Abstract
The consumption of indulgent, carbohydrate- and fat-rich foods is often used as a strategy to cope with negative affect because they provide immediate self-reward. Such dietary choices, however, can severely affect people’s health. One countermeasure could be to improve one’s emotion regulation ability. We used functional magnetic resonance imaging to examine the neural activity underlying the downregulation of incidental emotions and its effect on subsequent food choices. We investigated whether emotion regulation leads to healthier food choices and how emotion regulation interacts with the brain’s valuation and decision-making circuitry. We found that 1) the downregulation of incidental negative emotions was associated with a subsequent selective increase in decisions for tasty but also for healthy foods, 2) food preferences were predicted by palatability but also by the current emotional state, and 3) emotion regulation modulated decision-related activation in the ventromedial prefrontal cortex and ventral striatum. These results indicate that emotional states are indeed important for food choice and that the process of emotion regulation might boost the subsequent processing of health attributes, possibly via neural reward circuits. In consequence, our findings suggest that increasing emotion regulation ability could effectively modulate food choices by stimulating an incidental upvaluation of health attributes.
Keywords: affect, emotion regulation, fMRI, food choice, machine-learning, neuroimaging, reappraisal
Introduction
The goal to maintain a healthy diet can often be threatened by our spontaneous decisions in emotionally laden situations. For example, coming home after a long and stressful work day and rewarding oneself by ordering fast food to compensate for the negative emotions through the consumption of high caloric food is a situation most people have experienced (Macht 2008). This tendency to eat in response to emotional triggers in order to decrease an unpleasant emotional state, as opposed to satisfy a true physiological need for food, is referred to as “emotional eating” (Arnow et al. 1995; Devonport et al. 2019). This unhealthy behavior [i.e., greater consumption of sweet and high-fat foods (Camilleri et al. 2014) and frequent snacking and eating in response to stressors (O’Connor et al. 2008)] has been related to weight gain over time (Koenders and van Strien 2011) and difficulty with losing weight (Braden et al. 2016). However, there is also some empirical evidence that when being in a negative emotional state people choose healthy food to comfort themselves (Adriaanse et al. 2011). One possible explanation for the inconsistency of the results might be the ability to cope with negative affective states, that is, the capacity to regulate emotions (Gross 1998).
Poor emotion regulation ability has been linked to emotional eating (Crockett et al. 2015; Braden et al. 2018; Ferrell et al. 2020) and eating disorders such as binge eating and purging (Wedig and Nock 2010; Haedt-Matt and Keel 2011; Berg et al. 2013; Gianini et al. 2013). It has been argued that craving can be regarded as an emotional state (Hill 2007) and that regulating craving can be achieved by using the same regulation strategies as for other emotions (Kober et al. 2010; Giuliani et al. 2013). In particular, one emotion regulation strategy—cognitive reappraisal [i.e., the process by which one changes the meaning of a stimulus by altering its emotional impact (Gross 2002)]—has been demonstrated to effectively modulate not only craving for foods (Giuliani et al. 2013; Reader et al. 2018) but also the desire to overeat (Svaldi et al. 2012). Neuroimaging studies that used reappraisal to regulate the desire to consume craved or not craved energy-dense foods reported increased activation in top-down self-regulation regions, including the dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus (IFG), and dorsal anterior cingulate cortex (dACC) (Giuliani et al. 2014). This prefrontal control network was also found to be implicated when dieters with high self-control chose healthy over unhealthy food in an implicit emotion regulation task (Hare et al. 2009) and when heavy smokers explicitly regulated their craving for cigarettes and food (Kober et al. 2010). Another study found that active reappraisal of unhealthy food recruits the brain’s valuation system in combination with prefrontal cognitive control areas associated with emotion regulation (Hollmann et al. 2012; Yokum and Stice 2013). In the aforementioned studies, reappraisal was mainly implemented by instructing participants to focus on negative long-term health-related consequences associated with the food. Other studies instructed participants to explicitly focus on the healthiness of food items before making their decisions and showed that healthier food choice behavior was related to activity in neural systems involved in value computation, such as the ventromedial prefrontal cortex (vmPFC) and dlPFC (Hare et al. 2011; Hutcherson et al. 2012; Herwig et al. 2016; van Meer et al. 2017; Tusche and Hutcherson 2018). Taken together, these findings imply that emotion regulation increases the ability to inhibit appetitive motivation fueled by craving, which leads to a reduction of unhealthy food intake (Evers et al. 2010).
The studies discussed so far primarily targeted integral emotions—those that are normatively relevant to a decision because they are elicited by a component of the decision or would be influenced by an outcome of the decision (Lerner et al. 2015). However, these are not the only affective influences on judgment and decision-making. Incidental emotions—those elicited by internal (e.g., dispositional affect) or external (e.g., environmental) factors not normatively relevant to the decision (Loewenstein and Lerner 2003; Lerner et al. 2015)—also impact decision-making. For example, negative emotions elicited by a prior event influence eating behavior (Grunberg and Straub 1992; Garg et al. 2007; Garg and Lerner 2013). Given the well-established impact of incidental emotions on decision-making, it is imperative to consider the influence of emotion regulation of incidental emotions on food choices.
The present study therefore explicitly investigated whether the regulation of incidental negative emotions, as often encountered in everyday life, leads to more healthy and/or less unhealthy dietary choices. While previous studies have suggested such effects, several important questions remain unanswered. First, it is unclear whether regulating a temporary negative emotional state using reappraisal as a strategy would show an immediate transfer effect on a subsequent dietary decision and lead to more healthy choices. In addition, if such an effect could be confirmed, this could be due to a shift towards fewer unhealthy choices, or towards more healthy choices, or both. Each outcome would have different consequences for the interpretation of the underlying mechanism, and our study aimed to clarify this. Second, it is known that food decisions are mainly driven by taste and hunger levels (Furst et al. 1996; Raynor and Epstein 2003; Nederkoorn et al. 2009; Shepherd and Raats 2010), but the extent to which emotional state, amongst other food, emotion, dieting, and personality variables, determines food decisions has not been sufficiently explored (Leng et al. 2017). Our study addressed this question by using a machine-learning approach to predict food choices from a large battery of factors. Third, given the involvement of similar neural networks in emotion regulation, value processing, and decision-making (Hare et al. 2011; Hutcherson et al. 2012; Morawetz et al. 2019), we hypothesized that effects of emotion regulation on dietary decision-making would be moderated by the brain’s valuation network. The final aim of this study was therefore to use neuroimaging to test for a modulation of activation related to decision-making and health attribute processing depending on the preceding application of emotion regulation.
To address these questions, we collected behavioral and functional magnetic resonance imaging (fMRI) data in two independent samples of healthy, normal-weighted, and non-dieting participants. In the experiments, participants alternated between a standard emotion regulation task in which they had to downregulate their emotional responses to incidental, negative stimuli, followed by a food choice task in each trial. We investigated whether the ratio of healthy and unhealthy food decisions as well as the decision strength differed depending on engagement in emotion regulation. In addition, we used machine learning to predict food preferences from food-related (nutrient contents, sensory attributes) and decision-maker–related factors (demographic and psychological factors), including emotional state. Third, we used a general linear model (GLM) approach for fMRI data analysis to identify brain regions in which decision and health attribute processing was modulated by emotion regulation. Given the neuroimaging evidence presented above, the vmPFC is implicated in food valuation, and it should also be involved in health-related choices. We therefore hypothesized that the activity in the vmPFC should be modulated by emotion regulation during food choice as well as reflecting processing health attributes if these were found to drive choice. This is because the relative activity in this region has been linked to processing salient properties of choice options (e.g., healthiness and palatability) (Hare et al. 2009, 2011; van Meer et al. 2017).
Materials and Methods
Here we report two studies: a behavioral experiment to investigate the effect of emotion regulation on food choices, followed by a fMRI experiment, which served to replicate these findings and additionally investigate the neural correlates of this process. The two experiments used identical experimental paradigms with two independent samples that are therefore described together in this section.
Participants
Behavioral experiment: We tested 49 right-handed, healthy participants with normal or corrected to normal vision (41 female, mean age = 23.40 years; standard deviation [SD] = 6.51, mean body-mass-index, BMI = 21.40 ± 4.48; note: BMI could only be measured in n = 44).
fMRI experiment: We tested 36 right-handed, healthy participants with normal or corrected to normal vision. One participant was excluded due to technical problems with data acquisition. The final sample consisted of 35 participants (29 female, mean age = 23.17 years; SD = 3.44, mean BMI = 21.26 ± 2.38). The distribution of the BMI is shown in Supplementary Figure 1.
Participants had no history of eating disorders and no aversion or allergies to any of the items used in the experiment and were unrestrained eaters, which was assessed before the experiment using a questionnaire. They were told that the goal of the experiment was to study food preferences. Participants in both experiments gave written, informed consent to participate. The studies were approved by the local ethics committee of the Department of Education and Psychology at Freie Universität Berlin.
Stimuli
Stimuli for Emotion Regulation
Stimuli consisted of 140 aversive pictures (normative ratings on a Likert scale from 1 [very negative/very calm] to 9 [very positive/very arousing]: mean valence = 2.26, mean arousal = 6.25) from the International Affective Picture System (IAPS; Bradley and Lang 2007).
Stimuli for Food Choice
Images were selected from the Food-pics database (Blechert et al. 2014) (Supplementary Fig. 2). Food-pics comprises a large variety of foods along with detailed data on image characteristics, food contents, and normative ratings. We only used images high on valence (>55), familiarity (>90), and recognizability (>90) (maximal score 100; total of 212 images selected). This stimulus set was further divided by calories using median split, resulting in high caloric (n = 104) and low caloric (n = 110) food items. Next, this set was further reduced to 140 images which were equally matched on arousal, recognizability, familiarity, complexity, palatability, craving, and all other image characteristics (color, object size, brightness, contrast, complexity, normed complexity, and spatial frequency) using the toolbox for stochastic optimization of stimuli (Armstrong et al. 2012). The final set therefore consisted of 70 high caloric (average kcal/100 g = 279.90; i.e., >50 kcal/100 g) and 70 low caloric (average kcal/100 g = 30.32; i.e., <50 kcal/100 g) items.
The stimulus presentation and response recording were controlled by Presentation (Version 14.1, Neurobehavioral Systems). Inside the MRI scanner, visual stimuli were presented using dual display goggles (VisuaStim, MR Research).
Experimental Design and Procedure
Before each experiment, participants rated all selected food items on familiarity (on a 100-point scale from “not at all” to “extremely”), palatability (on a 100-point scale from “not at all” to “extremely”), and healthiness (on a 100-point scale from “very unhealthy” to “very healthy”) to enable a direct comparison to the normative data.
The experimental task consisted of two parts. The first part was a standard emotion regulation task, which has been adapted from previous studies (Morawetz, Bode, Baudewig, Jacobs, et al. 2016a; Morawetz, Bode, Baudewig, Kirilina, et al. 2016b). This was followed by a food choice task. Both tasks were explained in great detail to the participants in written format as well as verbally. Participants performed a short training session before the experiment and could ask questions if they were uncertain about any aspects of the task. Participants were instructed not to eat for 3 h before the experiment.
Emotion Regulation Task
In each trial, participants were asked to regulate their emotions in response to viewing one of the aversive pictures. Two task conditions were implemented (Fig. 1): In the “Look” condition, participants were presented with aversive pictures and were asked to view the stimuli attentively and allow themselves to experience/feel any emotional responses, which these might elicit without manipulating them. In the “Decrease” condition, participants viewed aversive images and were asked to actively reduce the intensity of negative emotions by distancing themselves from the image by becoming a detached observer, for example, through thinking that the depicted situation is not real, by reducing the personal relevance of the image, or by telling themselves that the depicted situation is “only a picture” (Ochsner et al. 2004; Eippert et al. 2007; Urry et al. 2009). Importantly, participants were told not to substitute negative emotions with positive emotions.
Figure 1.
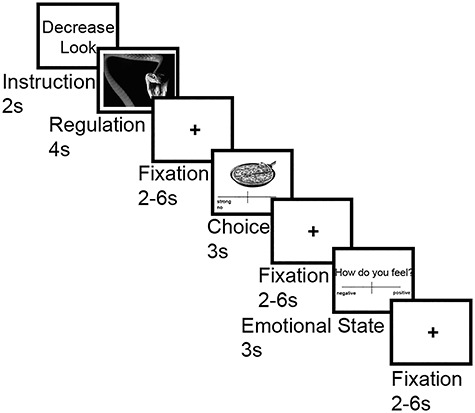
Experimental task design. In each trial, an emotion regulation task was followed by a food choice task. In the emotion regulation task, participants were instructed to either downregulate their emotions using reappraisal (Decrease) or maintain their emotional responses without regulating them (Look). After emotion regulation, participants indicated their preference for a depicted food item on a continuous scale from “strong yes” to “strong no.” At the end of each trial, participants indicated their current emotional state on a scale from “very negative” to “very positive”.
Food Choice Task
In each trial, the emotion regulation task was directly followed by the food choice task (Fig. 1). Participants were asked to decide whether they want to consume the food item that was shown by indicating the strength of their preferences using a continuous slider (from “strong no” to “strong yes,” arbitrarily scaled from −200 to +200). To make the decisions incentive compatible, participants were told that they were required to eat one of the chosen food items at the end of the experiment, pseudorandomly selected by taking into consideration their preference strength. Because participants did not know which trial would be selected, their optimal strategy was therefore to treat each decision as if it were the only one that counted to increase the probability to be given a desired item.
Task Procedures
In each trial the emotion regulation task was presented first, followed by the food choice task (Fig. 1). Each trial started with an instruction cue (2 s) indicating the experimental condition by displaying “Decrease” or “Look.” Subsequently, an image was presented for 4 s during which the instructed strategy had to be applied. This was followed by a fixation cross for a jittered duration of 2–6 s. After this, participants were presented with the food item on the top of the screen. The preference rating scale was presented below. Participants used a two-button fiber optic response pad (fORP, Cambridge Research Systems Ltd) to move a cursor along the scale to indicate their preference. The response window was 3 s. This was followed by another jittered fixation period for 2–6 s. Next, participants were asked to rate their current emotional state (from “extremely negative” to “extremely positive,” from −200 to +200) using the same response buttons to navigate the cursor. The response window for the rating was again 3 s. The cursor on both rating scales (preference and emotional state rating) was presented in the middle of the scale on each trial. Finally, a central fixation cross was presented for a jittered duration of 2–6 s, concluding the trial.
For both the behavioral and the fMRI experiment, participants performed five runs. Each run consisted of 28 trials, containing items that were balanced with respect to all food characteristics described above, and the same number of high and low caloric foods. The order of food items and aversive images were individually randomized. One trial lasted 24 s on average, and one run lasted about 12 min. A scanning/experimental session consisted of 140 trials, which resulted in ~1 h of scanning.
Questionnaires
After the experiment, participants completed several questionnaires. We measured the habitual use of emotion regulation strategies using the German version of the Emotion Regulation Questionnaire (ERQ, Abler and Kessler 2009), the ability to control emotions using the Emotional Competence Questionnaire (ECQ, Rindermann 2009), eating behavior using the Questionnaire of Eating Behavior (QEB, Diehl 2006), and nutrition attitudes using the Attitude to Healthy Nutrition (AHN, Diehl 2006).
fMRI Data Acquisition
Whole-brain functional and anatomical images were acquired using a 3.0 T Magnetom TrioTim MRI scanner (Siemens, Erlangen, Germany) using a 12-channel head coil. A high-resolution 3D T1-weighted data-set was acquired for each subject (176 sagittal sections, 1 × 1 × 1 mm3; 256 × 256 data acquisition matrix). Functional images were acquired using a T2*-weighted, gradient-echo echo planar imaging (EPI) pulse sequence recording 37 sections oriented parallel to the anterior and posterior commissure at an in-plane resolution of 3 × 3 × 3 mm3 (interslice gap = 0; TE = 30 ms; TR = 2 s; FA = 90°; FoV = 192 × 192 mm2; 64 × 64 data acquisition matrix). For each experimental run 340 whole-brain volumes were recorded.
Data Analyses
Behavioral Data
Emotional state, food choices, and preferences
Emotional state ratings were used to calculate regulation success scores. For this, the emotional state rating on each Decrease trial was divided by the average of all Look trials (serving as the baseline when emotions were experienced but not regulated) and divided by 100 (Morawetz, Bode, Baudewig, Jacobs, et al. 2016a; Morawetz, Bode, Baudewig, Kirilina, et al. 2016b; Morawetz et al. 2019). This score therefore provided a percentage estimate of how well emotions could be regulated in each trial. This measure served to check whether ER was successful.
Food choices were calculated as the proportion (in %) of chosen options, separately for palatability and health categories. For health, items were split into healthy and unhealthy using the scale midpoint of the individual ratings. For palatability, palatable and unpalatable food categories were defined by using the scale midpoint of the individual ratings. Note, however, that because items were pre-selected for having high valence scores, and food items’ valence is strongly determined by palatability (Blechert et al. 2014), there were only very few items with low palatability for each participant (Decrease: M = 10.24 SD = 7.27 trials; Look: M = 10.24 SD = 8.18 trials).
Food preferences were calculated as the raw scores for either rejecting (scores of 0 to −200) or choosing (scores of 1–200) an item. This provided a complementary continuous measure for food evaluations to the binary food choice categories.
The behavioral measures were subjected to ANOVAs, and SPSS Version 25 was used to perform the statistical tests. In the main analyses, we tested for 1) interactions between emotion regulation condition with health category and palatability category on food choices and 2) interactions between emotion regulation condition with health category and palatability category on food preferences.
Multivariable regression of preference strength
In addition, a model-based analysis was performed in Python (v3.7.4) to identify factors that predicted the preference for food items using a multivariable regression model. The factors stem from five sources: 1) demographic data (age, gender, hours without food), 2) ratings of familiarity, palatability, and healthiness for each item, 3) trial-by-trial emotional state ratings, 4) questionnaire data (ERQ, ECQ, AHN, and QEB), and 5) objective food item characteristics as taken from the Food-pics database (protein/100 g, fat/100 g, carbs/100 g, kcal/100 g), number of items in the image, total grams, total protein, total fat, total carbs, total kcal, color (red, green, blue), object size, brightness, contrast, complexity, spatial frequency, as well as normative ratings for complexity, recognizability, familiarity, valence, arousal, complexity, palatability, and craving.
For the regression model, “Extreme Gradient Boosted Trees” (XGBoost, scikit-learn library v0.21.2) was used, which is superior to most other techniques (Chen and Guestrin 2016). XGBoost implements performance optimized, regularized gradient-boosted decision trees. Gradient boosting is a method that improves the performance of a base prediction technique through reapplication of the technique on the error of the last application. This is repeated until no further improvement can be achieved. Such decision trees can capture linear and nonlinear relationships between factors and the prediction (Hastie et al. 2009), in our case the preference for food items. The model performance was estimated using a nested cross-validation (CV) procedure (Hastie et al. 2009). CV allows to assess the performance of the model that can be expected on new, unseen data and hence, the generalizability of the model performance. For the main CV loop, a five-time 10-fold partitioning of the data was chosen, resulting in 50 XGBoost models, each trained and tested on a different data-set. Feature scaling (z-scoring) and hyper-parameter tuning were carried out within the main CV loop. Hyper-parameter tuning is necessary to control model complexity and to avoid overfitting the data. For the hyper-parameter tuning, two inner CV loops were implemented. Both inner CV loops used a three-time 10-fold partitioning scheme and were carried out sequentially. The first inner CV loop was used to optimize the model complexity by tuning the tree’s depth. The following values for the depth were tested: 5, 6, 7, 8, 9, and 10. The best performing depth was used for the final model in the main CV loop and for the models in the second inner CV loop. The second inner CV loop was used to optimize the number of boosting rounds. The number of boosting rounds controls how often a new decision tree is trained on the error of the last decision tree. This parameter also controls the model complexity, but on a higher level than the tree’s depth. For this optimization, an early stopping procedure was implemented. If the performance of the model was not further increasing for 50 boosting rounds, the training was stopped. Early stopping was carried out in every CV loop, and the average number of boosting rounds was used for the final model in the main CV loop. After hyper-parameter tuning, a XGBoost model was trained in the main CV loop using the obtained hyper-parameter and the following constant parameters: learning rate = 0.01, gamma = 0.01, subsample = 0.63, and colsample_bynode = 0.33. These additional parameters again control model complexity.
The final model was tested on the respective holdout set of the main CV loop. The holdout set was not used in the inner CV loops. In each main CV loop, the following model performance metrics were computed: 1) the mean absolute error (MAE), 2) the root mean squared error (RMSE), 3) the Pearson correlation (CORR) between the predicted food preference ratings and the actual food preference ratings, and 4) the coefficient of determination (R2) of the model. For MAE and RMSE smaller values represent a better model fit, whereas for CORR and R2 higher values indicate a better model fit.
After obtaining sufficiently fitting models, we analyzed the contributions of single factors to the model’s performance. For nonlinear models as we used here, this is not as straightforward as for linear models. One common option is permutation feature importance testing, which works as follows: 1) A baseline R2 score is recorded by passing a validation set through the model. 2) The values of a single factor are permuted, and the validation set is passed back through the model. 3) The R2 score is then recomputed. 4) The importance of a factor is the difference between the baseline and the drop in overall R2 score caused by permuting a factor’s values (Breiman 2001). The permutation disentangles the relationship between a factor and the prediction (preference for food), that is, the drop in the model score is indicative of how much the model depends on that factor. We report the drop in R2 score for each factor normalized to the baseline R2 score. Hence, permutation feature importance values lie between 0 (not important, no change in R2 score) and 1 (very important, R2 changes to zero).
We also performed partial dependence analysis to investigate how the prediction of a model depends on a single factor (Hastie et al. 2009). Particularly, the partial dependence of a single factor corresponds to the average response of an estimator for each possible value of the single factor, while mitigating the influence of all other factors. This is achieved by replacing every single factor value by those of a defined grid and computing the average prediction. Intuitively, partial dependence can be interpreted as the expected prediction (preference for food) as a function of the values of the analyzed factor (Molnar 2019). Since the XGBoost model can capture nonlinear relationships, the partial dependence can be nonlinear too.
fMRI Data Analysis
Preprocessing
Functional imaging data analysis was performed using SPM12 (Wellcome Institute for Cognitive Neurology, London, UK). As interleaved slice acquisition was used, slice time correction was conducted (Sladky et al. 2011). In addition, standard preprocessing involved realignment to the mean image, spatial normalization to the standard EPI template (MNI template), and spatial smoothing with an 8-mm full width at half maximum (FWHM) isotopic Gaussian kernel.
General Linear Models
We used several GLMs to analyze the data
GLM1. A first general linear model (GLM) was estimated to identify neural networks supporting decision-making and emotion regulation. This model included the following regressors: instruction cue (duration 2 s), ER phase split by emotion regulation conditions (Decrease, Look) (duration 4 s), food choice phase split by type of food choice as function of regulation condition (Food-YesDecrease, Food-NoDecrease, Food-YesLook, Food-NoLook) (duration 3 s), and emotion rating phase (duration 3 s). This model included motion parameters as nuisance covariates. The regressors were convolved with a canonical form of the hemodynamic response. Contrast images of brain activations associated with decision-making across ER conditions (Food-YesDecrease + Food-YesLook > Food-NoDecrease + Food-NoLook; Food-NoDecrease + Food-NoLook > Food-YesDecrease + Food-YesLook) were calculated for each participant and used in a second-level analysis to identify decision-making–related regions. To identify ER-related regions, contrast images of brain activations associated with emotion regulation (Decrease>Look) and emotion reactivity (Look>Decrease) were produced for each participant. T-statistics for each voxel were thresholded at P < 0.05, corrected for multiple comparisons across whole brain with family-wise error rate (FWE) and at the cluster level.
GLM2. This model was designed to identify regions in which BOLD activity during the decision phase was parametrically related to preferences. The model included the following regressors: instruction cue (duration 2 s); ER phase with separate regressors for emotion duration regulation conditions (Decrease, Look) (duration 4 s); food choice phase regressor, which modeled the onsets of the phase (duration 3 s); and in addition a parametric regressor for preferences for the food item in each trial (duration 3 s). Finally, the emotion rating phase was modeled again (duration 3 s). The model included motion parameters as nuisance covariates. The regressors were convolved with a canonical form of the hemodynamic response.
GLM3. This model was designed to identify regions in which BOLD activity during the food choice phase was parametrically related to the healthiness of the food items. The model included the following regressors: instruction cue (duration 2 s), emotion regulation phase, split by conditions (Decrease, Look) (duration 4 s), food choice phase with an onset regressor (duration 3 s), and a parametric regressor for healthiness of the food item in each trial. The emotion rating phase was modeled again (duration 3 s). This model included motion parameters as nuisance covariates. The regressors were convolved with a canonical form of the hemodynamic response.
As the behavioral analysis of the food ratings revealed that participants rated most of the items as palatable (i.e., low variance in the palatability ratings) (Supplementary Fig. 3), a parametric regression using palatability of food items was of no experimental interest and, thus, not implemented in the fMRI analysis (Wood et al. 2008). Note, stimuli were selected based on calories (to provide a wide range of healthiness) and not on palatability.
GLM2–3 allowed searching for areas in which the BOLD response in the food decision phase parametrically varied with the magnitude of preference and healthiness, respectively. The following models GLM4–5 additionally allowed investigating preference and healthiness during the food choice phase as a function to the preceding emotion regulation condition.
GLM4. This GLM was similar in structure to GLM2. It included the following regressors: instruction cue (duration 2 s), ER phase split by emotion regulation conditions (Decrease, Look) (duration 4 s), and food choice phase onset regressors (duration 3 s) split by regulation condition (FoodDecrease, FoodLook), with a parametric regressor added that quantified the food choice preference for each trial. Finally, the emotion rating phase was again modeled (duration 3 s). The model included motion parameters as nuisance covariates.
GLM5. This GLM was again similar to GLM3, but it used parametric regressors for healthiness dependent on regulation condition in the food decision phase. This means, the regressors included instruction cue (duration 2 s), emotion regulation phase split by emotion regulation conditions (Decrease, Look) (duration 4 s), and food choice phase onset regressors (duration 3 s) split by regulation condition (FoodDecrease, FoodLook), with a parametric regressor added that quantified healthiness for each trial. Finally, the emotion rating phase was modeled (duration 3 s). The model again included motion parameters as nuisance covariates.
Region of Interest Analyses
To further test the modulating effect of emotion regulation on decision-making, region of interest (ROI) analyses were performed on regions that were both parametrically modulated by preference and healthiness (i.e., the overlap from the parametric effects of GLM2 and GLM3). This overlap was restricted to the vmPFC and the striatum (see Results for details), and ROIs were created, respectively, using the Marsbar (Version 0.44) toolbox for SPM12 (Brett et al. 2002) (Supplementary Fig. 6). Our empirical vmPFC ROI was additionally masked using the vmPFC mask from a meta-analysis on the value system (contrast: Decision Stage; left vmPFC: x = −7, y = 41, z = −5; right vmPFC: x = 7, y = 42, z = 2) (Bartra et al. 2013) to ensure that the vmPFC cluster was indeed restricted to this region (vmPFC ROI: x = 0, y = 40, z = −3; size = 6520 mm). Our empirical striatum ROI was masked using the anatomically derived mask of the caudate regions of WFU PickAtlas (Version 3.0.5b, https://www.nitrc.org/projects/wfu_pickatlas/, atlas = “human-atlas aal,” “L-caudate,” and “R-caudate”) (Maldjian et al. 2003). The ROIs for both hemispheres (left striatum ROI: x = −7, y = 11, z = −4, size = 200 mm; right striatum ROI: x = 7, y = 11, z = −4, size = 152 mm) were analyzed as one ROI. For each ROI, we applied the contrasts from GLM1 from the food choice phase for all combinations of food decisions by preceding emotion regulation condition (Food-YesDecrease, Food-NoDecrease, Food-YesLook, Food-NoLook). While from the definition of the ROI it was already clear that food preference modulated activity in these regions (hence the Food-Yes vs. Food-No contrast was circular and considered trivial), this approach nevertheless allowed us to test the independent contrast, that is, whether food preference was modulated in these regions depending on the preceding emotion regulation condition.
Results
The results are divided into three main parts. In the first part, we report the behavioral findings for each study separately, that is, online ratings, emotional state ratings, choices, and preferences. The choices and strength of preferences to consume the depicted foods are reported as a function of emotion regulation condition, subjectively perceived healthiness and palatability of the items. In the second part, the regression results of the machine-learning model are presented, in which both studies have been analyzed conjointly. Finally, in the third part, we report the fMRI findings.
Behavioral Data
Mauchly tests for sphericity showed that the sphericity assumptions were not violated for any of the ANOVAs.
Ratings of Food Items in Familiarity, Palatability, and Healthiness
First, we compared the ratings of familiarity, palatability, and healthiness of our studies with the norms (Supplementary Fig. 3, Table 1). The images were rated significantly less familiar in the fMRI study compared with the norms [t(278) = 5.56, P < 0.001] and the behavioral study [t(278) = 4.86, P < 0.001]. Note, however, that a familiarity of 97 out of 100 is still close to ceiling, rendering the differences practically meaningless. The food items were also rated higher on palatability in both the behavioral study and the fMRI study compared with the norms [behavioral study > norms: t(278) = 10.22, P < 0.001; fMRI > norms: t(278) = 14.48, P < 0.001]. Food items were also rated as more palatable in the fMRI study compared with the behavioral study [t(278) = 3.31, P < 0.001]. The food items did not differ significantly on the healthiness ratings in the behavioral study (M = 64.13, SD = 32.31) and the fMRI study (M = 67.26, SD = 34.17) [t(278) = −0.78, P = 0.43]. There are no normative ratings available on healthiness in the Food-pics database.
Table 1.
Ratings of food items
| Behavioral study | fMRI study | Norms | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Familiarity | 98.57 | 2.02 | 97.31 | 2.30 | 98.71 | 1.87 |
| Palatability | 74.44 | 8.42 | 77.63 | 7.64 | 65.04 | 6.88 |
| Healthiness | 64.13 | 32.31 | 67.26 | 34.17 | - | - |
| Familiarity | ||||||
| High caloric | 98.48 | 2.16 | 96.42 | 2.41 | 98.43 | 2.17 |
| Low caloric | 98.67 | 1.88 | 98.21 | 1.79 | 98.99 | 1.87 |
| Palatability | ||||||
| High caloric | 75.82 | 8.35 | 75.71 | 6.98 | 64.72 | 5.24 |
| Low caloric | 73.06 | 8.33 | 79.55 | 7.84 | 65.35 | 8.22 |
| Healthiness | ||||||
| High caloric | 54.53 | 36.96 | 42.30 | 32.86 | - | - |
| Low caloric | 73.73 | 23.46 | 92.21 | 3.05 | - | - |
Note: high caloric: >50 kcal/100 g according to norms. Low caloric: <50 kcal/100 g according to norms. M = mean; SD = standard deviation.
Behavioral study: n = 49; fMRI study: n = 35; norms: n = 638.
Next, we divided the food items into high and low caloric items based on the norm data (kcal/100 g) and compared the ratings of familiarity, palatability, and healthiness of the two samples (Supplementary Fig. 4, Table 1). As expected, high and low caloric food items did not differ significantly in palatability and familiarity within the behavioral study [palatability: t(138) = 1.95, P = 0.053; familiarity: t(138) = −0.53, P = 0.59]. However, the fMRI study rated low caloric food items as more familiar [t(138) = 4.97, P < 0.001] and palatable [t(138) = 3.06, P = 0.003]. Importantly, high caloric food items were indeed rated as significantly more unhealthy than low caloric food items in both the behavioral study [t(138) = −3.67, P < 0.001] and the fMRI study [t(138) = −12.65, P < 0.001]. This was confirmed by correlation analyses between the objective measure of calories (kcal/100 g) and the subjective measure of healthiness ratings (Supplementary Fig. 5). Healthiness was significantly negatively related to calories for both the behavioral (Pearson’s r = −0.43, P < 0.001) and the fMRI study (Pearson’s r = −0.73, P < 0.001).
Manipulation Check for Reappraisal
To test for the efficacy of emotion regulation, we compared the emotional state ratings of the Decrease condition with the Look condition. T-tests showed that Decrease resulted in less negative emotional state ratings compared with Look [behavioral study: t(48) = 3.40, P = 0.001; fMRI study: t(34) = 3.56, P = 0.001] (Table 2).
Table 2.
Emotional state rating results. Manipulation check
| Emotional state ratings | Behavioral study | fMRI study | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Decrease | 41.95 | 12.75 | 38.62 | 12.86 |
| Look | 38.14 | 12.78 | 34.40 | 14.13 |
| Emotional state ratings with respect to palatability | ||||
| Palatable | ||||
| Decrease | −26.02 | 51.45 | −26.52 | 48.85 |
| Look | −71.97 | 35.43 | −55.37 | 46.88 |
| Unpalatable | ||||
| Decrease | −27.83 | 47.82 | −37.51 | 58.46 |
| Look | −81.40 | 43.73 | −53.82 | 64.47 |
Note: M = mean; SD = standard deviation.
Manipulation Check for Emotions Felt Towards Food
As the emotional state was rated after the food choice, the ratings could potentially also be affected by the palatability of the presented food items (Desmet and Schifferstein 2008; Barthomeuf et al. 2009). Thus, we also analyzed the emotional state ratings with respect to palatability (Table 2). We did not observe a significant difference in emotional state ratings following palatable compared with unpalatable foods during the Decrease condition in either study [behavioral study: t(45) = 1.30, P = 0.19; fMRI study: t(34) = 1.99, P = 0.06]. During the Look condition, we found a significant difference in emotional state ratings following palatable compared with unpalatable foods in the behavioral study [t(43) = 3.00, P = 0.004], but not in the fMRI study [t(32) = −0.43, P = 0.66]. This potentially indicates only some small effects of palatability on emotional state ratings. Note that these results need to be interpreted with caution, given that the number of unpalatable items was very low. However, this also means that small effects of palatability on overall emotional state were neglectable, given that the majority of food items were perceived as tasty, and therefore any effect would have been equally present in most trials in both emotion regulation conditions.
Choices as a Function of Emotion Regulation and Individual Perceived Healthiness/Palatability
Healthiness. A repeated measures ANOVA was used with the dependent variable percentage of choices to consume food and emotion regulation (Decrease, Look) and healthiness (healthy, unhealthy) as independent variables for each study separately (Table 3). For the behavioral study, we found significant main effects for regulation, healthiness, and a significant interaction effect between emotion regulation and healthiness. The same results were observed for the fMRI study.
Table 3.
Choices as function of emotion regulation
| Effects | Behavioral study | fMRI study | ||
|---|---|---|---|---|
| Choices and healthiness | F(df = 48) | P | F(df = 34) | P |
| Regulation | 10.97 | 0.002 | 9.42 | 0.004 |
| Healthiness | 49.30 | <0.001 | 45.85 | <0.001 |
| Regulation × healthiness | 8.77 | .005 | 19.37 | <0.001 |
| Choices and palatability | ||||
| Regulation | 14.70 | <0.001 | 9.42 | 0.004 |
| Palatability | 272.99 | <0.001 | 175.76 | <0.001 |
| Regulation × palatability | 12.42 | 0.001 | 7.69 | <0.001 |
Note: df = degrees of freedom.
On average, participants preferred the healthy food items over the unhealthy ones irrespective of regulation condition in both studies. Moreover, in both samples, post hoc t-tests showed that participants wanted to consume the healthy food items more often after reappraisal compared with the Look condition (Table 4). Results are illustrated in Figure 2A for the fMRI study.
Table 4.
Paired t-tests of % choices to consume food items
| Study | t-test | t-value | P-value | Cohen’s d |
|---|---|---|---|---|
| % choices to consume food items as function of healthiness | ||||
| Behavioral study | Decreasehealthy > Decreaseunhealthy | 7.5 | <0.001 | 1.07 |
| Decreasehealthy > Lookhealthy | 3.58 | 0.001 | 0.51 | |
| Decreasehealthy > Lookunhealthy | 7.38 | <0.001 | 1.05 | |
| Decreaseunhealthy > Lookhealthy | -5.66 | <0.001 | -0.81 | |
| Decreaseunhealthy > Lookunhealthy | 1.46 | 0.149 | 0.21 | |
| Lookhealthy > Lookunhealthy | 6.05 | <0.001 | 0.87 | |
| fMRI study | Decreasehealthy > Decreaseunhealthy | 7.99 | <0.001 | 1.35 |
| Decreasehealthy > Lookhealthy | 4.01 | 0.001 | 0.68 | |
| Decreasehealthy > Lookunhealthy | 7.56 | <0.001 | 1.28 | |
| Decreaseunhealthy > Lookhealthy | -5.39 | <0.001 | -0.91 | |
| Decreaseunhealthy > Lookunhealthy | -0.004 | 0.407 | 0.00 | |
| Lookhealthy > Lookunhealthy | 5.37 | <0.001 | 0.91 | |
| % choices to consume food items as function of tastiness | ||||
| Behavioral study | Decreasetasty > Decreaseuntasty | 17.56 | <0.001 | 2.51 |
| Decreasetasty > Looktasty | 3.93 | <0.001 | 0.56 | |
| Decreasetasty > Lookuntasty | 17.07 | <0.001 | 2.53 | |
| Decreaseuntasty > Looktasty | -14.1 | <0.001 | -2.02 | |
| Decreaseuntasty > Lookuntasty | 1.18 | 0.99 | 0.17 | |
| Looktasty > Lookuntasty | 14.54 | <0.001 | 2.08 | |
| fMRI study | Decreasetasty > Decreaseuntasty | 13.38 | <0.001 | 2.34 |
| Decreasetasty > Looktasty | 3 | <0.001 | 0.51 | |
| Decreasetasty > Lookuntasty | 13.79 | <0.001 | 2.33 | |
| Decreaseuntasty > Looktasty | -11.78 | <0.001 | -1.99 | |
| Decreaseuntasty > Lookuntasty | 1.03 | 0.308 | 0.17 | |
| Looktasty > Lookuntasty | 11.85 | <0.001 | 2.00 | |
Notes: Bonferroni corrected for multiple comparisons. Significant results are indicated in bold.
Figure 2.
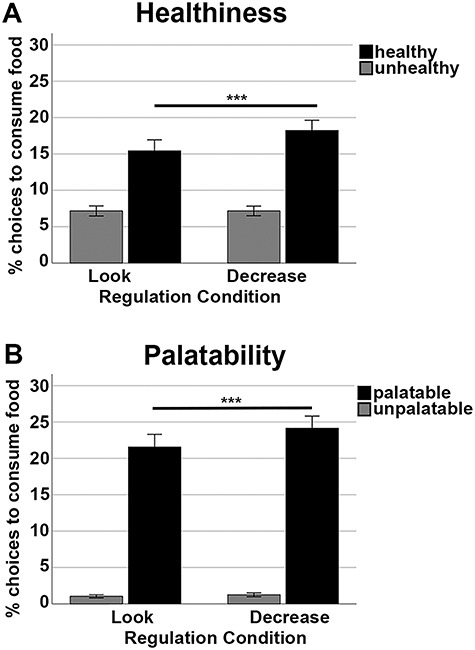
Behavioral results of the fMRI study. (A) Percentage of “yes” choices, that is, to consume the food item as a function of healthiness and regulation condition. (B) Percentage of consumption choices as a function of palatability and regulation condition.
Palatability. Using repeated measures ANOVA with the factors regulation (Decrease, Look) and palatability (palatable, unpalatable), we found a significant main effect for regulation and palatability as well as a significant interaction effect between regulation and palatability in both studies (Table 3).
In both studies, post hoc t-tests showed that on average participants preferred the palatable food items over the unpalatable ones irrespective of regulation condition. Moreover, participants wanted to consume the palatable food items more often after reappraisal compared with Look. All effects are reported in Table 4 and illustrated in Fig. 2B for the fMRI study. Note, however, that the trial numbers for low palatability items (based on the individual ratings) were only 7–9% in total, and therefore these results need to be interpreted with care.
Preference Strength as a Function of Emotion Regulation and Individual Perceived Healthiness/Palatability
Healthiness. In the behavioral study, using repeated measures ANOVA with the factors regulation (Decrease, Look), choice (yes, no), and healthiness (healthy, unhealthy), we found a significant main effect of healthiness and a significant main effect of choice on preference strength (Table 5). In addition, we observed a significant interaction effect between regulation and healthiness. In the fMRI study, we found a significant effect of regulation and a significant main effect of choice. In contrast to the behavioral study, no significant interaction effects were observed (Table 5).
Table 5.
Strength of preferences to consume food items as function of emotion regulation
| Effects | Behavioral study | fMRI study | ||
|---|---|---|---|---|
| Preference and healthiness | F | P | F | P |
| Regulation | 0.82 | 0.37 | 5.29 | 0.02 |
| Choice | 637.40 | <0.001 | 599.14 | <0.001 |
| Healthiness | 5.11 | 0.02 | 0.003 | 0.95 |
| Regulation × choice | 2.56 | 0.11 | 3.22 | 0.08 |
| Regulation × healthiness | 3.84 | 0.05 | 2.77 | 0.10 |
| Choice × healthiness | 2.23 | 0.14 | 0.25 | 0.61 |
| Regulation × choice × healthiness | 0.04 | 0.83 | <0.001 | 0.99 |
| Preference and palatability | ||||
| Regulation | 3.24 | 0.08 | 3.67 | 0.07 |
| Choice | 350.94 | <0.001 | 358.13 | <0.001 |
| Palatability | 17.61 | <0.001 | 15.80 | .002 |
| Regulation × choice | 0.15 | 0.69 | 0.01 | 0.92 |
| Regulation × palatability | 0.19 | 0.66 | 0.05 | 0.82 |
| Choice × palatability | 2.87 | 0.10 | 13.49 | 0.003 |
| Regulation × choice × palatability | 0.23 | 0.63 | 0.03 | 0.86 |
Note: df = degrees of freedom.
We then split the data by trials in which participants accepted items and rejected items and analyzed the preference strength ratings (which naturally differed by direction) separately. Post hoc t-tests revealed that in both studies, participants demonstrated a stronger preference for healthy foods after downregulating their emotions compared with the Look condition, while they showed no significant difference in preference when they declined to consume the food after emotion regulation compared with the Look condition. Detailed results from both studies are reported in Table 6 and illustrated in Figure 3A for the fMRI study.
Table 6.
Paired t-tests of preference strength
| Study | t-test | t(df = 48) | P | Cohen’s d |
|---|---|---|---|---|
| Strength of preference to consume food items as function of healthiness | ||||
| Behavioral study | Decreasehealthy_yes > Lookhealthy_yes | 4.10 | <0.001 | 0.59 |
| Decreasehealthy_no > Lookhealthy_no | 1.26 | 0.21 | 0.18 | |
| Decreaseunhealthy_yes > Lookunhealthy_yes | 0.15 | 0.88 | 0.02 | |
| Decreaseunhealthy_no > Lookhealthy_no | 1.32 | 0.19 | 0.19 | |
| t(df = 34) | P | Cohen’s d | ||
| fMRI study | Decreasehealthy_yes > Lookhealthy_yes | 3.80 | <0.001 | 0.64 |
| Decreasehealthy_no > Lookhealthy_no | 1.10 | 0.27 | 0.19 | |
| Decreaseunhealthy_yes > Lookunhealthy_yes | 1.60 | 0.11 | 0.28 | |
| Decreaseunhealthy_no > Lookhealthy_no | −0.09 | 0.92 | −0.02 | |
| Strength of preference to consume food items as function of palatability | ||||
| t(df = 48) | P | Cohen’s d | ||
| Behavioral study | Decreasetasty_yes > Looktasty_yes | 2.93 | 0.005 | 0.42 |
| Decreasetasty_no > Looktasty_no | 1.87 | 0.06 | 0.27 | |
| Decreaseuntasty_yes > Lookuntasty_yes | 1.13 | 0.27 | 0.24 | |
| Decreaseuntasty_no > Looktasty_no | −4.44 | <0.001 | −0.66 | |
| t(df = 34) | p | Cohen’s d | ||
| fMRI study | Decreasetasty_yes > Looktasty_yes | 3.43 | 0.002 | 0.58 |
| Decreasetasty_no > Looktasty_no | 2.00 | 0.05 | 0.34 | |
| Decreaseuntasty_yes > Lookuntasty_yes | 1.46 | 0.16 | 0.38 | |
| Decreaseuntasty_no > Looktasty_no | −1.63 | 0.11 | −0.28 | |
Note: Bonferroni corrected for multiple comparisons. Significant results are indicated in bold. Df = degrees of freedom.
Figure 3.
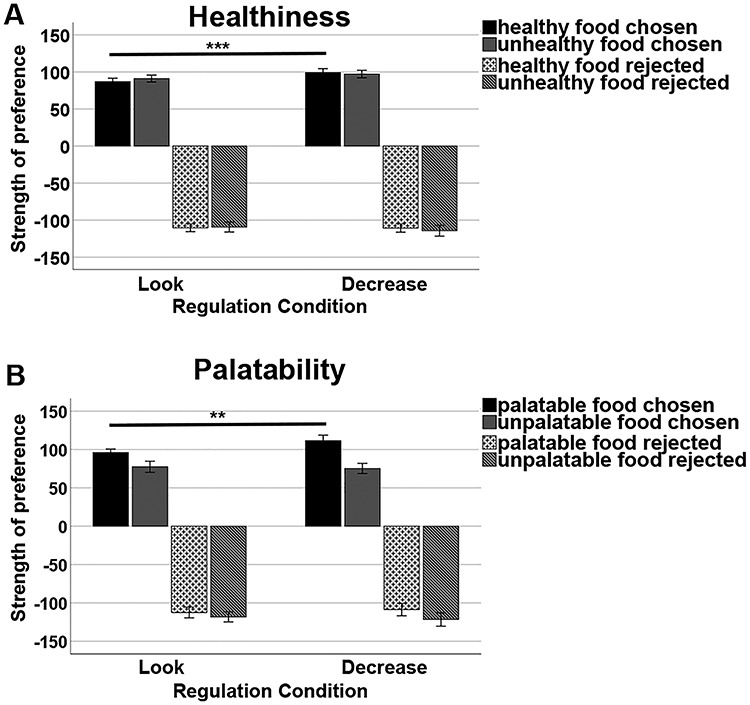
Behavioral results of the fMRI study. (A) Preference strength as a function of healthiness, choice, and regulation condition. (B) Preference strength as a function of palatability, choice, and regulation condition.
Palatability. In the behavioral study, using repeated measures ANOVA with the factors regulation (Decrease, Look), choice (yes, no), and palatability (palatable, unpalatable), we found both a significant main effect of choice and a significant main effect of palatability on preference strength. We did not observe any significant interaction effects (Table 5). In the fMRI study, we found a significant main effect of choice and a significant main effect of palatability. We also found a significant interaction effect between choice and palatability (Table 5).
Again, we split the data by trials in which participants accepted items and rejected items and analyzed the preference strength ratings separately. Post hoc t-tests revealed that in both studies, participants demonstrated a stronger preference for palatable foods after downregulating their emotions compared with the Look condition, while they showed no significant difference in preference when they declined to consume the food after emotion regulation compared with the Look condition. Detailed results from both studies are reported in Table 6 and illustrated in Figure 3B for the fMRI study.
Multivariable Prediction of Food Preferences
Given the high similarity of behavioral results between both studies, we pooled the data for both studies to maximize statistical power for the following analysis. We applied a machine-learning algorithm to predict food preference ratings from demographic data; online ratings of each food picture on familiarity, palatability, and healthiness; trial-by-trial emotional state ratings; descriptive data; and stimulus characteristics of each food picture.
First, we computed a correlation matrix (Supplementary Fig. 7) of all factors using Pearson’s correlations. On the one hand, this revealed a high correlation between the subscales of the questionnaires (QEB, AHN, ERQ, and ECQ), a high correlation between stimulus characteristics such as protein, fat, carbohydrates, and kcal and, finally, a high correlation between the norm ratings on palatability and craving. On the other hand, all factors showed low correlations with the preference for food items (bottom row in Supplementary Fig. 7). Due to its sensitivity to outliers, however, Pearson’s correlation can be a fall short to detect the existence of meaningful relationships between variables (Rousselet and Pernet 2012) and particularly if nonlinear relationships or more complex patterns of interdependent relationships exist. Thus, we used machine learning to predict the preference for food items from the full pattern of variables (factors) to establish which ones (if any) contribute to the prediction.
Next, we estimated the multivariate model and determined the model performance. The XGBoost model provided a good fit in accordance with conventional ranges of cutoff values (MAE = 57.64 ± 1.06 SD; RMSE = 74.11 ± 1.49 SD; CORR = 0.77 ± 0.01 SD; R2 = 0.59 ± 0.02 SD) (Fig. 4).
Figure 4.
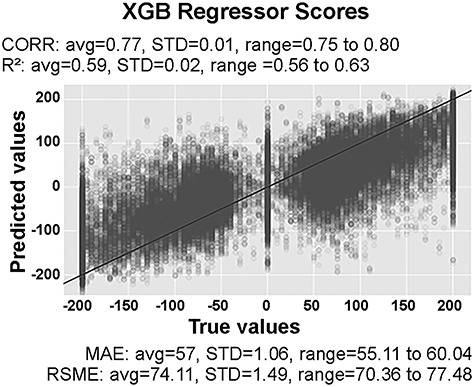
Model performance. True food ratings versus predicted food ratings. The black line (45° line) represents the theoretical optimal performance, and hence, all true ratings equal the predicted ratings. Ratings off the black line indicate prediction error. The farther away, the greater the error. Predictions were carried out with 50 extreme gradient-boosted (XGB) tree models in a cross-validation procedure. Average (avg), SD, minimum, and maximum of the 50 XGB model’s performance are reported in terms of their mean absolute error (MAE), root mean squared error (RMSE), correlation (CORR) between true values and predicted values, and R2 of true values and predicted values.
Third, we analyzed the contributions of single factors to the model performance, that is, which factors are important to predict food preferences. This was carried out by removing the connection between factor and target by permuting the factors’ values. Subsequently, the relative drop in model performance was computed. This analysis revealed that individual palatability and emotional state ratings were the most important factors for our models to predict food preferences (Fig. 5). Permuting these two factor values, hence, removing their predictive power, led to a median relative drop in R2 (model performance) of 0.287 and 0.285, respectively. Hence, the model explained 28.7 and 28.5% less variance in the food preference ratings. Also note that due to the permutation method used, these two factors can be assumed to independently contribute to the prediction and explain unique aspects of the variance. All other factors explained less than 5% variance of the food preference ratings.
Figure 5.
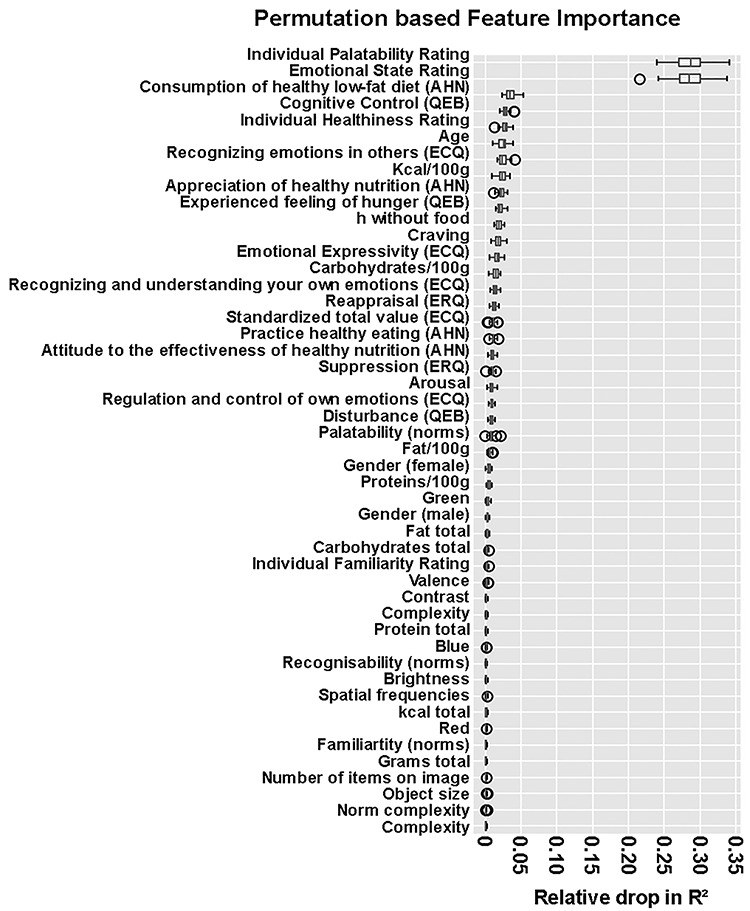
Distribution of the importance of factors for the extreme gradient-boosted (XGB) tree model’s predictions. Importance was estimated with a permutation procedure. First, a baseline R2 value was computed for an evaluation data-set. Second, the values of a single factor in the data-set were permuted. Third, a new R2 value was computed based on the permutated data-set. The relative drop in R2 caused by a single factor indicates the importance of features, that is, larger drops in R2 are related to higher feature importance. This procedure was repeated for each factor in each holdout data-set in the main cross-validation loop of the analysis, leading to 50 relative drops in R2 values per factor.
Finally, we performed partial dependence analysis to investigate how the prediction of a model depends on single factors (illustrated for the two most important factors in Fig. 6). We found an almost linear relationship between the two most predictive factors (palatability and emotional state ratings) and the preference ratings in our model (Fig. 6A,B). This means that foods that were rated as highly palatable were preferred stronger, while unpalatable foods were rejected stronger (Fig. 6A). Furthermore, a relatively more positive emotional state was related to increasing preference for foods (Fig. 6B).
Figure 6.
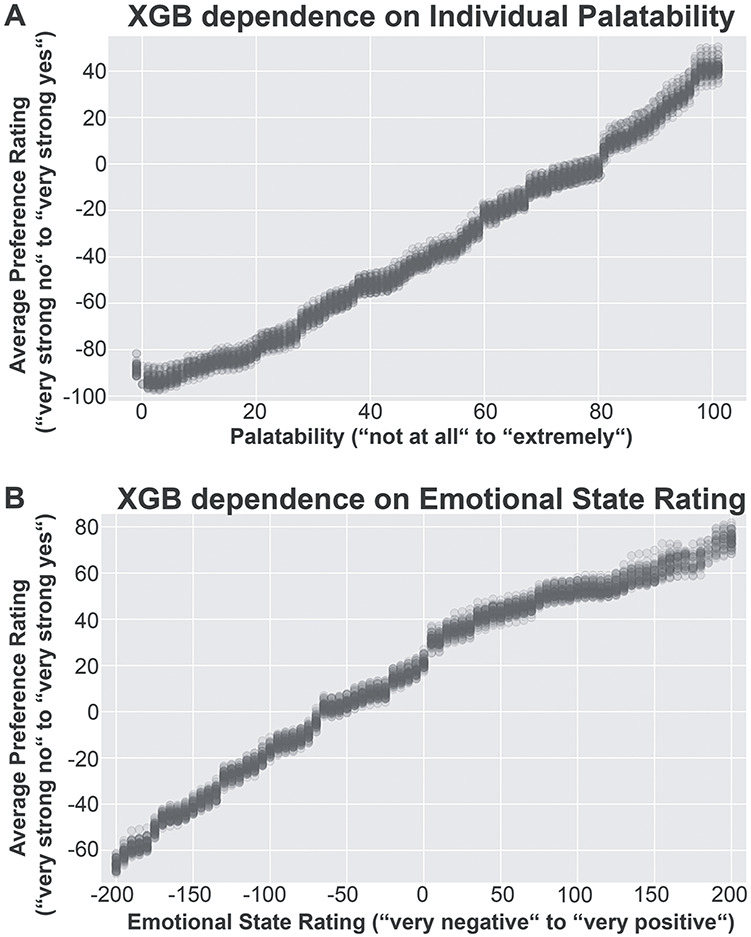
Distribution of the dependence of the extreme gradient-boosted (XGB) tree models’ predictions on the values of the factors. In a holdout data-set, the values of a single variable were replaced by a grid of possible values, and then this data-set was used to compute predictions. The influence of the other variables was mitigated by averaging over the model’s predictions. Average predictions over the value range of each factor were calculated for each holdout data-set in the main cross-validation loop of the analysis, leading to 50 average prediction per value per factor. The two best predictors are shown: (A) individual palatability and (B) emotional state rating.
Taken together, the machine-learning analyses revealed that food preferences were predominantly predicable by the palatability of the food and the emotional state (which was actively regulated by the participants), with both variables explaining unique aspects of the variance, rather than by objective features of the food such as calories, fat, and protein, and also not by descriptive measures of emotion regulation ability and attitudes towards nutrition. The difference in the relative drop of R2 between the two most important factors for our model and the remaining factors was substantial and emphasizes their importance.
fMRI Data
Emotion Regulation Network
To test for effects of emotion regulation, we contrasted the downregulation condition with the Look condition [Decrease>Look] based on GLM1. This revealed increased activity in several regions in the prefrontal cortex, supplementary motor area, and supramarginal gyrus, which is in line with previous studies (Morawetz et al. 2017) (Table 7). The reverse contrast did not show any significant clusters.
Table 7.
Regions implicated in emotion regulation
| Contrast | Region | Side | k | x | y | z | t-value | P-value |
|---|---|---|---|---|---|---|---|---|
| Decrease > Look | Supplementary motor area | R | 61 | 18 | 11 | 65 | 4.79 | 0.04 |
| Supplementary motor area | R | 18 | 26 | 62 | 3.28 | |||
| Supramarginal gyrus | R | 51 | 60 | -49 | 41 | 4.29 | 0.04 | |
| Middle frontal gyrus | R | 50 | 39 | 26 | 41 | 4.17 | 0.04 | |
| Look > Decrease | No significant clusters |
Note: P < 0.05 FWE corrected. Extent threshold, 10 voxels. L = left, R = right, k = cluster size.
Decision-Making Network
To identify the decision-making network, we contrasted trials in which participants decided to consume the depicted food with trials in which they rejected the food [Food-YesDecrease + Food-YesLook > Food-NoDecrease + Food-NoLook] based on GLM1. The results are shown in Figure 7 and Table 8. We observed enhanced activation in the ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), and striatum in accord with previous literature (Fig. 7, Table 8) (Bartra et al. 2013). The reverse contrast yielded increased activity in the lingual gyrus and the inferior frontal gyrus.
Figure 7.
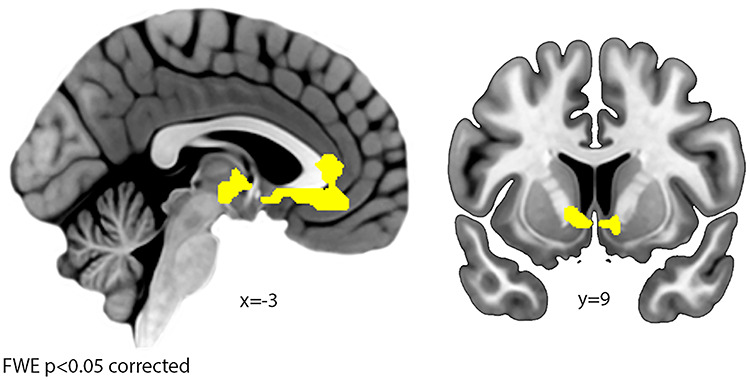
Decision-making network. Increased activation was found in the vmPFC (left), extending posterior into the cingulate cortex, and the striatum (right) for chosen versus rejected food items.
Table 8.
Regions implicated in choices
| Contrast | Region | Side | k | x | y | z | t-value | P-value |
|---|---|---|---|---|---|---|---|---|
| Food-YesDecrease + Food-YesLook > Food-NoDecrease + Food-NoLook | Fusiform gyrus | R | 272 | 18 | -67 | -7 | 6.01 | 0.001 |
| Calcarine | R | 15 | -76 | 2 | 5.92 | |||
| Medial orbitofrontal cortex | 276 | 0 | 26 | -7 | 5.08 | 0.001 | ||
| Medial orbitofrontal cortex | R | 3 | 35 | 5 | 4.74 | |||
| Medial orbitofrontal cortex | R | 3 | 17 | -7 | 4.71 | |||
| Food-NoDecrease + Food-NoLook > | Lingual gyrus | L | 726 | -9 | -70 | -7 | 10.08 | 0.001 |
| Food-YesDecrease + Food-YesLook | Inferior frontal gyrus | L | 119 | -51 | 32 | -7 | 5.01 | 0.001 |
Note: P < 0.05 FWE corrected. Extent threshold, 10 voxels. L = left, R = right, k = cluster size.
Parametric Analysis of Preferences and Healthiness During Decision-Making
We examined the parametric effects of preferences (GLM2) and healthiness (GLM3) during the decision-making phase, but independent of the preceding emotion regulation condition. For preference, we found effects of parametrically increased activation in the vmPFC and striatum (indicated in red in Fig. 8, Table 9). Activation during the food choice task in the vmPFC, fusiform gyrus, striatum, parahippocampal gyrus, anterior cingulate cortex (ACC), and superior medial frontal gyrus scaled negatively with healthiness (indicated in green in Fig. 8, Table 9). Thus, both regions that activated for decision-making and parametrically activated for preference (and for decision-making in general)—the vmPFC and the striatum—were also parametrically modulated by healthiness (overlap indicated in yellow in Fig. 8).
Figure 8.
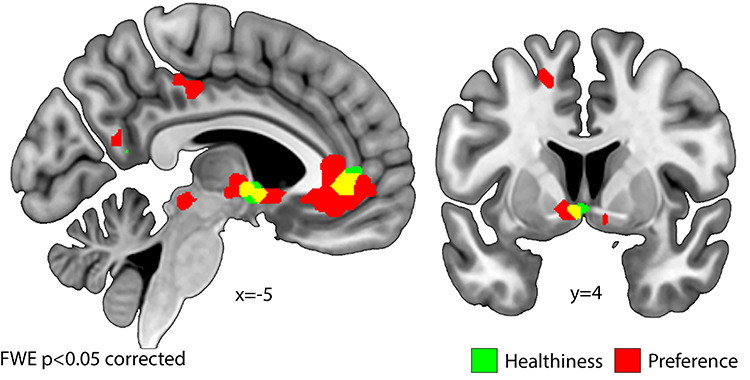
Activity in left vmPFC and right striatum was parametrically modulated by healthiness and preference at the time of decision-making. Voxels in green depict effects of the parametric regressor for healthiness and those in red of the parametric regressor of preference. The overlap of these contrasts is shown in yellow.
Table 9.
Regions correlated with food preferences, healthiness, and palatability ratings
| Contrast | Region | Side | k | x | y | z | t-value | P-value |
|---|---|---|---|---|---|---|---|---|
| Parametric preference pos | Anterior cingulate cortex | R | 536 | 6 | 29 | 11 | 6.35 | 0.001 |
| Anterior cingulate cortex | R | 6 | 35 | 2 | 5.9 | |||
| Caudate | 0 | -7 | 2 | 5.73 | ||||
| Lingual gyrus | R | 298 | 12 | -73 | -4 | 6.28 | 0.001 | |
| Calcarine | R | 15 | -79 | 2 | 5.91 | |||
| Parametric preference neg | Lingual gyrus | L | 441 | -12 | -70 | -7 | 8.44 | 0.001 |
| Calcarine | L | -9 | -85 | 11 | 6.88 | |||
| Cuneus | R | 12 | -88 | 41 | 4.68 | |||
| Parametric health pos | No significant clusters | |||||||
| Parametric health neg | Fusiform gyrus | L | 183 | -30 | -43 | -16 | 6.73 | 0.001 |
| Fusiform gyrus | R | 132 | 27 | -40 | -16 | 6.46 | 0.004 | |
| Middle occipital gyrus | L | 118 | -36 | -91 | 17 | 5.61 | 0.006 | |
| Middle occipital gyrus | R | 222 | 33 | -91 | 20 | 5.6 | 0.001 | |
| Middle occipital gyrus | R | 39 | -85 | 8 | 5.43 | |||
| Middle occipital gyrus | R | 33 | -85 | -1 | 4.74 | |||
| Inferior occipital gyrus | L | 110 | -45 | -70 | -7 | 4.95 | 0.006 | |
| Fusiform gyrus | L | -24 | -79 | -7 | 4.16 | |||
| Fusiform gyrus | L | -33 | -82 | -16 | 4.05 | |||
| Caudate | R | 67 | 3 | -1 | -4 | 4.88 | 0.03 | |
| Parahippocampal gyrus | R | 15 | -4 | -19 | 4.5 | |||
| Anterior cingulate cortex | L | 50 | -9 | 38 | -1 | 4.19 | 0.05 | |
| Superior medial frontal gyrus | L | -9 | 56 | 5 | 3.86 | |||
| Anterior cingulate cortex | L | -3 | 44 | 5 | 3.84 |
Note: P < 0.05 FWE corrected. Extent threshold, 10 voxels. Pos = positive correlation, neg = negative correlation, L = left, R = right, k = cluster size.
Next, we tested whether the parametric effects for preferences (GLM4) and healthiness (GLM5) during decision-making were different depending on whether participants engaged in emotion regulation beforehand or not. For healthiness, we found a positive parametric effect following Decrease in the supplementary motor area (SMA) and a negative parametric modulation in the fusiform gyrus (see Table 10, regions indicated with an *), but no effects were found following Look. No parametric effects could be found for preferences following either Decrease or Look when using FWE correction at whole-brain level.
Table 10.
Regions correlated with food preferences, healthiness, and palatability ratings as a function of preceding emotion regulation condition
| Regulation condition | Contrast | Region | Side | k | x | y | z | t-value | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Decrease | Parametric preference pos | No significant clusters | |||||||
| Decrease | Parametric preference neg | No significant clusters | |||||||
| Look | Parametric preference pos | No significant clusters | |||||||
| Look | Parametric preference neg | No significant clusters | |||||||
| Decrease | Parametric health pos | Supplementary motor area | L | 592 | −12 | −4 | 50 | 4.65 | <0.001* |
| 18 | −4 | 50 | 4.28 | ||||||
| −15 | −43 | 71 | 4.16 | ||||||
| Postcentral gyrus | R | 85 | 18 | −37 | 56 | 3.89 | 0.04 | ||
| 18 | −49 | 50 | 3.46 | ||||||
| 21 | −55 | 44 | 3.08 | ||||||
| Supramarginal gyrus | L | 79 | −54 | −28 | 14 | 3.71 | 0.04 | ||
| Decrease | Parametric health neg | Fusiform gyrus | L | 99 | −30 | −40 | −19 | 5.44 | 0.02* |
| −33 | −55 | −16 | 2.90 | ||||||
| Middle occipital gyrus | L | 72 | −33 | −94 | 14 | 4.18 | 0.05 | ||
| Middle occipital gyrus | R | 89 | 33 | −91 | 20 | 3.63 | 0.03 | ||
| 30 | −82 | 5 | 3.51 | ||||||
| 36 | −85 | −1 | 3.26 | ||||||
| Look | Parametric health pos | No significant clusters | |||||||
| Look | Parametric health neg | Fusiform gyrus | R | 1358 | 30 | −46 | −10 | 5.78 | <0.001* |
| −36 | −88 | 17 | 5.55 | ||||||
| −27 | −43 | −13 | 5.52 | ||||||
| Anterior cingulum | R | 167 | 6 | 35 | 23 | 3.79 | 0.006 | ||
| 15 | 32 | 41 | 3.60 | ||||||
| −6 | 29 | 23 | 3.03 | ||||||
| Anterior cingulum | L | 108 | −9 | 38 | −1 | 3.64 | 0.02 | ||
| −3 | 44 | 5 | 3.13 | ||||||
| 12 | 50 | 5 | 3.09 | ||||||
| Thalamus | L | 97 | −18 | 23 | 41 | 3.47 | 0.03 | ||
| −18 | 47 | 41 | 3.40 | ||||||
| −12 | 32 | 59 | 3.27 | ||||||
| Amygdala | R | 68 | 18 | −1 | −19 | 4.03 | 0.06 | ||
| 9 | −7 | −1 | 3.17 |
*Indicates significance at P < 0.001 FWE uncorrected.
Notes: P < 0.005 uncorrected. Extent threshold, 10 voxels. Pos = positive correlation, neg = negative correlation, L = left, R = right, k = cluster size.
Given that such indirect parametric effects can be expected to be weak, we further explored the data by applying a less stringent threshold of P < 0.005 (uncorrected) with an extent threshold of 10 adjacent voxels. At this very lenient threshold, there were clusters of activation in the SMA, postcentral gyrus, and supramarginal gyrus that scaled positively with healthiness after Decrease (Table 10). A negative parametric effect for healthiness was found in clusters within the visual cortex after both Decrease and Look. In addition, after Look activity in the anterior ACC and thalamus also scaled negatively with healthiness. Note, however, that these explorative analyses are only reported to allow for the generation of hypotheses but should not be strongly interpreted without replication.
vmPFC and Striatum Response as a Function of Choices
Finally, ROI analyses were conducted in the vmPFC and striatum, given that these regions displayed effects for decisions, preferences, as well as healthiness. In particular, we were interested in whether the decision-related effects in these regions would differ by preceding emotion regulation condition (as conceptualized in GLM1). We used a repeated measures ANOVA with the factors regulation (Decrease, Look) and choices (Yes, No).
For the vmPFC, no significant main effect for emotion regulation was found [F(1,32) = 0.65, P = 0.42] (the main effect for choice was used to define the ROI and therefore not analyzed), and the interaction for choice and emotion regulation was also not significant [F(1,32) = 1.29, P = 0.26]. However, follow-up t-tests showed that there was indeed a nested effect in one condition, as decreasing emotions significantly less reduced activity in the vmPFC when participants subsequently decided to consume the food items compared with rejection trials (Food-YesDecrease > Food-NoDecrease) [t(32) = 4.74, P < 0.001] (Fig. 9A displays the % signal change).
Figure 9.
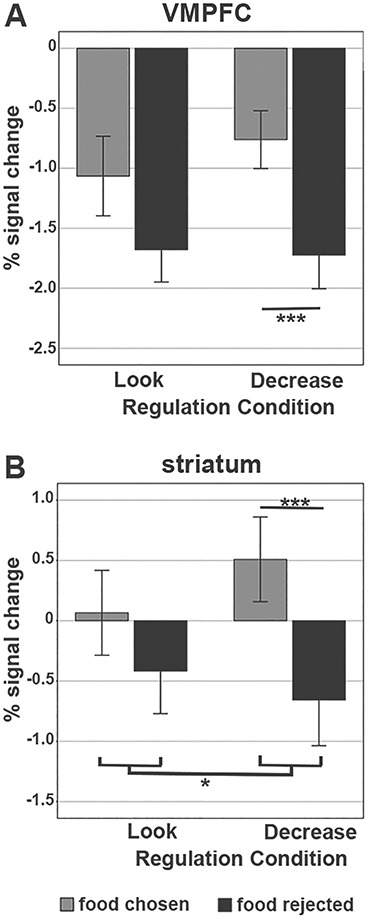
Mean activation of the vmPFC (A) and striatum (B) ROIs for chosen versus rejected food items during the choice phase as a function of preceding regulation condition. Displayed are parameter estimates (percent signal change) for each decision following Decrease and Look.
For the striatum, a significant main effect of emotion regulation was found [F(1,32) = 18.12, P < 0.001] as well as a significant interaction effect between regulation and choice [F(1,32) = 4.50, P = 0.04] (Fig. 9B). Overall, rejected food items were associated with a negative signal change in the striatum independent of the regulation condition, while chosen food items were related to an increase in response. The significant interaction means that the difference in activity between chosen and rejected food items differed significantly between Decrease and Look. There was a more pronounced difference in signal changes between chosen and rejected items after emotion regulation compared with the Look condition. Follow-up tests for both emotion regulation conditions separately showed that the magnitude of activation increase significantly differed between chosen and rejected items following Decrease (Food-YesDecrease > Food-NoDecrease) [t(32) = 4.59, P < 0.001], but not following Look. Taken together, these results suggest that decreasing emotions significantly modulated the activity in the vmPFC and striatum during consumption choices compared with rejection choices.
Discussion
The current study investigated how the regulation of incidental emotions impacts on food choices, and in this context we addressed two related open questions: does the regulation of incidental emotions lead to more healthy dietary decisions, and are such potential effects modulated by activity in the brain’s valuation system? In addition, in an exploratory manner, we aimed to identify factors that predict food choices based on behavioral and dispositional factors and tested the hypothesis that emotional state is indeed a key factor driving food preference.
Our hypothesis regarding the influence of emotion regulation on dietary decisions was partly confirmed. We found that the regulation of incidental emotions before the time of choice increased the percentage of choices for healthy foods, as well as the decision strength for desired foods (i.e., foods which were chosen), compared with no regulation. However, engaging in emotion regulation also promoted subsequent choices and stronger decision strength for palatable foods. Emotion regulation had no effect on the decision strength for rejected foods. These results have several interesting implications. Firstly, they suggest that when negative emotions are regulated, the desire for foods, which are palatable and healthy, is enhanced. Importantly, this implies a selective desire for food that is either palatable or healthy, but not a general increase in desire for all foods, because no effects were found for less healthy or less palatable foods. It has to be acknowledged that there were not many food items that were rated as unpalatable, due to our pre-selection of foods, which circumvented constructing categories, which represented all combinations of healthiness and palatability. However, the category of low-health foods was large enough to conclude that the observed bias for stronger preference after emotion regulation was not universal. Finding a stronger desire for palatable food might not be surprising, given that taste is often found to be the main driver for food preference (Furst et al. 1996; Shepherd and Raats 2010). An increased desire for healthy foods, while a large proportion of unhealthy foods was also rated as tasty, however, is of strong interest, because it suggests selective processing of health attributes following emotion regulation (Hare et al. 2011; Provencher and Jacob 2016; van Meer et al. 2017). One limitation here is that it is not clear whether healthy items would enjoy the same increase in choices if they were less palatable, given that all items in our study were rated as relatively high on palatability.
There are two possible explanations for this finding. Firstly, engaging in emotion regulation could have restored emotional equilibrium (at least to some extent) by reducing the impact of the negative emotion (Milyavsky et al. 2019). This view suggests that emotion regulation was not actually the factor which changed the choices and desire for food, but instead the negative emotional experience in the Look condition might initially have reduced the choice proportion for and desire to consume palatable and healthy food. In other words, our finding would suggest that the unregulated experience of a negative incidental emotion might restrain the desire to consume even palatable foods. This is in line with the idea of an activation of a Pavlovian fear response under emotional stress, which could suppress the activation of the parasympathetic regulated desire to eat (Wardle et al. 2000; Rodrigues et al. 2009; Yau and Potenza 2013). The negative emotional cue could potentially also have suppressed the processing of health attributes of stimuli to a certain degree that would otherwise make healthy foods even more attractive and, in turn, more strongly desired. Making decisions for healthy options has been suggested to require explicit attention to health aspects of foods (Hare et al. 2011; Provencher and Jacob 2016; van Meer et al. 2017), and experiencing negative emotions could have consumed the cognitive resources required to allocate such attention.
The second possible explanation is that the process of engaging in emotion regulation itself could have modulated food preferences. This explanation would assume that the experience of the incidental negative emotion might not have strongly shifted food preferences, but the cognitive process of regulating an inner state could have “spilled over” to more strongly regulate one’s food desires. Thus, the experience of self-control in one domain can have spillover effects to unrelated domains. This is plausible because of two reasons. Firstly, it has recently been suggested that a similar mechanism might drive changes in post-emotion regulation risk aversion (Morawetz et al. 2019). In this study, participants regulated incidental negative emotions before engaging in a risky choice task between a safe and a risky financial investment. It was found that participants showed reduced risk preference following emotion regulation but not following the experience of negative emotions, suggesting that the effect on risk-taking was due to engaging in the process of emotion regulation, and not due to the altered emotional state. Regulating emotions might have spilled over to regulating risk in the directly following task phase, which was also supported by a modulation of neural activity from the regulation phase into the decision phase, similar to our study. Unfortunately, the current study did not use a similar baseline condition to directly compare emotion experience with a neutral condition, which should be investigated in future studies. The second reason for the plausibility of this explanation is that we did not observe a decrease in the choices to consume unhealthy foods (which were still at large rated as tasty), but only for the healthy items; however, a general reduction of food choices might have been expected if the negative emotional state activated the fight-or-flight system. Arguably, such a suppression of the desire to consume foods because of a strong negative emotional state should have been expected to be more substantial and more general than observed here. Hence, it may be that, similar to the risky decision-making (Morawetz et al. 2019), the process of engaging in emotion regulation might have led to more controlled processing of food options, including an increase of attention to health attributes, leading to an increase of their relative weighting during value computation and, in turn, relatively stronger preferences for healthier foods.
While our study ultimately cannot fully differentiate between these two explanations, our additional results of the machine-learning approach provide evidence that both might have played a significant role. In addition to the standard behavioral analyses, we also used a data-driven approach to predict food preferences in a comprehensive regression model, considering individual factors, including emotional state, habitual use of regulation strategies, individual preferences, nutrition attitudes, as well as stimulus features (e.g., healthiness, palatability, calories, fat). The results show that besides palatability, which was expected to be the main driver for food preferences, emotional state emerged as the second most important and independent predictor in the model. This might provide support for the first explanation, suggesting that the actual emotional state modulated the trial-by-trial expression of foods preference. Interestingly, the effect of emotional state was much stronger than all other factors, including the hours without food, which had only a very low predictive power.
The fMRI results, however, could be interpreted as providing support for the second explanation, suggesting that emotion regulation per se modulated food preference by altering neural signals related to food choice and value computation. Firstly, we found that choice as well as healthiness and preference strength all modulated activation in overlapping areas in the vmPFC and striatum at the time of decision-making (Hare et al. 2009; Hutcherson et al. 2012; van Meer et al. 2017; Tusche and Hutcherson 2018; Maier and Hare 2019). It should be noted, however, that these effects were only significant in the ROI analysis and not at whole-brain level. Unfortunately, we did not have sufficient variance to reliably detect signals related to palatability, which was also expected to modulate activity in vmPFC (Hare et al. 2011; Maier and Hare 2019). Consistent with many previous studies, increased activity in the vmPFC and striatum is related to value computations at the time of choice (e.g., Plassmann et al. 2010; Levy and Glimcher 2011; Levy and Glimcher 2012; Bartra et al. 2013). This indicates that these regions encode the subjective value of a reward (Hare et al. 2009; Plassmann et al. 2010), in our case the value of the food items. Increased activity in the vmPFC has been found in food choice tasks (Killgore et al. 2003; Levy and Glimcher 2011), during the consumption of palatable foods (O’Doherty et al. 2002), administration of pleasant tastes (O’Doherty et al. 2001; Zald et al. 2002), and meal consumption (Del Parigi et al. 2002). The striatum has been suggested to be involved in the anticipation of primary rewards (O’Doherty et al. 2002), juice preferences (O’Doherty et al. 2006), meal pleasantness ratings (Small et al. 2003), subjective preferences of goods (Knutson et al. 2007), and food craving (Pelchat et al. 2004). The mere perception of food cues has been shown to activate the vmPFC as well as the striatum (Killgore et al. 2003; Simmons et al. 2005; Goldstone et al. 2009; Siep et al. 2009). In sum, this strongly indicates that the activation of the vmPFC and the striatum was associated with computing value signals and preferences and that health information was therefore implicated in the computation of value in our study (Hare et al. 2009, 2011; Hutcherson et al. 2012; Schmidt et al. 2018; Tusche and Hutcherson 2018). It should be noted, however, that these effects were only significant in the ROI analysis and not at whole-brain level. It should further be noted again that we lacked stimuli that varied in both health and taste attributes, and it remains unclear whether health-related activation would have been similarly modulated by emotion regulation in these areas for less palatable items.
Building on these findings, we then investigated whether the engagement in emotion regulation systematically modulated activation in these regions during the decision stage. The whole-brain analysis was unfortunately negative, but that could be expected given that we were interested in small and subtle effects of emotion regulation on parametric variables (Wood et al. 2008). The ROI analyses for the vmPFC and the striatum, however, were more conclusive and showed that the regulation of incidental emotions influenced subsequent activity during choice. In the striatum, we observed that activation differences for chosen and unchosen options were enhanced following emotion regulation as compared with emotion experience (in fact, in the vmPFC this difference only became significant after emotion regulation, not after emotion experience). Given the role of both regions in value and preference computation (Kable and Glimcher 2007; Knutson et al. 2007; Hutcherson et al. 2012; Bartra et al. 2013; Clithero and Rangel 2014; Grueschow et al. 2015), as discussed above, this is a strong neural evidence that the regulation process impacted value computation, as suggested by the second explanation. Emotion regulation via reappraisal therefore appears to have enhanced activation in the striatum for positive decisions, thereby increasing the value of the stimuli, suggesting a spillover of one regulation process to the next. This idea is in line with previous studies showing that cognitive control strategies such as thinking about the long-term consequences associated with eating high-fat foods have the potential to modulate food cravings by changing the activity in the striatum (Kober et al. 2010; Siep et al. 2012). In addition, the inhibition of hunger feelings has been shown to decrease activity in the striatum (Wang et al. 2009). The more likely interpretation, however, is again that this neural pattern could also be the result of the effect of emotion regulation (i.e., reflecting the emotional state) rather than the process of regulating. In other words, the neural patterns after emotion regulation could again be a reflection of a less negative emotional state, while in the Look condition, in which the negative emotion was experienced, the difference in neural signals for chosen and unchosen foods was reduced. Cognitive reappraisal could therefore neutralize the spillover effects of incidental affective states that would otherwise influence the computation of subjective value and the decision (Phelps et al. 2014), leading to clearer signal differences between chosen and unchosen options. The most significant limitation of our study is the lack of a condition in which participants did not experience negative emotions (e.g., by using neutral stimuli). This would have allowed us to test, for example, whether the activity in the striatum following unregulated emotional experience reflected the effect of the emotion per se and whether regulating negative emotions would re-establish activity levels similar to a neutral condition. Future studies will have to address these precise mechanisms using the appropriate baseline conditions and potentially also integrate the experience of positive emotions, as in previous studies (Maier and Hare 2019).
Conclusion
Regardless of the precise mechanism, the present study clearly demonstrates that the incidental emotional state, and the regulation thereof, preceding food choices can modulate striatum and vmPFC activity related to food preferences. An improved emotional state was linked to healthier decisions. Given that emotion regulation ability can be practiced (Schweizer et al. 2013; Denny and Ochsner 2014), cognitive reappraisal of emotions encountered during daily life might represent a promising regulatory strategy to promote healthy eating and reduce the risk for emotional eating. Furthermore, if these findings were to generalize, training of emotion regulation ability could positively affect other health-related behaviors such as a decrease in smoking and alcohol consumption (Oaten and Cheng 2006). Thus, our findings underscore the potential of interventions enhancing emotion regulation ability to decouple the link between negative emotional experience and the consumption of high caloric foods. As such, these findings have implications for our basic understanding of affect-regulatory mechanisms in the context of health and real-world appetitive behavior, as well as for studies that translate this work to clinical contexts.
Funding
Deutsche Forschungsgemeinschaft (Grant MO 2041/2-1) and Marie Skłodowska-Curie Action 795994 to C.M.; Australian Research Council Discovery Project (grant DP160103353 to S.B.). Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
References
- Abler B, Kessler H. 2009. Emotion regulation questionnaire – eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica. 55:144–152. [Google Scholar]
- Adriaanse MA, de Ridder DTD, Evers C. 2011. Emotional eating: eating when emotional or emotional about eating? Psychol Health. 26:23–39. [DOI] [PubMed] [Google Scholar]
- Armstrong BC, Watson CE, Plaut DC. 2012. SOS! An algorithm and software for the stochastic optimization of stimuli. Behav Res Methods. 44:675–705. [DOI] [PubMed] [Google Scholar]
- Arnow B, Kenardy J, Agras WS. 1995. The emotional eating scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 18:79–90. [DOI] [PubMed] [Google Scholar]
- Barthomeuf L, Droit-Volet S, Rousset S. 2009. Obesity and emotions: differentiation in emotions felt towards food between obese, overweight and normal-weight adolescents. Food Qual Prefer. 20:62–68. [Google Scholar]
- Bartra O, McGuire JT, Kable JW. 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, Wonderlich SA. 2013. Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. J Abnorm Psychol. 122:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Meule A, Busch NA, Ohla K. 2014. Food-pics: an image database for experimental research on eating and appetite. Front Psychol. 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden A, Flatt SW, Boutelle KN, Strong D, Sherwood NE, Rock CL. 2016. Emotional eating is associated with weight loss success among adults enrolled in a weight loss program. J Behav Med. 39:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden A, Musher-eizenman D, Watford T, Emley E. 2018. Eating when depressed, anxious, bored, or happy: are emotional eating types associated with unique psychological and physical health correlates? Appetite. 125:410–417. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. 2007. The International Affective Picture System (IAPS) in the Study of Emotion and Attention In: Allen JJB, Coan JA, editors. Handbook of emotion elicitation and assessment. Series in Affective Science. New York: Oxford University Press, pp. 29–46. [Google Scholar]
- Breiman L 2001. Random forests. Mach learn. 45:5–32. [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. 2002. Region of interest analysis using an SPM toolbox. Neuroimage. 16:abstract 497. [Google Scholar]
- Camilleri GM, Méjean C, Kesse-Guyot E, Andreeva VA, Bellisle F, Hercberg S, Péneau S. 2014. The associations between emotional eating and consumption of energy-dense snack foods are modified by sex and depressive symptomatology. J Nutr. 144:1264–1273. [DOI] [PubMed] [Google Scholar]
- Chen T, Guestrin C. 2016. XGBoost In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ‘16. New York: ACM Press, pp. 785–794. [Google Scholar]
- Clithero JA, Rangel A. 2014. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 9:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett AC, Myhre SK, Rokke PD. 2015. Boredom proneness and emotion regulation predict emotional eating. J Health Psychol. 20:670–680. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Gautier J, Chen K, Salbe A, Ravussin E, Reiman E, Tataranni P. 2002. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 967:389–397. [PubMed] [Google Scholar]
- Denny BT, Ochsner KN. 2014. Behavioral effects of longitudinal training in cognitive reappraisal. Emotion. 14:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet PMA, Schifferstein HNJ. 2008. Sources of positive and negative emotions in food experience. Appetite. 50:290–301. [DOI] [PubMed] [Google Scholar]
- Devonport TJ, Nicholls W, Fullerton C. 2019. A systematic review of the association between emotions and eating behaviour in normal and overweight adult populations. J Health Psychol. 24:3–24. [DOI] [PubMed] [Google Scholar]
- Diehl JM 2006. Fragebögen zur Erfassung ernährungs- und gewichtsbezogener Einstellungen und Verhaltensweisen. Questionnaires for the Assessment of Eating- and Weight-Related Attitudes and Behaviors.
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. 2007. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 28:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers C, Marijn Stok F, de Ridder DTD. 2010. Feeding your feelings: emotion regulation strategies and emotional eating. Personal Soc Psychol Bull. 36:792–804. [DOI] [PubMed] [Google Scholar]
- Ferrell EL, Watford TS, Braden A. 2020. Emotion regulation difficulties and impaired working memory interact to predict boredom emotional eating. Appetite. 144:104450. [DOI] [PubMed] [Google Scholar]
- Furst T, Connors M, Bisogni CA, Sobal J, Falk LW. 1996. Food choice: a conceptual model of the process. Appetite. 26:247–266. [DOI] [PubMed] [Google Scholar]
- Garg N, Lerner JS. 2013. Sadness and consumption. J Consum Psychol. 23:106–113. [Google Scholar]
- Garg N, Wansink B, Inman JJ. 2007. The influence of incidental affect on consumers’ food intake. J Mark. 71:194–206. [Google Scholar]
- Gianini LM, White MA, Masheb RM. 2013. Eating pathology, emotion regulation, and emotional overeating in obese adults with binge eating disorder. Eat Behav. 14:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Calcott RD, Berkman ET. 2013. Piece of cake. Cognitive reappraisal of food craving. Appetite. 64:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Tomiyama AJ, Mann T, Berkman ET. 2014. Neural systems underlying the reappraisal of personally craved foods. J Cogn Neurosci. 26:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl De Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G et al. 2009. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 30:1625–1635. [DOI] [PubMed] [Google Scholar]
- Gross JJ 1998. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 2:271–299. [Google Scholar]
- Gross JJ 2002. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 39:281–291. [DOI] [PubMed] [Google Scholar]
- Grueschow M, Polania R, Hare TA, Ruff CC. 2015. Automatic versus choice-dependent value representations in the human brain. Neuron. 85:874–885. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Straub RO. 1992. The role of gender and taste class in the effects of stress on eating. Heal Psychol. 11:97–100. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. 2011. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol Bull. 137:660–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T, Camerer CF, Rangel A. 2009. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 324:646–648. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. 2011. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 31:11077–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman JH. 2009. The elements of statistical learning: data mining, inference, and prediction. New York: Springer. [Google Scholar]
- Herwig U, Dhum M, Hittmeyer A, Opialla S, Scherpiet S, Keller C, Brühl AB, Siegrist M. 2016. Neural signaling of food healthiness associated with emotion processing. Front Aging Neurosci. 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ 2007. The psychology of food craving. Proc Nutr Soc. 66:277–285. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A. 2012. Neural correlates of the volitional regulation of the desire for food. Int J Obes. 36:648–655. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Plassmann H, Gross JJ, Rangel A. 2012. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci. 32:13543–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. 2007. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. 2003. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 19:1381–1394. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. 2007. Neural predictors of purchases. Neuron. 53:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. 2010. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenders PG, van Strien T. 2011. Emotional eating, rather than lifestyle behavior, drives weight gain in a prospective study in 1562 employees. J Occup Environ Med. 53:1287–1293. [DOI] [PubMed] [Google Scholar]
- Leng G, Adan RAH, Belot M, Brunstrom JM, De Graaf K, Dickson SL, Hare T, Maier S, Menzies J, Preissl H et al. 2017. The determinants of food choice Proc Nutr Soc. 76:316–327. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Li Y, Valdesolo P, Kassam KS. 2015. Emotion and decision making. Annu Rev Psychol. 66:799–823. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. 2011. Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci. 31:14693–14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. 2012. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 22:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Lerner JS. 2003. The role of affect in decision making In: Davidson R, Scherer K, Goldsmith D, editors. Handbook of Affective Sciences. Oxford: Oxford University Press, pp. 619–642. [Google Scholar]
- Macht M 2008. How emotions affect eating: a five-way model. Appetite. 50:1–11. [DOI] [PubMed] [Google Scholar]
- Maier SU, Hare TA. 2019. Greater BOLD signal during successful emotional stimulus reappraisal is associated with better dietary self-control. bioRxiv. 542712. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Milyavsky M, Webber D, Fernandez JR, Kruglanski AW, Goldenberg A, Suri G, Gross JJ. 2019. To reappraise or not to reappraise? Emotion regulation choice and cognitive energetics. Emotion. 19:964–981. [DOI] [PubMed] [Google Scholar]
- Molnar C. 2019. Partial dependence plot. In: Interpretable Machine Learning.
- Morawetz C, Bode S, Baudewig J, Jacobs AM, Heekeren HR. 2016a. Neural representation of emotion regulation goals. Hum Brain Mapp. 37:600–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Kirilina E, Heekeren HR. 2016b. Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cereb Cortex. 26:1923–1937. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, Heekeren HR. 2017. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 72:111–128. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Mohr PNC, Heekeren HR, Bode S. 2019. The effect of emotion regulation on risk-taking and decision-related activity in prefrontal cortex. Soc Cogn Affect Neurosci. 14:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Guerrieri R, Havermans RC, Roefs A, Jansen A. 2009. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int J Obes. 33:905–912. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Jones F, Conner M, McMillan B, Ferguson E. 2008. Effects of daily hassles and eating style on eating behavior. Heal Psychol. 27:S20–S31. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Buchanan T, Seymour B, Dolan R. 2006. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 49:157–166. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. 2001. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 85:1315–1321. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. 2002. Neural responses during anticipation of a primary taste reward. Neuron. 33:815–826. [DOI] [PubMed] [Google Scholar]
- Oaten M, Cheng K. 2006. Improved self-control: the benefits of a regular program of academic study. Basic Appl Soc Psych. 28:1–16. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 23:483–499. [DOI] [PubMed] [Google Scholar]
- Pelchat M, Johnson A, Chan R, Valdez J, Ragland J. 2004. Images of desire: food-craving activation during fMRI. Neuroimage. 23:1486–1493. [DOI] [PubMed] [Google Scholar]
- Phelps E a, Lempert KM, Sokol-Hessner P. 2014. Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci. 263–290. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. 2010. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 30:10799–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher V, Jacob R. 2016. Impact of perceived healthiness of food on food choices and intake. Curr Obes Rep. 5:65–71. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. 2003. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 40:15–24. [DOI] [PubMed] [Google Scholar]
- Reader SW, Lopez RB, Denny BT. 2018. Cognitive reappraisal of low-calorie food predicts real-world craving and consumption of high- and low-calorie foods in daily life. Appetite. 131:44–52. [DOI] [PubMed] [Google Scholar]
- Rindermann H 2009. Emotionale kompetenz fragebogen. Einschätzung emotionaler kompetenzen und emotionaler intelligenz aus selbst- und fremdsicht. Göttingen: Hogrefe. [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. 2009. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 32:289–313. [DOI] [PubMed] [Google Scholar]
- Rousselet G a, Pernet CR. 2012. Improving standards in brain-behavior correlation analyses. Front Hum Neurosci. 6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Tusche A, Manoharan N, Hutcherson C, Hare T, Plassmann H. 2018. Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. J Neurosci. 38:5799–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. 2013. Training the emotional brain: improving affective control through emotional working memory training. J Neurosci. 33:5301–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd R, Raats M. 2010. The psychology of food choice. CABI. [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. 2009. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal. Behav Brain Res. 198:149–158. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. 2012. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 60:213–220. [DOI] [PubMed] [Google Scholar]
- Simmons W, Martin A, Barsalou L. 2005. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 15:1602–1608. [DOI] [PubMed] [Google Scholar]
- Sladky R, Friston KJ, Tröstl J, Cunnington R, Moser E, Windischberger C. 2011. Slice-timing effects and their correction in functional MRI. Neuroimage. 58:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D, Jones-Gotman M, Dagher A. 2003. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 19:1709–1715. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Lackner HK, Zimmermann S, Naumann E. 2012. The effects of emotion regulation on the desire to overeat in restrained eaters. Appetite. 59:256–263. [DOI] [PubMed] [Google Scholar]
- Tusche A, Hutcherson CA. 2018. Cognitive regulation alters social and dietary choice by changing attribute representations in domain-general and domain-specific brain circuits. Elife. 7:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. 2009. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 47:852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer F, van der Laan LN, Viergever MA, Adan RAH, Smeets PAM. 2017. Considering healthiness promotes healthier choices but modulates medial prefrontal cortex differently in children compared with adults. Neuroimage. 159:325–333. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A et al. 2009. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci. 106:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z. 2000. Stress, dietary restraint and food intake. J Psychosom Res. 48:195–202. [DOI] [PubMed] [Google Scholar]
- Wedig MM, Nock MK. 2010. The functional assessment of maladaptive behaviors: a preliminary evaluation of binge eating and purging among women. Psychiatry Res. 178:518–524. [DOI] [PubMed] [Google Scholar]
- Wood G, Nuerk H-C, Sturm D, Willmes K. 2008. Using parametric regressors to disentangle properties of multi-feature processes. Behav Brain Funct. 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YHC, Potenza MN. 2013. Stress and eating behaviors. Minerva Endocrinol. 38:255–267. [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Stice E. 2013. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes. 37:1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D, Hagen M, Pardo J. 2002. Neural correlates of tasting concentrated quinine and sugar solutions. Neurophysiology. 87:1068–1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


