar W., Gurugubelli K.R., Mahadevappa V.H., Lepcha L., Choudhary A.K. Angiotensin-converting enzyme 2 (ACE2): COVID 19 gate way to multiple organ failure syndromes. Respir. Physiol. Neurobiol. 2021;283:103548. doi: 10.1016/j.resp.2020.103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandão S.C.S., Ramos J. de O.X., Dompieri L.T., Godoi E.T.A.M., Figueiredo J.L., Sarinho E.S.C., Chelvanambi S., Aikawa M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn K.M., Lee S.G., Kim H.J., Cheon S., Jeong H., Lee J., Kim I.S., Silwal P., Kim Y.J., Paik S., Chung C., Park C., Kim Y.S., Jo E.K. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Kor. Med. Sci. 2020;35:e343. doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 56.Becher P.M., Hinrichs S., Fluschnik N., Hennigs J.K., Klingel K., Blankenberg S., Westermann D., Lindner D. Role of Toll-like receptors and interferon regulatory factors in different experimental heart failure models of diverse etiology: IRF7 as novel cardiovascular stress-inducible factor. PloS One. 2018;13 doi: 10.1371/journal.pone.0193844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rackov G., Shokri R., De Mon M.Á., Martínez-A C., Balomenos D. The role of IFN-β during the course of sepsis progression and its therapeutic potential. Front. Immunol. 2017;8:493. doi: 10.3389/fimmu.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai B., Cui M., Zhu M., Su W.-L., Qiu M.-C., Zhang H. STAT1/3 and ERK1/2 synergistically regulate cardiac fibrosis induced by high glucose. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013;32:960–971. doi: 10.1159/000354499. [DOI] [PubMed] [Google Scholar]
- 59.V Glinsky G. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh C.C., Lee J., D'Souza K., Zhang W., Migrino R.Q., Thornburg K., Reaven P. Activator protein-1 and caspase 8 mediate p38α MAPK-dependent cardiomyocyte apoptosis induced by palmitic acid. Apoptosis. 2019;24:395–403. doi: 10.1007/s10495-018-01510-y. [DOI] [PubMed] [Google Scholar]
- 62.Herman J.G., Stadelman H.L., Roselli C.E. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int. J. Oncol. 2009;34:1319–1327. [PMC free article] [PubMed] [Google Scholar]
- 63.Lavrentyev E.N., Malik K.U. High glucose-induced Nox1-derived superoxides downregulate PKC-betaII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H106–H118. doi: 10.1152/ajpheart.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herranz N., Gallage S., Gil J. TORn about SASP regulation. Cell Cycle. 2015;14:3771–3772. doi: 10.1080/15384101.2015.1105694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tulotta C., Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Canc. 2018;25:R421–R434. doi: 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gales D., Clark C., Manne U., Samuel T. The chemokine CXCL8 in carcinogenesis and drug response. ISRN Oncol. 2013:859154. doi: 10.1155/2013/859154. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh A.K., Vaughan D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhuang W., Fan Z., Chu Y., Wang H., Yang Y., Wu L., Sun N., Sun G., Shen Y., Lin X., Guo G., Xi S. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front. Pharmacol. 2020;11:1066. doi: 10.3389/fphar.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., Luan X., Zhao Y., Xu J., Yuan W., Chen H. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.