Abstract
Electronic cigarette use is particularly prevalent in adolescents, but the effects of secondhand exposure to nicotine vapor in adolescents on the propensity to develop nicotine dependence and increase nicotine self-administration in adulthood are poorly known.
The present study explored the effects of nicotine vapor exposure on withdrawal-like states (hyperalgesia, spontaneous withdrawal signs, and locomotor activity) in adolescent rats and the vulnerability to acquire intravenous nicotine self-administration in adulthood.
Adolescent (postnatal day 38) rats were exposed to intermittent nicotine vapor (14 h/day) for 7 consecutive days in a range of doses (0, 0.4, and 7 mg/m3). The rats were tested for somatic, emotional, and motivational withdrawal symptoms. When the animals reached adulthood, they were allowed to self-administer nicotine (0.03 mg/kg/0.1 ml) intravenously in operant chambers for 1 h/day for 12 consecutive days.
Rats that were exposed to nicotine vapor presented moderate to severe signs of spontaneous withdrawal after the cessation of nicotine vapor. No effect on anxiety-like behavior was observed. Rats that were exposed to high levels of nicotine vapor in adolescence had lower pain thresholds and exhibited faster and higher acquisition of nicotine self-administration in adulthood.
Chronic exposure to nicotine vapor in adolescent rats produces a withdrawal-like state and facilitates the acquisition of intravenous nicotine self-administration in adulthood. These results suggest that exposure of adolescents to nicotine vapor may confer higher risk of developing nicotine dependence when they become adults.
Keywords: tobacco, abstinence, secondhand exposure, pain, dependence
Introduction
Electronic cigarettes (e-cigarettes) are battery-operated devices that are designed to deliver nicotine through vapor instead of smoke. Epidemiological population-based studies indicate that the rate of e-cigarette use across countries is increasing among both current and former smokers and dramatically increasing among nonsmokers, particularly among adolescents (https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes; accessed August 24, 2017). Recent clinical data suggest that e-cigarette use is most appealing and prevalent among adolescents. Findings from the 2014 National Youth Tobacco Survey showed that current e-cigarette use among high school students increased from 4.5% in 2013 to 13.4% in 2014, increasing from approximately 660,000 to 2 million students. In fact, e-cigarette use has surpassed the use of every other tobacco product overall, including conventional cigarettes, since 2011 to date.
Nicotine dependence is often characterized by the emergence of a nicotine abstinence syndrome after the cessation of chronic nicotine exposure. Such an abstinence syndrome has been characterized in both humans and rats and is associated with both somatic and motivational components (Shiffman & Jarvik, 1976; Hughes et al., 1991; Malin et al., 1992; Malin et al., 1993; Malin et al., 1994; Hildebrand et al., 1997; Epping-Jordan et al., 1998; Watkins et al., 2000). In rats, the somatic signs of nicotine withdrawal (both spontaneous withdrawal and withdrawal that is precipitated by the nicotinic acetylcholine receptor [nAChR] antagonist mecamylamine) include abdominal constrictions, facial fasciculation, ptosis, and hyperalgesia. The motivational components include anxiety-like behavior and higher motivation (craving) for nicotine (Bruijnzeel, 2016). However, unclear are the ways in which adolescent exposure to nicotine vapor affects the somatic and motivational aspects of nicotine dependence.
The first goal of the present study was to measure the effect of chronic exposure to nicotine vapor on key behaviors that are related to nicotine withdrawal (somatic signs, hyperalgesia, and anxiety-like behavior) in adolescent rats. The second goal was to test whether chronic exposure to nicotine vapor during adolescence produces long-lasting changes that confer greater vulnerability to self-administer intravenous nicotine in adulthood. Human studies have shown that the air concentrations of nicotine that are emitted by various brands of e-cigarettes range from 0.82 to 6.23 µg/m3 (Czogala et al., 2014). We tested the effects of two concentrations of nicotine vapor (0.4 and 7 mg/m3) over 7 days of exposure (14 h/day). The intermittent nicotine vapor exposure, with 14h on and 10h off, was used based on previous evidence (Gilpin et al., 2014; Xue et al., 2018;, McGinn et al., 2016; Baiamonte et al., 2014) showing that this protocol produces nicotine dependence with somatic signs and hyperalgesia when exposed to high levels of nicotine (7 mg/m3). This protocol is highly relevant to adolescent e-cig users, who often vape intermittently due to parental and school restrictions. Withdrawal in rats peaks 16h into withdrawal, but can be observed 10h into withdrawal (Grieder et al., 2010). Our overarching hypothesis is that repeated mild withdrawal (10h) are an important factor to the transition to addiction both in rats and humans and therefore it was important to introduce this parameter in our paradigm.
Materials and Methods
Animals
Male Wistar rats (n = 18) were obtained from Charles River (Wilmington, MA, USA) one week after their weaning period (postnatal day [PND] 28). The rats weighed 120–150 g upon arrival in the laboratory. The rats were tested during adolescence (PND 38 - PND 46; 200–220 g) except for nicotine self-administration that was performed later, during adulthood (PND 66 – PND 78; 300–330 g). The animals were group housed (3 rats/cage) in standard cages in a room with artificial lighting (12 h/12 h light/dark cycle, lights off at 8:00 AM) at a constant temperature (20–22°C) and humidity (45–55°) with food (2018 Teklad global 18% protein rodent diet; Teklad, Madison, WI) and water available ad libitum except during testing. The rats were handled twice for 5 min after arrival in the animal facility. The animal procedures met the guidelines of the National Institutes of Health and were approved by The Scripps Research Institute Institutional Animal Care and Use Committee (protocol no. 08–0015). All of the surgical procedures were performed under isoflurane anesthesia, and all necessary actions were taken to minimize suffering of the animals.
Nicotine vapor exposure
The rats (n = 6/group) were group housed in standard rat cages (3 rats/cage) and exposed daily from 9:00 AM to 11:00 PM to intermittent nicotine vapor (14 h ON/10 h OFF). Every cage had an air-tight transparent Plexiglas lid. (−) Nicotine ≥ 99% (GS), liquid 1.010 g/ml at 20 °C (Sigma-Aldrich) was used. For the entire duration of nicotine vapor exposure (14 h) and air exposure (10 h) for 7 consecutive days, the rats were left undisturbed in the nicotine-vapor chamber with food and water available ad libitum. Nicotine vapor was produced as previously described (George et al., 2010) to reach nicotine vapor concentrations of 0.4 and 7 mg/m3. The nicotine:air ratio was 0:20 L/min for the air control group, 1:19 L/min for the 0.4 mg/m3 nicotine group, and 10:10 L/min for the 7.0 mg/m3 nicotine group. Gas chromatography was used to analyze the level of nicotine in the test chamber. A glass tube (70 mm, 7 mm outer diameter) that contained two sections of XAD-4 (front = 80 mg, back = 40 mg) that were separated by silicate glass wool was used. The concentration (C) of nicotine was calculated as the following: C = Wf × Wb × Bf × Bb / V (mg/m3 = μg/L). Mass (μg) was corrected for desorption efficiency (DE). Wf is the sample front of the sorbent sections, and Wb is the sample back of the sorbent sections, and in the average media blank front (Bf) and back (Bb) sorbent sections. Air control rats were treated similarly to the nicotine-exposed rats, with the exception that the air that entered the chambers did not contain nicotine.
Withdrawal signs
After 7 days of intermittent nicotine vapor exposure (14 h ON/10 h OFF), the rats were maintained nicotine-free for 16–24 h and experienced spontaneous withdrawal in their home cages. On the test day, after 16 h of no exposure to nicotine vapor, the rats were placed inside a transparent cylinder (50 cm height × 30 cm diameter) and observed for 30 min. The number of wet dog shakes, front paw tremors, teeth chattering, genital licks, and abdominal constrictions was counted. The sum of the observation scores was used as a quantitative measure of withdrawal severity. Three experimenters who were blind to the treatment group scored the withdrawal signs. The average of the three measures was used for the analyses.
Open field test
The open field test was performed at 16–24 h of nicotine abstinence after 7 days of nicotine vapor exposure. Locomotor activity and anxiety-like behavior were evaluated in an opaque open field apparatus (100 cm × 100 cm × 40 cm) that was divided into 20 cm × 20 cm squares. On the experimental day, the animals were placed in the center of the open field, and locomotor activity (number of lines crossed), time in the center, entries into the center, and total distance traveled (in centimeters) were scored for 10 min. Behavior was videotaped, and AnyMaze software was used to analyze the recordings.
Mechanical nociception in the von Frey test
Mechanical nociception was evaluated using the von Frey test. The test was first performed 1 day before placing the animals into the nicotine exposure chamber to determine the baseline pain threshold of each rat. The second test was conducted after 7 days of nicotine vapor exposure at 16–24 h of nicotine withdrawal (immediately after withdrawal signs were scored and the locomotor activity test was performed). The hindpaw withdrawal threshold was determined using von Frey filaments, ranging from 3.63 to 125.89 g (Edwards et al., 2012; Xue et al., 2018). A test session began after 10 min of habituation to the testing environment. A series of von Frey filaments were applied in ascending order from below a wire mesh to the central region of the plantar surface of the left hindpaw, beginning with the lowest filament (3.63 g). The filament was applied until buckling of the hair occurred, and it was maintained for approximately 2 s. A withdrawal response was considered valid only if the hindpaw was completely removed from the mesh platform. The stimulus was incrementally increased until a positive response was observed and then decreased until a negative response was observed to determine a pattern of responses to apply to the statistical method of Dixon (Dixon, 1980). The 50% paw withdrawal threshold was calculated as the following: Xf + kδ, where Xf is the last von Frey filament employed, k is the Dixon value that corresponds to the response pattern, and δ is the mean difference between stimuli. Once the threshold was determined for the left hindpaw, the same testing procedure was applied to the right hindpaw after 5 min.
Intravenous catheterization
Chronic intravenous jugular catheters were implanted in all of the rats on PND 58 as described previously (Caine & Koob, 1993). The rats were anesthetized with 1–3% isoflurane in an oxygen mixture. Incisions were made to expose the right jugular vein. A catheter that was made from Micro-Renathane tubing (inner diameter, 0.020 inches; outer diameter, 0.037 inches; Braintree Scientific, Braintree, MA, USA) was subcutaneously implanted. After insertion into the vein, the proximal end of the catheter was anchored with surgical silk to the muscles under the vein. The distal end of the catheter was attached to a stainless-steel cannula that was bent at a 90° angle. The cannula was anchored in dental cement on the back of the rats. For 1 week after surgery, the rats were treated daily with 0.2 ml of the antibiotic Cefazolin (262 mg/ml). For the duration of the experiments, the catheters were flushed daily with 0.2–0.3 ml of heparinized saline solution. Body weight was monitored every other day. Catheter patency was confirmed at the end of the experiment with an injection of 0.2–0.3 ml of Brevital sodium solution (10 mg/ml). Catheter patency was assumed if there was an immediate loss of reflexes after the injection. The rats were given 1 week to recover from surgery before the self-administration experiments.
Operant training
The self-administration chambers consisted of operant conditioning chambers (Med Associates, St. Albans, VT, USA) that were enclosed in sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with two retractable levers that were located in the front panel. A plastic tube that was connected to the catheter before beginning the session delivered nicotine. An infusion pump was activated by responses on the right (“active”) lever, and responses on the left (“inactive)” lever were recorded but did not result in any programmed consequences. Activation of the pump, resulted in the delivery of 0.1 ml of nicotine solution (0.03 mg/kg/0.1 ml) in a fixed ratio-1 (FR-1) schedule of reinforcement for 1 hour, paired with the illumination of the chamber by the cue light. The delivery of nicotine solution was followed by a 20-s timeout (TO) period during which further lever presses did not result in any consequences. Nicotine solution was prepared fresh every other day from (−)Nicotine hydrogen tartrate salt (Sigma-Aldrich) based on the animals’ body weight, and the pH was adjusted to 7.3 with NaOH 1M.
Experimental design
The experimental design is summarized in Fig. 1. Adolescent rats (PND 37) were subjected to the von Frey test prior to the behavioral tests so that we could perform within-subjects analyses, based on the known between-subjects variability in pain thresholds in rats. The animals were equally divided into three groups (n = 6/group). They were placed in the nicotine vapor chambers and exposed daily to intermittent nicotine vapor (14 h ON/10 h OFF). Body weight was recorded every other day. After 7 days of intermittent nicotine exposure, the animals were returned to their home cages in the animal facility. Withdrawal scores, including teeth chattering, paw tremors, genital licking, wet-dog shakes and abdominal constrictions were recorded after 16 h of spontaneous withdrawal for 30 min starting at 3:00 PM the next day. After the assessment of somatic withdrawal signs, locomotor activity was assessed in a 10 min test at 16–24 h of nicotine withdrawal. The total distance traveled, number of entries into the central zone of the arena, and time spent in the central zone were recorded. Immediately after scoring spontaneous withdrawal signs and evaluating locomotor activity, the von Frey test was performed. On PND 58, the rats underwent intravenous catheter implantation surgery. They were allowed to recover for 1 week after surgery. Starting on PND 66, the rats were given daily 1-h access to nicotine self-administration (0.03 mg/kg/0.1ml) for 12 consecutive days.
Figure 1.
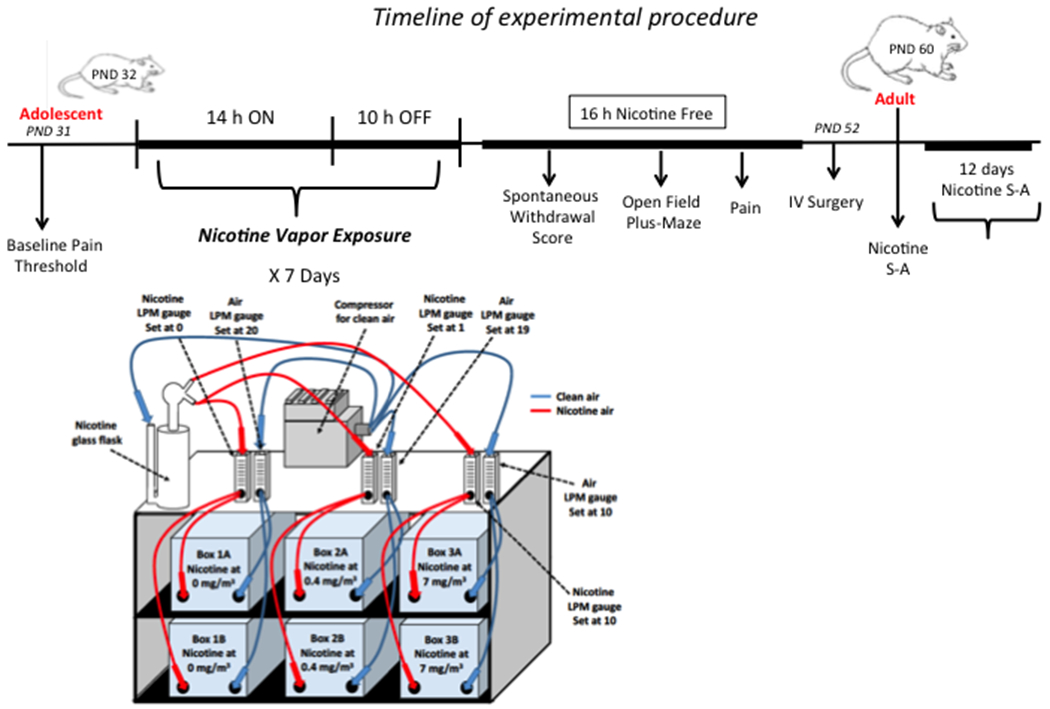
Timeline of the experiments and representative schematic diagram of the nicotine vapor machine.
Statistical analysis
The data were analyzed using mixed-factorial or one-way analysis of variance (ANOVA), followed by the Newman-Keuls post hoc test when appropriate. For individual means comparisons, Student’s paired or unpaired t-test was used. Data from the mechanical nociception test during spontaneous withdrawal were analyzed using Student’s t-test by comparing the withdrawal results with the rats’ baselines during the naive state. Values of p < 0.05 were considered statistically significant.
Results
Vapor exposure induced nicotine dependence in adolescent rats
The animals that were exposed to the highest concentration of nicotine vapor exhibited significant body weight loss compared with rats that were exposed to air and the low-nicotine-concentration of nicotine vapor (Fig. 2a). The mixed-factorial ANOVA, with group (passive nicotine dose) as the between-subjects factor and time as the within-subjects factor, revealed main effects of group (F2,15 = 34.2, p < 0.001) and time (F3,15 = 17.73, p < 0.001) and a significant group × time interaction (F6,45 = 17.434; p < 0.001). The Newman-Keuls post hoc test showed that rats that were exposed to the high concentration of nicotine vapor (7 mg/m3) exhibited a significant loss of body weight on day 3 (p < 0.05), day 5 (p < 0.01), and day 7 (p < 0 .01) compared with the air-exposed group.
Figure 2.
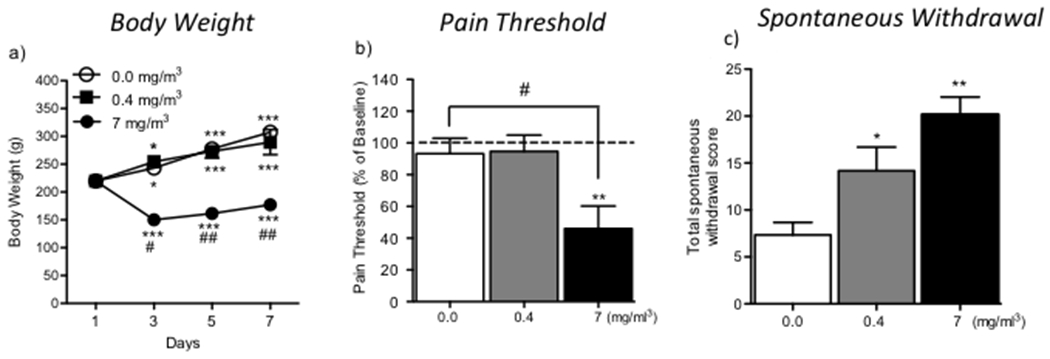
(a) Body weight of rats exposed to air (0.0 mg/m3) and nicotine vapor (0.4 and 7 mg/m3). *p < 0.05, ***p < 0.001, compared with day 1; #p < 0.05, ##p < 0.01, difference between air-exposed rats and nicotine (7 mg/m3)-exposed rats on days 3, 5, and 7. (b) Pain thresholds. The data are expressed as a percent difference from baseline. **p < 0.01, significant decrease in pain thresholds in nicotine (7 mg/m3)-exposed rats compared with their own baseline; #p < 0.05, difference between nicotine (7 mg/m3)-exposed rats compared with air-exposed rats. (c) Spontaneous withdrawal symptoms. *p < 0.05, **p < 0.01, compared with air-exposed rats.
Rats that were exposed to the highest concentration of nicotine vapor exhibited a decrease in mechanical thresholds during spontaneous withdrawal (t5 = 3.8, p < 0.01) relative to their own baseline pain threshold that was established prior to nicotine vapor exposure from (46.7 ± 7.1) g to (21.5 ± 4.3) g (Fig. 2b). The one-way ANOVA revealed that 7-day exposure to intermittent nicotine vapor significantly increased pain sensitivity (F2,15 = 5.73, p < 0.05) during nicotine withdrawal. Rats that were exposed to the highest concentration of nicotine vapor (7 mg/m3) were more sensitive to pain (p < 0.05) compared with air-exposed rats. The number of spontaneous withdrawal signs, measured 16–24 h after the cessation of nicotine vapor, is shown in Fig. 2c. The one-way ANOVA revealed that 7-day exposure to nicotine vapor induced significant spontaneous withdrawal signs after the discontinuation of intermittent nicotine vapor (F2,15 = 10, p < 0.0013). The Newman-Keuls post hoc test showed that both concentrations of nicotine vapor (0.04 and 7 mg/m3) significantly increased withdrawal scores (p < 0.05 and p < 0.01, respectively) compared with air-exposed rats.
Effect of nicotine vapor withdrawal on anxiety-like behavior
Immediately after the assessment of somatic withdrawal signs, locomotor activity was recorded over a 10-min period (Fig. 3). The ANOVA revealed a nonsignificant trend toward an overall difference in the time spent in the center (F2,15 = 0.36, p = 0.7; Fig. 3a), the number of entries into the center (F2,15 = 2.52, p = 0.11; Fig. 3b), and the total distance traveled (F2,15 = 3.43, p = 0.059; Fig. 3c) between the air- and nicotine-exposed groups. A trend toward a decrease in the number of entries into the center and total distance traveled was observed in nicotine-withdrawn animals compared with air-exposed controls.
Figure 3.
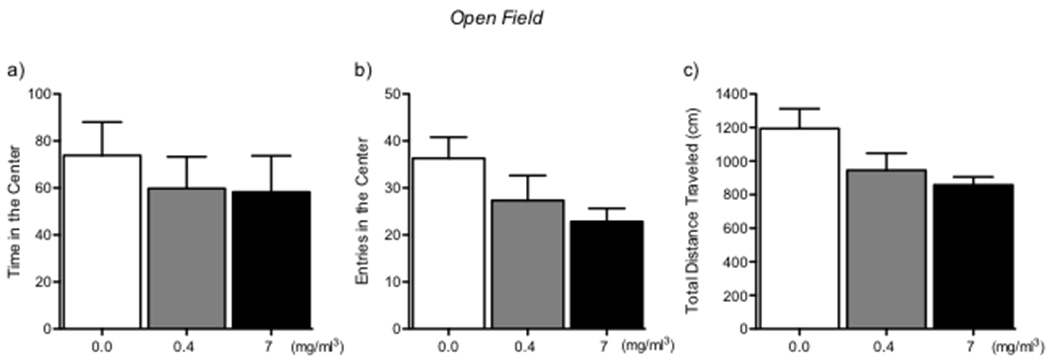
Open field test. (a) Time in center. (b) Entries into center. (c) Total distance traveled. No significant differences were observed between groups.
Effect of nicotine vapor exposure during adolescence on intravenous nicotine self-administration during adulthood
After completing the first set of experiments, the rats were left undisturbed in their home cages in the animal facility. On PND 58, the animals were implanted with intravenous catheters to assess nicotine self-administration. After 1 week of recovery from surgery, the animals were placed in operant chambers and allowed to self-administer nicotine (0.03 mg/kg/0.1ml) in 1 h daily sessions. Animals that were previously exposed to the highest concentration of nicotine (7 mg/m3) during adolescence exhibited higher nicotine intake during adulthood compared with rats that were exposed to air or low-concentration nicotine vapor during adolescence (Fig. 4a). The mixed-factorial ANOVA, with group (passive nicotine dose) as the between-subjects factor and time as the within-subjects factor, revealed main effects of group (F2,15 = 3.98, p < 0.05) and time (F10,15 = 3.88, p < 0.001) but no group × time interaction (F20,150 = 0.32, p > 0.05). The one-way ANOVA showed that total nicotine intake over the 12 sessions of intravenous nicotine self-administration was higher in animals that were previously exposed to 7 mg/m3 nicotine vapor during adolescence (F2,15 = 15.49, p < 0.0001; Fig. 4b). The Newman-Keuls post hoc test revealed that rats that were exposed to 7 mg/m3 nicotine vapor during adolescence exhibited higher intravenous nicotine intake in adulthood compared with the air-exposed group (p < 0.001).
Figure 4.
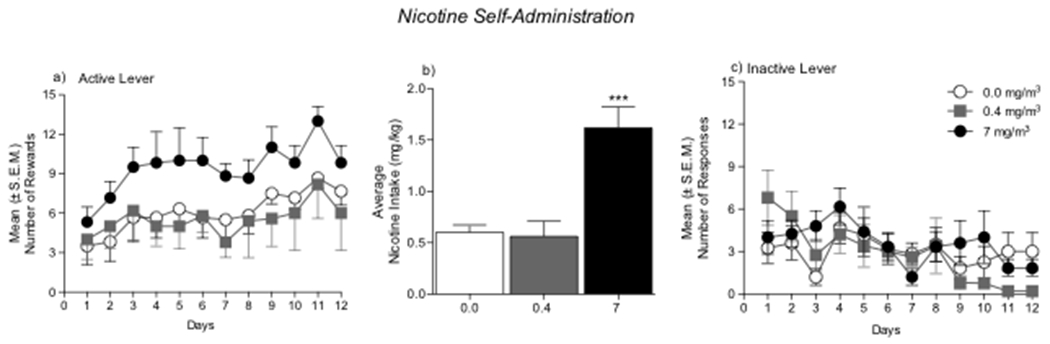
Intravenous nicotine self-administration in adulthood. (a) Number of nicotine rewards in 1 h sessions over 12 consecutive days. (b) Average number of nicotine rewards in air-exposed and nicotine-exposed rats. ***p < 0.001, compared with air-exposed rats. (c) Inactive lever responses. No differences were observed between groups.
Discussion
The present study evaluated the effects of nicotine vapor exposure on withdrawal-like states in adolescent rats and the propensity to initiate intravenous nicotine self-administration in adulthood. Chronic exposure to 0.4 mg/m3 nicotine vapor during adolescence increased somatic signs of nicotine withdrawal but was not associated with hyperalgesia, anxiety-like behavior, or the vulnerability to self-administer nicotine in adulthood. Chronic exposure to 7 mg/m3 nicotine vapor during adolescence increased somatic signs of nicotine withdrawal and increased hyperalgesia and the vulnerability to intravenously self-administer nicotine in adulthood.
We first tested whether intermittent exposure to low or high levels of nicotine vapor for 7 days in adolescent rats produces nicotine dependence. Withdrawal from nicotine induces somatic signs of withdrawal and negative affect in humans, leading to bradycardia, insomnia, gastrointestinal discomfort, increased appetite, weight gain, irritability, depressed mood, restlessness, anxiety, increased stress, and craving for tobacco (Hughes et al., 1991; Kallupi & George, 2017). In rodents, the cessation of nicotine administration or administration of nAChR antagonists in rats that are chronically exposed to nicotine results in the emergence of somatic signs of nicotine withdrawal, including abdominal constrictions, facial fasciculation, increased eye blinks, and ptosis (Malin et al., 1992; Malin et al., 1994; Hildebrand et al., 1997; Epping-Jordan et al., 1998). Our results showed that nicotine vapor exposure at levels that are sometimes observed in environments that enable a prolonged and high secondhand exposure to tobacco or e-cigarettes (Pellegrino et al., 2012; Evans & Hoffman, 2014; Schroeder & Hoffman, 2014) produced nicotine dependence, reflected by the emergence of spontaneous somatic signs of withdrawal. While there is a slight possibility that a fraction of nicotine was absorbed through the skin, toxicological studies have demonstrated that skin absorption represents less than 10% of the total dose received from combined skin and inhalation exposure (Hayes, 2010). These results are consistent with our previous findings (George et al., 2010) and further demonstrate that 1 week of exposure to nicotine vapor (0.4 mg/m3) is sufficient to produce mild signs of nicotine dependence in adolescent rats. We also observed a significant reduction of body weight and pronounced hyperalgesia in rats that were exposed to a high level of nicotine compared with rats that were exposed only to air or a low level of nicotine, suggesting that the high nicotine concentration resulted in a greater level of dependence, similar to observations in heavy smokers (Shiffman & Paty, 2006; Scheuermann et al., 2015). This finding is consistent with other studies that showed that high doses of nicotine reduce appetite and alter feeding patterns, typically resulting in a decrease in body weight (Grunberg, 1986; Grunberg et al., 1986; Blaha et al., 1998; Miyata et al., 1999; Bray, 2000; Zhang et al., 2001).
However, no significant alterations were seen in the activity levels following locomotor activity in the open field test. The withdrawal symptoms could have possibly interfered with measures of locomotor activity, like entries in the center zone and total distance traveled. Compared with nicotine-naive rats, nicotine-tolerant rats develop greater mechanical hyperagesia. Clinically, long-term smokers have a higher risk of suffering chronic pain (Ditre et al., 2011; Fishbain et al., 2013; Baiamonte et al., 2014; Cohen et al., 2015). Our data are consistent with our previous studies that reported the development of hyperalgesia in nicotine-dependent rats with extended access to nicotine self-administration (George et al., 2010; Cohen & George, 2013; Cohen et al., 2015). A few clinical studies have provided preliminary evidence that e-cigarettes may serve as a smoking cessation aid. These studies have reported the alleviation of craving and a decrease in nicotine withdrawal symptoms over time when these devices are used as substitutes for regular tobacco cigarettes (Dawkins et al., 2015; Lechner et al., 2015; Malas et al., 2016). However, more studies are needed to establish the long-term effects of the cessation of e-cigarette use. Unclear is whether e-cigarette use in adolescence produces dependence in humans. Several studies have investigated the potential differences of nicotine intake and relapse during adolescence and adulthood, using established rat models of nicotine self-administration (PMID: 17507913). Other studies have examined the rewarding properties of nicotine in adolescents compared with adults, using CPP and CTA procedures (Vastola et al, 2002; Belluzzi et al, 2004; Torrella et al, 2004; Wilmouth and Spear, 2004; Shram et al, 2006. Several investigators reported that adolescent rats may acquire nicotine self-administration faster than adult rats (Levin et al, 2003; Belluzzi et al, 2005; Chen et al, 2007), however, discrepancies related to the route of administration have been noticed. The present results demonstrate that 1 week of exposure to a wide range of concentrations of nicotine vapor (0.4–7 mg/m3) is sufficient to produce nicotine dependence. Such behavioral changes may contribute to the maintenance and progression of e-cigarette use through a mechanism of negative reinforcement. One limitation of our study is that we did not examine the blood nicotine levels. However, we have previously shown that this level of exposure leads to blood nicotine levels in the 10–100 ng/ml range and air nicotine concentration levels in the 1–10 mg/m3 range (George et al., 2010; Gilpin et al., 2014), condition that is very similar to human smoking. Moreover, other groups (Xue et al., 2018;, McGinn et al., 2016; Baiamonte et al., 2014) have replicated our original findings, showing that the 7 mg/m3 with 10LPM of nicotine vapor produced nicotine dependence with somatic signs and hyperalgesia similar to other animal models (minipump, intravenous self-administration) associated with blood nicotine level in the 10–100 ng/ml range. The present study was performed in male adolescent rats only, further studies in females and human studies are needed to clarify the effect of e-cigarette use on nicotine dependence in adolescence.
In a previous study, we found that chronic intermittent nicotine vapor inhalation produced escalation of nicotine self-administration and physical dependence in adult rats (Gilpin et al., 2014). However, the long-term consequences of adolescent exposure to nicotine vapor on intravenous self-administration in adulthood, was not previously investigated. The present results showed that exposure to high levels of nicotine (7 mg/m3) during adolescence facilitated the acquisition of intravenous nicotine self-administration in adulthood compared with rats that were exposed only to air or low nicotine levels. These results suggest that adolescent exposure to high-dose nicotine can either increase the positive reinforcing effects of nicotine or increase the negative reinforcing effects of withdrawal.
In summary, the present study demonstrated that chronic exposure to nicotine vapor in adolescence produced a withdrawal-like state and facilitated the acquisition of intravenous nicotine self-administration in adulthood. Secondhand exposure to e-cigarettes that contain high levels of nicotine may have long-term consequences on the vulnerability to develop nicotine dependence and the motivation for nicotine. Future animal studies may help elucidate the neurobiological changes that are associated with secondhand exposure to e-cigarettes.
Acknowledgements
The authors thank Michael Arends for proofreading the manuscript.
Funding and Disclosure
This work was supported by the National Institute on Drug Abuse (grant no. DA036691 to O.G.), National Institute on Alcohol Abuse and Alcoholism (grant no. AA022977 to O.G.), TRDRP (grant no. 27IR-0047 to O.G.) and Pearson Center for Alcoholism and Addiction Research.
The authors declare no competing financial interests.
References
- 1.American Psychiatric Association Baiamonte, B.A., Valenza M, Roltsch EA, Whitaker AM, Baynes BB, Sabino V & Gilpin NW (2014) Nicotine dependence produces hyperalgesia: role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA). Neuropharmacology, 77, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaha V, Yang ZJ, Meguid M, Chai JK & Zadak Z (1998) Systemic nicotine administration suppresses food intake via reduced meal sizes in both male and female rats. Acta Medica (Hradec Kralove), 41, 167–173. [PubMed] [Google Scholar]
- 3.Bray GA (2000) Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord, 24 Suppl 2, S8–17. [DOI] [PubMed] [Google Scholar]
- 4.Bruijnzeel AW (2016) Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caine SB & Koob GF (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science, 260, 1814–1816. [DOI] [PubMed] [Google Scholar]
- 6.Cohen A & George O (2013) Animal models of nicotine exposure: relevance to second-hand smoking, electronic cigarette use, and compulsive smoking. Front Psychiatry, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF & George O (2015) Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol, 20, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ & Sobczak A (2014) Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res, 16, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawkins L, Kimber C, Puwanesarasa Y & Soar K (2015) First- versus second-generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction, 110, 669–677. [DOI] [PubMed] [Google Scholar]
- 10.Ditre JW, Brandon TH, Zale EL & Meagher MM (2011) Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull, 137, 1065–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol, 20, 441–462. [DOI] [PubMed] [Google Scholar]
- 12.Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G & Koob GF (2012) Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology, 62, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epping-Jordan MP, Watkins SS, Koob GF & Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature, 393, 76–79. [DOI] [PubMed] [Google Scholar]
- 14.Evans SE & Hoffman AC (2014) Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control, 23 Suppl 2, ii23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishbain DA, Lewis JE, Bruns D, Meyer LJ, Gao J & Disorbio JM (2013) The prevalence of smokers within chronic pain patients and highest pain levels versus comparison groups. Pain Med, 14, 403–416. [DOI] [PubMed] [Google Scholar]
- 16.George O, Grieder TE, Cole M & Koob GF (2010) Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacol Biochem Behav, 96, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT & George O (2014) Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol, 19, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunberg NE (1986) Nicotine as a psychoactive drug: appetite regulation. Psychopharmacol Bull, 22, 875–881. [PubMed] [Google Scholar]
- 19.Grunberg NE, Bowen DJ & Winders SE (1986) Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl), 90, 101–105. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand BE, Nomikos GG, Bondjers C, Nisell M & Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology (Berl), 129, 348–356. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JR, Gust SW, Skoog K, Keenan RM & Fenwick JW (1991) Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry, 48, 52–59. [DOI] [PubMed] [Google Scholar]
- 22.Kallupi M & George O (2017) Nicotine Vapor Method to Induce Nicotine Dependence in Rodents. Curr Protoc Neurosci, 80, 8 41 41–48 41 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechner WV, Meier E, Wiener JL, Grant DM, Gilmore J, Judah MR, Mills AC & Wagener TL (2015) The comparative efficacy of first- versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction, 110, 862–867. [DOI] [PubMed] [Google Scholar]
- 24.Malas M, van der Tempel J, Schwartz R, Minichiello A, Lightfoot C, Noormohamed A, Andrews J, Zawertailo L & Ferrence R (2016) Electronic Cigarettes for Smoking Cessation: A Systematic Review. Nicotine Tob Res, 18, 1926–1936. [DOI] [PubMed] [Google Scholar]
- 25.Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL & Wilson OB (1994) The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl), 115, 180–184. [DOI] [PubMed] [Google Scholar]
- 26.Malin DH, Lake JR, Carter VA, Cunningham JS & Wilson OB (1993) Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl), 112, 339–342. [DOI] [PubMed] [Google Scholar]
- 27.Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS & Wilson OB (1992) Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav, 43, 779–784. [DOI] [PubMed] [Google Scholar]
- 28.Miyata G, Meguid MM, Fetissov SO, Torelli GF & Kim HJ (1999) Nicotine’s effect on hypothalamic neurotransmitters and appetite regulation. Surgery, 126, 255–263. [PubMed] [Google Scholar]
- 29.Pellegrino RM, Tinghino B, Mangiaracina G, Marani A, Vitali M, Protano C, Osborn JF & Cattaruzza MS (2012) Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig, 24, 279–288. [PubMed] [Google Scholar]
- 30.Scheuermann TS, Nollen NL, Cox LS, Reitzel LR, Berg CJ, Guo H, Resnicow K & Ahluwalia JS (2015) Smoking dependence across the levels of cigarette smoking in a multiethnic sample. Addict Behav, 43, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroeder MJ & Hoffman AC (2014) Electronic cigarettes and nicotine clinical pharmacology. Tob Control, 23 Suppl 2, ii30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiffman S & Paty J (2006) Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychol, 115, 509–523. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman SM & Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl), 50, 35–39. [DOI] [PubMed] [Google Scholar]
- 34.Watkins SS, Koob GF & Markou A (2000) Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res, 2, 19–37. [DOI] [PubMed] [Google Scholar]
- 35.Xue S, Kallupi M, Zhou B, Smith LC, Miranda PO, George O & Janda KD (2018) An enzymatic advance in nicotine cessation therapy. Chem Commun (Camb), 54, 1686–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Meguid MM, Miyata G, Varma M & Fetissov SO (2001) Role of hypothalamic monoamines in nicotine-induced anorexia in menopausal rats. Surgery, 130, 133–142. [DOI] [PubMed] [Google Scholar]


