Abstract
Background
Surgical smoke during operation is a well-known health hazard for medical staff. This study aimed to investigate the dynamics of surgical smoke during open surgery or laparoscopic surgery for colorectal disease.
Methods
This study quantitated particulate matter (PM) counts as part of surgical smoke in 31 consecutive patients who underwent colectomy at the Niigata City General Hospital using a laser particle counter. Particles were graded by size as ≤ 2.5 μm PM (PM2.5) or > 2.5 μm PM (large PM). Operative procedures were categorized as either open surgery (n = 14) or laparoscopic surgery (n = 17).
Results
The median patient age was 72 (range 41–89) years and 58.1% were male. The total PM2.5, PM2.5 per hour, and maximum PM2.5 per minute counts during operation were significantly higher in open surgery than in laparoscopic surgery (P = 0.001, P < 0.001, and P = 0.029, respectively). Large PM counts (total, per hour, and maximum per minute) were also higher in the open surgery group than in the laparoscopic surgery group. The maximum PM2.5 concentration recorded was 38.6 µm/m3, which is considered “unhealthy for sensitive groups” according to the U.S. Environment Protection Agency air quality index standards, if it was a 24-h period mean value.
Conclusion
Exposure to surgical smoke is lower during laparoscopic surgery than during open surgery for colorectal diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-021-08394-1.
Keywords: Surgical smoke, Particulate matter, Open surgery, Laparoscopic surgery
Surgical smoke is widely known to harbor particulates of blood, bacteria, and viruses, and of numerous chemicals such as benzene, formaldehyde, acrolein, and CO, among others [1–3], apart from carcinogenic and neurotoxic compounds [4]. Several reports have documented human papillomavirus (HPV) transmission to gynecologists or operating room nurse after laser ablation [5–7], and the presence of the hepatitis B virus (HBV) and human immunodeficiency virus have been confirmed in surgical smoke [8, 9]. Regrettably, most medical staff are not aware of the risks because of the low frequency of transmission; however, we think that this risk of infection should be noted, especially in preventing infection in the coronavirus disease-2019 (COVID-19) era.
While several reports on surgical smoke in the fields of urology, orthopedics, gynecology, and otolaryngology are available [10–14], there are only a few reports in the field of gastroenterological surgery [8, 15, 16]. Open surgery or laparoscopic surgery for gastroenterological diseases usually requires several hours and involves the frequent use of various devices, such as electric and ultrasonic scalpels, and sealing devices. Thus, it is extremely important to protect against surgical smoke during gastroenterological surgery. Here, we have investigated the dynamics of surgical smoke during colorectal surgery, with the aim of evaluating the differences in surgical smoke between open surgery and laparoscopic surgery.
Methods
Patients
This observational cohort study was conducted between June 2020 and August 2020 at the Niigata General City Hospital and enrolled 31 consecutive patients who required colectomy for colorectal disease. The cases of appendicitis were excluded and written informed consent was obtained from all 31 patients. This study was conducted in compliance with the Helsinki Declaration and Ethical Guidelines for Medical and Health Research Involving Human Subjects. This study was approved by the institutional review board at Niigata General City Hospital (20-022).
Surgical procedure
In our institution, as a rule, laparoscopic surgery is performed for colorectal disease, while open surgery is preferred in cases of perforation, large tumors, peritonitis, a history of multiple surgeries, and poor general condition. The use of energy devices, such as electric or ultrasonic scalpels, or vascular sealing devices, was left to the surgeon’s discretion. Laparoscopic procedures were multiport, with approximately four or five ports, and used the Olympus endoscopic system (VISERA ELITE II™). In this system, the surgical smoke was automatically evacuated using ultrasonic scalpel, but was not linked using electric scalpel. We did not use the complex integrated smoke evaluation system with ultra-low particulate air (ULPA) filters in this study. Specimens were removed through a small laparotomy incision.
Measurement of particulate matter
Particulate matter (PM) counts in surgical smoke in the operating room were enumerated using a dust monitor (Dylos air quality monitor DC170, SATOTECH, Japan), which is a laser particle counter that can measure particle sizes as > 0.5 and > 2.5 µm, in real-time. Here, particle size > 2.5 µm was defined as “large PM,” and data were recorded as 1-min average counts. The particles with a size ≤ 2.5 μm are defined as “PM2.5.” In this monitor, the counts of small particle (> 0.5 μm) display the counts including large particles (> 2.5 μm). Therefore, PM2.5 counts were calculated by subtracting large particle counts from small particle counts. The PM2.5 concentration (µm/m3) was estimated as described previously [17]. The concentration of PM2.5 resulting in adverse health outcomes was determined based on the U.S. Environment Protection Agency (USEPA) air quality index (AQI) specifications (Fig. 1) [18]. The dust monitor was placed on the patient’s head side and at the same height as the surgeon’s face during the operation.
Fig. 1.

Air quality index (AQI), according to the U.S. Environment Protection Agency
The environment in the operating rooms
All operations were performed in five operating rooms at our institution. The area of these rooms ranged between 34.5 and 47.6 m2 and the room volume was between 103.5 and 142.8 m3. All rooms were equipped with high-efficiency particulate air filters. The airflow was 3360–4680 m3/h and the air exchange rate was 42–49 times/h. We also determined PM counts (PM2.5 and large PM) in each operating room before preparing for the surgeries.
Endpoint
We investigated PM counts (PM2.5 and large PM) during colorectal surgery, and the operative procedures were categorized as open surgery (n = 14) or laparoscopic surgery (n = 17). The total PM2.5, PM2.5 per hour, and maximum PM2.5 per minute counts were compared between the two groups.
Statistical analysis
Data are reported as the chi-squared test or Fisher’s exact test was used for categorical variables, while the Mann–Whitney U test or the Kruskal–Wallis test was used to analyze the numerical variables. Statistical analyses were performed using IBM SPSS Statistics 22 (IBM Japan, Inc., Tokyo, Japan). All analyses were two-sided, and P-values less than 0.05 were considered statistically significant.
Results
Clinical characteristics
The median patient age was 72 years (range 41–89 years), 58.1% were male, and the median body mass index was 21.3 kg/m2. All patients underwent colectomy for colorectal disease, which included colon cancer (n = 17; 54.8%), rectal cancer (n = 7; 22.6%), perforation of the colon (n = 3; 9.7%), malignant lymphoma (n = 1; 3.2%), Crohn’s disease (n = 1; 3.2%), ulcerative colitis (n = 1; 3.2%), and necrosis of the colon (n = 1; 3.2%). The operations were performed in five operating rooms. The median operating time was 192 min. There were no significant differences in patient characteristics between the two groups (Table 1).
Table 1.
Patient characteristics
| Variables | All (n = 31) | Open surgery (n = 14) | Laparoscopic surgery (n = 17) | P value |
|---|---|---|---|---|
| Age (years)* | 72 (41–89) | 70 (47–89) | 73 (41–86) | 0.33 |
| Sex | 1.00 | |||
| Male | 18 | 8 | 10 | |
| Female | 13 | 6 | 7 | |
| Height (cm)* | 162.0 (140.0–174.0) | 162.5 (140.0–174.0) | 157.1 (140.0–170.4) | 0.83 |
| Weight (kg)* | 55.6 (33.6–76.2) | 51.3 (37.3–76.2) | 57.3 (33.6–73.7) | 0.53 |
| Body mass index (kg/m2)* | 21.3 (15.3–30.8) | 20.8 (16.0–27.2) | 21.3 (15.3–30.8) | 0.35 |
| Body surface area (m2)* | 1.6 (1.2–1.9) | 1.5 (1.2–1.9) | 1.6 (1.2–1.8) | 0.71 |
| Diseases | 0.07 | |||
| Colon cancer | 17 | 6 | 11 | |
| Rectal cancer | 7 | 1 | 6 | |
| Perforation of the colon | 3 | 3 | 0 | |
| Malignant lymphoma | 1 | 1 | 0 | |
| Crohn’s disease | 1 | 1 | 0 | |
| Ulcerative colitis | 1 | 1 | 0 | |
| Necrosis of the colon | 1 | 1 | 0 | |
| Operating room | 0.08 | |||
| A | 1 | 1 | 0 | |
| B | 1 | 1 | 0 | |
| C | 1 | 0 | 1 | |
| D | 22 | 7 | 15 | |
| E | 6 | 5 | 1 | |
| Operating time (min)* | 192 (87–509) | 172 (87–509) | 201 (131–257) | 0.58 |
*Median (range)
The environment of the operating rooms
The mean PM counts (counts/m3/min) for all the operating rooms are shown in Table 2. There was no significant difference in PM counts, for both PM2.5 and large PM, in any of the operating rooms before preparing for the operation.
Table 2.
The counts of PM in the operating rooms before surgery preparation
| Operating room | PM2.5* | P-value | Large PM* | P-value |
|---|---|---|---|---|
| A | 353.4 ± 335.2 | 0.760 | 0 ± 0 | 0.564 |
| B | 353.4 ± 335.2 | 353.4 ± 335.2 | ||
| C | 706.7 ± 447.0 | 0 ± 0 | ||
| D | 706.7 ± 670.4 | 706.7 ± 670.4 | ||
| E | 706.7 ± 670.4 | 0 ± 0 |
PM particulate matter
*Mean (counts/m3/min) ± standard error
Intraoperative dynamics of particulate matter
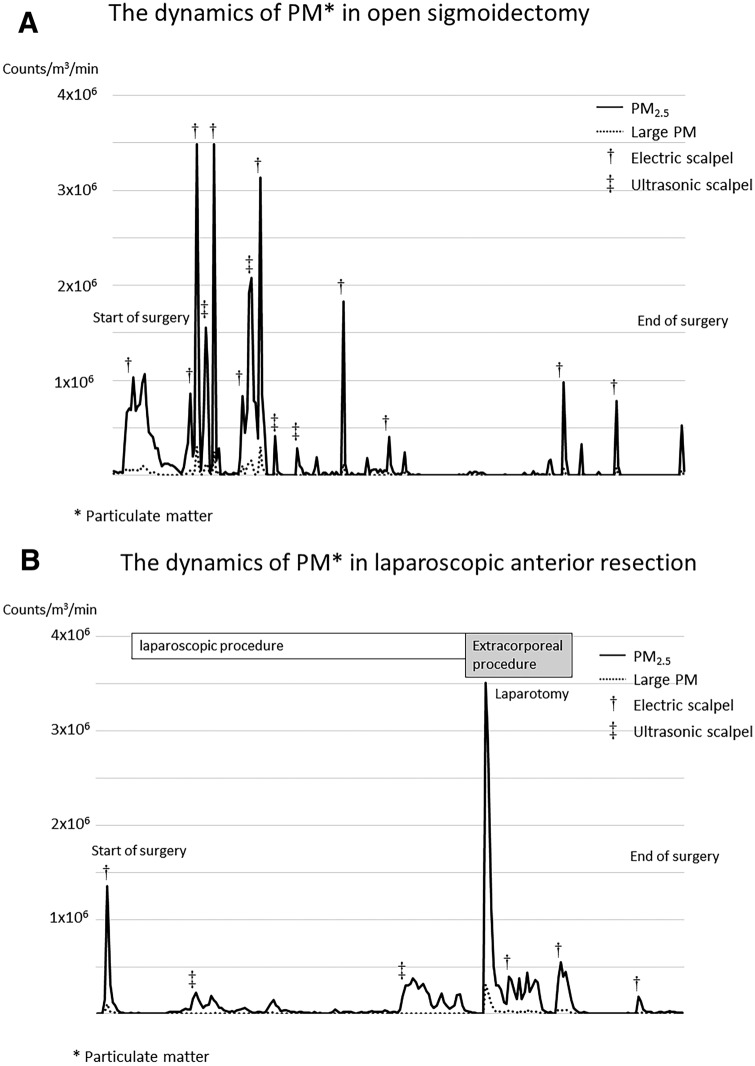
The dynamics of PM counts (PM2.5 and large PM) in representative laparoscopic and open operations are shown in Fig. 2A and B, respectively. The graph in Fig. 2A shows changes in PM counts during open surgery in a 71-year-old man who underwent sigmoidectomy for locally advanced sigmoid colon cancer, while Fig. 2B represents the case of laparoscopic surgery in a 73-year-old man who underwent anterior resection for rectosigmoid colon cancer. As can be seen from the graphs, open surgery led to frequent increases in PM counts, which corresponded to the use of various devices during the operation. In contrast, PM was rarely detected during laparoscopic procedures and PM counts increased mainly during port insertion and extracorporeal procedures.
Fig. 2.
Dynamics of particulate matter (PM) counts in representative cases. A Open surgery. B Laparoscopic surgery
Comparison of PM counts between open and laparoscopic surgeries
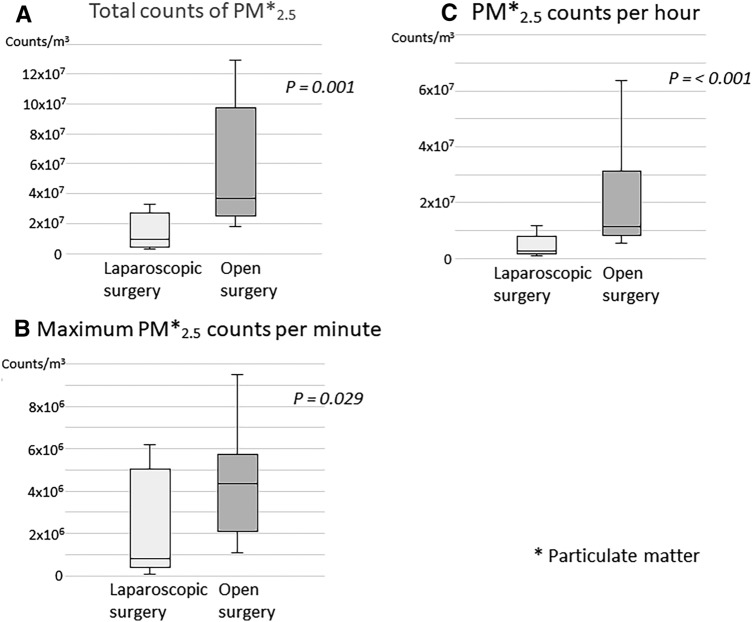
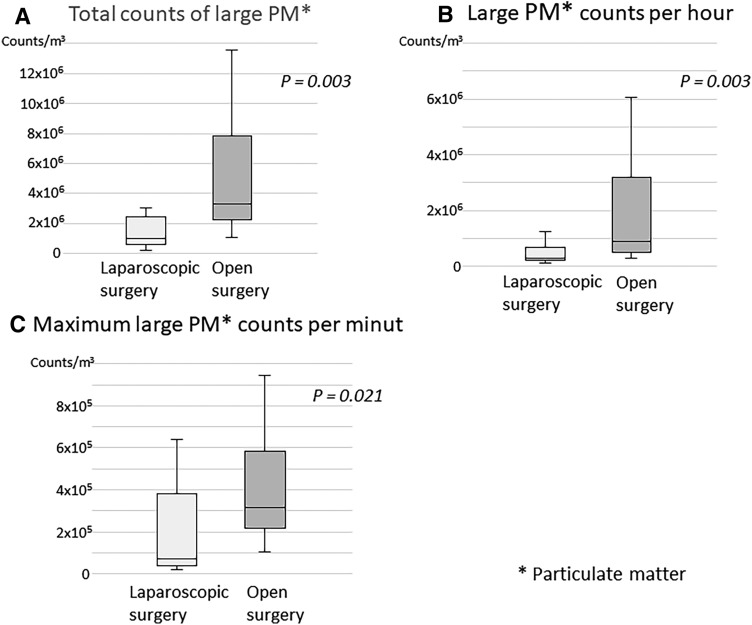
The results of PM 2.5 counts during the operation are shown in Fig. 3, and were significantly higher in the open surgery group than in the laparoscopic surgery group (P = 0.001) (Fig. 3A). Further, PM2.5 counts per hour and the maximum PM2.5 counts per minute were also significantly higher in the open surgery group than in the laparoscopic surgery group (P = 0.003) (Fig. 3B, C). Large PM counts (total, per hour, and maximum per minute counts) were also higher in the open surgery group than in the laparoscopic surgery group (Fig. 4). In this study, the maximum PM2.5 concentration recorded was 38.6 µm/m3, which according to the AQI of the USEPA can be characterized as “unhealthy for sensitive groups,” if it was a 24-h period average value.
Fig. 3.
Comparison of PM2.5 counts between open surgery and laparoscopic surgery. A Total counts of PM2.5. Total counts of PM2.5 during surgery were significantly higher in the open surgery than in the laparoscopic surgery (P = 0.001). B PM2.5 counts per hour. PM2.5 counts per hour during surgery were also significantly higher in the open surgery group than in the laparoscopic surgery group (P < 0.001). C Maximum PM2.5 counts per minute. Maximum PM2.5 counts per minute during surgery were also significantly higher in the open surgery group than in the laparoscopic surgery group (P = 0.029)
Fig. 4.
Comparison of large PM counts between open surgery and laparoscopic surgery. A Total counts of large PM. Total counts of large PM during surgery was significantly higher in the open surgery group than in the laparoscopic surgery group (P = 0.003). B Large PM counts per hour. Large PM counts per hour during surgery were significantly higher in the open surgery than in the laparoscopic surgery (P = 0.003). C Maximum large PM counts per minute. Maximum large PM counts during surgery were significantly higher in open surgery than in laparoscopic surgery (P = 0.021)
Discussion
Several studies have reported on surgical smoke in the fields of urology, orthopedics, otolaryngology, breast surgery, and gynecology [10–14, 19]; however, there are only few reports in the field of gastroenterological surgery [8, 15, 16]. Gastroenterological surgery often continues for several hours and uses various devices. Further, both open surgery and laparoscopic surgery may be performed. During the beginning of the COVID-19 pandemic, surgical societies quickly recommended avoiding laparoscopy because of SARS-CoV-2 virus transmission risk through surgical smoke; however, the review of Mintz et al. suggested that laparoscopic surgery carries a lower risk of transmission through surgical smoke than open surgery [1]. The Endoscopic and Laparoscopic Surgeons of Asia recommendation for minimally invasive surgery states that there was no evidence to recommend or prohibit laparoscopic surgery compared to open surgery [20]. Additionally, both the Society of American Gastrointestinal and Endoscopic Surgeons and the European Association for Endoscopic Surgeons have stated that the risk of aerosol transmission during laparoscopic surgery in the COVID-19 pandemic remains unknown [21].
Here, we have revealed that there are differences in PM counts between open surgery and laparoscopic surgery, with laparoscopic surgery producing lower PM counts than open surgery. It is remarkable that almost no occurrence of surgical smoke was observed during laparoscopic procedures.
The terms “smoke,” “plume,” “aerosol,” and “vapor” have been used with similar meanings and we have used “surgical smoke” in this paper. The risks of surgical smoke have been recognized for a long time [22], but remain neglected by surgeons because of low infectivity rates [1]. In contrast, during the COVID-19 pandemic, aerosols produced by open surgery or laparoscopic surgery have received greater attention.
The risk of viral transmission through surgical smoke has been reported in several reviews [1–3, 23], and HPV transmission is the most widely reported infection [5–7]. Zhou et al. have demonstrated that the positive rate of HPV DNA in surgical smoke was 29.9% in loop electrosurgical excision procedures [24], while Kwak et al. have reported that the HBV was detected in the surgical smoke in 10 of 11 patients who underwent laparoscopic or robotic surgery [8]. Even though the SARS-CoV-2 virus has never been found in surgical smoke till date, as its presence has been reported in blood and stools, the risk of viral transmission cannot be excluded [25].
The size of surgical smoke particles varies from 0.07 to 6.5 µm, depending on the device [20]. It has been reported that small particles (0.07 µm) are produced by electrocautery, while large particles (0.35–6 µm) are produced by an ultrasonic scalpel [20, 26, 27]. According to the Aerosol Consensus Statement, particles that are 5 μm or larger in size reach the nasopharynx, those sized 2–5 μm can be delivered to the airway, and those smaller than 3 μm reach the pulmonary parenchyma [28]. We reveal that both PM2.5 and large PM were significantly more abundant in open surgery than in laparoscopic surgery.
Surgical smoke has been reported to cause respiratory diseases such as emphysema, bronchial asthma, and chronic bronchitis [20]. Many studies have used USEPA AQI standards [17, 18], which state that PM concentrations of more than 35.5 µm/m3 over a 24-h period are considered “unhealthy for sensitive groups.” The maximum PM2.5 concentration was 38.6 µm/m3 in this study, but it was not 24-h period value. That the PM2.5 concentration in this study did not reach a value that can cause immediate health damage reduces the cause for concern; nonetheless, exposure to surgical smoke should be avoided as much as possible.
We revealed that using an electric scalpel ejected more particles than using an ultrasonic device (Fig. 2A, B). Ott et al. reported that blood and tissue particles increased significantly using an ultrasonic device [27]. Regarding HPV, it is reported that electric scalpel had a lower transmission risk through surgical smoke than laser [23], but the risks for COVID-19 transmission are not clear.
Tokuda et al. have described treatment possibilities against surgical smoke and report that the concentration of total volatile organic compounds and formaldehyde could be reduced by using evacuation systems [23]. Several associations have provided recommendations for laparoscopic surgery, and these include maintaining lower pneumoperitoneum pressure, minimizing the use of energy devices, avoiding long dissecting times, frequent suction to avoid the accumulation of smoke, and safety evacuation before trocar removal or laparotomy [24, 25]. In laparoscopic surgery, attention to surgical smoke is important during the extracorporeal procedure performed through a small laparotomy incision. It appears that high levels of PM can be released even in laparoscopic surgery. Thus, surgeons and operating room personnel should attempt to minimize any risk of transmission through surgical smoke during both open and laparoscopic surgeries [29].
The use of more complex integrated endoscopic systems with ULPA filters is recommended during the COVID-19 pandemic [24], and ULPA filters can remove 99.99% of the particles that measure 0.12 μm or more in diameter [24]. However, as the risk of escape from the pneumoperitoneum around the trocar has been reported when using these systems [30], further studies on the risk of transmission through surgical smoke when using these systems may be necessary [30].
Despite the above, there are some limitations to this study. First, this was a small, single-institution study that was limited to procedures pertinent to colorectal disease. Second, we evaluated PM numbers using a dust monitor, and we did not analyze the components of surgical smoke. In principle, the measurement before surgery was performed in a situation where no one came in and out but the effects of the door opening/closing while preparing surgical equipment cannot be denied. Moreover, since the content analysis has not been performed, the details of the difference between PM2.5 and large PM are not clear. Third, there was a bias between open surgery and laparoscopic surgery groups.
Nevertheless, we describe the dynamics of surgical smoke during colorectal surgery and show significant differences in PM2.5 and large PM counts between open surgery and laparoscopic surgery. Additionally, our results reveal that PM2.5 and large PM are rarely produced during laparoscopic procedures. Therefore, we consider that this study is clinically significant, especially in the era of the COVID-19 pandemic.
Thus, laparoscopic surgery for colorectal diseases may be superior to open surgery from the viewpoint of surgical smoke.
Supplementary Information
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Disclosures
Hitoshi Kameyama, Tetsuya Otani, Toshiyuki Yamazaki, Akira Iwaya, Hiroaki Uehara, Rina Harada, Motoharu Hirai, Masaru Komatsu, Akira Kubota, Tomohiro Katada, Kazuaki Kobayashi, Daisuke Sato, Naoyuki Yokoyama, Shirou Kuwabara, Yuki Tanaka, and Kimihiko Sawakami have no conflict of interests or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mintz Y, Arezzo A, Boni L, Baldari L, Cassinotti E, Brodie R, Uranues S, Zheng M, Fingerhut A. The risk of COVID-19 transmission by laparoscopic smoke may be lower than for laparotomy: a narrative review. Surg Endosc. 2020;26:1–8. doi: 10.1007/s00464-020-07652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karuppal R, Surendran S, Patinharayil G, Muhammed Fazil VV, Marthya A. It is time for a more cautious approach to surgical diathermy, Especially in COVID-19 outbreak: a schematic review. J Orthop. 2020;20:297–300. doi: 10.1016/j.jor.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett WL, Garber SM. Surgical smoke—a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979–987. doi: 10.1007/s00464-002-8584-5. [DOI] [PubMed] [Google Scholar]
- 4.Al Sahaf OS, Vega-Carrascal I, Cunningham FO, McGrath JP, Bloomfield FJ. Chemical composition of smoke produced by high-frequency electrosurgery. Ir J Med Sci. 2007;176:229–232. doi: 10.1007/s11845-007-0068-0. [DOI] [PubMed] [Google Scholar]
- 5.Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425–427. doi: 10.1007/BF01463570. [DOI] [PubMed] [Google Scholar]
- 6.Calero L, Brusis T. Laryngeal papillomatosis—first recognition in Germany as an occupational disease in an operating room nurse. Laryngorhinootologie. 2003;82:790–793. doi: 10.1055/s-2003-44546. [DOI] [PubMed] [Google Scholar]
- 7.Rioux M, Garland A, Webster D, Reardon E. HPV positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54. doi: 10.1186/1916-0216-42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak HD, Kim SH, Seo YS, Song KJ. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857–863. doi: 10.1136/oemed-2016-103724. [DOI] [PubMed] [Google Scholar]
- 9.Baggish MS, Poiesz BJ, Joret D, Williamson P, Refai A. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197–203. doi: 10.1002/lsm.1900110302. [DOI] [PubMed] [Google Scholar]
- 10.Wang HK, Mo F, Ma CG, Dai B, Shi GH, Zhu Y, Zhang HL, Ye DW. Evaluation of fine particles in surgical smoke from a urologist’s operating room by time and by distance. Int Urol Nephrol. 2015;47:1671–1678. doi: 10.1007/s11255-015-1080-3. [DOI] [PubMed] [Google Scholar]
- 11.Yeganeh A, Hajializade M, Sabagh AP, Athari B, Jamshidi M, Moghtadaei M. Analysis of electrocautery smoke released from the tissues frequently cut in orthopedic surgeries. World J Orthop. 2020;11:177–183. doi: 10.5312/wjo.v11.i3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Sawakami K, Shoji S, Ishikawa S, Kusabe Y, Wakui J, Sakai Y, Kawase H, Okumura G, Yamashita H, Segawa H. Generation of surgical smoke in spinal surgery. Med J Niigata City Gen Hosp. 2019;40:18–23. [Google Scholar]
- 13.Liu Y, Song Y, Hu X, Yan L, Zhu X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J Cancer. 2019;10:2788–2799. doi: 10.7150/jca.31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo-Yánez M, Calvo-Henríquez C, Lechien JR, Fakhry N, Ayad T, Chiesa-Estomba CM. Is the ultrasonic scalpel recommended in head and neck surgery during the COVID-19 pandemic? State-of-the-art review. Head Neck. 2020;42:1657–1663. doi: 10.1002/hed.26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W, Zhu H, Zhang N, Dong D, Wang S, Ren F, Xiang J, Wu R, Lv Y. Characterization of the PM2.5 concentration in surgical smoke in different tissues during hemihepatectomy and protective measures. Environ Toxicol Pharmacol. 2019;72:103248. doi: 10.1016/j.etap.2019.103248. [DOI] [PubMed] [Google Scholar]
- 16.Sagar PM, Meagher A, Sobczak S, Wolff BG. Chemical composition and potential hazards of electrocautery smoke. Br J Surg. 1996;83:1792. doi: 10.1002/bjs.1800831241. [DOI] [PubMed] [Google Scholar]
- 17.Semple S, Ibrahim AE, Apsley A, Steiner M, Turner S. Using a new, low-cost air quality sensor to quantify second-hand smoke (SHS) levels in homes. Tob Control. 2015;24:153–158. doi: 10.1136/tobaccocontrol-2013-051188. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Environmental Protection Agency (2018) Technical assistance document for the reporting of Daily Air quality–the air quality index (AQI). https://www.airnow.gov/sites/default/files/2020-05/aqi-technical-assistance-document-sept2018.pdf. Accessed 6 Aug 2020
- 19.Tokuda Y, Okamura T, Maruta M, Orita M, Noguchi M, Suzuki T, Matsuki H. Prospective randomized study evaluating the usefulness of a surgical smoke evacuation system in operating rooms for breast surgery. J Occup Med Toxicol. 2020;15:13. doi: 10.1186/s12995-020-00259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shabbir A, Menon RK, Somani J, So JBY, Ozman M, Chiu PWY, Lomanto D. ELSA recommendations for minimally invasive surgery during a community spread pandemic: a centered approach in Asia from widespread to recovery phases. Surg Endosc. 2020;34:3292–3297. doi: 10.1007/s00464-020-07618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis N, Dort J, Cho E, Feldman L, Keller D, Lim R, Mikami D, Phillips E, Spaniolas K, Tsuda S, Wasco K, Arulampalam T, Sheraz M, Morales S, Pietrabissa A, Asbun H, Pryor A. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. 2020;34:2327–2331. doi: 10.1007/s00464-020-07565-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowbray NG, Ansell J, Horwood J, Cornish J, Rizkallah P, Parker A, Wall P, Spinelli A, Torkington J. Safe management of surgical smoke in the age of COVID-19. Br J Surg. 2020 doi: 10.1002/bjs.11679,May3,2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoshi K, Kobayashi K, Kinoshita K, Tomizawa Y, Hasegawa S, Sakai Y. Health risks associated with exposure to surgical smoke for surgeons and operation room personnel. Surg Today. 2015;45:957–965. doi: 10.1007/s00595-014-1085-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q, Hu X, Zhou J, Zhao M, Zhu X, Zhu X. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019;11:3643–3654. doi: 10.2147/CMAR.S201975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavan N, Crestani A, Abrate A, Nunzio C, Esperto F, Giannarini G, Galfano A, Gregori A, Liguori G, Bartoletti R, Porpiglia F, Simonato A, Trombetta C, Tubaro A, Ficarra V, Novara G, Research Urology Network (RUN) Risk of virus contamination through surgical smoke during minimally invasive surgery: a systematic review of the literature on a neglected issue revived in the COVID-19 pandemic era. Eur Urol Focus. 2020;6:1058–1069. doi: 10.1016/j.euf.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinsohn PA, Jewett DL, Balzer L, Bennett CH, Seipel P, Rosen A. Aerosols created by some surgical power tools: particle size distribution and qualitative hemoglobin content. Appl Occup Environ Hyg. 1991;6:773–776. doi: 10.1080/1047322X.1991.10389727. [DOI] [Google Scholar]
- 27.Ott DE, Moss E, Martinez K. Aerosol exposure from an ultrasonically activated (Harmonic) device. J Am Assoc Gynecol Laparosc. 1998;5:29–32. doi: 10.1016/S1074-3804(98)80007-8. [DOI] [PubMed] [Google Scholar]
- 28.American College of Chest Physicians Aerosol consensus statement. Consensus conference on aerosol delivery. Chest. 1991;100:1106–1109. doi: 10.1378/chest.100.4.1106. [DOI] [PubMed] [Google Scholar]
- 29.Vourtzoumis P, Alkhamesi N, Elnahas A, Hawel JE, Schlachta C. Operating during COVID-19: Is there a risk of viral transmission from surgical smoke during surgery? Can J Surg. 2020;63:E299–E301. doi: 10.1503/cjs.007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalli J, Khan MF, Nolan K, Cahill RA. Laparoscopic pneumoperitoneum escape and contamination during surgery using the Airseal insufflation system: video vignette. Colorectal Dis. 2020 doi: 10.1111/codi.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.