Abstract
Background and aims
Retrospective studies have shown that angiotensin-converting-enzyme (ACE) inhibitors are associated with a reduced risk of complications and mortality in persons with novel coronavirus disease 2019 (COVID-19). Thus, we aimed to examine the efficacy of ramipril, an ACE-inhibitor, in preventing ICU admission, mechanical ventilation and/or mortality while also minimizing the risk of transmission and use of personal protective equipment (PPE).
Methods
RAMIC is a multicenter, randomized, double-blind, allocation-concealed, placebo-controlled trial comparing the efficacy of treatment with ramipril 2.5 mg orally daily compared to placebo for 14 days. The study population includes adult patients with COVID-19 who were admitted to a hospital or assessed in an emergency department or ambulatory clinic. Key exclusion criteria include ICU admission or need for mechanical ventilation at screening, use of an ACE inhibitor or angiotensin-receptor-II blocker within 7 days, glomerular filtration rate < 40 mL/min or a systolic blood pressure (BP) < 100 mmHg or diastolic BP < 65 mmHg. Patients are randomized 2:1 to receive ramipril (2.5 mg) or placebo daily. Informed consent and study visits occur virtually to minimize the risk of SARS-CoV-2 transmission and preserve PPE. The primary composite endpoint of ICU admission, invasive mechanical ventilation and death are adjudicated virtually.
Conclusions
RAMIC is designed to assess the efficacy of treatment with ramipril for 14 days to decrease ICU admission, mechanical ventilator use and mortality in patients with COVID-19 and leverages virtual study visits and endpoint adjudication to mitigate risk of infection and to preserve PPE (ClinicalTrials.gov, NCT04366050).
Keywords: SARS-CoV2, Covid-19, Mechanical ventilation, Virtual visit
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; RAAS, renin-angiotensin-aldosterone system; AKI, acute kidney injury; ITT, Intention-to-Treat; DSMB, data safety monitoring board; GFR, estimated glomerular filtration rate; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; CMP, comprehensive metabolic panel; FDA, Food and Drug Administration;; IND, investigational new drug.
1. Introduction
As of September 2020, the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 25 million people globally and caused more than 800,000 deaths [1]. Therapeutic options remain limited, especially for those with mild-moderate disease and/or in the ambulatory setting. Thus far, only Remdesivir, and one corticosteroid, dexamethasone, have demonstrated efficacy in preliminary published reports [2,3]. The need for IV administration and subsequent clinical trials demonstrating no convincing benefit limit the broad use of Remdesivir [4]. In addition, the mortality benefit with dexamethasone was only seen in those requiring supplemental oxygen or mechanical ventilation. Thus, additional therapeutics are needed to decrease morbidity and mortality of COVID-19 patients.
Among COVID-19 cases, pre-existing comorbidities such as hypertension, diabetes, obesity, and cardiovascular disease are associated with higher risk of developing severe symptoms and mortality [5]. Initially, the continued use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) was controversial in the setting of COVID-19. This controversy stems from the idea that use of ACEIs and ARBs might increase the expression of angiotensin converting enzyme 2 (ACE2) [[6], [7], [8]], the cellular receptor and necessary entry point for SARS-CoV-2 infection [9]. Multiple clinical studies have subsequently demonstrated that the use of ACEIs or ARBs are not significantly associated with increased risk of SARS-CoV-2 infection or disease severity [[10], [11], [12]].
Similarly, critical review of the mechanisms contributing to SARS-CoV-2 pathobiology support a possible benefit from ACEIs and ARBs [13]. In vivo studies have demonstrated that ACE2 expression/activity is down-regulated following infection with the original SARS-CoV, resulting in excessive activation of the renin-angiotensin-aldosterone system (RAAS), and exacerbated response to angiotensin II, including its effects on the lungs and vasculature [[14], [15], [16]]. Cellular interactions that drive COVID-19 pathobiology may result from dysregulation of angiotensin signaling induced by effects of SARS-CoV-2 infection on multiple cell types in the lung (e.g., epithelial cells, endothelial cells and fibroblasts), likely mediated via Angiotensin II Receptor type 1 [17] . Increased angiotensin signaling in these cells increases inflammation, epithelial disruption, endothelial permeability, cell death and fibrosis. A mechanistic rationale thus exists for the administration of ACEIs/ARBs to blunt such pathological effects in COVID-19. Meta-analyses of observational, retrospective studies of ACEI/ARB use in COVID-19 patients imply that these drugs may have a survival benefit without increased risk of infection [[18], [19], [20]]. Therefore, administration of ACEIs/ARBs may be beneficial by blunting these enhanced responses to angiotensin II, thereby preventing or mitigating acute lung injury, risk of acute respiratory distress syndrome, endothelitis, and other features of COVID-19 [17].
Ramipril is an ACEI that is approved by the FDA for the treatment of hypertension, to reduce the risk of heart failure and death after myocardial infarction and to reduce the risk of myocardial infarction, stroke, and death from cardiovascular events but has not been studied in SARS-CoV-2 infected patients. The suppression of the RAAS pathway by ramipril decreases tissue and circulating ACE1 activity, thereby mitigating angiotensin II-mediated vasoconstrictive, pro-inflammatory, and pro-oxidative effects. Such findings led us to design of RAMIC, a randomized, double-blind, placebo-controlled trial of ramipril for the treatment of COVID-19. Our goal was to examine the efficacy of ramipril in preventing intensive care unit (ICU) admission, mechanical ventilation and/or mortality during and after treatment for 14 days.
2. Methods
2.1. Study design
RAMIC (ClinicalTrials.gov, NCT04366050) is a phase 2B, prospective, randomized, double-blind, allocation-concealed, placebo-controlled, multicenter study evaluating the effect of once-daily ramipril (2.5 mg orally) versus placebo for 14 days. This study is designed to include approximately 560 adult patients with SARS-CoV-2 infection who present to an ambulatory visit, emergency department or are hospitalized outside of the ICU at up to 15 clinical sites.
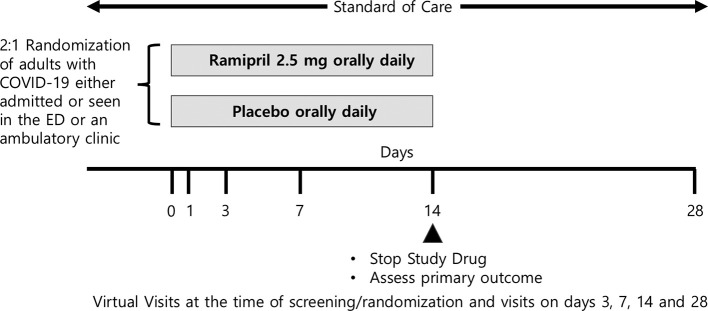
The primary outcome is a composite of mortality, ICU admission or use of invasive mechanical ventilation by day 14 (Fig. 1 ).
Fig. 1.
RAMIC study design.
2.2. Endpoint rationale
Based on early clinical reports of a bimodal time from the onset of symptoms to requirement for mechanical ventilation with modes at 3–4 days and 9 days, day 14 was chosen to assess the composite primary outcome–a direct measure of clinically meaningful endpoints. In addition, this primary endpoint can be adjudicated accurately and be assessed remotely.
2.3. Dosing rationale
A dose of 2.5 mg ramipril was chosen to balance reliable RAAS blockade for patients who are ACEI and ARB naïve and maintain safety by minimizing the risk of hypotension and acute kidney injury (AKI) in patients with COVID-19. Pharmacodynamic studies suggest that single doses of 2.5 mg produce ~60–80% inhibition of ACE [21]. Given the exploratory nature of the study there is no a priori reason to select one ACEI over another, however, ramipril has a long safety profile and is well tolerated at low doses making it an appropriate choice. The 2.5 mg daily dose is comparable to initial dosing of ramipril used to treat normotensive patients or those with controlled hypertension at risk for major cardiovascular events [22]. Due to the short duration of the trial and to decrease the risk of adverse events, dose titration was not incorporated.
2.4. Study objectives and duration
In addition to the primary objective, secondary objectives evaluate additional endpoints that include length of hospitalization, need for re-admission, development of shock and AKI. In addition, time-to-event data and a 28-day composite outcome will be evaluated (Table 1 ). Exploratory objectives include evaluation of biomarkers of RAAS blockade (Angiotensinogen, Ang II, ACE, ACE2, Angiotensin 1–7, Adrenaline, Aldosterone, Renin, Prorenin) in a subset of patients and examination for racial differences in treatment response to ramipril based on emerging data of racial differences in COVID-19 outcomes and existing data supporting differential response to ACEI by race [23]. The pre-specified study follow-up is 28 days.
Table 1.
Study objectives.
| Primary objective |
| To examine the efficacy of ramipril 2.5 mg orally daily over 14 days versus placebo in patients with SARS-CoV-2 infection in improving survival and reduction in need for admission to an intensive care unit (ICU) or to receive invasive mechanical ventilation. |
| Secondary objectives |
| Proportion of patients needing continued hospitalization at day 14 |
| Proportion of patients needing ICU admission |
| Proportion of patients needing invasive mechanical ventilation |
| Time to mortality |
| Time to ICU admission |
| Time to discharge from the hospital |
| Need for hospitalization among outpatients or rehospitalization among those discharged |
| Proportion of patients developing hypotension and needing pressor support |
| Proportion of patients developing septic shock, defined as sepsis with hypotension requiring vasopressors to maintain MAP ≥65 and serum lactate >2 mmol/L after fluid resuscitation (Sepsis-3 JAMA 2016) |
| Acute kidney injury defined by KDIGO guidelines, increase in serum creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine ≥1.5 times baseline within 7 days. |
| Exploratory objectives |
| To examine the efficacy of ramipril in improving biomarkers of the renin-angiotensin-aldosterone axis |
| To examine racial differences in response to treatment with ramipril in patients with SARS-Cov-2 infection |
2.5. Sample size justification and statistical analysis
Statistical analyses will compare the primary outcomes between the treatment and placebo arms. Given the limited preliminary data, it is not possible to predict confidently the spontaneous or therapeutic response rate. In the primary analysis, we assume that the 14 day mortality/ICU admission/ventilator use of the placebo and ramipril-treatment groups to be 22% and 12%, respectively, based on early published observational data [24] and the proportion of patients in the placebo and ramipril-treatment groups to be 1:2. Using Whittemore's formula, we estimate that 510 patients–340 receiving ramipril and 170 receiving placebo—are required to detect effects with a power 80% (or higher) with a Type I error rate of alpha = 0.05. To account for dropout, we include an additional 10% enrollment of subjects (to 560).
The primary outcome will first be analyzed using a 2 × 2 contingency table (treatment by outcome) and testing via Fisher's exact test. This analysis will be followed by logistic regression, controlling for covariates in a stepwise fashion: logistic regression with 1) treatment group only; 2) treatment group, age and sex; 3) treatment group, age, sex and self-reported ethnicity; 4) treatment group, age, sex and ethnicity, hypertension and diabetes. We will test for significance of covariates in nested models using Likelihood Ratio tests. Finally, we will examine time to event with censoring (by the end of the follow-up period) using Cox Proportional Hazards models in a similar fashion. The primary analyses will be Intention-to-Treat (ITT) and dropouts are considered non-responders. Per Protocol analyses excluding patients who drop out before the week 2 evaluation will be performed as well. Secondary outcomes will be analyzed in a similar fashion, using logistic regression (for dichotomous outcomes), linear regression (for continuous outcomes), and Cox models (time-to-event outcomes). Secondary analyses will also include covariates in the regressions, including baseline hypertension, diabetes, race/ethnicity, sex and age.
An interim analysis will occur after approximately 50% of the planned enrollment of the trial and will be reviewed by an independent data safety monitoring board (DSMB). Analysis will evaluate safety and futility. The overall study duration is driven by time-to-target enrollment and anticipated to be less than one year.
3. Study procedures
3.1. Eligibility criteria
Patients aged 18 years or older with SARS-CoV-2 infection diagnosed with PCR within 7 days of randomization or with a clinical presentation consistent with infection (fever or cough or shortness of breath) with positive IgM serology who are currently hospitalized or with a visit to the emergency department or ambulatory clinic are eligible for the study. Key exclusion criteria include mechanical ventilation, ICU care, estimated glomerular filtration rate (GFR) < 40 mL/min, systolic BP < 100 mmHg or diastolic BP < 65 mmHg, serum potassium ≥5.1 mEq/l, pregnancy or breastfeeding. Complete inclusion and exclusion criteria are outlined in Table 2 .
Table 2.
RAMIC inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years Currently hospitalized or with a visit to an emergency department or urgent care Willing and able to provide written informed consent prior to performing study procedures Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)-2 infection confirmed by polymerase chain reaction (PCR) test ≤7 days before randomization OR Clinical presentation consistent with SARS-CoV-2 infection (fever or cough or shortness of breath) with positive IgM serology |
Participation in any other clinical trial of an experimental treatment for COVID-19 (compassionate use of hydroxychloroquine, chloroquine, azithromycin or emergency use authorization of remdesivir outside of a clinical trial is allowed) Requiring mechanical ventilation at screening Requiring ICU care at admission NSAID use within 12 h of randomization or requiring continued NSAID use during this triala Alanine Aminotransferase (ALT) or aspartate aminotransferase (AST) > 5 X upper limit of normal (ULN) Estimated GFR < 40 mL/minb History of serum creatinine ≥2 mg/dl in the previous 28 days Systolic BP < 100 mm hg or diastolic BP < 65 mm hg Hypersensitivity to an ACE inhibitor History of angioedema Outpatient use of ACE inhibitor or Angiotensin II receptor blocker in the last 7 days History of renal artery stenosis Serum potassium ≥5.1 mEq/l Pregnancy or breastfeeding Use of aliskiren, amifostine, lithium, sacubitril, within 7 days |
If a participant took an NSAID prior to presentation they could still be screened for the study and randomized 12 h after the dose of NSAID with counseling to avoid further NSAID use.
GFR of 40 mL/min is the threshold for dose reduction of ramipril.
3.2. Recruitment and virtual consent
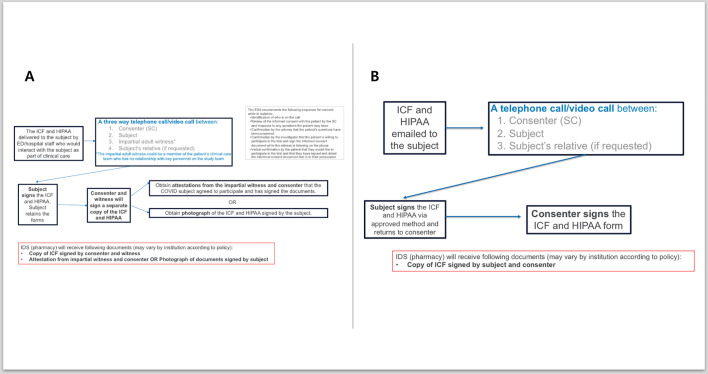
Candidates for the study can undergo informed consent by videoconference or telephone, using either a paper consent form or an electronic consent form. A paper copy of the consent form can be provided by a health care provider caring for the patient and if using a paper consent form, informed consent must occur in the presence of a co-signing witness. To minimize the risk of transmission of SARS-CoV-2, a picture of the signed consent can be sent to the study team via secure email or an attestation can be signed by an impartial witness and the consenter indicating that the subject agreed to participate and has signed the documents. If a photograph is sent to the study team, it is printed and maintained with source documents. The paper consent is retained by the study participant. Alternatively, electronic methods may be used to consent subjects. An electronic copy of the signed consent will be provided to the subject (Fig. 2 ). Any method of electronic consent in accordance with FDA guidance and with local institutional IRB approval could be used. An example of an acceptable method of electronic consent in this trial is DocuSign, which provides an auditable signature with timestamp and provides all parties with electronic copies of the completed consent form.
Fig. 2.
Remote Consent Process for RAMIC: A) Patient receives paper consent and sends a photograph or obtains impartial witness attestations or B) electronic consent and signature using IRB approved platform.
3.3. Study blinding
During the conduct of the study, patients and investigators are blinded to individual treatment assignment. There is no provision for emergency unblinding as treatment for any anticipated adverse events (AEs) would not be affected by treatment assignment and the study drug would be stopped. Allocation concealment was accomplished by randomization using permuted block randomization by the UCSD Investigational Drug Service. All 14 doses of study drug were dispensed in opaque bottles for nursing administration for inpatients or self-administration for outpatient participants.
3.4. Study assessments
Study subjects will attend a virtual information session during which the primary consent document, and the UCSD-HIPAA forms are reviewed, discussed and signed. Structured case report forms will be used to review concomitant medication use, past medical history, review and record blood tests: complete blood count (CBC with differential and platelet count), prothrombin time, comprehensive metabolic panel [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total serum bilirubin, alkaline phosphatase, albumin, total protein, plasma glucose, sodium, chloride, potassium, bicarbonate, blood urea nitrogen, creatinine, calcium], lactic acid, C-reactive protein, D-dimer, ferritin, and procalcitonin, if available. CBC and comprehensive metabolic panel (CMP) are required and will be obtained by the study if not obtained as part of routine clinical care. Lactic acid, C-reactive protein, D-dimer, ferritin, procalcitonin, troponin and brain natriuretic peptide will be recorded only if available for routine clinical care. If needed, a pregnancy test will be obtained to confirm pregnancy status. Data on imaging, if available, will be extracted to record chest x-ray and computed tomography results to characterize the disease severity of the study population. Vital signs including heart rate, BP, oxygen saturation and the use of supplemental oxygen, body temperature, and respiratory rate will be recorded during screening and where available, on subsequent virtual visits. At a single study site biobanking and biomarker assessment at baseline, day 7 and day 14 will be performed to assess cytokines and markers of the RAAS pathway. A structured symptom assessment, outcome assessment, review of concomitant medications, treatment adherence and review for AEs will be performed at all study visits. All study visits will be performed virtually by telephone or videoconference.
3.5. Premature discontinuation of study drug
Patients meeting a primary endpoint (invasive mechanical ventilation, transfer to the ICU [or death]) will stop ramipril/placebo. In addition, patients who discontinue treatment based on toxicity rules defined below may stop ramipril/placebo. Additional data on secondary endpoints will continue to be collected and the schedule of events will be completed when possible.
3.6. Post-treatment phase
At the end of the 14-day treatment phase, patients will be followed for 14 days off-treatment until day 28. They will receive a phone visit to assess changes in clinical status related to the primary and secondary outcomes.
3.7. Safety monitoring
Safety will be assessed on a continuous basis. AEs are any unfavorable or unintended sign, symptoms or diagnosis that occurs in a study participant independent of attribution. All AEs will be recorded on case report forms within 72 h if the AEs meet any of the following criteria: ≥ Grade 3 toxicity modified from the Common Terminology Criteria version 4.0 for this trial or those that lead to cessation of ramipril/placebo, regardless of toxicity. Dose modifications will not occur in this trial. The DSMB will review interim data, including AEs, to ensure the safety of participants.
3.8. Human studies and patients
This study is in full compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol has been approved by a central institutional review board. The trial is available on clinicaltrials.gov (NCT04366050) and approved by a Food and Drug Administration (FDA) investigational new drug (IND) application.
4. Discussion
RAMIC initiated patient enrollment in May 2020, soon after recognition of the global pandemic and amid controversy regarding the role of ACEI and ARB in COVID-19 patients. Multiple observational studies subsequently confirmed the absence of harm [10,24] and potential for benefit [17,24] associated with ACEI and ARB use in COVID-19, including a detailed review of how ACEI can modulate the deleterious effects of the RAAS pathway in COVID-19 [25]. Such drugs could fill a critical need for an easy to administer, inexpensive and safe treatment for COVID-19 [17]. Innovations in the trial design include the virtual consenting process and study visits in order to limit the risk of spread of SARS-CoV-2 and to conserve personal protective equipment (PPE).
The primary objectives of this study are to reduce clinically important outcomes: ICU admission, invasive mechanical ventilator use and mortality. Decreasing ICU admission and mechanical ventilator use are critical for conserving medical resources during surges in health care utilization. These highly impactful outcomes can be readily adjudicated remotely, limiting the need for in-person assessment. Additional long-term outcomes are not defined in this protocol but if effective, ramipril may mitigate potential long-term pulmonary and cardiovascular sequelae, a possibility that will warrant further study.
The trial design leveraged guidance from the FDA to obtain informed consent without increasing the risk of exposure. The guidance document entitled, FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency, was initially published in March 2020. Pathways for either paper or electronic consent will facilitate informed consent virtually or via telephone. In addition, with clear outcomes and utilization of a drug with a well-established safety profile, all study visits can be performed virtually. The dose selection balances possible benefit, while minimizing the risk of harm. Similar doses have been studied in normotensive patients at risk for cardiovascular outcomes [22]. A study of an ARB, losartan, for the treatment of COVID-19 in hospitalized (NCT04312009) and ambulatory (NCT04311177) patients, will test if alternative approaches to RAAS blockade are beneficial in COVID-19 patients. Future studies may be required to evaluate higher doses of RAAS blockade and use in patients with more advanced disease who are excluded from this study.
Design and implementation of a clinical trial during a rapidly evolving pandemic presents unique challenges for recruitment including consideration of competing therapeutic trials and the evolving standard of care. Due to limitations on face-to-face contact, the study team leveraged secure videoconference with the investigator, study coordinator and prospective patients to address patient concerns and increase participant comfort with the study team. Furthermore, many institutions established committees to review clinical trials and limit potentially overlapping trials, which, in some instances, delayed site initiation. Implementation of the trial required coordination among investigators for multiple trials to prevent overlapping enrollment. Future trials can leverage the innovation electronic consent process outlined in FDA guidance and operationalized in this trial (Fig. 2), along with secure video conference to facilitate enrollment and minimize the burden of patient visit.
5. Conclusions
RAMIC is a clinical trial designed to study the efficacy of an ACEI, ramipril, to decrease ICU admission, requirement for mechanical ventilation and death. This study repurposes a drug with longstanding safety data and a putative mechanistic hypothesis for benefit in COVID-19. By obtaining informed consent virtually and performing virtual study visits, RAMIC minimizes exposure and resource utilization. The results should provide valuable information regarding the efficacy of ACEI in reducing morbidity and mortality in COVID-19 patients.
Author contributions
Study concept and design: VA, WT, DS, AM, RM, VT, JY, KS, PI, SC, LR, RL.
Drafting of the manuscript: VA, RL.
Critical revision of the manuscript: VA, WT, DS, AM, RM, VT, JY, KS, PI, SC, LR, RL.
Approved final submission: VA, WT, DS, AM, RM, VT, JY, KS, PI, SC, LR, RL.
Funding
The study was supported by an investigator-initiated study grant from Pfizer Inc. to RL. RL receives additional funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), and NIDDK (R01DK106419, and P30DK120515), and DOD PRCRP (CA170674P2). VA is supported by NIDDK (K23DK119460). RLM Receives funding from NIDDK UAB/UCSD O'Brien center for AKI research DK079337. AM is funded by NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. DS: receives support from the John and Mary Tu Foundation.
Declaration of Competing Interest
Dr. Malhotra reports funding related to medical education from Livanova, Equillium and Corvus. ResMed provided a philanthropic donation to UCSD. Rohit Loomba: Potential conflict of interest for Rohit Loomba: Dr. Loomba serves as a consultant or advisory board member for Bird Rock Bio, Celgene, Enanta, GRI Bio, Madrigal, Metacrine, NGM, Sanofi, Arrowhead Research, Galmed, NGM, GNI, NovoNordisk, Merck, Siemens, Pfizer, Gilead. Glympsebio, In addition, his institution has received grant support from Allergan, BMS, BI, Daiichi-Sankyo Inc., Eli-Lilly, Galectin, Galmed, GE, Genfit, Intercept, Janssen Inc., Madrigal, Merck, NGM, Pfizer, Prometheus, Siemens, and Sirius. He is also co-founder of Liponexus Inc. Paul Insel is not currently but within the past 3 years has served as a consultant or received research support from Merck, Pfizer, and Bristol Myers Squibb. Davey Smith serves a consultant for Arena Pharmaceuticals, Nitto Pharmaceuticals and Bayer. He also serves on the Scientific Advisory Board of Safe Aloha and FluxErgy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2021.106330.
Appendix A. Supplementary data
Supplementary material
References
- 1.WHO, WHO Coronavirus Disease (COVID-19) Dashboard 2020. https://covid19.who.int Accessed September 4 2020.
- 2.Beigel J., Tomashek K., Dodd L., Mehta A., Zingman B., Kalil A., Hohmann E., Chu H., Luetkemeyer A., Kline S., de Castilla D. Lopez, Finberg R., Dierberg K., Tapson V., Hsieh L., Patterson T., Paredes R., Sweeney D., Short W., Touloumi G., Lye D., Ohmagari N., Oh M., Ruiz-Palacios G., Benfield T., Fätkenheuer G., Kortepeter M., Atmar R., Creech C., Lundgren J., Babiker A., Pett S., Neaton J., Burgess T., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H. Remdesivir for the treatment of Covid-19 - preliminary report. New England J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Karim Q. Abdool, Alejandria M.M., García C. Hernández, Kieny M.P., Malekzadeh R., Murthy S., Reddy K.S., Periago M. Roses, Hanna P. Abi, Ader F., Al-Bader A.M., Alhasawi A., Allum E., Alotaibi A., Alvarez-Moreno C.A., Appadoo S., Asiri A., Aukrust P., Barratt-Due A., Bellani S., Branca M., Cappel-Porter H.B.C., Cerrato N., Chow T.S., Como N., Eustace J., García P.J., Godbole S., Gotuzzo E., Griskevicius L., Hamra R., Hassan M., Hassany M., Hutton D., Irmansyah I., Jancoriene L., Kirwan J., Kumar S., Lennon P., Lopardo G., Lydon P., Magrini N., Maguire T., Manevska S., Manuel O., McGinty S., Medina M.T., Rubio M.L. Mesa, Miranda-Montoya M.C., Nel J., Nunes E.P., Perola M., Portolés A., Rasmin M.R., Raza A., Rees H., Reges P.P.S., Rogers C.A., Salami K., Salvadori M.I., Sinani N., Sterne J.A.C., Stevanovikj M., Tacconelli E., Tikkinen K.A.O., Trelle S., Zaid H., Røttingen J.A., Swaminathan S. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 2021 Feb 11;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020 Apr 7;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario C., Jessup J., Chappell M., Averill D., Brosnihan K., Tallant E., Diz D., Gallagher P. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20) doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 7.Klimas J., Olvedy M., Ochodnicka-Mackovicova K., Kruzliak P., Cacanyiova S., Kristek F., Krenek P., Ochodnicky P. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal mas receptor in spontaneously hypertensive rats. J. Cell. Mol. Med. 2015;19(8) doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soro-Paavonen A., Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L., Sandholm N., Thomas M., Groop P. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012;30(2) doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 9.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807) doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fosbøl E., Butt J., Østergaard L., Andersson C., Selmer C., Kragholm K., Schou M., Phelps M., Gislason G., Gerds T., Torp-Pedersen C., Køber L. Association of Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020 Jul 14;324(2):168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;382(25) doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds H., Adhikari S., Pulgarin C., Troxel A., Iturrate E., Johnson S., Hausvater A., Newman J., Berger J., Bangalore S., Katz S., Fishman G., Kunichoff D., Chen Y., Ogedegbe G., Hochman J. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382(25) doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin. Pharmacol. & Ther. 2020;108(2):236–241. doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A., Liu D., Qin C., Jiang C., Penninger J. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8) doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105(22) doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Penninger J., Li Y., Zhong N., Slutsky A. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4) doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sriram K., Insel P. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020 Nov;177(21):4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X., Zhu Y., Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020;76(2):e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Long C., Xiong Q., Chen C., Ma J., Su Y., Hong K. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID-19, inflammation level, severity, and death in patients with COVID-19: A rapid systematic review and meta-analysis. Clin. Cardiol. 2020 Aug 5 doi: 10.1002/clc.23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baral R., White M., Vassiliou V.S. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr. Atheroscler. Rep. 2020;22(10):61. doi: 10.1007/s11883-020-00880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Griensven J.M.T., Schoemaker R.C., Cohen A.F., Luus H.G., Seibert-Grafe M., Röthig H.-J. Pharmacokinetics, pharmacodynamics and bioavailability of the ACE inhibitor ramipril. Eur. J. Clin. Pharmacol. 1995;47(6):513–518. doi: 10.1007/BF00193704. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, Ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000;342(3) doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 23.Moran A., Simon J.A., Shiboski S., Pickering T.G., Waters D., Rotter J.I., Lyon C., Nickerson D., Yang H., Saad M., Hsueh W., Krauss R.M. Differential effects of ramipril on ambulatory blood pressure in African Americans and Caucasians. Am. J. Hypertens. 2007;20(8):884–891. doi: 10.1016/j.amjhyper.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Zhu L., Cai J., Lei F., Qin J., Xie J., Liu Y., Zhao Y., Huang X., Lin L., Xia M., Chen M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y., Chen J., She Z., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X., Wang X., Touyz R., Xia J., Zhang B., Huang X., Yuan Y., Loomba R., Liu P., Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126(12) doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sriram K., Loomba R., Insel P.A. Targeting the renin-angiotensin signaling pathway in COVID-19: unanswered questions, opportunities, and challenges. Proc. Natl. Acad. Sci. U. S. A. 2020;117(47):29274–29282. doi: 10.1073/pnas.2009875117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material