Abstract
Introduction
Hydroxychloroquine (HCQ)/Chloroquine (CQ) has been evaluated for treatment and prophylaxis against SARS-CoV-2 infection in various studies with conflicting results. We performed a systematic review to synthesize the currently available evidence over the efficacy and safety of HCQ/CQ therapy alone against SARS-CoV-2 infection.
Methods
We searched Embase, PubMed, Web of Science, and Cochrane central for randomized controlled trials (RCTs) and prospective cohort studies published until October 15, 2020 and assessing the efficacy of HCQ alone against SARS-CoV-2 infection. We included studies evaluating HCQ/CQ alone as intervention and placebo/standard care as a control group. Retrospective studies and studies using other drugs (namely azithromycin, corticosteroids, immunomodulators, etc.) we excluded. Thirteen RCTs and three prospective cohort studies were included in this review. We pooled data using a random-effect model.
Results
Pooled data from 12 studies (9917 participants) showed that HCQs increase mortality as compared to placebo/standard of care (RR 1.10; 95% CI:1.00–1.20). Hydroxychloroquine did not reduce the need for hospitalization in out-patients (RR 0.57; 95% CI 0.31–1.02). HCQ group has a significantly higher rate of any adverse event (RR 2.68; 95% CI 1.55–4.64), as compared to the control group. Also, using HCQ for prophylaxis against SARS-CoV-2 infection did not reduce the risk of acquiring SARS-CoV-2 infection (RR 1.04; 95% CI 0.58–1.88).
Conclusions
HCQ therapy for COVID-19 is associated with an increase in mortality and other adverse events. The negative effects are more pronounced in hospitalized patients. Therefore, with the available evidence, HCQ should not be used in prophylaxis or treatment of patients with COVID-19.
Keywords: COVID-19, Coronavirus, Mortality, Prophylaxis, Therapy
1. Introduction
With close to 53 million cases and 1.3 million deaths globally, the coronavirus disease 2019 (COVID-19) pandemic has caused unprecedented damage and continues to wreak havoc, plaguing healthcare systems globally [1]. Despite being almost a year since the pandemic commenced, a conclusively efficacious and safe therapy for COVID-19 still evades us. Numerous antiviral and immunomodulatory drugs have been tried, but none has shown unequivocal evidence of efficacy and safety across the studies in COVID-19 [2]. Chloroquine (CQ) and Hydroxychloroquine (HCQ) are one such group of immunomodulatory agents that were considered to have antiviral property against SARS-CoV-2 and were used extensively without any strong evidence [3]. Several mechanisms have been proposed for their role in COVID-19. These mechanisms include: (i) Direct antiviral action (by inhibiting viral attachment/biosynthesis of sialic acid/inhibition of replication and transcription); (ii) Attenuation of disease progression by anti-inflammatory action (by inhibiting TNF- α, Interleukins, and Th17 differentiation); and (iii) Anti-thrombotic effect (by decreased platelet aggregation, inhibiting the membrane binding of clotting proteins, and improvement of endothelial relaxation) [4,5].
Hydroxychloroquine formed a part of the national guidelines for prophylaxis in some countries like India, based on preliminary evidence [6]. Recently, HCQ/CQ has been evaluated as prophylactic and therapeutic agents using rigorous study designs with varying results [[7], [8], [9], [10], [11], [12]]. Serious concerns were raised over the adverse cardiovascular effects associated with HCQ use [13,14]. However, the long multidecadal experience of its safety (comparable doses) as an immunomodulator in rheumatic diseases arose the possibility of confounding by the underlying disease and the effect of concomitant therapy such as macrolides [15]. Recently few reviews summarized the available evidence, however, they included studies with heterogeneous intervention and control groups [[16], [17], [18]]. Therefore, the evidence of safety and efficacy of HCQ/CQ alone against COVID-19 is unclear.
We aimed to systematically synthesize the evidence over the efficacy and safety of HCQ or CQ alone as compared to placebo or no active intervention in adults with COVID-19.
2. Material and methods
2.1. Search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines during the conduct of this systematic review [19]. A predefined specific search strategy was developed for each electronic database (Supplementary Table 1). Three investigators (JM, JK, AY) independently performed a literature search in the Web of Science, PubMed, EMBASE, and Cochrane central register of controlled trials for original articles published between December 01, 2019, to October 15, 2020. We used keywords related to the study population (exposed to patients with SARS-CoV-2 infection or having RT-PCR confirmed SARS-CoV-2 Infection), and intervention (Hydroxychloroquine or Chloroquine) for literature search. The electronic search was also supplemented by a hand search of the bibliography of the included studies and relevant review articles. We did not use any language restriction. The studies in non-English language were converted to English using google translator and relevant data was extracted.
2.2. Study selection
The protocol for this systematic review was registered at the PROSPERO database (CRD42020201750). Criteria mentioned in the above protocol were used for the assessment of the eligibility of the studies. Studies investigating the role of Hydroxychloroquine or Chloroquine as a therapeutic or prophylactic agent for SARS-CoV-2 infection were considered eligible for the review. Initially, two researchers (JK, JM) independently screened the titles and abstracts for eligibility. Later three authors (AY, JM, JK) examined the full-text articles for inclusion and exclusion criteria. Studies were included if they met all the following criteria: (i) Population-studies enrolling patients with clinically suspected or RT-PCR-confirmed SARS CoV-2 infection (therapeutic studies) or their contacts (prophylaxis studies), (ii) Intervention-HCQ or chloroquine (CQ) along with standard care for prophylaxis or treatment. (iii) Comparison-control group should receive standard care alone (that does not include antivirals or immunomodulators) or placebo. We included prospective cohort studies or randomized/non-randomized controlled trials. Considering the rapidly evolving evidence and the long-time taken to get a study published after peer-review, we also included pre-print studies after rigorous methodology checks. The methodology check comprised of inclusion of the latest version of the manuscript uploaded on pre-print server, ensuring that the trial protocol was registered with one of the registries and is accessible to public, and the authors followed standard reporting guidelines for presenting the results. We also checked the comments associated with the pre-print version (which act like a peer-review) to ensure that there are no serious concerns associated with data-quality or results.
We excluded: (i) studies comparing HCQ or CQ with active control or comparator group (like azithromycin, Lopinavir/ritonavir, other immunomodulators), (ii) studies using any other antiviral or immune-modulator drug along with HCQ/CQ in the intervention arm, (iii) study designs like case-series, case-control or retrospective cohort study, (iv) studies reporting about other serotypes of coronavirus or testing methods other than RT-PCR, (v) narrative or systematic reviews, (vi) Conference proceedings, (vii) editorial, perspective, etc. not meeting the inclusion criteria. If a study used more than one intervention and provides clear data on various interventions, we used data of HCQ/CQ alone and the control group only. Studies in which other interventions were not differentiated but HCQ/CQ was the primary intervention, were also included. Similarly, in the case of a mixed population (virologically confirmed and clinically suspected COVID-19), if the study provided separate data for confirmed and suspect COVID-19, we used data of RT-PCR confirmed patients only. However, if no such data was provided and the majority were confirmed COVID-19, data of all the patients were included.
2.3. Data extraction and quality assessment
A well-structured, standardized proforma was used for data extraction. Two investigators [JM, JK] independently extracted data from the full text of the eligible studies. Data extraction included information about: Author’s name, year of publication, journal, study design, study setting, study methodology, study population, inclusion and exclusion criteria, baseline demographic characteristics, details of intervention and control group, case definition of SARS-CoV-2 infection and contacts, intervention (dosage, duration, frequency, and co-administration of other therapy if any), and outcomes (mortality, the proportion of patients recovered, time to clinical recovery, time to viral clearance, the proportion of participants requiring hospitalization, the proportion of patients developing severe disease, duration of hospital stay, duration of ICU stay, the proportion of acquiring infection among exposed ones, adverse effects, etc.). Any disagreement between the two investigators was resolved through discussion with the third investigator (SJ). A researcher (AY) independently rechecked the extracted data for its accuracy and completeness. The quality of the RCTs was assessed using the Cochrane risk of bias tool [20], while for observational studies, the Newcastle Ottawa scale [21] was used. Two investigators (JM, JK) independently assigned an overall risk of bias to each eligible study, and if they disagreed, another researcher (SJ) was called to resolve the discrepancy.
2.4. Data synthesis and statistical analysis
We provided a quantitative synthesis of primary and secondary outcomes. The dichotomous outcomes are reported as risk ratio (RR) with 95% CI and continuous data as mean difference (MD) with 95% CI. We pooled the data using the random-effects model. Heterogeneity among studies was assessed by inspection of the forest plot as well as using the Chi-square test on Cochrane’s Q statistics and I2 statistics. The publication bias was assessed by Funnel plot asymmetry. We used RevMan 5.3 for statistical analysis. We also graded the level of evidence using GRADEpro software. For GRADE evidence, only randomized controlled trials were used.
3. Results
3.1. Selection and characteristics of included studies
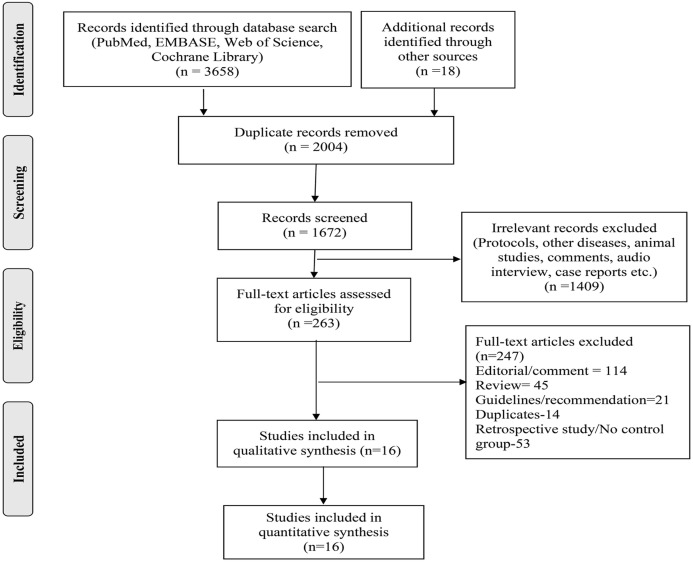
We screened a total of 3676 records and identified 16 studies [[8], [9], [10], [11], [12],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]] eligible for this systematic review (Fig. 1 ). Of these 16 studies, 13 were randomized clinical trials (10 therapeutic and 3 prophylaxis), and the rest three were prospective cohort studies (all therapeutic). Fifteen studies used HCQ as an intervention whereas one used Chloroquine. These studies represented various countries with different treatment protocols and varying standards of care (Table 1 ). Most therapeutic trials (10 out of 13) were done in hospitalized patients with varying degrees of severity. Only three out of 13 therapeutic studies used placebo in the control groups (in the rest 10, the control group received standard care only), whereas all three prophylactic trials used placebo in their control arm. The dosage and duration of the intervention (HCQ/CQ) and outcomes varied greatly across the trials. Among cohort studies, two out of three were done in the severe disease population and used HCQ as the intervention. Whereas one study used CQ as an intervention and was done in patients with mild to moderate disease. The detailed characteristics of eligible studies are reported in Table 1. The quality of the studies was assessed using the Cochrane risk of bias tool for RCTs (Fig. 2 ) and the Newcastle–Ottawa scale for observational studies (Supplementary Table S2). We intend to assess publication bias using funnel plot; however, the number of studies was insufficient for the same (only 6 RCT and 2 non-RCT reported mortality). The Cochrane handbook recommends using tests for funnel plot asymmetry only when there are at least 10 studies included in the meta-analysis, as with fewer studies, the power of test becomes too low to detect real asymmetry.
Fig. 1.
Prisma flow chart.
Table 1.
Characteristics of the studies included in the systematic review.
| Author (Year) | Country/Setting | Number of Patientsc |
Intervention/Exposure | Control | Outcome/s | |
|---|---|---|---|---|---|---|
| HCQ | Control | |||||
| HCQ therapy: RCTs | ||||||
| Abd-Elsalam S et al. [11] (2020) | Egypt/Hospitalized (all categories) | 97 | 97 | HCQ 800 mg on day 1followed by 400 mg daily for 15 days. | SOC | Number of patients with cure or death in 4 weeks |
| Cavalcanti A.B. et al. [9] (2020) | Brazil/Hospitalized (Mild to moderate) | 159 (221) | 173 (227) | HCQ 800 mg daily for 7 daysa | SOC | Primary: Ordinal outcome at 15 days Secondary: mortality, ventilation, length of stay, adverse events, etc. |
| Chen J et al. [23](2020) | China/Hospitalized (all categories) | 15 | 15 | HCQ 400 mg daily for 5 days | SOC | Primary: Viral clearance on day 7/death within 2 weeks. Secondary: serious adverse events |
| Chen Z et al. [28](2020) | China/Hospitalized (mild cases) | 31 | 31 | HCQ 400 mg daily for 5 days | SOC | Primary: Severe adverse event Secondary: Clinical, radiological, and pulmonary recovery |
| Lofgren SM et al. [31] (2020) | USA/Non-hospitalizedb | 576 (658) | 563 (654) | HCQ 1400 mg on day 1 followed by 600 mg daily for the next 4 days | Placebo | Self-reported adverse effects including death (internet-based) |
| Mitjà O et al. [32] (2020) | Spain/Non-hospitalized | 136 (169) | 157 (184)c | HCQ 800 mg on day 1 followed by 400 mg daily for the next 6 days | SOC | Primary: reduction in viral load on day 3 and 7 Secondary: Disease progression (death, hospitalization, ventilation) |
| RECOVERY Collaborative group [12] (2020) | UK/Hospitalized (all categories)b | 1561 | 3155 | HCQ 1600 mg on day 1, followed by 800 mg daily for the next 9 days/discharge | SOC | Primary: Mortality within 28 days Secondary: Composite outcome mechanical ventilation and/or death |
| Skipper CP et al. [25] (2020) | USA and Canada/Non-hospitalizedb | 212 (244) | 211 (247) | HCQ 1400 mg on day 1 followed by 600 mg daily for the next 4 days | Placebo | Primary: ordinal change in symptom severity score Secondary: Incidence of all hospitalizations, deaths, and adverse events (internet-based) |
| Tang W et al. [10] (2020) | China/Hospitalized (mild to moderate) | 75 | 75 | HCQ 1200 mg daily for 3 days followed by 800 mg daily for the remaining 2-3 weeks | SOC | Primary: Virological recovery by 28 days, Secondary: Adverse events |
| WHO Solidarity trial [22] (2020) | Multinational/Hospitalized (all categories) | 947 (954) | 906 (909) | HCQ 2400 mg on day 1 followed by 400 mg for the next 9 days | SOC | Mortality and serious adverse events (reported) |
| HCQ therapy: cohort studies | ||||||
| Grimaldi Da et al. [26] (2020) | France and Belgium/Hospitalized (Severe) | 220 | 85 | HCQ used in variable dose (400-800 mg/day) for variable duration (5-10 days) | SOC | Primary: ventilator-free days at day 28. Secondary: Survival, adverse events. |
| Huang M et al. [27] (2020) | China/Hospitalized (Mild to moderate) | 197 | 176 | CQ250-500 mg mg daily | SOC | Primary: Time to viral clearance. Secondary: hospital stay, and adverse events. |
| Karolyi M et al. [24] (2020) | Austria/Hospitalized (Severe) | 20 | 89 | HCQ 800 mg on day 1, followed by 400mgdaily. | SOC | In-hospital mortality, ICU admission, length of stay, viral clearance, and adverse events |
| HCQ Prophylaxis | ||||||
| Abella BS et al. [29] (2020) | USA/Health care workers (pre-exposure) | 66 | 66 | HCQ 600 mg daily for 8 weeks | Placebo | Primary: Incidence of SARS-CoV-2infection Secondary: Adverse events |
| Boulware DR et al. [8] (2020) | USA and Canada/moderate to high-risk exposure (post-exposure) | 414 | 407 | HCQ 2000 mg on day 1 followed by 600 mg daily for 4 more days | Placebo | Primary: Incidence of either laboratory-Confirmed/clinical compatible Covid-19 within 14 days |
| Rajasingham R et al. [30] (2020) | USA and Canada/Healthcare workers (pre-exposure) | 494 | 989 | HCQ 400 mg once weekly or twice weekly for 12 weeks. | Placebo | Primary: Incidence of either laboratory-Confirmed/clinical compatible Covid-19 within 14 days |
Abbreviations: HCQ- Hydroxychloroquine, SOC- Standard of care, ICU- Intensive care unit, RT-PCR- Reverse Transcriptase Polymerase chain reaction.
We included only the HCQ group.
The patient may or may not be RT-PCR proven but were clinically suspected to have COVID-19 (applicable for treatment studies).
Number in () indicates the number of patients randomized to two arms.
Fig. 2.
Risk of bias summary of included randomized controlled trials.
4. Efficacy and safety of hydroxychloroquine in the treatment of SARS-CoV-2 infection
4.1. All-cause mortality
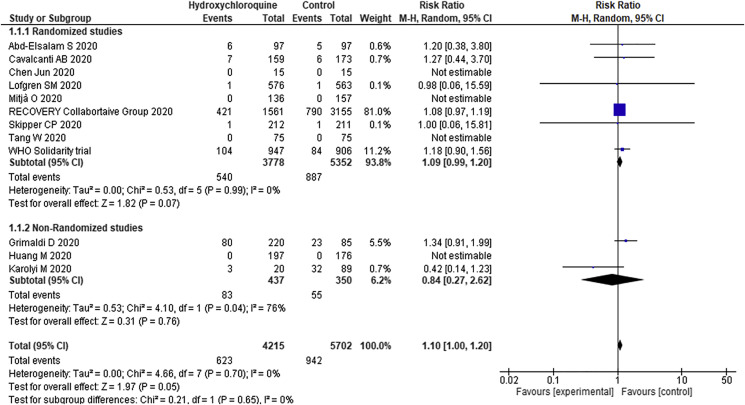
Nine RCTs (9130 patients) and three cohort studies (787 patients) reported data on all-cause mortality. The overall mortality rate was 14.7% and 16.5% in the HCQ and control group, respectively. Treatment with HCQs as compared to the control group increases the risk of mortality (RR 1.10; 95% CI 1.00–1.20) (Fig. 3 ) Among hospitalized patients (8062 patients) also, HCQ increases the risk of mortality (RR 1.10; 95% CI 1.00–1.20). On the other hand, in prospective observational studies (3 studies, 787 patients) there was no significant difference in mortality (RR 0.84; 95% CI 0.27–2.62) between HCQ and control group. On subgroup analysis, there was no significant effect of HCQ over mortality in non-hospitalized cases (RR- 0.99; 95% CI 0.14–6.98).
Fig. 3.
Forest Plot showing the comparison of mortality among patients receiving hydroxychloroquine and those receiving standard of care.
4.2. Secondary outcomes
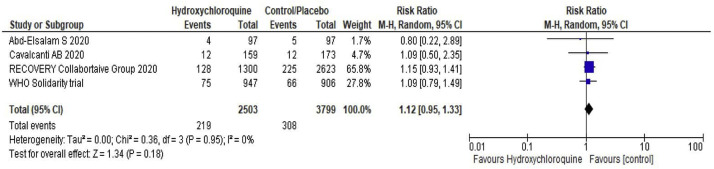
HCQ neither reduces the need for hospitalization in out-patients (RR 0.57; 95% CI 0.31–1.02) nor the duration of the hospital stay (Table 2 ). Among the patients who were not on mechanical ventilation at the time of randomization, HCQ does not reduce the need for mechanical ventilation (RR 1.12; 95% CI 0.95–1.33) (Fig.4 ). Two RCTs reported the proportion of patients requiring admission to the intensive care unit and did not find any significant difference between the HCQ and control group (Table 2). However, in cohort studies, the use of HCQ is associated with a significant increase in the need for ICU admission (RR-38.57; 95% CI 2.16–689.10). Three RCT’s done in outpatients did not find any significant effect of HCQ on need for hospitalization (RR 0.57; 95% CI 0.32–1.02). A single small trial showed a positive impact of HCQ over radiological improvement (RR 1.47; 95% CI 1.02–2.11), however the same is not translated into clinical recovery (RR 1.19; 95% CI 0.72–1.95). Though in a single prospective cohort, HCQ has a significantly higher viral recovery rate, the same was not seen in randomized controlled trials (Table 2).
Table 2.
Primary and secondary outcomes.
| Outcome | Study design | No. of studies (subjects) | Risk ratio/mean difference. (95% CI) | Heterogeneity (I2) p-value | Quality of evidence |
|---|---|---|---|---|---|
| All-cause mortality (Overall) | RCT | 9 (9130) | 1.09 (0.99–1.20) | 0%, 0.96 | Moderate |
| Prospective Cohort | 3 (787) | 0.84 (0.27–2.62) | 76%, 0.04 | ||
| All-cause mortality (Hospitalized) | RCT | 6 (7275) | 1.09 (0.99–1.20) | 0%, 0.05 | Moderate |
| Prospective Cohort | 3 (787) | 0.84 (0.27–2.62) | 76%, 0.04 | ||
| All-cause mortality (Non-Hospitalized) | RCT | 3 (1855) | 0.99 (0.14–6.98) | 0%, 0.99 | Low |
| Need for hospitalization (only for outpatient trials) | RCT | 3 (1855) | 0.57 (0.32–1.02) | 0%, 0.51 | Low |
| Need for mechanical ventilation (post-randomization) | RCT | 4 (6302) | 1.12 (0.95–1.33) | 0%, 0.95 | Low |
| Need for ICU admission (post-randomization/enrolment) | RCT | 2 (224) | 0.85 (0.40–1.79) | – | Very low |
| Prospective Cohort | 2 (482) | 38.6(2.2–689.1) | – | ||
| Clinical Recovery | RCT | 2 (4910) | 1.19 (0.72–1.95) | 89%, 0.003 | Very low |
| Radiological recovery | RCT | 1 (62) | 1.47 (1.02–2.11) | – | Very low |
| Virological Recovery by day 28 of illness | RCT | 2 (180) | 0.98 (0.89–1.09) | 0%, 0.49 | Very low |
| Prospective Cohort | 1 (373) | 1.21 (1.11–1.31) | – | ||
| Progression to severe disease | RCT | 1 (150) | 3.00 (0.12–72.5) | – | Very low |
| Any adverse event | RCT | 8 (4645) | 2.00 (1.32–3.01) | 80%, <0.01 | Low |
| Prospective Cohort | 1 (373) | 0.83 (0.61–1.14) | – | ||
| Serious adverse events | RCT | 8 (4645) | 1.21 (0.91–1.62) | 0%, 0.97 | Low |
| Time to clinical recovery | RCT | 2 (374) | 0.40 (−0.67 - 1.46) | 68%, 0.08 | Very low |
| Time to virological recovery | RCT | 2 (344) | 0.16 (−1.44,1.75) | 85%, 0.01 | Very low |
| Prospective Cohort | 2 (482) | −4.66 (−7.99 -1.32) | 75%, 0.05 | ||
| Duration of hospital stay | RCT | 2 (526) | −0.17 (−0.80 -0.46) | 0%, 0.69 | Very low |
| Prospective Cohort | 2 (482) | −1.00 (−2.02 - 0.02) | 0%, 1.00 | ||
| HCQs Prophylaxis | |||||
| Confirmed COVID-19 | RCT | 3 (2429) | 1.04 (0.58–1.88) | 0%,0.91 | Moderate |
| Need for hospitalization | RCT | 2 (2304) | 0.64 (0.28–1.47) | 0%, 0.75 | Low |
| Any adverse events | RCT | 3 (2315) | 1.87 (1.39–2.51) | 67%,0.05 | Moderate |
Fig 4.
Forest plot showing the comparison of the need of mechanical ventilation among patients receiving hydroxychloroquine vs standard of care.
4.3. Adverse events
HCQ group has a significantly higher rate of any adverse event (41.3% vs. 16.4%; RR 2.68; 95% CI 1.55–4.64), as compared to the control group. This increased risk of adverse events was also observed in the subgroup of non-hospitalized patients (RR 2.71; 95% CI 1.29–5.68). However, we did not find any significant difference for serious adverse events (any life-threatening event/cardiac arrhythmia as defined by individual studies) between HCQ and standard care group (3.8% vs.2.6%; RR 1.21 95% CI 0.91–1.62).
4.4. Efficacy and safety of hydroxychloroquine in preventing SARS-CoV-2 infection
Use of HCQ for prophylaxis (pre- or post-exposure) against SARS-CoV-2 infection neither reduces the risk of acquiring SARS-CoV-2 infection (RR 1.04; 95% CI 0.58–1.88) nor the need for hospitalization (RR 0.64; 95% CI 0.28–1.47). On the other hand, it increases the risk for any adverse event (RR 1.87; 95% CI 1.39–2.51). None of the trials reported any serious adverse event associated with HCQ use as prophylaxis.
5. Discussion
In this meta-analysis, we have pooled key efficacy and safety outcomes of the use of HCQ or CQ alone for the treatment and prophylaxis of COVID-19. All except one used HCQ in the intervention arm. The use of HCQ alone for the treatment of COVID-19 was found to increase the risk of all-cause mortality (moderate-quality evidence). On subgroup analysis, this increase in mortality was predominantly seen in hospitalized patients (moderate-quality evidence) but not in non-hospitalized patients (low-quality evidence). Also, HCQ use led to a significantly higher adverse event rate (low-quality evidence), but the serious adverse event rate was similar to the control group (very-low quality evidence). There was no significant difference in other secondary efficacy outcomes (low to very low-quality evidence).
A subgroup analysis of the primary outcome as per the disease severity found the effect on mortality to be limited to trials involving patients of all types of disease severity. Separate subgroup analysis for mild, moderate, and severe disease severity was not feasible due to a lack of reporting of separate data for different disease categories. In the trials including patients with mild-to-moderate disease, there was a trend towards increased mortality, though it did not reach statistical significance [9,10,23]. This difference could be due to the low event rate in the later population.
We explored the possible reasons for increased mortality in the HCQ group and did not find any difference in baseline characteristics like age, disease severity, the timing of initiation of therapy after symptom onset, care given other than the intervention, etc. among the two groups. Furthermore, we excluded studies employing combination therapy of HCQ/CQ with antivirals, macrolides, and other active agents in either control or intervention group, therefore, the confounding effect of the other drugs (macrolides, corticosteroids, immunomodulators, etc.) or any other therapy is unlikely. Also, there was a trend towards the increased need for mechanical ventilation (low-quality evidence) in patients treated with HCQ, for reasons not clearly understood. Besides, treatment with HCQ did not reduce the need for ICU admission (very-low quality evidence), progression to severe disease, or duration of hospital stay in patients with COVID-19. Taken together, these data highlight that the use of HCQ may preferably be avoided in patients hospitalized with COVID-19.
Interestingly, there was no increase in mortality in non-hospitalized patients of COVID-19 treated with HCQ (low-quality evidence). Also, there was a trend towards a reduction in the need for hospitalization in outpatients treated with HCQ (low-quality evidence). Taken together, this may point to a possible benefit of HCQ in this cohort of patients, which needs to be addressed separately in dedicated randomized controlled trials.
Although one small study reported better radiological recovery with HCQ (very-low quality evidence), the impact over clinical recovery was not observed (very-low quality evidence) [28]. The pooling of data on virological recovery with HCQ yielded conflicting results. The data from relatively large prospective cohort studies reported higher (low-quality evidence) and faster (very-low quality) virological clearance with HCQ, but the same was not consistently seen in RCTs. The virological clearance may have significant public health importance, with a faster clearance potentially helping in curbing transmission and achieving disease control. However, with the current evidence, we are unsure about its effect over virological clearance, a fundamental basis on which it was widely used even before the clinical evidence emerged.
Furthermore, a higher rate of adverse events in the HCQ group (low-quality evidence) is worrisome, even though the pooled risk of serious adverse events (including cardiac arrhythmias) was not different (very-low quality evidence). Dose-adverse effect correlation could not be attempted due to the wide variability in the dosing regimens used in different studies. This data suggest that the high rates of cardiac toxicity noted in a few observational studies of HCQ may, at least in part, be because of concomitant therapies like azithromycin, and frequently observed cardiac involvement by SARS-CoV-2 [33].
Although case-control and unpublished studies from India reported positive results with HCQ prophylaxis in healthcare workers, none of the three RCTs to this end found any reduction in the incidence of COVID-19 with HCQ prophylaxis (moderate-quality evidence) [[6], [7], [8],29,30]. Besides, adverse events were also found to be increased (moderate-quality evidence), though serious adverse events (including cardiac arrhythmias) were not seen. Taken together, HCQ should not be used in prophylaxis against COVID-19.
We have registered protocol for this systematic review in PROSPERO and followed it rigorously during the conduct of this systematic review. We have included only randomized clinical trials and prospective cohort studies with controlled groups comparing HCQ/CQ alone with placebo/control to avoid selection bias and confounding effects of other interventions. This systematic review also has a few limitations. First, included trials enrolled patients with mild to severe disease and did not report separate data for individual disease severity hence, we could not assess the effect of HCQ separately on patients with severe COVID-19 for which the evidence is most needed. Although, we did attempt a separate analysis comparing mild-moderate disease patients with all types of disease severity (including severe ones). Second, we could find only limited data for most of the secondary outcomes hence, the overall quality for these outcomes was low to very-low. Third, with the available data, we could not assess the dose-response relationship of HCQ. However, the findings were consistent across all trials, and it is unlikely that any given dosage has a protective effect against COVID-19.
6. Conclusion
Moderate quality evidence suggests that the use of hydroxychloroquine as a treatment for COVID-19 is associated with a considerable increase in mortality and other adverse events. The negative effects are more pronounced in hospitalized patients. Therefore, based on current evidence the HCQ should be avoided for prophylaxis or treatment of COVID-19 in hospitalized patients. However, the positive effects of HCQ over the need for hospitalization without any increase in mortality in out-patients (mild disease) warrants further exploration.
Data Availability Statement
Data will be made available on request.
Financial support
None.
Author’s contribution
Concept and design: JM, JK; Acquisition, analysis, or interpretation of data: JM, JK, AY, SJ; Drafting of the manuscript: JK, JM SJ; Critical revision of the manuscript for important intellectual content: JM, JK, SJ. JK had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.02.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.COVID-19 situation update worldwide, as of 27 October 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 2.Ali M.J., Hanif M., Haider M.A., Ahmed M.U., Sundas F., Hirani A., et al. Treatment options for COVID-19: a review. Front Med. 2020;7:480. doi: 10.3389/fmed.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathy S., Dassarma B., Roy S., Chabalala H., Matsabisa M.G. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int J Antimicrob Agents. 2020;56:106028. doi: 10.1016/j.ijantimicag.2020.106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quiros Roldan E., Biasiotto G., Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res. 2020;158:104904. doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee P., Anand T., Singh K.J., Rasaily R., Singh R., Das S., et al. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459–467. doi: 10.4103/ijmr.IJMR_2234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 8.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abd-Elsalam S., Esmail E.S., Khalaf M., Abdo E.F., Medhat M.A., Abd El Ghafar M.S., et al. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am J Trop Med Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L., et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5:1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morand E.F., McCloud P.I., Littlejohn G.O. Continuation of long term treatment with hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 1992;51:1318–1321. doi: 10.1136/ard.51.12.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das R.R., Jaiswal N., Dev N., Jaiswal N., Naik S.S., Sankar J. Efficacy and safety of anti-malarial drugs (chloroquine and hydroxy-chloroquine) in treatment of COVID-19 infection: a systematic review and meta-analysis. Front Med. 2020;7:482. doi: 10.3389/fmed.2020.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A.K., Singh A., Singh R., Misra A. Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashour Z., Riaz M., Garbati M.A., AlDosary O., Tlayjeh H., Gerberi D., et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76:30–42. doi: 10.1093/jac/dkaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PRISMA-P Group. Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. 2020. The newcastle–ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. [Google Scholar]
- 22.WHO Solidarity Trial Consortium. Pan H., Peto R., Henao-Restrepo A.-M., Preziosi M.-P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ Med Sci. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karolyi M., Pawelka E., Mader T., Omid S., Kelani H., Ely S., et al. Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19 patients : results from a real-life patient cohort. Wien Klin Wochenschr. 2020 doi: 10.1007/s00508-020-01720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M., et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19 : a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimaldi D., Aissaoui N., Blonz G., Carbutti G., Courcelle R., Gaudry S., et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies: the COVADIS multicentre observational study. Ann Intensive Care. 2020;10:131. doi: 10.1186/s13613-020-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang M., Li M., Xiao F., Pang P., Liang J., Tang T., et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. National Science Review. 2020;7:1428–1436. doi: 10.1093/nsr/nwaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medrxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 29.Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I., et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis Among health care workers: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajasingham R., Bangdiwala A.S., Nicol M.R., Skipper C.P., Pastick K.A., Axelrod M.L., et al. Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofgren S.M., Nicol M.R., Bangdiwala A.S., Pastick K.A., Okafor E.C., Skipper C.P., et al. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. Open Forum Infect Dis. 2020;7:ofaa500. doi: 10.1093/ofid/ofaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitjà O., Corbacho-Monné M., Ubals M., Tebe C., Peñafiel J., Tobias A., et al. Hydroxychloroquine for early treatment of adults with mild covid-19: a randomized-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson A., Kirresh A., Conway S., White L., Ahmad M., Little C. Hydroxychloroquine use in COVID-19: is the risk of cardiovascular toxicity justified? Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.