Abstract
Purpose:
STM 434 is a soluble receptor ligand trap targeting activin A, a protein in the TGF-β family that plays important roles in growth, differentiation, and cancer cachexia. This study evaluated the safety, antitumor activity, and metabolic effects of STM 434 in a first-in-human, multicenter phase I clinical trial (NCT02262455).
Experimental Design:
Patients with advanced solid tumors were enrolled in 8 dose cohorts ranging from 0.5 mg/kg Q4W to 8 mg/kg Q2W via a 3+3 dose escalation design. The primary endpoint was maximum tolerated dose. Second endpoints included safety, pharmacokinetics, and response. As Activin A is implicated in metabolism and muscle function, changes in key metabolic parameters including lead body mass and 6-minute walk test, were serially measured.
Results:
32 patients were treated on study. The most common treatment-related adverse events were fatigue (41%) as well as mucocutaneous bleeding complications including epistaxis (34%) and gingival bleeding (22%), likely related to off-target inhibition of bone morphogenetic protein 9 (BMP9). STM 434 treatment resulted in the expected FSH level decreases in most patients and in metabolic parameter changes, including an increase in total lean body mass and 6-minute walk test distance. No responses were observed in the 30 evaluable patients, but the stable disease rate in patients with granulosa cell ovarian cancer was 10/12 (80%).
Conclusions:
While no direct antitumor efficacy was documented, potentially clinically meaningful dose-related metabolic effects, including treatment of cancer cachexia, were observed that support further exploration of Activin A inhibitors that limit BMP9 blockade.
INTRODUCTION
Activins are members of the transforming growth factor-beta (TGFβ) superfamily, which also includes TGF-β, inhibins, nodals, bone morphogenetic proteins (BMPs), myostatin, and growth and differentiation factors (GDFs). The activin family, in particular, is believed to play an important role in regulating growth of both normal tissues and tumors (1,2). Activin A, which signals through the high affinity activin type 2B receptor (ActR2B or ACVR2B), leads to downstream SMAD signaling and modulation of gene transcription. Activin A expression is tightly regulated through numerous mechanisms, including by endogenous inhibitors inhibin and follistatin, that inhibit Activin A by blocking access to the activin receptor binding site (1).
Overexpression of activin A has been associated with shortened survival in ovarian, colon, gastric, breast, non-small cell lung cancers, as well as neuroblastoma (3). Likewise, functional experiments have shown that activin A promotes ovarian tumor proliferation and invasion (4). Experimental data also support a role for activin signaling in mediating cancer stem cell survival (5,6).
Beyond exerting a direct role in modulating cancer growth, activins and other ligands of ActR2B including myostatin, are also believed to play an important role in skeletal muscle homeostasis (7–9). In animal models, overexpression of activin A or deficiency of its counter regulator, inhibin, induces a profound cachexia syndrome (10). Similarly, perturbations in activin expression are believed to play a key role in the wasting syndromes observed in several human cancers (11).
Activin A overexpression has been best studied in serous, clear cell and sex cord stromal (granulosa cell) ovarian cancers. In these tumor types, malignant epithelial cells are believed to be the primary source of activin A production. Secreted activin A can be detected in both the serum and body fluid of affected patients (12–14). Consistent with the hypothesis that activin biology in important in gynecologic cancer, germline mutations in genes encoding activin and inhibin subunits (INHBA and INHA) have been described in women with young onset ovarian cancers, raising the question that in some settings, alteration of this pathway may play a role in tumorigenesis and inherited mutations may confer cancer susceptibility risk (15). These mutations are believed to disrupt activin and inhibin production, altering the ratio of activin and inhibin in favor of epithelial ovarian cell growth.
Additional data suggest that mutations more commonly associated with distinct ovarian cancer histologies including BRCA1 (serous), ARID1A (endometriosis associated clear cell and endometrioid), and FOXL2 (granulosa cell) may promote dysregulation in activin signaling and may further enhance dependence on this pathway (16,17). For example, downregulation of BRCA1 indirectly enhances activin signaling through downregulation of the activin antagonist follistatin alongside upregulation of ActR2B, with the net effect of establishing an autocrine loop feeding cell proliferation (16). Moreover, the tumor suppressor activity of ARID1A is mediated through SMAD3, a key transducer of activin signaling (17,18). Similarly, adult granulosa cell tumors (GCTs) harbor pathognomonic FOXL2 C134W mutations that drive tumorigenesis in part through upregulation of ActR2B pathway signaling (19–23). In addition, FOXL2 C134W inhibits activin A transcriptional activation of follistatin, the beta-subunit of follicle-stimulating hormone.
Reduced follistatin in granulosa cells expressing FOXL2 C134W may contribute to tumor formation as a direct result of the loss of activin antagonism (24).
The importance of activin to a wide variety of human physiologic mechanisms makes it an important drug target. Given the potential multifaceted role of activin A signaling in tumor proliferation, cancer stem cell survival, and cancer cachexia, STM 434, a soluble receptor-Fc fusion protein targeting human activin A was developed. STM 434 is a ligand trap that binds Activin A with high potency, thereby inhibiting activin A signaling. STM 434 also binds and neutralizes other ligands of the endogenous activin type 2B receptor (ActR2B), including myostatin, which are implicated in cancer cachexia and skeletal muscle wasting. Here, we describe the first-in-human phase I experience of STM 434 in patients with granulosa cell ovarian cancer and other advanced solid tumors.
PATIENTS AND METHODS
Study Oversight
This was an open-label, multicenter, single-arm, first-in-human phase I study. The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. The protocol was approved by institutional review boards within each institution, and all patients provided written informed consent before undergoing any study procedures. The study was designed by the lead investigators and the sponsor, Atara Biotherapeutics. The sponsor collected and analyzed the data in conjunction with the authors.
Study Design and Endpoints
The primary endpoint of the study was to define the maximum tolerated dose (MTD) of STM 434 injection administered as monotherapy in subjects with ovarian cancer or other advanced solid tumors. Secondary endpoints included evaluating the incidence of adverse events as assessed according to the common terminology criteria for adverse events (CTCAE) v4.03 (NCI CTCAE v4.03 2010), pharmacokinetics, overall response rate (ORR), and progression-free survival (PFS). Exploratory objectives included measuring changes in multiple metabolic parameters including change from baseline in lean body mass and 6-minute walk test distance (6MWD).
Eligible patients were age ≥ 18 years with Eastern Cooperative Group performance score 0–1, with measurable disease using RECIST v1.1 (25). Patients were required to have recurrent metastatic or locally advanced disease considered refractory or intolerant to standard treatment, tumors for which no standard treatment was available, or serous ovarian, fallopian tube, primary peritoneal cancers, granulosa cell tumors, or clear cell tumors considered platinum refractory/resistant or platinum intolerant.
Treatment Plan
The study incorporated a 3 + 3 dose escalation design to define an MTD with intravenous (IV) STM 434 and evaluate the safety, tolerability, and pharmacokinetic (PK) profiles of IV STM 434 at 5 planned dose levels (0.25 mg/kg, 0.5 mg/kg, 1 mg/kg, 2 mg/kg, and 4 mg/kg IV). STM 434 was initially administered intravenously every 4 weeks. After pharmacokinetic data from the first dosing cohort (0.25 mg/kg) showed a shorter than anticipated half-life, the dosing schedule was subsequently modified to a once every 2 week (Q2W) interval. In addition to the planned doses, the protocol was amended to study a dose of 8 mg/kg Q2W, due to lower than anticipated exposures predicted to yield anti-catabolic effects and to further investigate the potential for antitumor activity. Patients were treated with STM 434 until disease progression based on radiographic measurements according to RECIST v1.1, unacceptable toxicity, or withdrawal of consent.
Dose limiting toxicities were defined as any grade ≥ 3 (according to CTCAE v 4.03) non-hematologic toxicity (excluding toxicities which could be definitively attributed to other underlying disease), any grade ≥ 4 hematologic toxicity lasting 7 days, febrile neutropenia, or grade 3 thrombocytopenia with active bleeding. The DLTs monitoring period included the first 28 days of treatment, regardless of dosing schedule. At least three patients were required to be evaluable for DLTs at each dose level prior to further escalation. “Backfill” slots were also permitted at doses that had been declared safe while newer doses levels were under evaluation. Dose escalation continued until the MTD was identified based on all available safety data. Intrasubject dose escalation, to the next highest dosing level previously declared as safe, was permitted provided the patient had not had a DLT or evidence of radiographic disease progression.
Assessments
Response assessments were conducted according to RECIST v1.1 with tumor measurements obtained by computed tomography (CT) and/or magnetic resonance imaging (MRI). Assessments were performed at baseline and then approximately every 8 weeks. Duration of therapy was recorded from the day of the first dose to the last dose of STM 434 administered. Type and duration of prior oncologic therapies were captured in the study database. Tumor markers, as applicable, were measured at baseline and then every other cycle. Body composition (lean body mass) was measured by dual energy absorptiometry (DXA) scan at baseline and then every other cycle. Six-minute walk tests (6MWT), a measure of muscle function, were performed at baseline and then with each subsequent cycle (26). Serum tumor biomarkers, activin A and follicle stimulating hormone (FSH), were measured every cycle, and laboratory parameters, including complete blood count and comprehensive metabolic profile, were measured at baseline and with each cycle. ECOG performance status was assessed at baseline, with each cycle, and at end of study. Safety was monitored continuously throughout the study period.
Efficacy and Safety Analyses
Progression free survival was estimated using the Kaplan-Meier methods. Ninety-five percent confidence intervals were calculated using the binomial exact test. Safety analyses were descriptive. The safety analysis population included all subjects who received at least 1 dose of STM 434. The efficacy analysis population included all subjects who received at least 1 cycle of therapy (corresponding to either one or two doses of STM 434, depending on whether they were enrolled to a Q4W or Q2W dosing cohort, respectively).
Pharmacokinetic Analyses
Pharmacokinetic (PK) parameters, terminal half-life, clearance, mean residence time, volume of distribution, and volume of steady state were estimated for patients according to a predefined PK schedule. PK analyses were summarized using descriptive statistics.
RESULTS
Patient Characteristics
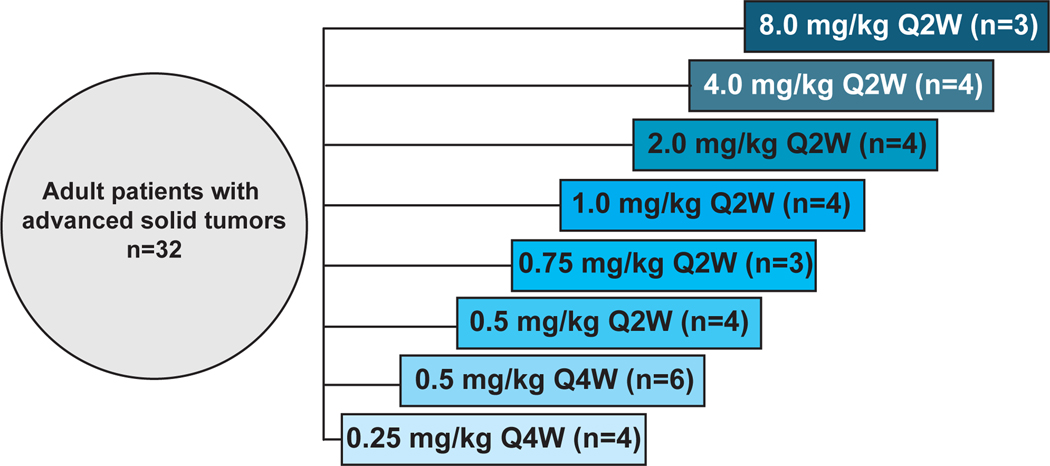
Between October 17, 2014 and January 13, 2017, a total of 32 subjects were enrolled to one of 8 dosing cohorts (Figure 1). Baseline demographics and disease characteristics are presented in Table 1. The 32 subjects enrolled in the study were a median of 61 years of age (range: 41 to 79 years). Primary cancer types included 20 patients with ovarian cancer (13 granulosa, 7 epithelial), 3 with colorectal, 2 with pancreas, and 7 with other solid tumors. Patients had received a median of 3 prior lines of therapy (range: 1–11).
Figure 1: STM 434: Dose escalation cohorts (3+3 design).
Schema depicting the dose escalation design of the first-in-human, phase I clinical trial of STM 434. Abbreviations: Q2W, every 2 weeks; Q4W, every 4 weeks.
Table 1: Patient baseline characteristics.
The clinical and pathologic features of the 32 patients with ovarian granulosa cell tumors and other advanced solid tumors who received STM 434 are summarized. Data are presented as N (%) unless indicated otherwise.
| Baseline Characteristic | All Patients (n=32) |
|---|---|
| Age at screening | |
| Median (range), years | 61 (41–79) |
| <18 | 0 (0) |
| ≥18–65 | 22 (69) |
| ≥65–85 | 10 (31) |
| ≥85 | 0 (0) |
| ECOG Performance Status 0–1 [n (%)] | 32 (100) |
| Prior therapies [median (range)] | 3 (1–11) |
| Sex and child-bearing potential1 [n (%)] | |
| Male | 6 (19) |
| Female | 26 (81) |
| Childbearing potential | 0 (0) |
| Post-menopausal | 10 (38) |
| Surgically sterile | 18 (69) |
| Ethnicity [n (%)] | |
| Hispanic or Latino | 7 (22) |
| Non-Hispanic or Latino | 24 (75) |
| Not reported | 1 (3) |
| Race1 [n (%)] | |
| White | 28 (88) |
| Black/African American | 2 (6) |
| Asian | 1 (3) |
| Not reported | 1 (3) |
| Tumor type [n (%)] | |
| Ovarian granulosa cell | 13 (41) |
| Epithelial ovarian | 7 (22) |
| Colon | 3 (9) |
| Pancreas | 2 (6) |
| Other solid tumor2 | 7 (22) |
More than one response can be provided for Race and Childbearing Potential and, as such, the percentage may total more than 100%.
Squamous cell carcinoma (2), chondrosarcoma (1), urachal (1), leiomyosarcoma (1), renal cell carcinoma (1), thymic carcinoma (1)
Safety and Tolerability
A total of 32 patients were evaluable for safety. The most common treatment emergent adverse events (TE-AE), regardless of attribution, was fatigue, occurring in 13 (41%) of subjects at grade 1 and 2 severity. Epistaxis occurred in 11 (34%) of subjects and in one subject (8 mg/kg Q2W cohort) was grade 3 in severity. Gingival bleeding, peripheral edema, and headache each occurred in 7 subjects each (22%) but only at grade 1 and 2. Grade 1 and 2 TE-AEs occurring at >10% frequency and all Grade 3 TE-AEs, regardless of frequency, are summarized in Table 2. No treatment-related grade 4 adverse events were observed, and no deaths occurred on study or within 30 days after the last dose of STM 434.
Table 2: Grade 1/2 treatment-emergent adverse events occurring in >10% of subjects and Grade 3 TEAEs regardless of frequency.
Listed below are adverse events reported in at least 10% of the patients (n=32) with advanced solid tumors who received STM 434 and that were deemed by the investigators to be related to study drug. No treatment-emergent grade 4 or 5 events were reported.
| Adverse Event, n (%) | Grade 1/2 | Grade 3 | All Grades (n=32) |
|---|---|---|---|
| Fatigue | 13 (41) | 0 | 13 (41) |
| Epistaxis | 10 (31) | 1 (3)1 | 11 (34) |
| Gingival bleeding | 7 (22) | 0 | 7 (22) |
| Headache | 7 (22) | 0 | 7 (22) |
| Peripheral edema | 7 (22) | 0 | 7 (22) |
| Abdominal pain | 4 (13) | 2 (6)2 | 6 (19) |
| Diarrhea | 6 (19) | 0 | 6 (19) |
| Pyrexia | 6 (19) | 0 | 6 (19) |
| Rash, maculo-papular | 6 (19) | 0 | 6 (19) |
| Anemia | 3 (9) | 2 (6)3 | 5 (16) |
| Decreased appetite | 4 (13) | 0 | 4 (13) |
| Dyspnea | 4 (13) | 0 | 4 (13) |
| Nausea | 4 (13) | 0 | 4 (13) |
8 mg/kg Q2W cohort
0.25 mg/kg Q4W and 0.5 mg/kg Q2W cohorts
2.0 mg/kg Q2W cohort
Nine patients (28%) experienced serious adverse events (SAEs), with the following SAEs occurring in one subject each (a single subject may have had more than 1 event): abdominal abscess, abdominal pain, anemia, ascites, acute cholecystitis, dehydration, epistaxis, gastritis, hemorrhagic ascites, infection, pain, and pyrexia. Of these 9 SAEs, 4 were deemed related to STM 434: anemia and epistaxis (8 mg/kg Q2W cohort), hemorrhagic ascites (0.5 mg/kg Q4W cohort) and gastritis (8 mg/kg Q2W cohort).
Three patients withdrew from STM 434 treatment as a result of at least possibly STM 434-related AEs including: grade 2 dyspnea on exertion in the 0.5 mg/kg Q4W cohort, grade 3 hemorrhagic ascites in the 0.5 mg/kg Q4W cohort, grade 1 abdominal wall hemorrhage in the 4 mg/kg Q2W cohort, In some cases, the observed bleeding diathesis persisted for weeks after discontinuation of STM 434.
Dose Limiting Toxicity and Maximum Tolerated Dose
A total of 5 DLTs (all grade 3) were observed in 3 patients: 1) hemorrhagic ascites lasting 2 months (0.5 mg/kg Q4W), 2) stomatitis and gastritis (8 mg/kg Q2W), and 3) epistaxis and increased alkaline phosphatase (8 mg/kg Q2W). As 2 subjects experienced DLTs in the 8 mg/kg Q2W cohort, this was considered to have exceeded the MTD and 4mg/kg Q2W was formally determined to be the MTD. However, given the overall safety profile of the agent with bleeding complications observed at dose levels as low as 0.5mg/kg Q2W, the safety review committee recommended against proceeding with dose expansion.
Pharmacokinetic Analysis
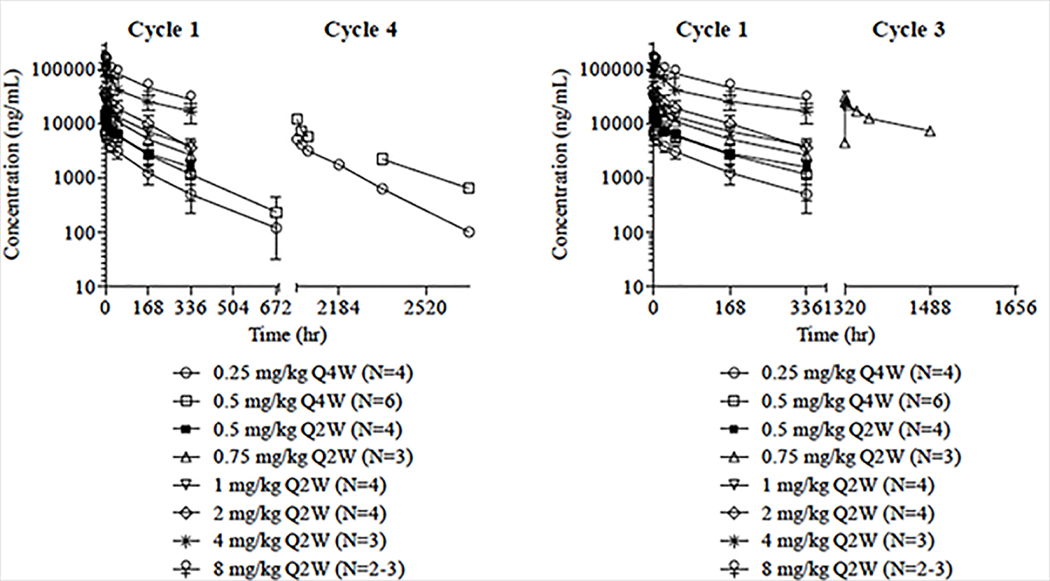
The PK evaluable population consisted of 31 subjects. Data from Cohort 1 (0.25 mg/kg Q4W) showed a half-life and clearance that was 2-fold higher in humans compared to observations in rodent and monkey preclinical studies. Therefore, based on the half-life of 5.0 days, the safety committee determined that Q2W dosing should be administered. Further PK data from Cohort 2 (0.5 mg/kg Q4W) confirmed the half-life observations from Cohort 1. Therefore, subsequent cohorts were administered at a Q2W schedule to optimize drug exposure. STM 434 exhibited approximately linear PK over a dose range of 0.25 mg/kg to 8 mg/kg with little inter-subject variability (Figure 2). Across dose cohorts, the half-life of STM 434 in Cycle 1 ranged from 5.0 days in the 0.25 mg/kg Q4W, 0.5 mg/kg Q2W, and 2 mg/kg Q2W cohorts) to 7.6 days in the 8 mg/kg Q2W cohort. Mean clearance in Cycle 1 was 0.3 mg/kg in all cohorts except for the 0.25 mg/kg Q4W, 0.5 mg/kg Q4W, and 0.5 mg/kg Q2W cohorts, in which it was 0.4 mg/hr/kg and the 2 mg/kg Q2W cohort, which was 0.5 mg/hr/kg.
Figure 2: Pharmacokinetics of STM 434.
Exposure to STM 434, as measured by the maximum concentration (Cmax) and area under the concentration-time curve from 0 to 336 hours (AUC 0–336 hr) and 320–1448 hours (AUC 320–1448 hr) appears to increase in a linear manner with doses from 0.25 mg/kg to 8 mg/kg. Abbreviations: Q2W, every 2 weeks; Q4W, every 4 weeks.
Exposure to STM 434, as measured by the maximum concentration (Cmax) and area under the concentration-time curve from 0 to 336 hours (AUC 0–336 h), appeared to increase in a linear manner with doses from 0.25 mg/kg to 8 mg/kg (Figure 2). In the 0.25 mg/kg Q4W cohort, the mean Cmax in Cycle 1 was 7.3 μg/mL and increased with dose and frequency to 149.7 mg/mL in the 8 mg/kg Q2W cohort. The mean AUC 0–336 h in Cycle 1 increased approximately linearly from 577.5 (179.04) hr*μg/mL in the 0.25 mg/kg Q4W cohort to 18,566.7 (1960.44) hr*μg/mL in the 8 mg/kg Q2W cohort. The subjects who experienced DLTs were not outliers for PK exposure. For the study population as a whole, subjects had a mean (SD) of 8.0 (8.72) weeks of therapy with STM 434, entered a mean (SD) of 2.8 (2.03) cycles, and had a mean (SD) cumulative drug exposure of 7.8 (8.59) mL (Supplemental Table 1).
Response, Duration, and Discontinuation of Therapy
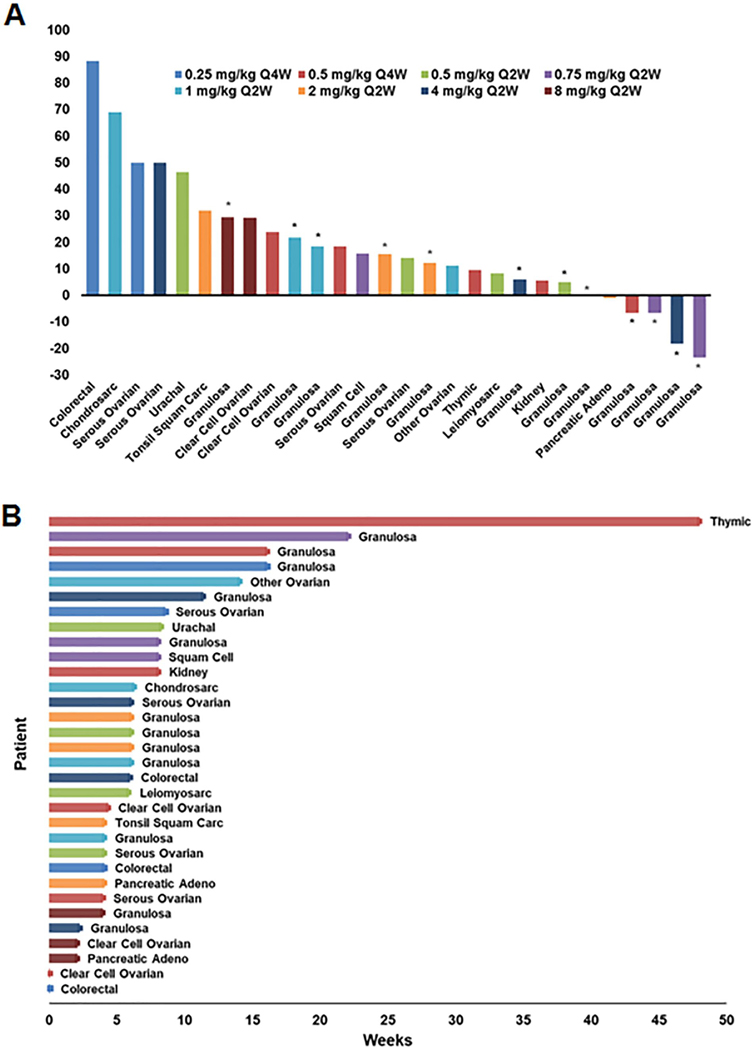
The efficacy population, defined as having received a minimum of 1 cycle of STM 434, consisted of 30 patients. Sixteen (53.3%) patients experienced a best response of stable disease and 12 (40%) had progressive disease according to RECIST. Two (6.7%) patients were non-evaluable by RECIST but discontinued prior to their first response assessment due to clinical deterioration and were counted as non-responders. The best percent change in target lesions observed in each patient are shown in Figure 3A. Duration of therapy is shown for all 32 patients enrolled on the study (Figure 3B). Among the 30 patients in the efficacy population the median duration of therapy was 6 weeks (range: 2–48). One patient, with thymic cancer enrolled in the 0.5 mg/kg Q4W cohort, continued on study with a best response of stable disease for twelve cycles (48 weeks). Specifically, among the patients with granulosa cell tumors, 10/12 achieved a best response of stable disease. However, the median duration of therapy for these 10 patients was only 7.1 weeks (range: 2.3–22.1). The median ratio of duration of STM 434 therapy to duration of prior therapy was 1.08. Seven patients discontinued therapy due to either disease progression of lack of clinical benefit as judged by the PI, 2 discontinued due to an adverse event, and 1 patient withdrew consent.
Figure 3: Greatest change in target lesions and duration of therapy.
A) Waterfall plotting showing the greatest change in target lesions by RECIST v1.1 criteria. Only patients with baseline and at least one post-baseline assessment of target lesion are included. Two patients in the efficacy population are not included in this figure. Colors (shown in legend) represent the different dose cohorts. Asterisks indicate that the tumor type is granulosa ovarian. B) Swimmer plot showing duration of therapy for the 32 patients who received STM 434.
Pharmacodynamics and Metabolic Response to Therapy
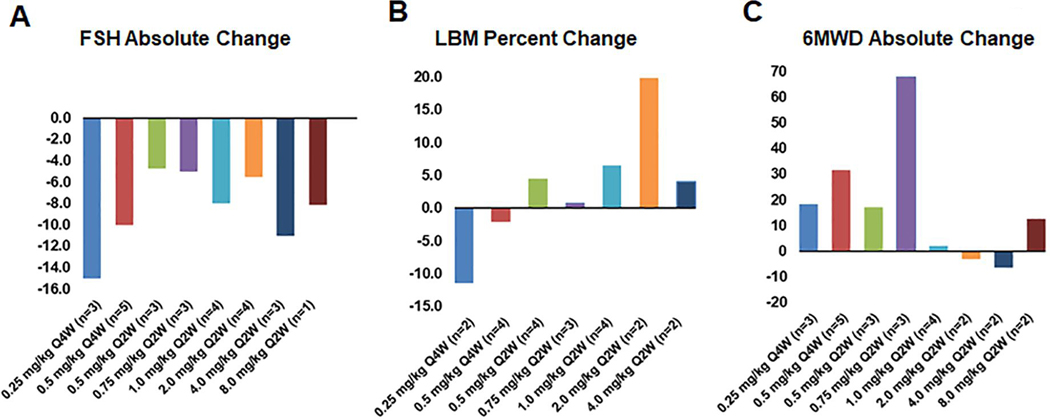
Activins function biologically to enhance biosynthesis and secretion of FSH. Therefore, to confirm on target and pathway inhibition by STM 434, FSH levels were measured at baseline (pre-treatment) and at defined intervals with each cycle. As expected, STM 434 induced a reciprocal decrease in FSH in most subjects. FSH decreased by a mean (SD) of 8.1 (11.45) mIU/mL for all cohorts (n=27) from baseline to the last available post-baseline observation. The change in FSH for each cohort is shown in Figure 4A.
Figure 4: Pharmacodynamic and metabolic response to therapy.
A) Mean absolute change in FSH compared to baseline. B) Mean percent change in total lean body mass (LBM) compared to baseline. C) Mean absolute change in 6 Minute Walk Test Distance (6MWD) in meters.
Other metabolic parameters of activin A inhibition were also measured. As activin A overexpression is implicated in muscle wasting and cancer cachexia, lean body mass and six-minute walk distance were also assessed. Lean body mass increased by a mean percent (SD) of 3.00 (10.4) (n=21) (Figure 4B). The 6-minute walk distance increased by a mean (SD) of 20.0 (60.2) meters (n=22) (Figure 4C).
To explore whether the changes observed in lean body mass and 6-minute walk distance were reflected in other biologic and functional parameters of performance, we also examined changes in laboratory parameters (Supplemental Table 2) and ECOG performance status (Supplemental Table 3). The mean (SD) changes from baseline to end of treatment for albumin, hemoglobin, and absolute lymphocyte count were −0.2 g/dL (0.2), −0.8 g/dL (1.1), and 188.3/μL (979.5), respectively. In terms of ECOG performance status, at baseline, 23/32 patients had an ECOG score of 0, with the remaining 9 patients with an ECOG score of 1. At the end of study, ECOG score was only available on 13 patients, of which 8 patients had ECOG score of 0 and 5 patients had an ECOG score of 1. Among these 13 patients who had both baseline and end of study ECOG scores, 10 patients had stable ECOG scores at baseline to end of treatment, 1 patient’s ECOG score improved from 1 to 0, and 2 patients’ ECOG score worsened from 0 to 1.
Consistent with the hypothesis that STM 434 may have the potential to alter fatty metabolism, one patient with ovarian granulosa cell cancer enrolled in the 0.75 mg/kg Q2W cohort was noted to have complete radiographic resolution of hepatic steatosis previously observed on her baseline CT scan after 2 months of treatment (Supplemental Figure 2). No other changes in her clinical status, dietary behavior, or concomitant medications could otherwise explain this change.
DISCUSSION
We report results from a first-in-human, first-in-class study evaluating the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of STM 434 in 32 patients with advanced solid tumors, including 12 patients with ovarian granulosa cell tumors. Multiple episodes of mucocutaneous bleeding syndrome mimicking hereditary hemorrhagic telangiectasia occurred at several dose levels among patients treated with STM 434. This safety signal was ultimately hypothesized to be related to off-target inhibition of BMP9, a related ligand of ActR2B, the target of STM 434. Preclinical toxicity studies had identified bleeding diathesis as one potential risk of STM 434. Specifically, in a study of cynomolgus monkeys of the STM 434 surrogate STM 217, 3 of 7 monkeys treated with 3 mg/kg and 2 of 6 monkeys treated with 10 mg/kg developed red muzzle discharge consistent with epistaxis, compared to 0/7 control monkeys. One monkey in the 10 mg/kg group also developed significant bleeding from an ischial pad lesion 7 weeks after starting treatment. Platelet counts were normal in the monkeys, and it was hypothesized that the bleeding was the result of inhibition of bone morphogenetic protein 9 (BMP9), another member of the TGFβ superfamily that serves as a ligand for the activin receptor-like kinase-1 (ACVRL1 or ALK1). ACVRL1 bears significant homology to ActR2B, the target of STM 434. Mutations in activin receptor-like kinase-1 (ACVRL1) have been reported in hereditary hemorrhagic telangiectasia (HHT) which is associated with a similar spectrum of mucocutaneous bleeding disorders as well as capillary telangiectasias (27). Interestingly, one patient with ovarian granulosa cell tumor treated at the 0.75 mg/kg Q2W dose developed diffuse telangiectasias on the upper chest, back, and face (Supplemental Figure 1). The telangiectasias resolved 3 months following cessation of STM 434.
No definitive evidence of anti-tumor activity was observed with STM 434, including in patients with granulosa cell tumor in which activin A biology was expected to play a significant biological role. Due to the overall safety profile of STM 434 as well as the absence of clear efficacy, the decision was made to discontinue further development of STM 434.
Nevertheless, STM 434 demonstrated the expected on-target pathway inhibition, with a reciprocal decrease in FSH for most patients. In addition, treatment with STM 434 led to increased lean body mass and in most cohorts, an increase in six-minute walk test distance. Most patients experienced stable ECOG performance status at baseline and at the end of study. Other metabolic effects that implicate activins potential role in regulating fat metabolism were observed. This included the complete radiographic resolution of fatty liver after 2 months of STM 434 therapy in one patient with ovarian granulosa cell cancer. These metabolic effects of activin inhibition, specifically the potential to increase lean body and physical stamina, may have an important role in supportive care, including in preventing and diminishing cancer cachexia. While the toxicity observed with this particular agent render it unacceptable for use in cancer cachexia reversal, these data suggest that activin pathway inhibitors may play a potentially important supportive care role in oncology and provide a better-informed opportunity to develop a second-generation agent. However, it will be critical that future drugs targeting this pathway avoid off-target BMP9 inhibition and the bleeding diathesis it appears to induce.
In summary, we report data from the first in human, phase I dose escalation study evaluating STM 434, an activin inhibitor, as treatment for patients with advanced cancers. Though STM 434 did not lead to tumor responses, metabolic effects that may have a future role in treating cancer cachexia, were observed.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
This is the first-in-human clinical trial of STM 434, a soluble receptor ligand trap targeting activin A, which plays an important role in growth, differentiation, and cancer cachexia. Though treatment with STM 434 did not result in clinical response, predicted metabolic effects of Activin A inhibition, including increase in total lean body mass and 6-minute walk test, were observed. This provides a rationale for further exploration of activin A inhibitors as metabolic modulators in other clinical settings, including improving muscle wasting and cancer cachexia.
ACKNOWLEDGEMENTS:
The clinical trial was supported by Atara Biotherapeutics and an institutional Core Grant.
Footnotes
CONFLICTS OF INTEREST:
DM: consultant: Atara Biotherapeutics, Chugai Pharma, Cytom X Therapeutics, Boehringer Ingelheim, AstraZeneca, Puma Biotechnology, LOXO. CH, WH: employment, stock ownership: Atara Biotherapeutics. VM: consultant: Eisai. KC: Research Funding: AstraZeneca. DR: consultant: Eli Lilly, Boehringer Ingelheim; research funding: Abbvie, Ascentage, Asana, Celgene, Constellation, FivePrime, GSK, Eisai, Macrogenics, Merck; travel, accommodations, expenses: Takeda, Asana. JT, NC, JL: no disclosures.
REFERENCES
- 1.Loomans HA, Andl CD. Intertwining of Activin A and TGFβ Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion. Cancers (Basel). 2014;7:70–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomans HA, Andl CD. Activin receptor-like kinases: a diverse family playing an important role in cancer. Am J Cancer Res. 2016;6:2431–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Watkinson J, Varadan V, Anastassiou D. Multi-cancer computational analysis reveals invasion-associated variant of desmoplastic reaction involving INHBA, THBS2 and COL11A1. BMC Med Genomics. 2010;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steller MD, Shaw TJ, Vanderhyden BC, Ethier J-F. Inhibin resistance is associated with aggressive tumorigenicity of ovarian cancer cells. Mol Cancer Res. 2005;3:50–61. [PubMed] [Google Scholar]
- 5.Lonardo E, Hermann PC, Mueller M-T, Huber S, Balic A, Miranda-Lorenzo I, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46. [DOI] [PubMed] [Google Scholar]
- 6.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–90. [DOI] [PubMed] [Google Scholar]
- 7.Argilés JM, Orpí M, Busquets S, López-Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov Today. 2012;17:702–9. [DOI] [PubMed] [Google Scholar]
- 8.Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA. 1994;91:8817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. [DOI] [PubMed] [Google Scholar]
- 12.Cobellis L, Reis FM, Luisi S, Danero S, Pirtoli L, Scambia G, et al. High concentrations of activin A in the peritoneal fluid of women with epithelial ovarian cancer. J Soc Gynecol Investig. 2004;11:203–6. [DOI] [PubMed] [Google Scholar]
- 13.Menon U, Riley SC, Thomas J, Bose C, Dawnay A, Evans LW, et al. Serum inhibin, activin and follistatin in postmenopausal women with epithelial ovarian carcinoma. BJOG. 2000;107:1069–74. [DOI] [PubMed] [Google Scholar]
- 14.Welt CK, Lambert-Messerlian G, Zheng W, Crowley WF, Schneyer AL. Presence of activin, inhibin, and follistatin in epithelial ovarian carcinoma. J Clin Endocrinol Metab. 1997;82:3720–7. [DOI] [PubMed] [Google Scholar]
- 15.Tournier I, Marlin R, Walton K, Charbonnier F, Coutant S, Théry J-C, et al. Germline mutations of inhibins in early-onset ovarian epithelial tumors. Hum Mutat. 2014;35:294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karve TM, Preet A, Sneed R, Salamanca C, Li X, Xu J, et al. BRCA1 regulates follistatin function in ovarian cancer and human ovarian surface epithelial cells. PLoS ONE. 2012;7:e37697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan B, Wang T-L, Shih I-M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SP, Köbel M, Senz J, Morin RD, Clarke BA, Wiegand KC, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360:2719–29. [DOI] [PubMed] [Google Scholar]
- 20.Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol. 2003;206:93–111. [DOI] [PubMed] [Google Scholar]
- 21.Pisarska MD, Bae J, Klein C, Hsueh AJW. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology. 2004;145:3424–33. [DOI] [PubMed] [Google Scholar]
- 22.McConechy MK, Färkkilä A, Horlings HM, Talhouk A, Unkila-Kallio L, van Meurs HS, et al. Molecularly Defined Adult Granulosa Cell Tumor of the Ovary: The Clinical Phenotype. J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagida S, Anglesio MS, Nazeran TM, Lum A, Inoue M, Iida Y, et al. Clinical and genetic analysis of recurrent adult-type granulosa cell tumor of the ovary: Persistent preservation of heterozygous c.402C>G FOXL2 mutation. PLOS ONE. 2017;12:e0178989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTavish KJ, Nonis D, Hoang YD, Shimasaki S. Granulosa cell tumor mutant FOXL2C134W suppresses GDF-9 and activin A-induced follistatin transcription in primary granulosa cells. Mol Cell Endocrinol. 2013;372:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 26.ATS Statement. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 27.Seo J, Chu H, Lee JS, Kim DY. Mucocutaneous Telangiectasia as a Diagnostic Clue of Hereditary Hemorrhagic Telangiectasia: An Activin Receptor-Like Kinase-1 Mutation in a Korean Patient. Ann Dermatol. 2016;28:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.