Abstract
Coronavirus disease-19 (COVID-19), a devastating respiratory illness caused by SARS-associated coronavirus-2 (SARS-CoV-2), has already affected over 64 million people and caused 1.48 million deaths, just 12 months from the first diagnosis. COVID-19 patients develop serious complications, including severe pneumonia, acute respiratory distress syndrome (ARDS), and or multiorgan failure due to exaggerated host immune response following infection. Currently, drugs that were effective against SARS-CoV are being repurposed for SARS-CoV-2. During this public health emergency, food nutraceuticals could be promising prophylactic therapeutics for COVID-19. Curcumin, a bioactive compound in turmeric, exerts diverse pharmacological activities and is widely used in foods and traditional medicines. This review presents several lines of evidence, which suggest curcumin as a promising prophylactic, therapeutic candidate for COVID-19. First, curcumin exerts antiviral activity against many types of enveloped viruses, including SARS-CoV-2, by multiple mechanisms: direct interaction with viral membrane proteins; disruption of the viral envelope; inhibition of viral proteases; induce host antiviral responses. Second, curcumin protects from lethal pneumonia and ARDS via targeting NF-κB, inflammasome, IL-6 trans signal, and HMGB1 pathways. Third, curcumin is safe and well-tolerated in both healthy and diseased human subjects. In conclusion, accumulated evidence indicates that curcumin may be a potential prophylactic therapeutic for COVID-19 in the clinic and public health settings.
Keywords: COVID-19, SARS-CoV-2, Curcumin, Antiviral, Immunomodulator, Therapeutics
COVID-19, SARS-CoV-2, curcumin, antiviral, immunomodulator, therapeutics
1. Introduction
Coronavirus disease-19 (COVID-19) is a dreadful respiratory illness caused by a newly discovered coronaviruses (CoV) strain known as SARS-CoV-2. SARS-CoV-2 much resembles SARS-associated coronavirus (SARS-CoV) that caused the SARS pandemic in 2003. It was first detected in Wuhan city, Hubei province, China, in December 2019, and as of Dec 02, 2020, over 64 million people are diagnosed with COVID-19, with around 1.48 deaths reported across the world (Source: Johns Hopkins University). WHO has declared COVID-19 as a global pandemic, and the prediction is that the number of deaths due to COVID-19 will further worsen in the coming months (Abais et al., 2015; Wu et al., 2020b). In most cases, COVID-19 patients exhibit fever, dry cough, dyspnoea, fatigue, and myalgia (Chen et al., 2020c; Huang et al., 2020; Wu et al., 2020a). However, in severe cases, COVID-19 patients develop fatal complications such as severe pneumonia, acute respiratory distress syndrome (ARDS), septic shock, arrhythmia, and acute cardiac injury (Wang et al., 2020). Other than the management with ventilator support and other supportive care, there are no effective treatments available for COVID-19. Therefore, there is an urgent need for the discovery and development of therapeutics for COVID-19.
The discovery and development of an effective therapeutic agent against SARS-CoV-2 is a time-consuming process. Alternatively, repurposing of already-licensed pharmaceuticals may provide a faster path for developing effective therapeutics for COVID-19. First, effective drugs against SARS-CoV were tested against SARS-CoV-2 in the laboratory and clinical settings, and the results were very encouraging. These studies prompted WHO to initiate a large global mega trial called ‘SOLIDARITY’ for testing four therapeutics for COVID-19: i) remdesivir, an inhibitor of RNA-dependent RNA Polymerase; ii) antimalarial drugs chloroquine and hydroxychloroquine; iii) anti-retroviral drugs, lopinavir-ritonavir that are HIV protease inhibitors, and iv) immunomodulatory agent, interferon-beta. Additionally, vaccine trials (Amanat and Krammer, 2020) and convalescent plasma therapy (Bloch et al., 2020; Shen et al., 2020) for SARS-CoV-2 are also under investigation; the outcomes are very promising. So far, some vaccination trials for COVID-19 have shown to be safe and effective in inducing robust humoral and cellular responses in the participants (Jackson et al., 2020; Ramasamy et al., 2020; Walsh et al., 2020). Although it is good news, it is likely to take many months to vaccinate the entire global population.
Along with repurposing approved drugs, scientists are also actively seeking safe, natural products with antiviral pharmacological activity as potential prophylactic therapeutics for COVID-19. Turmeric (Curcuma longa) is a perennial herbaceous, rhizomatous plant of the ginger family Zingiberaceae. It is widely used in Ayurveda, Siddha, and traditional Chinese medicines for its medicinal properties such as antiviral, analgesic, antimicrobial, antiproliferative, and anti-inflammatory activity (Aggarwal and Sung, 2009; Karimi et al., 2019; Padmanaban and Rangarajan, 2016; Patel et al., 2020). Turmeric is also the most popular spice across the globe, especially in India. Turmeric's medicinal properties are primarily attributed to three main curcuminoids-curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcumin (diferuloylmethane) is the most abundant bioactive curcuminoid in turmeric. It elicits diverse pharmacological activities, including antioxidant, anti-inflammatory, anti-bacterial, antiviral, and immunomodulatory activity (Aggarwal and Sung, 2009; Karimi et al., 2019; Padmanaban and Rangarajan, 2016; Patel et al., 2020). Curcumin has been an effective antiviral agent against many enveloped viruses, including respiratory viruses such as influenza A virus and Respiratory Syncytial Virus (Praditya et al., 2019). More importantly, curcumin is also reported to be effective against SARS-CoV in in vitro studies (Wen et al., 2007). Additionally, findings from animal models suggest that curcumin supplementation intervenes in several respiratory diseases, in particular, acute respiratory distress syndrome (ARDS), acute lung injury, pneumonia, pulmonary fibrosis, and sepsis by modulating inflammation and oxidative stress (Lelli et al., 2017; Venkatesan et al., 2007). US Food and Drug Administration (FDA) has approved curcuminoids as "Generally Recognized As Safe" (GRAS). Several clinical studies have documented the tolerability and safety profile of curcumin both in healthy subjects and patients. In the present review, we have discussed i) broad-spectrum antiviral activity of curcumin against enveloped viruses including SARS-CoV-2; ii) immunomodulatory activity of curcumin against infectious ARDS; iii) safety profile. Because curcumin as a food component is consumed widely, we provide a strong case for testing curcumin as a promising prophylactic, therapeutic candidate for the treatment of COVID-19 in clinical and or public health settings.
2. Pathophysiology of COVID-19
COVID-19 is caused by beta-coronavirus, and its genome sequence is 79% similar to SARS-associated coronavirus (SARS-CoV) (Lu et al., 2020); therefore, it was named SARS-CoV-2. SARS-CoV-2 is an enveloped virus with positive sense, single-strand RNA genome of size ~30 kb. Like other coronaviruses, the SARS-CoV-2 genome encodes for four major structural proteins-spike (S) protein, membrane protein, envelope protein, and nucleocapsid protein (Fehr and Perlman, 2015). The membrane protein is the predominant structural protein that helps in the virion assembly by interacting with other structural proteins and maintains the viral shape. Nucleocapsid protein interacts with the viral genome and regulates the replication process. Envelope protein is the smallest structural protein, which helps in viral maturation, and budding. The spike protein protrudes from the surface of the SARS-CoV-2 and attaches to angiotensin-converting enzyme 2 (ACE2) protein expressed by target lower airway cells (ciliated epithelial cells and type II pneumocytes) and mediates the entry of the virus into the cell (Hoffmann et al., 2020; Walls et al., 2020). The Spike protein is a homotrimer, and each monomer of spike protein consists of S1 and S2 subunit. The S1 subunit consists of the receptor-binding domain that interacts with ACE2 (Tai et al., 2020). The SARS-CoV-2 spike protein binding affinity for ACE2 correlates well with the rate of transmissibility and severity of the diseases (Hoffmann et al., 2020; Walls et al., 2020; Zhou et al., 2020).
The pathogenesis of COVID-19 is yet to be completely understood; however, to a great extent, it resembles to severe acute respiratory syndrome (SARS) pandemic. Common symptoms associated with COVID-19 patients were fever, dry cough, myalgia, fatigue, and dyspnoea. The average time period from onset of symptoms to hospital admission was 7 days, and all patients with COVID-19 were associated with mild-to severe-pneumonia (Huang et al., 2020). The systemic inflammation, as indicated by plasma levels of multiple interleukins including IL2, IL7, IL6, IL10, GCSF, IP10, MCP1, MIP1A, and TNFα, was elevated in COVID-19 patients as compared to healthy subjects, which further increased in ICU admitted patients (Chen et al., 2020a; Huang et al., 2020). Also, ICU admitted COVID-19 patients showed a immunosuppressive phenotype characterized by severe lymphopenia and lower IFN-gamma levels (Chen et al., 2020a). Few COVID-19 patients in ICU also developed secondary infections. Histopathological analysis showed typical ARDS features, including an influx of mononuclear cells, including monocytes and lymphocytes, into air spaces, bilateral diffuse alveolar damage, and desquamation of alveolar epithelial cells, and hyaline membrane formation (Xu et al., 2020). The mortality was mainly due to respiratory failure caused by ARDS and multiorgan failure (Du et al., 2020; Huang et al., 2020).
3. Antiviral effects of curcumin against enveloped viruses including SARS-CoV-2
A large body of evidence has documented curcumin's direct antiviral activity against several enveloped viruses (Praditya et al., 2019), including SARS-CoV (Table 1). Wen et al. (2007) used cytopathic effects of SARS-CoV in Vero 6 cells as a cell-based assay to screen phytochemicals against SARS-CoV. They reported that curcumin (at 20 μM and 40μM) showed significant anti-SARS-CoV activity. The same study reported that curcumin inhibits SARS-CoV 3CL protease activity, which is vital for viral replication. Few studies have utilized computational modelling tools and predicted that curcumin interacts with S protein and ACE2 protein and potentially intervenes in viral entry into lung cells (Pandey et al., 2020). Based on these pleiotropic effects of curcumin, it is postulated that curcumin may directly intervene SARS-CoV-2 entry and or replication and prevent infection (Soni et al., 2020). 4-octyl-itaconate, a pharmacological agonist of transcription factor NRF2 was reported to repress SARS-CoV-2 replication in lung cells by an independent interferon mechanism (Olagnier et al., 2020). Furthermore, the same study revealed that the activation of the NRF2 pathway by another structurally dissimilar agonist dimethyl fumarate inhibited SARS-CoV-2 replication, suggesting that NRF2 agonists are potential drug candidates for repressing SARS-CoV-2 replication (Olagnier et al., 2020). We (Pandey et al., 2011; Thimmulappa et al., 2008) and others (Fattori et al., 2015; Jiménez-Osorio et al., 2016; Li et al., 2016) have previously reported that curcumin is a promising NRF2 agonist, and administration of curcumin activates NRF2 pathway in lungs of mice (Garg et al., 2008; Shen et al., 2015; Thimmulappa et al., 2008). So, it is conceivable that curcumin may also exert antiviral activity against SARS-COV-2 by activating the NRF2 pathway.
Table 1.
Antiviral effects of curcumin against enveloped viruses, which causes human diseases.
| S.No | Virus Name | Study design (dose) | Human Disease | Mode of action | Reference |
|---|---|---|---|---|---|
| 1 | SARS-CoV | In vitro Study (20 & 40 μM) | Severe acute respiratory syndrome | Inhibit SARS-CoV 3CL protease | (Wen et al., 2007) |
| 2 | Influenza A virus | In vitro Study (30 μM) | Respiratory illness | Disrupts virus envelope; Inhibits haemagglutinin activity; Abrogates NF-κB signaling | (Chen et al., 2013; Dai et al., 2018; Ou et al., 2013) |
| 3 | Respiratory syncytial virus | In vitro Study (10 μg/ml) | Respiratory illness | Inhibits viral replication and budding | (Obata et al., 2013) |
| 4 | Herpes simplex virus type 1 | In vitro Study (30 μg/ml) | Cold sores | Not Reported | (Zandi et al., 2010) |
| 5 | Japanese encephalitis virus | In vitro Study (30 μM) | Encephalitis | Disrupts of viral envelope | (Chen et al., 2013) |
| 6 | HIV | In vitro Study (30 & 40 μg/ml) | AIDS | Inhibit HIV proteases & HIV-integrase | (Mazumder et al., 1995; Sui et al., 1993; Vajragupta et al., 2005) |
| 7 | Hepatitis B & C virus |
In vitro Study (40 μM) In vitro Study (20 μM) |
Liver disease | Downregulates PGC-1α; Inhibit RNA replication & viral assembly | (Anggakusuma et al., 2014; Rechtman et al., 2010) |
| 8 | Zika | In vitro study (5 μM) | Zika Fever | Inhibit virus binding to host cell | (Mounce et al., 2017) |
| 9 | Chikungunya virus | In vitro study (5 μM) | Chikungunya fever | Inhibit virus binding to host cell | (Mounce et al., 2017) |
| 10 | Dengue virus | In vitro study (52.97 μg/ml) | Dengue fever | Disrupt viral envelope | (Chen et al., 2013; Nabila et al., 2020) |
A recent study employed genome-wide screening using CRISPR-Cas9 and discovered HMGB1 as an important pro-viral host factor in determining SARS-CoV-2 infection (Wei et al., 2020). HMGB1 is a non-histone nuclear protein that binds with DNA and regulates transcription (Harris et al., 2012). However, under stress conditions such as viral infection, the intracellular expression of HMGB1 is markedly elevated, and it is also secreted into the extracellular milieu (Harris et al., 2012). The extracellular HMGB1 function as a danger-associated molecular pattern further augments inflammatory responses by binding to Toll-like receptors and or activating inflammasome complex (Harris et al., 2012). Wei et al. (2020) observed that SARS-CoV-2 infection in Vero-E6 cells increased intracellular and extracellular levels of HMGB1. Furthermore, the authors found that genetic disruption of HMGB1 repressed SARS-CoV-2 replication in Vero cells. Mechanistic studies revealed that HMGB1 down-regulates transcriptional expression of ACE2 epigenetically, and the study concluded that HMGB1 antagonist might be a potential drug candidate for protecting from SARS-CoV-2 susceptibility (Wei et al., 2020). Several studies have demonstrated that curcumin pre-treatment represses HMGB1 expression (Da et al., 2019; Gu et al., 2015) and dampens HMGB1 mediated proinflammatory responses (Cheng et al., 2018; Wang et al., 2012). Therefore, curcumin supplementation may potentially protect from SARS-CoV-2 infection by down-regulating ACE2 expression.
Curcumin was also effective against the respiratory virus, influenza A virus (IAV), and respiratory syncytial virus (RSV), which are the common causal agents of acute respiratory infections in the community (Chen et al., 2013; Dai et al., 2018; Ou et al., 2013). Curcumin inhibits IAV hemagglutinin's binding, a homotrimeric membrane glycoprotein, with host cell receptors (Ou et al., 2013). Curcumin also impairs the replication and budding of RSV in human nasal epithelial cells by inhibiting NF-κB and cyclooxygenase 2 signaling, implicated in RSV replication and budding (Obata et al., 2013). Mounce et al. (2017), reported antiviral effects of curcumin against the Zika and Chikungunya virus. The investigators demonstrated that curcumin or its derivatives attenuated the Zika and Chikungunya virus's infectivity by modifying its surface proteins, which resulted in blockage of its binding and entry into the host cells (Mounce et al., 2017). Curcumin and its analogs were also effective in inhibiting HIV replication by targeting HIV protease (Sui et al., 1993), HIV integrase (Mazumder et al., 1995; Vajragupta et al., 2005), and HIV tat protein (Ali and Banerjea, 2016). Anggakussuma et al. (Anggakusuma et al., 2014) studied the antiviral effects of curcumin against the hepatitis C virus. They observed that curcumin impaired virus binding and fusion with cell membranes by affecting the viral envelope's fluidity. Zen et al. (Chen et al., 2013) assessed whether curcumin's broad-spectrum antiviral activity is mediated by disruption of the enveloped virus's membrane structure. To address this, the authors evaluated the antiviral activity of curcumin against IAV, and other RNA enveloped virus (New castle disease virus, Dengue virus, Japanese encephalitis virus), and DNA enveloped (PRV swine herpes virus, vaccinia virus) in Vero cells. The authors observed that the curcumin effectively inhibited plaque formation when the virus was in-direct contact with the agent (during pre-and co-treatment experimental regimen); however, its antiviral activity was lost when Vero cells were treated post-infection. Furthermore, curcumin was ineffective against the non-enveloped virus, enterovirus 71. The authors concluded that curcumin's intrinsic ability to disrupt the membrane integrity of enveloped viruses was the common mechanism for inhibiting the viral infectivity (Chen et al., 2013).
Emerging evidence suggests that antiretroviral drug lopinavir-ritonavir, a protease inhibitor exerts antiviral activity against SARS-CoV-2 (Choy et al., 2020). Unfortunately, combination lopinavir-ritonavir in COVID-19 patients showed no clinical benefits (Cao et al., 2020); however, the results of other on-going trials with lopinavir-ritonavir awaited. Hoffmann et al. (2020) reported that camostat mesylate, an inhibitor of serine protease TMPRSS2, effectively blocked the entry of SARS-CoV-2. Lopinavir/ritonavir (Chu et al., 2004; Yao et al., 2020) and camostat mesylate were also effective against SARS-associated coronavirus (Zhou et al., 2015). In vitro studies have demonstrated the potency of curcumin to function as an HIV protease inhibitor (Sui et al., 1993).
In summary, curcumin exerts antiviral activity against the enveloped virus by multiple mechanisms such as direct interaction with viral membrane proteins, disruption of the viral envelope, inhibition of viral proteases, and modulating host factors NF-κB, NRF2 and or HMGB1 pathways. Given that in vitro studies have confirmed the antiviral activity of curcumin against SARS-CoV, it is tempting to speculate that curcumin may also attenuate SARS-CoV-2 infectivity by mechanisms as summarized above.
4. Immunomodulatory activity of curcumin suppress cytokine release syndrome and mitigates progression to ARDS
The death of severely ill COVID-19 patients is associated with respiratory failure and or multiorgan failure caused by ARDS and septic shock. ARDS and or sepsis's pathogenesis involves an early hyperactivated inflammatory response characterized by a "cytokine storm" (Chen et al., 2020a, 2020c). A positive association between cytokine release syndrome (CRS) and the severity of illness and mortality among COVID-19 patients is reported by many studies (Tang et al., 2020). Immunomodulatory drugs are likely to effectively mitigate ARDS progression or sepsis by dampening early inflammatory response following infectious insults (Tang et al., 2020; Yadav et al., 2017). A recently concluded clinical trial found that dexamethasone, a widely used immunomodulatory drug, effectively improved the survival of severely ill hospitalized COVID-19 patients compared to placebo (Horby et al., 2020; Tomazini et al., 2020). This finding has renewed the interest in finding safer and more effective immunomodulatory therapeutics to treat COVID-19. Analysis of lung transcriptome and the circulatory inflammatory cytokines profiles of COVID-19 patients indicated that NF-κB, inflammasome, IL-6 trans-signaling, and HMGB1 signaling may be driving the CRS (El-Hachem et al., 2020; Lee et al., 2020). We present several lines of evidence to illustrate how pleiotropic effects of curcumin could dampen CRS and mitigate the progression to ARDS and or sepsis in COVID-19 patients.
Curcumin administration via oral route alleviated reovirus 1/L-induced-ARDS in mice that recapitulate the clinical features of ARDS (Avasarala et al., 2013). The same study reported that curcumin lowered interleukins levels such as IL6, MCP1, and IFN gamma by abrogating NF-κB activation in the lungs (Avasarala et al., 2013). In most cases, the clinical course of respiratory viral infection is exacerbated by secondary bacterial infections. Curcumin also effectively protects against lethal bacterial pneumonia (Zhang et al., 2019). Direct delivery of water-soluble curcumin into the lungs of mice infected with Klebsiella pneumonia dramatically improved the survival and reduced lung and blood bacteriemia compared to the vehicle-treated group. The improved survival of the infected mice treated with curcumin was associated with significantly reduced levels of inflammatory mediators (IL1β, TNFα, IL6, KC) in bronchoalveolar lavage fluid, lung tissue, and serum as well as diminished oxidative tissue injury as compared to vehicle treatment (Zhang et al., 2019). Most studies have revealed that curcumin alleviates viral-induced pulmonary inflammation by abrogating the activation of NF-κB signalling (Zhang et al., 2019) potentially by inhibiting upstream IKKβ kinase activity (Jobin et al., 1999; Kim et al., 2011; Xu and Liu, 2017).
Cytokines, especially IL1β and IL6, were markedly elevated in severely ill COVID patients admitted to intensive care units compared to stable COVID-19 patients (Conti et al., 2020; McElvaney et al., 2020). These observations have implicated inflammasome activation in driving CRS (Cauchois et al., 2020; Lee et al., 2020). Several studies have found the potency of curcumin to block inflammasome activation through Nod-like receptor family, pyrin domain-containing 3 (NLRP3), and inhibit secretion of mature IL-1β (Yin et al., 2018)) and abrogate pulmonary inflammation (Liu et al., 2018; Zhang et al., 2019).
A growing body of evidence suggests that a higher circulatory IL6 level is predictive of severity of illness and mortality in COVID-19 patients (Nasonov and Samsonov, 2020), and abrogating IL6 signalling by JAK inhibitors is advocated to be an additional way to mitigate the progression to ARDS in COVID-19 patients (Nasonov and Samsonov, 2020). Although the direct role of IL6 per se in acute lung injury is conflicting, IL6 is implicated in-hyperactivation of immune responses; vascular dysfunction leading to multiorgan failure; fibrotic responses by trans signalling mechanisms in which the complex of IL6 with soluble IL6 receptor (SIL-6R) promote pro-inflammatory cytokine expression in T helper (Th)1 cells, monocytes and stromal cells (fibroblasts and endothelial cells) via activation of downstream JAK/STAT3 signaling (Barkhausen et al., 2011; Scheller et al., 2014). Selective blockade of IL6 trans-signaling pathway in macrophages dampened inflammatory cytokine secretion and protected from polymicrobial sepsis in mouse models (Barkhausen et al., 2011). CRS also mediates endothelial injury and increases the risk for coagulopathy in COVID-19 patients, further worsening the prognosis. Hospitalized COVID-19 patients are associated with elevated levels of plasminogen activator inhibitor-1 (PAI-1). Kang et al. (2020), reported that IL-6 trans-signaling plays a crucial role in endothelial dysfunction during microbial infection resulting in secretion of PAI-1, promoting thrombosis. The same researchers showed blockade of IL-6 trans-signaling by Tocilizumab decreased endothelial dysfunction and circulatory levels of PAI-1 in COVID-19 patients. Curcumin effectively blocked IL6 trans-signaling (Bharti et al., 2003; Hahn et al., 2018; Weissenberger et al., 2010) and inhibited the progression of inflammatory diseases (Zhao et al., 2016; Zhu et al., 2017). Curcumin is also reported to exert anticoagulant activity by reducing TNF alpha-induced endothelial tissue factor (Pendurthi et al., 1997)) and by directly inhibiting thrombin activity (Kim et al., 2012). Chen et al. reported that curcumin administration significantly reduced plasma fibrinogen and fibrin deposition in kidney in rat model of LPS-induced endotoxemia (Bierhaus et al., 1997).
Circulatory levels of HMGB1 positively correlate with severe illness in COVID-19 patients (Chen et al., 2020b). Extracellular HMGB1 functions as an inflammatory cytokine and plays a crucial role in the pathogenesis of acute lung injury and/ARDS (Andersson et al., 2020) by upregulating inflammation. In mouse models, curcumin administration downregulated HMGB1 expression and attenuated LPS-induced acute lung injury (Cheng et al., 2018). Moreover, curcumin supplementation also protected from HMGB1 mediated vascular injury and hepatitis (Wang et al., 2012) in mouse models.
Besides inhibiting proinflammatory signals, curcumin also upregulates anti-inflammatory responses. Curcumin administration alleviated acute lung injury induced in cecal ligation and puncture-induced sepsis model, and it was accompanied by elevated numbers of regulatory T cells (FOXP3+ T-reg) and M2 Macrophages (Chai et al., 2020). T-reg and M2 macrophages suppress activated immune cells (CD4+, CD8+ T cells) via TGF-β and IL-10 secretion and facilitate inflammation resolution (Chai et al., 2020). In vitro studies revealed that curcumin enhanced the differentiation of T-reg cells and the polarization of M2 macrophages (Chai et al., 2020). These findings suggest that curcumin treatment may improve the resolution of pulmonary inflammation and tissue repair by increasing T-reg and M2 macrophages. In the later stages of COVID-19, some severely ill patients develop myelosuppression characterized by a lower number of blood neutrophils and monocytes, which increases the risk for secondary infections (Liao et al., 2020). Curcumin has been shown to potentiate myelopoiesis in mouse models, although the underlying mechanisms are not clear (Vishvakarma et al., 2012). Few studies have also revealed that curcumin mediates immunomodulatory activity by affecting other molecular targets such as NADPH oxidase, and NRF2. Besides direct antioxidant activity, curcumin dampens reactive oxygen species (ROS) generation by inhibiting NADPH oxidase activity (Derochette et al., 2013; Huang et al., 2015). ROS generated by NADPH oxidase activity is implicated in over-activation of the innate immune response, inflammation, tissue injury, and death during sepsis (Bernard et al., 2014; Kong et al., 2010). Curcumin also activates transcription factor NRF2, a master regulator of cellular antioxidant defenses, including heme oxygenase-1 and GSH-biosynthesizing enzymes (Dai et al., 2018; Pandey et al., 2011). Activation of the NRF2 pathway in shown to mitigate oxidative tissue injury in the lungs (Rangasamy et al., 2004; Thimmulappa et al., 2006a), inhibits pulmonary inflammation (Harvey et al., 2011), protects from an acute lung injury, ARDS, and or sepsis (Cuadrado et al., 2019; Kong et al., 2011; Thimmulappa et al., 2006a, 2006b, 2007; Yamamoto et al., 2018).
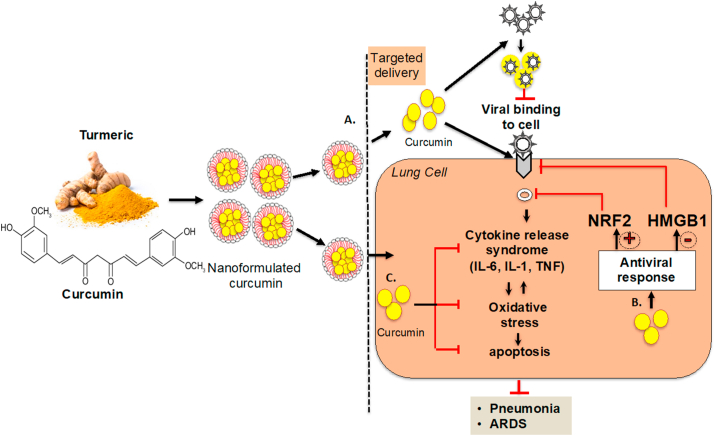
As illustrated in Figure 1, a large body of evidence provides a strong rationale that curcumin supplementation could alleviate cytokine release syndrome, oxidative stress, apoptosis, and ensuing tissue injury following viral infection; thus, it could be a promising therapeutic for COVID-19.
Figure 1.
The schematics representing the potential mechanisms by which curcumin may be effective against COVID-19 (A) Antiviral activity of curcumin against SARS-CoV-2 mediated by disrupting the viral envelope, S protein and or ACE2, which prevents the entry of the virus into the cells. B. Curcumin induces antiviral responses by positively regulating NRF2 and repressing ACE2 expression by negatively regulating HMGB1. C. Curcumin mediates immunomodulatory responses by inhibiting cytokine response syndrome and oxidative stress and thus mitigating the progression to pneumonia and or ARDS following SARS-CoV-2 infection.
5. Safety profile, bioavailability, and clinical efficacy of curcumin
In ayurvedic medicine, turmeric has been employed to cure a broad spectrum of common ailments, and similar usage has been noted in Chinese traditional medicine. Clinical trial in asthmatics using turmeric extract was associated with better disease control than placebo (Manarin et al., 2019). Because of its diverse pharmacological activities and FDA approval as "Generally Recognized As Safe," curcumin has been evaluated to treat various human diseases in over 100 clinical trials (www.clinicaltrial.gov). We have summarized the selected clinical trials conducted in the last 10 yr in Table 2, and these human trials were conducted both in healthy subjects and diseased patients. Almost all the clinical trials have revealed that curcumin supplementation is safe and well-tolerated even at doses as high as 8 g/day. There is significant accumulated evidence suggesting curcumin or curcumin containing nutraceuticals mitigate various human diseases by exerting anti-oxidant and anti-inflammatory actions. Randomized clinical trials (RCT) have shown that curcumin, when supplemented as adjunctive therapy maintained and or induced remission in ulcerative colitis patients (Coelho et al., 2020). Hanai et al. (2006) reported that curcumin supplementation (two doses of 1g/day) for six months as an adjunct therapy in patients with stable ulcerative colitis was well tolerated and significantly protected from remission and improved the quality of life as compared to placebo. Lang et al. (2015) conducted a multicentre RCT in patients with mild to moderate ulcerative colitis. They observed that patients receiving curcumin (3g/day) and mesalamine showed significant clinical remission compared to the placebo plus mesalamine group. In an RCT, Campbell et al. (2019) reported beneficial effects of curcumin in young adults with high-risk for cardiovascular diseases (body mass index >30). Curcumin supplementation (500 mg/day) for 12 weeks significantly reduced serum levels of homocysteine and concomitantly increased HDL-cholesterol (HDL-C) levels, however, there was no improvement in blood pressure as compared to placebo (Campbell et al., 2019). Panahi et al. (2015) compared lipid profiles in patients with metabolic syndrome at baseline and eight weeks after curcuminoids supplementation (1g/day). When compared to baseline, curcuminoid supplementation for eight weeks significantly increased HDL-C as well as reduced serum levels of LDL-C, total cholesterol, triglycerides, and lipoprotein(a) (Panahi et al., 2014)). Nanoformulation of curcumin was also evaluated in patients with rheumatoid arthritis. Administration of curcumin nanomicelle (40mg, 3 times/day) to rheumatoid arthritis patients for 12 weeks reduced the clinical disease score (tender joint count and swollen joint count) compared to baseline. In another RCT, Amalraj et al. (2017) also reported that supplementation of highly bioavailable curcumin formulation reduced the clinical symptoms in patients with rheumatoid arthritis as compared to placebo. Furthermore, supplementation of curcumin at high doses showed clinical improvement in gastrointestinal disorders, namely in gall-bladder, gastric ulceration, irritable bowel disease, tropical pancreatitis, and biliary motility (reviewed in (Gupta et al., 2013).
Table 2.
Completed clinical trials of curcumin in healthy and diseased subjects with various disorders.
| Sl. No | Disease condition | Dose | Sample size; Study design; study Duration | Study Outcome | Reference |
|---|---|---|---|---|---|
| 1 | Rheumatoid arthritis | 1.2 g/day | N = 18; double blind, cross over study; 15 days | Significant improvement in pain and physical function scores | S. D. Deodhar et al., Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 71, 632–634 (1980). |
| 2 | Osteoarthritis (Knee) | 1.5 g/day | N = 367; Randomized, multicenter study; 30 days | No significant Changes | V. Kuptniratsaikul et al., Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging 9, 451–458 (2014). |
| 3 | Osteoarthritis (Knee) | 0.5 g twice per day | N = 120, Randomized, Single blind, multicenter study; 42 days | Significant improvement in pain and physical function scores | K. Madhu et al., Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology 21, 129–136 (2013). |
| 4 | Osteoarthritis (Knee) | 0.5 g twice per day | N = 40, Randomized, double-blind placebo-controlled parallel trial; 42 days |
Significant improvement in pain and physical function scores | Y. Panahi et al., Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res 28, 1625–1631 (2014). |
| 5 | Metabolic disorders | 0.5 g twice per day | N = 117; Randomized controlled trial; 56 days | Significant decrease in inflammatory cytokines (MCP-1, TGF-β, IL-6 and TNF-α) | Y. Panahi et al., Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr 34, 1101–1108 (2015). |
| 6 | Psoriasis | 2g/day | N = 63, Randomized, double-blind, placebo-controlled; 84 days |
Reduction in PASI Score (erythema, scaling and induration of lesions) and serum IL-22 titer | E. Antiga et al., Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. Biomed Res Int 2015, 283634 (2015). |
| 7 | Scalp Psoriasis | Turmeric tonic, twice per day | N = 40; Randomized, <!--Soft-enter Run-on-- > double-blind, placebo-controlled; 63 days |
Reduction in PASI Score (erythema, scaling and induration of lesions) and improvement | P. Bahraini et al., Turmeric tonic as a treatment in scalp psoriasis: A randomized placebo-control clinical trial. J Cosmet Dermatol 17, 461–466 (2018) |
| 8 | Radiation dermatitis | 6g/day | N = 30; Randomized, double-blind, placebo-controlled; Until completion of radiotherapy |
Reduction of Radiation dermatitis severity scores | J. L. Ryan et al., Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res 180, 34–43 (2013). |
| 9 | Healthy (Aged adults) | 1.5g/day BCM-95®CG (Biocurcumax™) | N = 160; Randomized, double-blind, placebo-controlled trial; 365 days | No significant differences in clinical parameters and cognitive measures | S. R. Rainey-Smith et al., Curcumin and cognition: a randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br J Nutr 115, 2106–2113 (2016). |
| 10 | Healthy (Aged adults) | Longvida® Optimized Curcumin, in 1 month dose of 400 mg | N = 60; Randomized, double-blind, placebo-controlled Phase 3/4 trial; 30 days | Improvisation of alertness and<!--Soft-enter Run-on-- > contentedness; as well as decreases levels of LDL cholesterol | K. H. Cox et al., Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol 29, 642–651 (2015). |
| 11 | Alzheimer Disease | 0.09 g curcumin in theracurmin; twice per day | N = 46, Randomized, double-blind, Two groups; 540 days | Improvement in Buschke selective reminding test, visual memory and attention. | G. W. Small et al., Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am J Geriatr Psychiatry 26, 266–277 (2018). |
| 12 | Anxiety and depression | 1g/day | N = 50, Randomized, double- blind blind, placebo-controlled study; 56 days | Significant decrease Inventory of Depressive Symptomatology score | A. L. Lopresti et al., Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur Neuropsychopharmacol 25, 38–50 (2015). |
| 13 | Anxiety and depression | 1g/day | N = 56, Randomized, double-blind, placebo-controlled study; 56 days | Significant decrease Inventory of Depressive Symptomatology score | A. L. Lopresti et al., Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord 167, 368–375 (2014). |
| 14 | Anxiety and depression | 1g/day | N = 108; Randomized, double-blind, placebo-controlled study; 42 days | Significant decrease in antidepressent behavioral response, along with decrease in inflammatory cytokines in the plasma | J. J. Yu et al., Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J Clin Psychopharmacol 35, 406–410 (2015). |
| 15 | Anxiety and depression | 1g/day (C3 Complex formula) | N = 30; Double blind, cross over trail; 30 days | Significant decrease Beck Anxiety Inventory scale | H. Esmaily et al., An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin J Integr Med 21, 332–338 (2015). |
| 16 | Anxiety and depression | 0.5 g/day | N = 40; Randomized, double-blind, placebo-controlled study; 35 days | Reduction in rapid depressive symptoms reduction | J. Bergman et al., Curcumin as an add-on to antidepressive treatment: a randomized, double-blind, placebo-controlled, pilot clinical study. Clin Neuropharmacol 36, 73–77 (2013). |
| 17 | Anxiety and depression | 1 g/day | N = 60; Randomized controlled trail; 42 days | Reduction in Hamilton depression rating scale | J. Sanmukhani et al., Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res 28, 579–585 (2014). |
| 18 | Cardiovascular disorder (Hypercholestrolemic) | 0.2 g/day | N = 70; Randomized, double-blind, placebo-controlled study; 28 days | Reduction in total cholesterol | J. J. A. Ferguson et al., Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 82, 22–35 (2018). |
| 19 | Cardiovascular disorder (Obese) | 0.5 g/day (C3 complex capsules) | N = 30; Randomized, double-blind, cross over study; 30 days | Reduction in pro-oxidant-anti-oxidant balance and serum Triglycerol | A. Sahebkar et al., Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother Res 27, 1883–1888 (2013). |
| 20 | Metabolic syndrome | 1.89 g/day | N = 65; Randomized, double-blind study; 84 days | Reduction in low density lipoprotein cholesterol and triglycerol and triglycerol/high density lipoprotein cholesterol ratio | Y. S. Yang et al., Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother Res 28, 1770–1777 (2014). |
| 21 | Metabolic syndrome | 2.4 g/day and 1.5 g/day | N = 250; Randomized, double-blind, placebo-controlled study; 56 days | Improved Body Mass Index, body fat and waste circumference | F. Amin et al., Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome - a double blind randomized controlled trial - TAK-MetS trial. Complement Ther Med 23, 165–174 (2015). |
| 22 | Inflammatory Bowel Disease (mild to moderate active ulcerities colities) | 3 g/day | N = 50; Randomized, double-blind, placebo-controlled study; 30 days | Improvement in clinical symptoms | A. Lang et al., Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol 13, 1444–1449.e1441 (2015). |
| 23 | Hepatoprotective effects | 0.237 g/day | N = 60; Randomized, double-blind, placebo-controlled study; 84 days | Reduction in alanine aminotransferase, Aspartate amino-transferase levels | S. W. Kim et al., The effectiveness of fermented turmeric powder in subjects with elevated alanine transaminase levels: a randomised controlled study. BMC Complement Altern Med 13, 58 (2013). |
| 24 | non-alcoholic fatty liver disease | 0.07 g/day | N = 80; Randomized, double-blind, placebo-controlled study; 56 days | Reduction in weight and body mass index and improvement liver ultrasonographic findings | S. Rahmani et al., Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother Res 30, 1540–1548 (2016). |
| 25 | Chronic kidney disorder | 0.32 g/day | N = 101, Randomized, double-blind, placebo-controlled study; 56 days | Reduction in lipid peroxidation and increase in antioxidant activity | A. S. Jiménez-Osorio et al., The Effect of Dietary Supplementation With Curcumin on Redox Status and Nrf 2 Activation in Patients With Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J Ren Nutr 26, 237–244 (2016). |
| 26 | Chronic prostatitis/Chronic pelvic pain syndrome | 0.2 g | N = 60; Randomized, single-blind, placebo-controlled study, Phase II; 30 days | Improvement in chronic Chronic Prostatitis Symptom Index | G. Morgia et al., A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III. Arch Ital Urol Androl 89, 110–113 (2017). |
| 27 | Type II Diabetis Mellitus | 0.3 g/day | N = 100; Randomized double-blind, placebo-controlled study; 84 days | Reduction in fasting blood glucose and free fatty acid levels | L. X. Na et al., Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res 57, 1569–1577 (2013). |
| 28 | Type II Diabetis Mellitus | 2 g/day | N = 60, Open label randomized clinical study; 28 days | Decrease in fasting blood glucose, low density lipoprotein cholestrol and anti-inflammatory molecule hs C-reactive protein | N. Maithili et al., Efficacy of Turmeric as Adjuvant Therapy in Type 2 Diabetic Patients. Indian J Clin Biochem 30, 180–186 (2015). |
| 29 | Type II Diabetis Mellitus | 0.08 g/day (nano-formulation) | N = 70, Randomized double blind, placebo control add-on clinical study; 90 days | Reduction in fast blood glucose, triglycerol, glycated hemoglobin a1c, and body mass index | H. R. Rahimi et al., The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6, 567–577 (2016). |
| 30 | Type II Diabetis Mellitus | 0.45 g/day | N = 8, Open-label, randomized control study; 11 days | Decrease in glucose, low density lipoprotein cholestrol, very low density lipoprotein cholestrol and triglycerol | P. Neerati et al., Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother Res 28, 1796–1800 (2014). |
| 31 | Obesity (female) | 2.8 g/day | N = 62, Randomized, doubled-blind, placebo-controlled, crossover study; 70 days | No significant changes in clinical parameters | F. Di Pierro et al., Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci 19, 4195–4202 (2015). |
| 32 | Obesity | 1 g/day | N = 30, Randomized, doubled-blind, cross over study; 28 days | Reduction in inflammatory cytokines, and vascular endothelial growth factor | S. Ganjali et al., Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. ScientificWorldJournal 2014, 898361 (2014). |
| 33 | Beta-thalassemia | 0.5 g/day | 60 | Reduction in oxidative stress, iron levels and increase in hemoglobin concentration and antioxidant level. | O. U. Yanpanitch et al., Treatment of β-Thalassemia/Hemoglobin E with Antioxidant Cocktails Results in Decreased Oxidative Stress, Increased Hemoglobin Concentration, and Improvement of the Hypercoagulable State. Oxid Med Cell Longev 2015, 537954 (2015). |
| 34 | Beta-thalassemia | 1 g/day | N = 68, Randomized, doubled-blind, controlled clinical study; 84 days | Reduction in total & direct bilirubin content. Increase in antioxidant activity, hemoglobin, ferritin, iron, and catalase activity. | E. Mohammadi et al., An investigation of the effects of curcumin on iron overload, hepcidin level, and liver function in β-thalassemia major patients: A double-blind randomized controlled clinical trial. Phytother Res 32, 1828–1835 (2018). |
| 35 | Breast Cancer (metastatic) | 0.5–8 g/day | N = 14, open-label, Phase I clinical trial; 7 days | Reduction in carcinoembryonic antigen tumor marker; Vascular endothelial growth factor showing anti-angiogenic effect | M. Bayet-Robert et al., Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9, 8–14 (2010). |
| 36 | Pancreatic cancer | 0.2–0.4 g/day | N = 16, Phase I clinical trial | Improved fatigue- and association with quality of life scores | M. Kanai et al., A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol 71, 1521–1530 (2013). |
Except for a few trials demonstrating clinical benefits, most curcumin trials showed no significant clinical benefits (White et al., 2019). Inadequate clinical efficacy of curcumin is attributed to poor bioavailability at the target site due to low absorption, instability at physiological pH, rapid hepatic metabolism, and systemic clearance (Liu et al., 2016). Upon oral consumption, a large portion of the ingested curcumin is excreted in feces, and the intestines absorb a relatively smaller amount. The absorbed curcumin is rapidly metabolized by the liver yielding an aqueous-soluble moiety eliminated by the kidney (Pan et al., 1999) and therefore, plasma levels of curcumin remains below detection limits. In contrast, the plasma levels of curcumin metabolite, curcumin-O-glucuronide were detected by 30 min after curcumin intake by healthy human subjects and reached to a maximum concentration of 29 ng/ml by 2.57h (Cheng et al., 2019). Although curcumin levels of were undetectable, plasma PK levels of curcumin-O-glucuronide corresponded with pharmacodynamic (PD) responses of curcumin such as increase in NRF2 regulated antioxidant gene expression and suppression of histone deacetylase (HDAC) 1, HDAC2, HDAC3 and HDAC4 in blood cells. The study underscored the potential role of curcumin metabolite in mediating the PD responses of curcumin. Intense research is being underway to improve curcumin's bioavailability by developing a formulation to increase its solubility, stability, and absorption (Jyoti et al., 2019; Liu et al., 2016). The formulations such as water-soluble curcumin, curcumin nanomicelle, and curcumin plus piperine have increased the bioavailability of curcumin and improved the clinical efficacy of curcumin.
6. Nanoformulation of curcumin for pulmonary delivery: an alternative delivery approach
Broad-spectrum antiviral activity and immunomodulatory activity provides a strong rationale for testing curcumin for COVID-19 treatment. However, low bioavailability is the major obstacle in attaining the therapeutic potential of oral curcumin. In contrast, pulmonary delivery of curcumin will overcome this limitation and offers several advantages: direct delivery of high concentration of curcumin to the site of infection; direct contact of curcumin with the virus SARS-CoV-2; direct deposition into lower airways and alveolar region; larger surface area for deposition and absorption; lower intra- and extracellular detoxification enzymatic activity in the pulmonary system (Borghardt et al., 2018). The nanotechnology-based formulation has dramatically eased drug delivery to the pulmonary system (Selvaraj et al., 2018; Smola et al., 2008). Scientists have developed curcumin encapsulated nano-carriers such as liposomes (De Leo et al., 2018), niosomes (Obeid et al., 2019), lipid complexation (Gupta and Dixit, 2011), micro/nano-emulsions (Sood et al., 2014; Yu and Huang, 2012) and polymeric nanoparticles (Umerska et al., 2018). This nanoformulated curcumin could be delivered in dry powder, nebulizer, solution, nasal spray or gel (Sood et al., 2014). Curcumin at physiological pH of 7.4 is unstable with a shelf life of 10 min, and the pH of the respiratory tract ranges from 7.2 to 7.4. The nanoformulation of curcumin would be protected from exposure to alkaline pH and thereby improve curcumin's stability at the target site. Furthermore, nanoformulations of curcumin could also be engineered for longer-retention, sustained release, and penetration across the mucus barrier (Sung et al., 2007). Clinical studies of liposomal curcumin, microparticle curcumin, and micelle curcumin by oral delivery have been found to be safe (Yallapu et al., 2015) and shown to be effective in reducing markers of oxidative stress (Helli et al., 2021). Animal studies have shown that inhalable curcumin effectively mitigates lethal bacterial pneumonia (Zhang et al., 2019). FDA has already approved inhalable powder of antibiotic tobramycin, and the nebulized liposomal formulation of ciprofloxacin was found to effective in reducing Pseudomonas aeruginosa infection in a phase III clinical trials for non-CF bronchiectasis (Haworth et al., 2019).
In summary, accumulated evidence suggests that the pulmonary delivery of nanoformulated curcumin such as liposomal curcumin would help in deposition of curcumin in the lower airways at a higher concentration, which may inhibit SARS-CoV-2 infectivity and concomitantly mitigate pulmonary inflammation and the progression to ARDS.
7. Conclusions
There is an urgent need for therapeutics against COVID-19 outbreak. Besides vaccine trials, few therapeutics such as remdesivir and or interferon-beta based on prior knowledge of antiviral activity against SARS-CoV, are in clinical practice for the treatment of COVID-19. Recognizing the public health emergency, perhaps it is imperative to evaluate phytochemical curcumin for management or treatment of COVID-19 in a randomized clinical trial because i) it is relatively safe; ii) it shows broad-spectrum antiviral activity against enveloped viruses; iii) it may suppress SARS-CoV-2 infection by directly modifying spike protein and or ACE2 and inducing host antiviral responses by targeting NRF2 and HMGB1; iv) it exerts immunomodulatory activity by blocking NF-κB, inflammasome, HMGB1, and IL-6 driven inflammatory responses; v) it dampens ROS production by inhibiting NADPH oxidase and alleviates oxidative tissue injury by increasing antioxidant defenses by modulating NRF2.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
RKT acknowledges the support from the Department of Biotechnology Ramalingaswami Re-entry Fellowship. We acknowledge the support of DST-FIST to the Centre of Excellence in Molecular Biology and Regenerative Medicine, Dept. of Biochemistry, JSS Medical College, Mysore and Dept. of Pharmaceutics, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Ooty, Nilgiris, Tamil Nadu.
Contributor Information
Rajesh K. Thimmulappa, Email: rajeshkt@jssuni.edu.in.
Gowthamarajan Kuppusamy, Email: gowthamsang@gmail.com.
References
- Abais J.M., Xia M., Zhang Y., Boini K.M., Li P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxidants Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Ali A., Banerjea A.C. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016;6:27539. doi: 10.1038/srep27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalraj A., Varma K., Jacob J., Divya C., Kunnumakkara A.B., Stohs S.J., Gopi S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: a randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food. 2017;20:1022–1030. doi: 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26:42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggakusuma, Colpitts C.C., Schang L.M., Rachmawati H., Frentzen A., Pfaender S., Behrendt P., Brown R.J., Bankwitz D., Steinmann J. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- Avasarala S., Zhang F., Liu G., Wang R., London S.D., London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PloS One. 2013;8 doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhausen T., Tschernig T., Rosenstiel P., van Griensven M., Vonberg R.P., Dorsch M., Mueller-Heine A., Chalaris A., Scheller J., Rose-John S. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011;39:1407–1413. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- Bernard K., Hecker L., Luckhardt T.R., Cheng G., Thannickal V.J. NADPH oxidases in lung health and disease. Antioxidants Redox Signal. 2014;20:2838–2853. doi: 10.1089/ars.2013.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti A.C., Donato N., Aggarwal B.B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Zhang Y., Quehenberger P., Luther T., Haase M., Müller M., Mackman N., Ziegler R., Nawroth P.P. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb. Haemostasis. 1997;77:772–782. [PubMed] [Google Scholar]
- Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Canc. Res. J. 2018;2018:2732017. doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M.S., Ouyang A., I M.K., Charnigo R.J., Westgate P.M., Fleenor B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: a double- blinded, randomized, controlled trial. Nutrition. 2019;62:135–139. doi: 10.1016/j.nut.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchois R., Koubi M., Delarbre D., Manet C., Carvelli J., Blasco V.B., Jean R., Fouche L., Bornet C., Pauly V. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl. Acad. Sci. U. S. A. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y.S., Chen Y.Q., Lin S.H., Xie K., Wang C.J., Yang Y.Z., Xu F. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020;125:109946. doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.Y., Chen D.Y., Wen H.W., Ou J.L., Chiou S.S., Chen J.M., Wong M.L., Hsu W.L. Inhibition of enveloped viruses infectivity by curcumin. PloS One. 2013;8 doi: 10.1371/journal.pone.0062482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Li W., Wang L., Lin T., Poiani G., Wassef A., Hudlikar R., Ondar P., Brunetti L., Kong A.N. Pharmacokinetics, pharmacodynamics, and PKPD modeling of curcumin in regulating antioxidant and epigenetic gene expression in healthy human volunteers. Mol. Pharm. 2019;16:1881–1889. doi: 10.1021/acs.molpharmaceut.8b01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K., Yang A., Hu X., Zhu D., Liu K. Curcumin attenuates pulmonary inflammation in lipopolysaccharide induced acute lung injury in neonatal rat model by activating peroxisome proliferator-activated receptor γ (PPARγ) pathway. Med. Sci. Mon. 2018;24:1178–1184. doi: 10.12659/MSM.908714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Yin-Lam Wong A., Kaewpreedee P., Sia S.F., Chen D., Yan Hui K.P., Wing Chu D.K., Wai Chan M.C., Pak-Hang Cheung P., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M.R., Romi M.D., Ferreira D., Zaltman C., Soares-Mota M. The use of curcumin as a complementary therapy in ulcerative colitis: a systematic review of randomized controlled clinical trials. Nutrients. 2020;12 doi: 10.3390/nu12082296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Caraffa A., Gallenga C.E., Ross R., Kritas S.K., Frydas I., Younes A., Ronconi G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/20-1-E. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- Da W., Zhang J., Zhang R., Zhu J. Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibiton of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 2019;33 doi: 10.1177/2058738419861600. 2058738419861600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Gu L., Su Y., Wang Q., Zhao Y., Chen X., Deng H., Li W., Wang G., Li K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018;54:177–187. doi: 10.1016/j.intimp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- De Leo V., Milano F., Mancini E., Comparelli R., Giotta L., Nacci A., Longobardi F., Garbetta A., Agostiano A., Catucci L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules. 2018;23 doi: 10.3390/molecules23040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derochette S., Franck T., Mouithys-Mickalad A., Ceusters J., Deby-Dupont G., Lejeune J.P., Neven P., Serteyn D. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem. Biol. Interact. 2013;206:186–193. doi: 10.1016/j.cbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hachem N., Eid E., Nemer G., Dbaibo G., Abbas O., Rubeiz N., Zeineldine S., Matar G.M., Bikorimana J.P., Shammaa R. Integrative transcriptome analyses empower the anti-COVID-19 drug arsenal. iScience. 2020;23:101697. doi: 10.1016/j.isci.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori V., Pinho-Ribeiro F.A., Borghi S.M., Alves-Filho J.C., Cunha T.M., Cunha F.Q., Casagrande R., Verri W.A. Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-κB activation. Inflamm. Res. 2015;64:993–1003. doi: 10.1007/s00011-015-0885-y. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- Gu Q., Guan H., Shi Q., Zhang Y., Yang H. Curcumin attenuated acute Propionibacterium acnes-induced liver injury through inhibition of HMGB1 expression in mice. Int. Immunopharmacol. 2015;24:159–165. doi: 10.1016/j.intimp.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Gupta N.K., Dixit V.K. Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J. Pharm. Sci. 2011;100:1987–1995. doi: 10.1002/jps.22393. [DOI] [PubMed] [Google Scholar]
- Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y.I., Kim S.J., Choi B.Y., Cho K.C., Bandu R., Kim K.P., Kim D.H., Kim W., Park J.S., Han B.W. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018;8:6409. doi: 10.1038/s41598-018-23840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama Y., Mitsuyama K., Sata M. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Harris H.E., Andersson U., Pisetsky D.S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- Harvey C.J., Thimmulappa R.K., Sethi S., Kong X., Yarmus L., Brown R.H., Feller-Kopman D., Wise R., Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C.S., Bilton D., Chalmers J.D., Davis A.M., Froehlich J., Gonda I., Thompson B., Wanner A., O'Donnell A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir. Med. 2019;7:213–226. doi: 10.1016/S2213-2600(18)30427-2. [DOI] [PubMed] [Google Scholar]
- Helli B., Gerami H., Kavianpour M., Heybar H., Hosseini S.K., Haghighian H.K. Endocr Metab Immune Disord Drug Targets; 2021. Curcumin nanomicelle improves lipid profile, stress oxidative factors and inflammatory markers in patients undergoing coronary elective angioplasty; A randomized clinical trial. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.L., Chen P.Y., Wu M.J., Tai M.H., Ho C.T., Yen J.H. Curcuminoids modulate the PKCδ/NADPH oxidase/reactive oxygen species signaling pathway and suppress matrix invasion during monocyte-macrophage differentiation. J. Agric. Food Chem. 2015;63:8838–8848. doi: 10.1021/acs.jafc.5b04083. [DOI] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Osorio A.S., García-Niño W.R., González-Reyes S., Álvarez-Mejía A.E., Guerra-León S., Salazar-Segovia J., Falcón I., Montes de Oca-Solano H., Madero M., Pedraza-Chaverri J. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: a pilot study. J. Ren. Nutr. 2016;26:237–244. doi: 10.1053/j.jrn.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Jobin C., Bradham C.A., Russo M.P., Juma B., Narula A.S., Brenner D.A., Sartor R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- Jyoti J., Anandhakrishnan N.K., Singh S.K., Kumar B., Gulati M., Gowthamarajan K., Kumar R., Yadav A.K., Kapoor B., Pandey N.K. A three-pronged formulation approach to improve oral bioavailability and therapeutic efficacy of two lipophilic drugs with gastric lability. Drug Deliv. Transl. Res. 2019;9:848–865. doi: 10.1007/s13346-019-00635-0. [DOI] [PubMed] [Google Scholar]
- Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi A., Ghodsi R., Kooshki F., Karimi M., Asghariazar V., Tarighat-Esfanjani A. Therapeutic effects of curcumin on sepsis and mechanisms of action: a systematic review of preclinical studies. Phytother Res. 2019;33:2798–2820. doi: 10.1002/ptr.6467. [DOI] [PubMed] [Google Scholar]
- Kim D.C., Ku S.K., Bae J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012;45:221–226. doi: 10.5483/bmbrep.2012.45.4.221. [DOI] [PubMed] [Google Scholar]
- Kim S.G., Veena M.S., Basak S.K., Han E., Tajima T., Gjertson D.W., Starr J., Eidelman O., Pollard H.B., Srivastava M. Curcumin treatment suppresses IKKβ kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin. Canc. Res. 2011;17:5953–5961. doi: 10.1158/1078-0432.CCR-11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Thimmulappa R., Craciun F., Harvey C., Singh A., Kombairaju P., Reddy S.P., Remick D., Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am. J. Respir. Crit. Care Med. 2011;184:928–938. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Thimmulappa R., Kombairaju P., Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A., Salomon N., Wu J.C., Kopylov U., Lahat A., Har-Noy O., Ching J.Y., Cheong P.K., Avidan B., Gamus D. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2015;13:1444–1449. doi: 10.1016/j.cgh.2015.02.019. e1441. [DOI] [PubMed] [Google Scholar]
- Lee S., Channappanavar R., Kanneganti T.D. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41:1083–1099. doi: 10.1016/j.it.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli D., Sahebkar A., Johnston T.P., Pedone C. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol. Res. 2017;115:133–148. doi: 10.1016/j.phrs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Li W., Suwanwela N.C., Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., Gao Y., Cai L., Wang Z., Yin P. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Guo W., Zhu Y., Peng S., Zheng W., Zhang C., Shao F., Hang N., Kong L., Meng X. Targeting peroxiredoxin 1 by a curcumin analogue, AI-44, inhibits NLRP3 inflammasome activation and attenuates lipopolysaccharide-induced sepsis in mice. J. Immunol. 2018;201:2403–2413. doi: 10.4049/jimmunol.1700796. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: problems and advancements. J. Drug Target. 2016;24:694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manarin G., Anderson D., Silva J.M.E., Coppede J.D.S., Roxo-Junior P., Pereira A.M.S., Carmona F. Curcuma longa L. ameliorates asthma control in children and adolescents: a randomized, double-blind, controlled trial. J. Ethnopharmacol. 2019;238:111882. doi: 10.1016/j.jep.2019.111882. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Raghavan K., Weinstein J., Kohn K.W., Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., Ní Choileáin O., Clarke J., O'Connor E., Hogan G. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce B.C., Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Nabila N., Suada N.K., Denis D., Yohan B., Adi A.C., Veterini A.S., Anindya A.L., Sasmono R.T., Rachmawati H. Antiviral action of curcumin encapsulated in nanoemulsion against four serotypes of Dengue virus. Pharm. Nanotechnol. 2020;8:54–62. doi: 10.2174/2211738507666191210163408. [DOI] [PubMed] [Google Scholar]
- Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed. Pharmacother. 2020;131:110698. doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Kojima T., Masaki T., Okabayashi T., Yokota S., Hirakawa S., Nomura K., Takasawa A., Murata M., Tanaka S. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PloS One. 2013;8 doi: 10.1371/journal.pone.0070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M.A., Khadra I., Albaloushi A., Mullin M., Alyamani H., Ferro V.A. Microfluidic manufacturing of different niosomes nanoparticles for curcumin encapsulation: physical characteristics, encapsulation efficacy, and drug release. Beilstein J. Nanotechnol. 2019;10:1826–1832. doi: 10.3762/bjnano.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier D., Farahani E., Thyrsted J., Blay Cadanet J., Herengt A., Idorn M., Hait A., Hernaez B., Knudsen A., Iversen M.B. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J.L., Mizushina Y., Wang S.Y., Chuang D.Y., Nadar M., Hsu W.L. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013;280:5829–5840. doi: 10.1111/febs.12503. [DOI] [PubMed] [Google Scholar]
- Padmanaban G., Rangarajan P.N. Curcumin as an adjunct drug for infectious diseases. Trends Pharmacol. Sci. 2016;37:1–3. doi: 10.1016/j.tips.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Pan M.H., Huang T.M., Lin J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- Panahi Y., Hosseini M.S., Khalili N., Naimi E., Majeed M., Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015;34:1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Panahi Y., Khalili N., Hosseini M.S., Abbasinazari M., Sahebkar A. Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Compl. Ther. Med. 2014;22:851–857. doi: 10.1016/j.ctim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Pandey M.K., Kumar S., Thimmulappa R.K., Parmar V.S., Biswal S., Watterson A.C. Design, synthesis and evaluation of novel PEGylated curcumin analogs as potent Nrf2 activators in human bronchial epithelial cells. Eur. J. Pharmaceut. Sci. 2011;43:16–24. doi: 10.1016/j.ejps.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Pandey P., Rane J.S., Chatterjee A., Kumar A., Khan R., Prakash A., Ray S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.S., Acharya A., Ray R.S., Agrawal R., Raghuwanshi R., Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020;60:887–939. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- Pendurthi U.R., Williams J.T., Rao L.V. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler. Thromb. Vasc. Biol. 1997;17:3406–3413. doi: 10.1161/01.atv.17.12.3406. [DOI] [PubMed] [Google Scholar]
- Praditya D., Kirchhoff L., Brüning J., Rachmawati H., Steinmann J., Steinmann E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019;10:912. doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., Yamamoto M., Petrache I., Tuder R.M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtman M.M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y., Halpern Z., Shlomai A. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. FEBS Lett. 2010;584:2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Scheller J., Garbers C., Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Selvaraj K., Gowthamarajan K., Karri V.V.S.R. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018;46:2088–2095. doi: 10.1080/21691401.2017.1420073. [DOI] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T., Jiang T., Long M., Chen J., Ren D.M., Wong P.K., Chapman E., Zhou B., Zhang D.D. A curcumin derivative that inhibits vinyl carbamate-induced lung carcinogenesis via activation of the Nrf2 protective response. Antioxidants Redox Signal. 2015;23:651–664. doi: 10.1089/ars.2014.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola M., Vandamme T., Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int. J. Nanomed. 2008;3:1–19. [PMC free article] [PubMed] [Google Scholar]
- Soni V.K., Mehta A., Ratre Y.K., Tiwari A.K., Amit A., Singh R.P., Sonkar S.C., Chaturvedi N., Shukla D., Vishvakarma N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020;886:173551. doi: 10.1016/j.ejphar.2020.173551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S., Jain K., Gowthamarajan K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Biointerfaces. 2014;113:330–337. doi: 10.1016/j.colsurfb.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Sui Z., Salto R., Li J., Craik C., Ortiz de Montellano P.R. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993;1:415–422. doi: 10.1016/s0968-0896(00)82152-5. [DOI] [PubMed] [Google Scholar]
- Sung J.C., Pulliam B.L., Edwards D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25:563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R.K., Fuchs R.J., Malhotra D., Scollick C., Traore K., Bream J.H., Trush M.A., Liby K.T., Sporn M.B., Kensler T.W. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxidants Redox Signal. 2007;9:1963–1970. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R.K., Rangasamy T., Alam J., Biswal S. Dibenzoylmethane activates Nrf2-dependent detoxification pathway and inhibits benzo(a)pyrene induced DNA adducts in lungs. Med. Chem. 2008;4:473–481. doi: 10.2174/157340608785700199. [DOI] [PubMed] [Google Scholar]
- Thimmulappa R.K., Scollick C., Traore K., Yates M., Trush M.A., Liby K.T., Sporn M.B., Yamamoto M., Kensler T.W., Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., Avezum A., Lopes R.D., Bueno F.R., Silva M.V.A.O. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umerska A., Gaucher C., Oyarzun-Ampuero F., Fries-Raeth I., Colin F., Villamizar-Sarmiento M.G., Maincent P., Sapin-Minet A. Polymeric nanoparticles for increasing oral bioavailability of curcumin. Antioxidants (Basel) 2018;7 doi: 10.3390/antiox7040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajragupta O., Boonchoong P., Morris G.M., Olson A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 2005;15:3364–3368. doi: 10.1016/j.bmcl.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Punithavathi D., Babu M. Protection from acute and chronic lung diseases by curcumin. Adv. Exp. Med. Biol. 2007;595:379–405. doi: 10.1007/978-0-387-46401-5_17. [DOI] [PubMed] [Google Scholar]
- Vishvakarma N.K., Kumar A., Kant S., Bharti A.C., Singh S.M. Myelopotentiating effect of curcumin in tumor-bearing host: role of bone marrow resident macrophages. Toxicol. Appl. Pharmacol. 2012;263:111–121. doi: 10.1016/j.taap.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Nie H., Li K., Zhang Y.X., Yang F., Li C.B., Wang C.F., Gong Q. Curcumin inhibits HMGB1 releasing and attenuates concanavalin A-induced hepatitis in mice. Eur. J. Pharmacol. 2012;697:152–157. doi: 10.1016/j.ejphar.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Alfajaro M.M., DeWeirdt P.C., Hanna R.E., Lu-Culligan W.J., Cai W.L., Strine M.S., Zhang S.M., Graziano V.R., Schmitz C.O. Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell. 2020 doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger J., Priester M., Bernreuther C., Rakel S., Glatzel M., Seifert V., Kögel D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin. Canc. Res. 2010;16:5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., Lee C.K., Chang S.T., Kuo C.J., Lee S.S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- White C.M., Pasupuleti V., Roman Y.M., Li Y., Hernandez A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;146:104280. doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Liu L. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway. Inf. Other Respir. Viruses. 2017;11:457–463. doi: 10.1111/irv.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H., Thompson B.T., Gajic O. Fifty years of research in ARDS. Is acute respiratory distress syndrome a preventable disease? Am. J. Respir. Crit. Care Med. 2017;195:725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- Yallapu M.M., Nagesh P.K., Jaggi M., Chauhan S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17:1341–1356. doi: 10.1208/s12248-015-9811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020 doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Guo Q., Li X., Tang T., Li C., Wang H., Sun Y., Feng Q., Ma C., Gao C. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- Yu H., Huang Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012;60:5373–5379. doi: 10.1021/jf300609p. [DOI] [PubMed] [Google Scholar]
- Zandi K., Ramedani E., Mohammadi K., Tajbakhsh S., Deilami I., Rastian Z., Fouladvand M., Yousefi F., Farshadpour F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010;5:1935–1938. [PubMed] [Google Scholar]
- Zhang B., Swamy S., Balijepalli S., Panicker S., Mooliyil J., Sherman M.A., Parkkinen J., Raghavendran K., Suresh M.V. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. Faseb. J. 2019;33:13294–13309. doi: 10.1096/fj.201901047RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.M., Xu R., Huang X.Y., Cheng S.M., Huang M.F., Yue H.Y., Wang X., Zou Y., Lu A.P., Liu D.Y. Curcumin suppressed activation of dendritic cells via JAK/STAT/SOCS signal in mice with experimental colitis. Front. Pharmacol. 2016;7:455. doi: 10.3389/fphar.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Nunneley J.W., Barnard D., Pöhlmann S., McKerrow J.H., Renslo A.R. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Zhang C., Weng Q., Ye B. Curcumin protects against acute renal injury by suppressing JAK2/STAT3 pathway in severe acute pancreatitis in rats. Exp. Ther. Med. 2017;14:1669–1674. doi: 10.3892/etm.2017.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.