Abstract
Background
With a unique influenza season occurring in the midst of a pandemic, there is interest in assessing the role of the influenza vaccine in COVID-19 susceptibility and severity.
Methods
In this retrospective cohort study, patients receiving a laboratory test for COVID-19 were identified. The primary outcome was comparison of positive COVID-19 testing in those who received the influenza vaccine versus those who did not. Secondary end points in patients testing positive for COVID-19 included mortality, need for hospitalization, length of stay, need for intensive care, and mechanical ventilation.
Results
A total of 27,201 patients received laboratory testing for COVID-19. The odds of testing positive for COVID-19 was reduced in patients who received an influenza vaccine compared to those who did not (odds ratio 0.76, 95% CI 0.68-0.86; P < .001). Vaccinated patients testing positive for COVID-19 were less likely to require hospitalization (odds ratio, 0.58, 95% CI 0.46-0.73; P < .001), or mechanical ventilation (odds ratio, 0.45, 95% CI 0.27-0.78; P = .004) and had a shorter hospital length of stay (risk ratio, 0.76, 95% CI 0.65-0.89; P < .001).
Conclusion
Influenza vaccination is associated with decreased positive COVID-19 testing and improved clinical outcomes and should be promoted to reduce the burden of COVID-19.
Key Words: COVID-19, Influenza vaccination
Introduction
The novel coronavirus of 2019 (COVID-19) was first identified in Wuhan, China in December 2019, and was declared a public health emergency of international concern within one month. As of February 2021, more than 106 million confirmed cases of COVID-19 and over 2.3 million deaths have been reported globally.1 The clinical spectrum of illness caused by COVID-19 is broad, with severity of disease ranging from mild symptoms to acute respiratory distress syndrome with rapid deterioration.2 Pre-existing cerebrovascular, liver, kidney and gastrointestinal diseases, as well as hypertension, diabetes, COPD, and age greater than 60 confer higher susceptibility to infection by COVID-19 and greater risk of mortality with infection.3 , 4 Importantly, patients with pre-existing cardiovascular risk factors are more likely to experience severe disease resulting from both direct and indirect cardiovascular complications of COVID-19, including myocarditis, arrhythmias, and venous thromboembolism.5
Clinical trials of dexamethasone6 , 7 and Remdesivir8 have shown a reduction in complications in very ill COVID-19 patients, and while effective vaccines against COVID-19 from both Pfizer-BioNTech and Moderna have been approved for use in the United States, they are not yet broadly available, making it imperative to explore the effects of currently available medical interventions that may lessen the susceptibility to and burden of disease.
With the influenza season upon us, there is interest in exploring the relationship between influenza vaccination and COVID-19 susceptibility and disease severity. Recent studies have suggested that prior vaccination to pathogens such as tuberculosis and influenza may confer some protection against COVID-19.9, 10, 11, 12, 13, 14 An analysis of over 92,000 COVID-19 patients in a nonpeer reviewed study from Brazil found a 17% reduced odds of mortality, 8% lower odds of need for intensive care treatment and 18% lower odds of invasive respiratory support in those who received an influenza vaccine.12 Separate epidemiologic studies in Italy and the United States found a correlation between increased vaccination rates in those aged greater than 65 years and decreased rates of COVID-19 deaths across different regions.11 , 13 Using data from patients tested for COVID-19 within the Michigan Medicine healthcare system, we explored the relationship between influenza vaccination and positive COVID-19 testing. For COVID-19 positive patients, we compared disease severity and mortality risk between those who were vaccinated and unvaccinated against influenza.
METHODS
We searched over 4.5 million unique patient charts within the Michigan Medicine healthcare system. The search was performed using DataDirect, an online tool which enables access to clinical data with search filter functions based on ICD-9 and ICD-10 coding. We limited our search to include only patients with a COVID-19 laboratory test ordered and resulted in the Michigan Medicine electronic medical record through July 15, 2020. Testing was first processed through the Michigan Department of Health and Human Services on February 27, 2020, with the first positive test resulting on March 10, 2020. Patient charts were searched for record of influenza vaccination between August 1, 2019 and July 15, 2020 to capture the entire date range of influenza vaccine availability from the beginning of the prior influenza season. From the onset of the pandemic, our institution's testing criteria followed directly along with updated Center for Disease Control and Prevention recommendations. Any patient with symptoms of COVID-19, close exposure or recent high-risk activity could call the screening hotline or their healthcare provider and would be referred for testing. Starting on April 30, 2020, all patients being admitted to Michigan Medicine were screened for COVID-19, regardless of symptoms or recent exposure. The study was deemed exempt by the University of Michigan Institutional Review Board (HUM00183952).
Record of influenza vaccination is inputted into a patient's chart by the provider at the time of administration if done during a clinic or hospital encounter. For those who received an influenza vaccination outside the Michigan Medicine healthcare system, immunization data is readily available through the Michigan Care Improvement Registry (MICR), which is routinely accessed and updated by a clerk or medical assistant every time a patient makes an appointment or checks-in to any encounter. A patient's vaccination record is also updated by a nurse whenever a patient is transferred or admitted to the hospital.
For patients with multiple positive COVID-19 test results, the date of their first positive test was used as their positive test date. For patients with no positive COVID-19 tests, the date of their last negative test result was used as their test date. There were 14 patients who received the influenza vaccine after their COVID-19 test date and were therefore included in the ‘no vaccine’ group. Patient baseline characteristics and clinical outcomes were obtained from the electronic medical record.
The primary outcome of interest was COVID-19 positivity, with rates compared between groups of patients based on influenza vaccination status. Baseline patient characteristics, including age, gender, race, and presence of co-morbidities were also compared by COVID-19 status and influenza vaccination status. For those with a positive COVID-19 test result, we compared clinical outcomes, including need for hospitalization, length of stay, need for intensive care, need for mechanical ventilation and mortality. Time to death was calculated as the time from first positive COVID-19 test to date of death. Patients who were still alive at the end of the data collection window were censored for death at that time. We also explored if testing for other respiratory pathogens (including Adenovirus, Human Metapneumovirus, Haemophilus influenzae, Human Rhinovirus-Enterovirus, Influenza A, Influenza B, Parainfluenza Virus, and Respiratory Syncytial Virus) was performed at the same time as COVID-19 testing and evaluated for the presence of a co-infection. The temporal relationship between influenza vaccination and COVID-19 testing with COVID-19 positivity and clinical outcomes was also determined.
Influenza vaccination rates and baseline characteristics were first summarized using descriptive statistics. Independent predictors of influenza vaccination were determined using a multivariable logistic regression model with a stepwise selection procedure. The association between influenza vaccination and COVID-19 status was assessed using a multivariable logistic regression model, adjusting for baseline patient characteristics, with results expressed as odds ratios (ORs) with 95% confidence intervals (CIs).
Models to assess clinical outcomes utilized inverse probability of treatment weighting by baseline covariates to control for confounding.15 Weighted logistic regression models were used to assess the associations between influenza vaccination status and the need for hospitalization, mechanical ventilation and need for intensive care in those who were COVID-19 positive, with results expressed as ORs with 95% CIs. A weighted Cox proportional hazards model was employed to evaluate mortality differences between vaccinated and unvaccinated COVID-19 positive patients with results expressed as hazards ratios (HRs) with 95% CIs. The association of influenza vaccination status with length of stay in hospitalized COVID-19 positive patients was assessed using a weighted negative binomial model, with results expressed as rate ratios (RRs) with 95% CIs. For those who were vaccinated, the relationship between timing of vaccination and COVID-19 testing with each outcome was assessed by including a continuous covariate for the number of days from vaccination to COVID-19 test. This model additionally controlled for the timing of COVID-19 testing with respect to the beginning of test availability to account for changing COVID-19 positivity rates over time. There was a small amount of missing data on patient comorbidities, including chronic pulmonary disease, congestive heart failure, diabetes, hypertension, age, race and ethnicity, and a larger amount of missing data on BMI and smoking status. Missing values were included in all descriptive summaries and multivariable analyses as their own category for categorical covariates. A small number of patients (n = 14) had more than one hospitalization during the study time frame in which they tested positive for COVID-19. For these patients, their index hospitalization was used in assessing the length of stay, mechanical ventilation, and need for intensive care outcomes. Four COVID-19 positive patients were still hospitalized at the time of data collection and were therefore excluded from in the analysis of length of stay. Due to collinearity of chronic pulmonary disease, congestive heart failure, diabetes, and hypertension, these variables were combined into a single indicator for the presence of any of these comorbidities in the multivariable and propensity score weighted models. A 2-sided P value <.05 was used to indicate significance. All analyses were performed in SAS, version 9.4 (SAS Institute Inc.).
Results
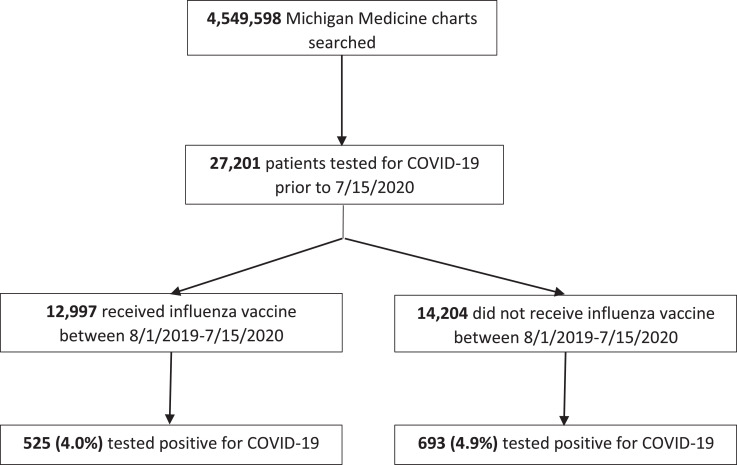
The search yielded a total of 27,201 patients who received a COVID-19 test in the Michigan Medicine healthcare system. Of these, 1,218 (4.5%) tested positive for COVID-19 and 25,983 (95.5%) tested negative. A total of 12,997 (47.8%) patients had a documented influenza vaccination during the prior flu season and 14,204 (52.2%) patients did not. In patient who received the influenza vaccine, there was a significant reduction in the odds of testing positive for COVID-19 compared to those who did not receive the vaccine (odds ratio 0.82, 95% CI 0.73-0.92; P < .001) Flow diagram of search results is shown in Figure 1 .
Fig 1.
Study Flow Diagram of Search Results for Included Patients. A total of 4,549,598 unique patient charts were searched within the Michigan Medicine health care system using DataDirect. The search was limited to include only patients who received a laboratory test for COVID-19 within the Michigan Medicine healthcare system. Record of influenza vaccination between August 1, 2019 to July 15, 2020 was then obtained.
Of the 27,201 patients tested for COVID-19, 4209 (15.46%) were also tested for other respiratory pathogens. Of these, 202 (4.80%) were positive for any respiratory pathogen and 46 (1.1%) were positive for influenza. Patients who received an influenza vaccination were more likely to receive testing for other respiratory pathogens (16.70% vs 14.33%, P < .001), however there was no significant difference in positive testing for respiratory pathogens or influenza alone between those who received and did not receive an influenza vaccination (4.24% vs 5.40%, P = .08 for all pathogens, and 0.92% vs 1.27% for influenza, P = .27). Patients who were COVID-19 positive were more likely to receive testing for other respiratory pathogens (57.14% vs 13.52%, P < .001). No patients who tested positive for COVID-19 were also positive for influenza, and the majority of positive cases of other respiratory pathogens were in COVID-19 negative patients (5.57% vs 0.68%, P < .001, in COVID-19 negative vs. COVID-19 positive patients, respectively).
Higher rates of comorbid conditions were seen in patients testing positive for COVID-19, including chronic pulmonary disease (19.8% vs 14.6%, P < .001), congestive heart failure (10.3% vs 7.8%, P = .003), any diabetes (21.5% vs 9.8%, P < .001), complicated diabetes (8.5% vs 2.9%, P < .001), uncomplicated diabetes (13.1% vs 7.0%, P < .001), any hypertension (36.0% vs 22.5%, P < .001), complicated hypertension (14.4% vs 6.0%, P < .001), and uncomplicated hypertension (21.6% vs 16.5%, P < .001). Additionally, older patients and African Americans were more likely to test positive versus negative for COVID-19 (50.7 years vs 47.1 years, P < .001 and 35.5% vs 11.4%, P < .001, respectively). A comparison of baseline characteristics stratified by COVID-19 status is seen in Table 1 .
Table 1.
Patient characteristics and associations with COVID-19
| Variable* | COVID-19 Negative (n = 25,983) | COVID-19 Positive (n = 1,218) | Entire cohort (n = 27,201) | P value† |
|---|---|---|---|---|
| Influenza Vaccine, n (%) | 12,472 (48.0) | 525 (43.1) | 12,997 (47.8) | <.001 |
| Women, n (%) | 1,4512 (55.9) | 649 (53.3) | 1,5161 (55.7) | .08 |
| Age, Mean (SD) | 47.07 (22.21) | 50.69 (18.67) | 47.23 (22.07) | <.001 |
| Age, n (%) | <.001 | |||
| <35 | 8143 (31.3%) | 276 (22.7%) | 8419 (31.0%) | |
| 35-49 | 4532 (17.4%) | 276 (22.7%) | 4808 (17.7%) | |
| 50-64 | 6098 (23.5%) | 349 (28.7%) | 6447 (23.7%) | |
| ≥65 | 6597 (25.4%) | 308 (25.3%) | 6905 (25.4%) | |
| Race, n (%) | ||||
| African American | 2,972 (11.4) | 432 (35.5) | 3,404 (12.5) | <.001 |
| Caucasian | 20,386 (78.5) | 617 (50.7) | 21,003 (77.2) | |
| Other | 1,841 (7.1) | 113 (9.3) | 1,954 (7.2) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 824 (3.2) | 27 (2.2) | 851 (3.1) | .08 |
| Non-Hispanic or Latino | 23,951 (92.2) | 1,111 (91.2) | 25,062 (92.1) | |
| Chronic Pulmonary Disease, n (%) | 3,806 (14.6) | 241 (19.8) | 4,047 (14.9) | <.001 |
| Congestive Heart Failure, n (%) | 2,032 (7.8) | 125 (10.3) | 2,157 (7.9) | .003 |
| Diabetes, n (%) | 2,556 (9.8) | 262 (21.5) | 2,818 (10.4) | <.001 |
| Complicated Diabetes, n (%) | 749 (2.9) | 103 (8.5) | 852 (3.1) | <.001 |
| Uncomplicated Diabetes, n (%) | 1,807 (7.0) | 159 (13.1) | 1,966 (7.2) | <.001 |
| Hypertension, n (%) | 5,847 (22.5) | 438 (36.0) | 6,285 (23.1) | <.001 |
| Complicated Hypertension, n (%) | 1,549 (6.0) | 175 (14.4) | 1,724 (6.3) | <.001 |
| Uncomplicated Hypertension, n (%) | 4,298 (16.5) | 263 (21.6) | 4,561 (16.8) | <.001 |
| BMI, Mean (SD) | 28.23 (9.10) | 32.42 (12.28) | 28.39 (9.28) | <.001 |
| BMI, n (%) | <.001 | |||
| <18.5 | 1713 (6.6%) | 17 (1.4%) | 1730 (6.4%) | |
| 18.5-24.9 | 4856 (18.7%) | 117 (9.6%) | 4973 (18.3%) | |
| 24.9-29.9 | 5097 (19.6%) | 219 (18.0%) | 5316 (19.5%) | |
| 30-39.9 | 5014 (19.3%) | 264 (21.7%) | 5278 (19.4%) | |
| >40 | 1424 (5.5%) | 118 (9.7%) | 1542 (5.7%) | |
| Elixhauser Score, Mean (SD) | 1.54 (2.34) | 2.53 (3.19) | 1.59 (2.39) | <.001 |
| Smoking Status, n (%) | ||||
| Current Smoker | 1,678 (6.5%) | 40 (3.3%) | 1,718 (6.3%) | .002 |
| Former Smoker | 3,635 (14.0%) | 175 (14.4%) | 3,810 (14.0%) | |
| Tobacco Use | 91 (0.4%) | 2 (0.2%) | 93 (0.3%) | |
| Never Smoker | 6,836 (26.3%) | 348 (28.6%) | 7,184 (26.4%) |
Proportions vary because of missing data on comorbid covariates chronic pulmonary disease, congestive heart failure, diabetes, complicated diabetes, uncomplicated diabetes, hypertension, complicated hypertension, and uncomplicated hypertension (n = 251, 0.9%), age (n = 622, 2.3%), race (n = 840, 3.1%), and ethnicity (n = 1,288, 4.7%), and a larger amount of missing data on BMI (n=8,362, 30.7%) and smoking status (n=14,396, 52.9%).
P values are shown from Chi-square tests for categorial variables and Wilcoxon rank sum tests for continuous variables comparing probability of positive COVID-19 test.
Patients receiving an influenza vaccine tended to have more comorbidities than those in the unvaccinated group, including higher rates of chronic pulmonary disease (16.4% vs 13.4%, P < .001), congestive heart failure (9.2% vs 6.7%, P < .001), diabetes (11.1% vs 9.7%, P = .001), and hypertension (23.9% vs 22.3%, P = .01). Patients receiving an influenza vaccine also tended to be older (48.4 years vs 46.1 years, P < .001), female (61.0% vs 50.9%, P < .001), and Caucasian (80.1% vs 74.6%, P < .001). A comparison of baseline characteristics stratified by influenza vaccination status is seen in Table 2 . A multivariable logistic regression model with a stepwise variable selection found that significant independent predictors of influenza vaccination included age, BMI, gender, Elixhauser score, race, smoking status, and the presence of chronic pulmonary disease, congestive heart failure, diabetes or hypertension (Appendix Table A.1).
Table 2.
Baseline patient characteristics by influenza vaccination status
| Variable* | No flu vaccine (n = 14,204) | Flu vaccine (n = 12,997) | P value† |
|---|---|---|---|
| COVID-19 Positive | 693 (4.9%) | 525 (4.0%) | <.001 |
| Women, n (%) | 7,231 (50.9) | 7,930 (61.0) | <.001 |
| Age, Mean (SD) | 46.09 (22.30) | 48.44 (21.76) | <.001 |
| Age, n (%) | <.001 | ||
| <35 | 4563 (32.1%) | 3856 (29.7%) | |
| 35-49 | 2395 (16.9%) | 2413 (18.6%) | |
| 50-64 | 3381 (23.8%) | 3066 (23.6%) | |
| >=65 | 3337 (23.5%) | 3568 (27.5%) | |
| Race, n (%) | |||
| African American | 1,966 (13.8) | 1,438 (11.1) | <.001 |
| Caucasian | 1,0597 (74.6) | 10,406 (80.1) | |
| Other | 933 (6.6) | 1021 (7.9) | |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 463 (3.3) | 388 (3.0) | .07 |
| Non-Hispanic or Latino | 1,2845 (90.4) | 1,2217 (94.0) | |
| Chronic Pulmonary Disease, n (%) | 1,910 (13.4) | 2,137 (16.4) | <.001 |
| Congestive Heart Failure, n (%) | 958 (6.7) | 1199 (9.2) | <.001 |
| Diabetes, n (%) | 1,381 (9.7) | 1,437 (11.1) | .001 |
| Complicated Diabetes, n (%) | 378 (2.7%) | 474 (3.6%) | <.001 |
| Uncomplicated Diabetes, n (%) | 1,003 (7.1) | 963 (7.4) | .43 |
| Hypertension, n (%) | 3,174 (22.3) | 3,111 (23.9) | .01 |
| Complicated Hypertension, n (%) | 808 (5.7) | 916 (7.0) | <.001 |
| Uncomplicated Hypertension, n (%) | 2,366 (16.7) | 2,195 (16.9) | .99 |
| BMI, Mean (SD) | 28.08 (9.36) | 28.79 (9.16) | <.001 |
| BMI, n (%) | |||
| <18.5 | 1125 (7.9%) | 605 (4.7%) | |
| 18.5-24.9 | 2837 (20.0%) | 2136 (16.4%) | |
| 24.9-29.9 | 2971 (20.9%) | 2345 (18.0%) | |
| 30-39.9 | 2834 (20.0%) | 2444 (18.8%) | |
| >40 | 866 (6.1%) | 676 (5.2%) | |
| Elixhauser Score, Mean (SD) | 1.56 (2.28) | 1.61 (2.51) | <.001 |
| Smoking Status, n (%) | |||
| Current Smoker | 1137 (8.0) | 581 (4.5) | <.001 |
| Former Smoker | 1,864 (13.1) | 1,946 (15.0) | |
| Tobacco Use | 62 (0.4) | 31 (0.2) | |
| Never Smoker | 3,796 (26.7) | 3,388 (26.1) |
Proportions vary because of missing data on comorbid covariates chronic pulmonary disease, congestive heart failure, diabetes, complicated diabetes, uncomplicated diabetes, hypertension, complicated hypertension, and uncomplicated hypertension (n = 251), age (n = 622), BMI (n = 8,362), ethnicity (n = 1,288), race (n = 840), and smoking status (n = 14,396).
P values are shown from Chi-square tests for categorial variables and Wilcoxon rank sum tests for continuous variables comparing probability of receiving an influenza vaccine.
On multivariable logistic regression analysis, the association between influenza vaccine and COVID-19 status remained significant after controlling for ethnicity, race, gender, age, BMI, Elixhauser score, smoking status and the combined metric for chronic pulmonary disease, congestive heart failure, diabetes, and hypertension (OR, 0.76; 95% CI, 0.68-0.86, P < .001; Table 3 ).
Table 3.
Association of influenza vaccine and positive COVID-19 test: multivariable analysis
| Variable | Comparator | Odds Ratio (95% CI) | P-value |
|---|---|---|---|
| Influenza vaccination | Yes vs No | 0.76 (0.68, 0.86) | <.0001 |
| Chronic Pulmonary Disease, Congestive Heart Failure, Diabetes or Hypertension | Yes vs No | 1.28 (1.06, 1.53) | <0.001 |
| Unknown vs No | 0.22 (0.09, 0.56) | ||
| Ethnicity | Non-Hispanic/Latino vs Hispanic/Latino | 1.55 (1.02, 2.33) | 0.02 |
| Unknown vs Hispanic/Latino | 2.01 (1.21, 3.32) | ||
| Age | 35-49 vs <35 | 1.54 (1.28, 1.84) | <0.001 |
| 50-64 vs <35 | 1.48 (1.24, 1.76) | ||
| ≥65 vs <35 | 1.25 (1.03, 1.50) | ||
| Unknown vs <35 | 0.63 (0.31, 1.27) | ||
| BMI | 18.5-24.9 vs <18.5 | 1.95 (1.14, 3.32) | <0.001 |
| 25-29.9 vs <18.5 | 3.11 (1.85, 5.24) | ||
| 30-39.9 vs <18.5 | 3.50 (2.09, 5.87) | ||
| >40 vs <18.5 | 4.26 (2.48, 7.31) | ||
| Unknown vs <18.5 | 5.92 (3.56, 9.82) | ||
| Elixhauser Score | Per Unit Increase | 1.15 (1.12, 1.18) | <.001 |
| Gender | Female vs Male | 0.85 (0.76, 0.96) | .01 |
| Race | Caucasian vs African American | 0.23 (0.20, 0.27) | <.001 |
| Other vs African American | 0.56 (0.44, 0.70) | ||
| Unknown vs African American | 0.46 (0.32, 0.67) | ||
| Smoking Status | Former Smoker vs Current Smoker | 1.88 (1.32, 2.69) | <.001 |
| Tobacco User vs Current Smoker | 0.79 (0.19, 3.38) | ||
| Never Smoker vs Current Smoker | 2.52 (1.79, 3.53) | ||
| Unknown vs Current Smoker | 3.02 (2.15, 4.24) |
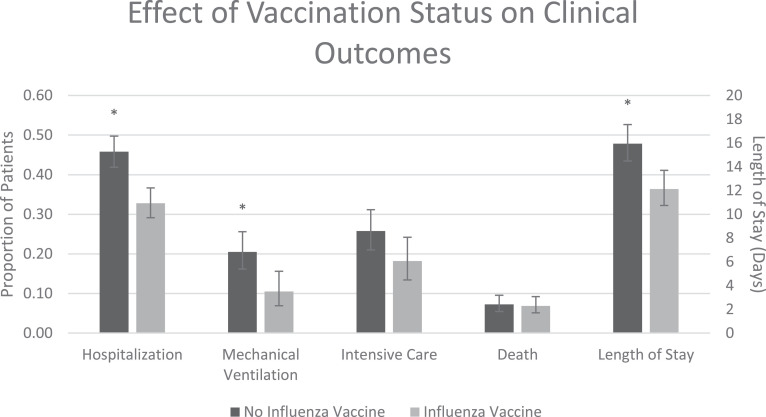
In patients with a positive COVID-19 test, we compared clinical outcomes between those who did and did not receive an influenza vaccine, which is reported in Appendix Table A.2. Of the 1,218 patients testing positive for COVID-19, 505 (41.5%) required hospitalization with 182 (36.0%) of the hospitalized patients requiring mechanical ventilation and an overall average (SD) length of stay of 10 (14.3) days. Overall, 90 (7.4%) COVID-19 positive patients died within the time frame of our data. The median follow-up time from the date of first positive COVID-19 testing to death or censoring date was 96 days (IQR 59-106) and the median time to death for those who died was 9.5 days (IQR 5-19). Baseline patient characteristics stratified by clinical outcomes are shown in Appendix Table A.3. After adjustment for baseline covariates including ethnicity, race, gender, age, BMI, Elixhauser score, smoking status and the combined metric for chronic pulmonary disease, congestive heart failure, diabetes, and hypertension, we found that vaccinated patients were less likely to require hospitalization (OR, 0.58; 95% CI, 0.46-0.73, P < .001), less likely to require mechanical ventilation if hospitalized (OR, 0.45; 95% CI, 0.27-0.78, P = .004), and had a shorter length of stay (RR, 0.76; 95% CI, 0.65-0.89, P < .001). No significant differences in mortality or in need for intensive care were seen between the vaccinated and unvaccinated groups (HR, 0.84; 95% CI, 0.51-1.36, P = .47 and OR 0.64; 95% CI, 0.41-1.00, P = .05, respectively). A graph comparing adjusted probabilities of COVID-19 clinical outcomes based on influenza vaccination status is represented in Figure 2 .
Fig 2.
Effect of Influenza Vaccination Status on COVID-19 Clinical Outcomes. Total cohort includes all patients who tested positive for COVID-19 (n = 1,218) and is stratified by those who did (n = 525) or did not (n = 693) receive the influenza vaccine. Mechanical ventilation, intensive care and length of stay were assessed only for those who were hospitalized (n = 505). The proportion of patients requiring hospitalization, mechanical ventilation, intensive care as well as mortality rates are represented by values on the primary Y-axis. Average length of stay (days) is represented on the secondary Y-axis. All clinical outcomes reported are adjusted for baseline covariates including ethnicity, race, gender, age BMI, Elixhauser score, smoking status and the combined metric for chronic pulmonary, congestive heart failure, diabetes, and hypertension. *P < .05.
The median time from influenza vaccination to COVID-19 testing was 225 days (IQR 188-257), with the majority of patients receiving their influenza vaccine in September 2019 and October 2019 (n = 8,861, 68.2%). The total number of COIVD-19 tests performed continually increased from March 2020 to July 15th, 2020, with the positivity rate of COVID-19 tests decreasing from a maximum of 29.0% in March to a minimum of 0.9% in June. For patients who received an influenza vaccination, those vaccinated a longer time from their date of COVID-19 testing were less likely to test positive for COVID-19 (P < .001), however this association was nonsignificant after controlling for the overall decreasing rate of COVID-19 test positivity (P = .57). No association was found between the timing of influenza vaccination and COVID-19 clinical outcomes (Appendix Table A.4).
Discussion
The results of our study indicate that influenza vaccination presents no harmful effect on COVID-19 susceptibility or increased disease severity, and points to a possible association between the vaccine and decreased risk of COVID-19 and improved clinical outcomes. There has been recent speculation about potential protection from COVID-19 conferred by the influenza vaccine,10 and we have further strengthened this suggestion through the use of retrospective chart review and patient level data. Consistent with previous research,3 , 4 we found higher rates of COVID-19 in older patients and in those with preexisting comorbidities. Recent data has also suggested an association between influenza vaccination and reduced mortality from COVID-19,11, 12, 13 as well as a decreased need for intensive care treatment and invasive respiratory support.12 The results of our study lend support to the decreased need for mechanical ventilation in COVID-19 patients who received the influenza vaccine, as well as identify a lower rate of hospitalizations and length of stay in those who were vaccinated. Rates of infection with other respiratory pathogens in our study cohort was low, and no patients were identified as having both COVID-19 and influenza, resulting in minimal confounding of our results by COVID-19 and influenza co-infection. Additionally, we found that outcomes were independent of the length of time between influenza vaccination and COVID-19 testing. This is a slight contradiction to a recent report from Italy demonstrating the greatest protection against COVID-19 in elderly patients who received the vaccine in close proximity to COVID-19 exposure as compared to several months before.14 While we did see decreased mortality in the influenza vaccinated group, the association was non-significant. This may be due to a small sample size and the low number of deaths observed.
While we were able to control for many patient comorbidities known to be associated with COVID-19 risk in our analyses, the observed protective association between the vaccine and COVID-19 may be confounded by differences in health and social behaviors or disparate socioeconomic factors between those in the vaccinated and unvaccinated groups. A decrease in all respiratory viral infections was seen in a number of countries in 2020, likely due to interventions such as physical distancing, mask wearing, community education and lockdowns.16 , 17 In the state of Michigan, the first positive case of COVID-19 was on March 10th, 2020, with school closures, banning of large group gatherings and restrictions on visiting healthcare and residential facilities put into place March 13th, with closure of most public places by March 16th. On March 23rd, an official “stay at home” order was issued and on April 26th a mask mandate put into effect. Given the rapid implementation of these restrictions following the first positive cases which extended into June, 2020, the data from our cohort is largely confined within these strict public health interventions. Discrepancies in adherence to these guidelines between influenza vaccinated and unvaccinated patients could bias the observed association. A prospective study accounting for these differences is needed to explore the possible protective effect of the influenza vaccine on COVID-19 susceptibility and outcomes.
Another factor to consider is the impact of the so-called healthy user effect, which refers to the observation that the reduced risk in patients who receive the influenza vaccine may be independent of protection against the influenza virus and rather due to bias from the healthier patient population who typically receive preventative therapies.18 When controlling for several variables that could impact the risk of developing pneumonia, the association between influenza vaccination and protection against adverse effects becomes insignificant.19
While our descriptive study cannot discern mechanisms, a hypothetical, yet plausible immunologic mechanism that could explain the apparent protective effects of influenza vaccine against COVID-19 is a process called trained immunity.20 Classically, vaccinations activate an adaptive immune response via T-helper cells to produce a memory cellular (macrophages, NK cells) and humoral (antibody mediated) response to destroy antigen-presenting cells on repeat exposure to a similar antigen. However, emerging data on epigenetic and metabolic reprogramming of innate immune cells suggests that exposure to a second, non-specific stimulus could trigger a targeted and heightened proinflammatory response.21 , 22 This “heterologous immunity” could explain the nonspecific cross-reactivity that vaccines have against unrelated pathogens. Trained immunity has been demonstrated with the heterogenous effect of the bacilli Calmette-Guérin (BCG) vaccine against infectious diseases such as yellow fever or malaria, and even certain malignancies.22 Additionally, recent research from the Netherlands found that the quadrivalent inactivated influenza vaccine was able to induce an improved cytokine response after stimulation of immune cells with SARS-CoV-2.23 Further, the measles vaccine has been shown to have survival benefits beyond the expected protection against the measles virus alone, as suggested by a 30% reduction in all-cause mortality in vaccinated children, with only 4% explained by prevention of measles-related deaths.24 More studies are necessary to understand any potential role of trained immunity with influenza vaccine and COVID-19 infection.
Prior to approval of the Pfizer-BioNTech and Moderna COVID-19 vaccines, the potential of non-COVID-19 vaccinations to curb infection rates was explored. Improved outcomes correlating with BCG administration has been postulated25 and decreased SARS-CoV-2 infection rates have been seen in individuals who recently received a Polio, Haemophilus Influenza type B (HIB), Measles-Mumps-Rubella (MMR), Varicella, pneumococcal conjugate (PCV13), Geriatric Flu, Hepatitis A or Hepatitis B vaccine.26 Our study is the first of our knowledge to explore the association between the standard influenza vaccine and COVID-19.
We are in the midst of a unique influenza season taking place during a global pandemic, placing additional strain on health care systems as well as creating the potential for COVID-19 and influenza co-infection.27 While demonstrated to be effective and safe,28 , 29 the influenza vaccine remains underutilized.30 , 31 Recent suggestions of an increased risk of other respiratory viruses following influenza vaccination32 threaten to further diminish use of the influenza vaccine. This is especially of concern in the elderly and in those with preexisting medical conditions who experience increased morbidity and mortality with influenza infection33 and are also at an increased risk of severe infection with COVID-19.3 , 4 In particular, influenza has been associated with an increased risk of myocarditis,34 aortic dissection,35 myocardial infarction, stroke, and death36 in those with cardiovascular disease, and administration of the influenza vaccination has been shown to decrease the risk of major adverse cardiovascular events in this population.37 Annual influenza vaccination has therefore been identified as an important intervention in reducing the risk of cardiovascular events in those with coronary and other atherosclerotic vascular disease, designated as a class IB recommendation in the updated AHA/ACC guidelines.38 The increased risk of cardiovascular complications due to COVID-19 in these patients,5 combined with a potential protective effect against COVID-19 lend further support to the importance of the influenza vaccine in this population.
While the largest benefit to health from the influenza vaccine comes from prevention of influenza, the ancillary potential benefit of COVID-19 protection may provide enough impetus for hesitant patients to get vaccinated. Even if the direct link between the prevention of COVID-19 and the influenza vaccine is minimal, through an overall reduction in the number of patients presenting to their providers with viral-like symptoms necessitating work up for COVID-19 or requiring hospitalization for complications of influenza, vaccination will preserve healthcare resources for those with COVID-19. Patient education and widespread promotion of the influenza vaccine are therefore necessary to increase vaccine uptake and reduce the burden of both COVID-19 and influenza.
Study Limitations
All data for this study was obtained from information from electronic medical records. Influenza vaccination records for patients who primarily receive their healthcare within the state of Michigan is reliably documented and updated into a patient's chart regularly, however vaccinations administered out-of-state may not be updated into their medical record.
Clinical outcomes such as need for hospitalization, length of stay, need for intensive care, need for mechanical ventilation and mortality pertain specifically to the patient demographic admitted to our medical institution – a 1,000 bed, tertiary center with a specialized 32-bed isolation unit. Therefore, results from this study cannot be extrapolated to smaller hospitals in more of a community setting. In addition, patients who tested positive for COVID-19 within our healthcare system and were subsequently admitted in a separate medical institution would not be captured in our search. However, the purposes of this study was to compare COVID-19 infection rates and clinical outcomes based on influenza vaccination status within a single cohort, and not to report on absolute statistics, which we appreciate may be skewed in our population.
Finally, the search was conducted in the midst of the COVID-19 pandemic, with new cases still being reported daily. We therefore may not have captured the full extent of outcomes for those recently diagnosed with COVID-19 and cannot predict how future case rates will affect our results. However, with the peak of the influenza season encroaching, there is a sense of urgency to help healthcare providers and patients make better informed medical decisions.
Conclusion
In this electronic medical records based retrospective cohort study, we found a significant reduction in the odds of testing positive for COVID-19 in patients who received an influenza vaccine compared to those who did not receive the vaccine. In addition, in patients who tested positive for COVID-19, those who previously received an influenza vaccine had significantly better clinical outcomes. Future prospective studies are needed to establish a causal relationship between the influenza vaccine and COVID-19 susceptibility and severity. Until the COVID-19 vaccine becomes widely available, the influenza vaccine should be promoted to reduce the burden of disease during this pandemic.
Acknowledgment
We would like acknowledge the Data Office for Clinical & Translational Research of the University of Michigan Medical School for providing free access to DataDirect software. We would also like to thank staff members at DataDirect, who assisted in the design of our electronic medical record search strategy.
Footnotes
Conflicts of interest: None to report.
Funding: No funding provided.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2021.02.012.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49:108–118. [PubMed] [Google Scholar]
- 3.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 7.The WHOREAfC-TWG Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. New Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor E, Teh J, Kamat AM, Lawrentschuk N. Bacillus Calmette Guérin (BCG) vaccination use in the fight against COVID-19 - what's old is new again? Future Oncol. 2020;16:1323–1325. doi: 10.2217/fon-2020-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem ML, El-Hennawy D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marín-Hernández D, Schwartz RE, Nixon DF. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J Med Virol. 2021;93:64–65. doi: 10.1002/jmv.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink G, Orlova-Fink N, Schindler T, et al. Inactivated trivalent influenza vaccine is associated with lower mortality among Covid-19 patients in Brazil [e-pub ahead of print]. BMJ Evid Based Med. 10.1136/bmjebm-2020-111549, Accessed February 26, 2021. [DOI] [PubMed]
- 13.Zanettini C, Omar M, Dinalankara W, et al. medRxiv; 2020. Influenza vaccination and COVID19 mortality in the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragni P, Marino M, Formisano D, et al. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines. 2020;8:675. doi: 10.3390/vaccines8040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39:423–427. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 17.Tan JY, Conceicao EP, Sim XYJ, Wee LEI, Aung MK, Venkatachalam I. Public health measures during COVID-19 pandemic reduced hospital admissions for community respiratory viral infection. J Hosp Infect. 2020;106:387–389. doi: 10.1016/j.jhin.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35:337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 19.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Pinho MP, Buckley P, et al. CD8+ T cell cross-reactivity against SARS-CoV-2 conferred by other coronavirus strains and influenza virus. Med Let CDC & FDA. 7 June 2020 doi: 10.3389/fimmu.2020.579480. 204 Business Insights: Essentials Web 2 Aug 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debisarun PA, Struycken P, Domínguez-Andrés J, et al. medRxiv; 2020. The effect of influenza vaccination on trained immunity: impact on COVID-19. 2020.2010.2014.20212498. [Google Scholar]
- 24.Donzelli A, Schivalocchi A, Giudicatti G. Non-specific effects of vaccinations in high-income settings: How to address the issue? Hum Vaccin Immunother. 2018;14:2904–2910. doi: 10.1080/21645515.2018.1502520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinger D, Blass I, Rappoport N, Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. Vaccines (Basel) 2020;8:378. doi: 10.3390/vaccines8030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlowski C, Puranik A, Bandi H, et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. medRxiv. 2020:2020.2007.2027.20161976. [DOI] [PMC free article] [PubMed]
- 27.Antony SJ, Almaghlouth NK, Heydemann EL. Are coinfections with COVID-19 and influenza low or underreported? An observational study examining current published literature including three new unpublished cases. J Med Virol. 2020;92:2489–2497. doi: 10.1002/jmv.26167. [DOI] [PubMed] [Google Scholar]
- 28.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 29.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheney MK, John R. Underutilization of influenza vaccine: a test of the health belief model. SAGE Open. 2013;3 [Google Scholar]
- 31.Grandhi GR, Mszar R, Vahidy F, et al. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2021;6:87–91. doi: 10.1001/jamacardio.2020.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff GG. Influenza vaccination and respiratory virus interference among Department of Defense personnel during the 2017-2018 influenza season. Vaccine. 2020;38:350–354. doi: 10.1016/j.vaccine.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keilman LJ. Seasonal Influenza (Flu) Nurs Clin North Am. 2019;54:227–243. doi: 10.1016/j.cnur.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Kodama M. Influenza myocarditis. Circ J. 2010;74:2060–2061. doi: 10.1253/circj.cj-10-0833. [DOI] [PubMed] [Google Scholar]
- 35.Ashur C, Norton E, Farhat L, et al. Higher admission rates and in-hospital mortality for acute type A aortic dissection during influenza season: a single center experience. Sci Rep. 2020;10:4723. doi: 10.1038/s41598-020-61717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madjid M, Naghavi M, Litovsky S, Casscells SW. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 2003;108:2730–2736. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 37.Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. Jama. 2013;310:1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 38.Smith SC, Jr., Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.