Abstract
BACKGROUND & AIMS:
We created and validated a clinical decision support tool (CDST) to predict outcomes of vedolizumab therapy for ulcerative colitis (UC).
METHODS:
We performed logistic regression analyses of data from the GEMINI 1 trial, from 620 patients with UC who received vedolizumab induction and maintenance therapy (derivation cohort), to identify factors associated with corticosteroid-free remission (full Mayo score of 2 or less, no subscore above 1). We used these factors to develop a model to predict outcomes of treatment, which we called the vedolizumab CDST. We evaluated the correlation between exposure and efficacy. We validated the CDST in using data from 199 patients treated with vedolizumab in routine practice in the United States from May 2014 through December 2017.
RESULTS:
Absence of exposure to a tumor necrosis factor (TNF) antagonist (+3 points), disease duration of 2 y or more (+3 points), baseline endoscopic activity (moderate vs severe) (+2 points), and baseline albumin concentration (+0.65 points per 1 g/L) were independently associated with corticosteroid-free remission during vedolizumab therapy. Patients in the derivation and validation cohorts were assigned to groups of low (CDST score, 26 points or less), intermediate (CDST score, 27–32 points), or high (CDST score, 33 points or more) probability of vedolizumab response. We observed a statistically significant linear relationship between probability group and efficacy (area under the receiver operating characteristic curve, 0.65), as well as drug exposure (P < .001) in the derivation cohort. In the validation cohort, a cutoff value of 26 points identified patients who did not respond to vedolizumab with high sensitivity (93%); only the low and intermediate probability groups benefited from reducing intervals of vedolizumab administration due to lack of response (P = .02). The vedolizumab CDST did not identify patients with corticosteroid-free remission during TNF antagonist therapy.
CONCLUSIONS:
We used data from a trial of patients with UC to develop a scoring system, called the CDST, which identified patients most likely to enter corticosteroid-free remission during vedolizumab therapy, but not anti-TNF therapy. We validated the vedolizumab CDST in a separate cohort of patients in clinical practice. The CDST identified patients most likely to benefited from reducing intervals of vedolizumab administration due to lack of initial response. ClinicalTrials.gov no: NCT00783718
Keywords: Prognostic Factor, Response to Treatment, Personalized Medicine, Biologic
In phase 3 randomized controlled trials, vedolizumab (VDZ) has been proven efficacious for achieving clinical remission, corticosteroid-free remission (CSFREM), and mucosal healing in ulcerative colitis (UC).1 In clinical practice, pooled rates for clinical response and remission by week 22 were 51% (95% confidence interval [CI], 43%–61%) and 30% (95% CI, 24%–36%), respectively.2 Studies have identified predictors of treatment outcomes for VDZ3; however, the optimal approach to integrating predictors into routine clinical practice is uncertain.
Waljee et al4 recently developed a machine learning algorithm for predicting CSFREM with VDZ in UC. This tool was limited by lack of external validation, need for 6 weeks of therapy before determining risk for treatment failure, and difficulty of bedside implementation. There is a need for well-validated, drug-specific, easy-to-use prediction models and clinical decision support tools (CDSTs) to help guide clinicians in the use of VDZ therapy for UC.
We addressed this gap by deriving a prediction model and CDST using the GEMINI 1 VDZ clinical trial dataset for the outcome of CSFREM. We explored correlations between measured VDZ exposure, rapidity in onset of action, and overall efficacy across predicted probability groups in the GEMINI 1 trial, and the CDST was subsequently validated in an external routine practice cohort of UC patients treated with VDZ. To confirm the drug-specific nature of this model, we assessed the performance of the CDST for predicting treatment outcomes in patients with UC treated with tumor necrosis factor (TNF) antagonist therapy in a similar routine clinical practice setting. Our intent was to create a CDST that will help clinicians optimize the use of VDZ therapy specifically for individual patients.
Materials and Methods
This study is reported according to the TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) statement and the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) statement.5,6 All authors had access to the study results and reviewed and approved the final manuscript.
Data Sources and Participants
Data from the GEMINI 1 trial were used to derive the prediction model and VDZ-CDST.1 Patients from GEMINI 1 trial (n = 620) were included if they had received VDZ induction therapy and were assigned to receive VDZ during maintenance therapy, irrespective of week 6 response status. Placebo-treated patients were excluded. Data from the Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases (VICTORY) Consortium cohort (VDZ: n = 199; TNF antagonist: n = 123) were used to externally validate the prediction model and VDZ-CDST (Supplementary Material).7
Outcome Definitions
The primary objective was to develop and validate a VDZ-specific prediction model and CDST for achieving CSFREM. Secondary objectives were to assess whether the VDZ-CDST was able to predict differences in measured VDZ exposure and onset of action (reductions in partial Mayo score and fecal calprotectin) within the GEMINI 1 trial derivation cohort and differences in colectomy rates and response to VDZ interval shortening within the VICTORY validation cohort. These secondary objectives were designed to explore the exposure-efficacy relationship for VDZ in UC (Supplementary Material).
CSFREM in the GEMINI 1 trial was defined as a full Mayo score of ≤2, with no subscore >1, and being off corticosteroids at 52 weeks. CSFREM in the VICTORY cohort was defined as achieving complete resolution of UC-related symptoms (rectal bleeding, urgency, stool frequency), a Mayo endoscopic subscore of 0 or 1, and being off corticosteroids at 26 weeks. Colectomy status was also assessed at 26 weeks. We chose 26 weeks as the time point for validation based on prior clinical observations that patients may need up to 26 weeks to achieve clinical remission and mucosal healing with VDZ. This time point was also judged to be the maximal acceptable duration for clinicians and patients to attempt a therapeutic trial of VDZ.7,8
Statistical Analysis
VDZ Model and CDST Derivation: GEMINI 1 Trial Cohort.
A multivariable logistic regression prediction model was built from the GEMINI 1 trial cohort data with CSFREM as the dependent variable. Baseline variables with P value <.15 on univariable analyses were included after assessment for collinearity, clinical importance, and interpretability. A backward model selection approach with a P value threshold of .15 for inclusion was used. Interaction terms were assessed individually and included in the final model if they had a P value of <.10 on both the univariable and multivariable analyses. A sensitivity analysis was performed replacing albumin with calculated individual-patient VDZ drug clearance profiles based on measured drug exposure to determine whether this modification better predicted CSFREM (Supplementary Material).9,10
The prediction model was transformed into a CDST, and prognostic scores were calculated by summing the points for all predictors present for each patient.11 The GEMINI 1 trial cohort subjects were split into quartiles using the VDZ-CDST, and cutoff points were determined for patients with low (lowest quartile of CSFREM rates), intermediate (middle 2 quartiles of CSFREM rates), or high (highest quartile of CSFREM rates) probability of achieving CSFREM with VDZ therapy. We assessed changes in fecal calprotectin, partial Mayo score, and differences in measured VDZ concentrations across probability groups throughout the 52-week GEMINI 1 trial study (exposure-efficacy relationship) (Supplementary Material).9,10
To control for type I error when comparing probability groups, a closed test procedure was used. Each of the pairwise comparisons was conducted at the .05 level, with no P value adjustments if the hypothesis “all probability groups equal” was first rejected at the .05 level. If the omnibus comparison was not significant at the .05 level, the subsequent comparisons were not made. Finally, the cutoff points were applied to the GEMINI 1 trial intention-to-treat (ITT) population to understand how the probability of achieving CSFREM with VDZ compared with study participants receiving placebo and to understand whether the prediction model was truly predicting outcomes with VDZ or only a patient’s inherent likelihood of responding to any therapy (ie, placebo) (Supplementary Material).
VDZ Model and CDST Validation: VDZ-Treated VICTORY Cohort.
External validation of the model and CDST was conducted in the VICTORY cohort. Discriminative ability was assessed by receiver operating characteristic curve analysis. Calibration of the model was evaluated using a calibration curve, a joint hypothesis test using a likelihood ratio, and the Hosmer-Lemeshow goodness-of-fit test. The overall performance of the models was evaluated with the Nagelkerke R2 and the Brier score (Supplementary Material).12
The sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of the VDZ-CDST scoring tool to identify patients with a low or high probability of achieving CSFREM or requiring colectomy were calculated after grouping patients into 3 groups according to predicted risk. In UC patients who underwent VDZ interval shortening for insufficient response (n = 28), we assessed whether response to VDZ interval shortening varied across VDZ-CDST predicted probability groups. The decision to undergo shortening was made by providers without prior knowledge of the VDZ-CDST scoring tool.
VDZ-CDST Drug-Specific Assessment: TNF Antagonist–Treated VICTORY Cohort.
The VDZ-CDST cutoff points were applied to patients treated with TNF antagonists in the VICTORY cohort. The proportion of TNF antagonist–treated UC patients who achieved CSFREM or required colectomy by week 26 across the VDZ-CDST–defined probability groups was compared to the proportion of VDZ-treated UC patients who achieved CSFREM or required colectomy by week 26 within defined probability groups.
Results
Patient Characteristics
Of the 437 VDZ-treated UC patients within the VICTORY cohort, 85 were excluded for missing baseline albumin values, and 153 were excluded because they had no endoscopic follow-up after starting VDZ. There were no significant differences in the VICTORY cohort patients included or excluded from the current analyses (Supplementary Table 1). Compared with the VICTORY cohort, participants in the GEMINI 1 trial derivation cohort had shorter disease duration (P < .01), were less often exposed to prior TNF antagonist therapy (P < .01), more often had severe disease on baseline endoscopy (P < .01), and had lower baseline albumin concentrations (P < .01) (Table 1).
Table 1.
Comparison of Demographics Between the GEMINI 1 and the VICTORY Cohorts
| GEMINI 1 Trial Cohort | VICTORY Cohort | ||
|---|---|---|---|
| Vedolizumab Derivation Cohort (n = 620) | Vedolizumab Validation Cohort (n = 199) | P value | |
| Female | 256 (41) | 104 (52) | <.01 |
| Smoker (never) | 380 (61) | 144 (72) | <.01 |
| Age, y | 40.1 ± 13 | 41.5 ± 17.3 | .23 |
| Body mass index, kg/m2 | 25.1 ± 5.6 | 25.3 ± 5.83 | .66 |
| Disease duration, y | 5.0 (2.3–9.1) | 6.0 (2–12) | <.01 |
| Disease duration <2 y | 120 (20) | 31 (16) | .25 |
| Prior hospitalization | 211 (34) | 55 (28) | .10 |
| Prior TNF antagonist exposure | 311 (50) | 135 (68) | <.01 |
| Prior TNF antagonist failure | 266 (43) | 117 (59) | <.01 |
| Extensive baseline disease | 308 (50) | 112 (56) | .12 |
| Baseline moderate endoscopic disease | 278 (45) | 126 (63) | <.01 |
| Baseline albumin, g/L | 37 ± 4.96 | 39.4 ± 5.41 | <.01 |
| Concomitant corticosteroids only | 226 (36) | 69 (35) | .67 |
| Concomitant IMMs only | 114 (18) | 36 (18) | 1.00 |
| Concomitant corticosteroids and IMMs | 99 (16) | 49 (25) | <.01 |
Values are n (%), mean ± SD, or median (interquartile range).
IMM, immunomodulator; TNF, tumor necrosis factor; VICTORY, Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases.
Variable Selection
Factors significantly (P < .05) associated with increased probability of achieving CSFREM with VDZ were disease duration (odds ratio [OR], 1.04 per year), no previous TNF antagonist exposure (OR, 1.84), no previous TNF antagonist failure (OR, 1.88), baseline endoscopic activity (moderate vs severe: OR, 1.57), baseline stool frequency (nonsevere [partial Mayo score 0–2] vs severe [partial Mayo score 3]: OR, 1.70), and baseline albumin (OR, 1.08) (Supplementary Table 2). Disease duration was transformed into a binary categorization (≥2 years vs <2 years), and previous TNF antagonist exposure was used instead of previous TNF antagonist failure for further model building. Baseline endoscopy was used as a metric for disease activity instead of stool frequency because it was considered more objective (Supplementary Material).
Model Building
Variables identified for potential inclusion were (1) disease duration (≥2 years vs <2 years), (2) previous TNF antagonist exposure (no vs yes), (3) baseline endoscopy (moderate vs severe), (4) baseline albumin (absolute value), and (5) sex (female vs male). Sex was observed to have a significant relationship to VDZ clearance (P < .001)13 and was deemed an indirect predictor of treatment outcomes through correlation with the known covariates of drug clearance: height and weight. Accordingly, sex was dropped from the model. Baseline characteristics for patients with short (<2 years) or longer (≥2 years) disease duration are described in Supplementary Table 3. Patients with longer disease duration were more likely to have been exposed to TNF antagonists before initiation of VDZ therapy (53% vs 38%). Despite this observation, patients with longer disease duration were more likely to respond to VDZ. This finding is of interest because multiple studies have documented a relatively poor prognosis for the use of biologics in patients who have previously had TNF antagonist failure. Therefore, this variable was thought to be a true predictor and was retained in the model. The other 3 variables have been previously identified in the literature and were deemed clinically and biologically relevant.3
The final model equation is as follows (Table 2):
Table 2.
Final Multivariable Model for Corticosteroid-Free Remission With VDZ After 52 Weeks of Therapy
| Variable | Odds ratio | 95% Cl |
|---|---|---|
| Previous TNF antagonist exposure (no vs yes) | 1.758 | 1.194–2.587 |
| Disease duration (≥2 y vs <2 y) | 1.689 | 1.018–2.803 |
| Baseline endoscopy (moderate vs severe) | 1.447 | 0.991–2.114 |
| Baseline albumin | 1.067 | 1.024–1.112 |
CI, confidence interval; TNF, tumor necrosis factor; VDZ, vedolizumab.
An example calculation is provided in the Supplementary Material.
Model Performance and Validation
The discrimination ability in the derivation cohort was 0.65 and on external validation it was 0.64 (95% CI, 0.50–0.77). During external validation the model explained approximately 20.8% of variation (Nagelkerke R2 = 0.10; Brier score 0.18, maximum Brier score 0.22). There was poor calibration (likelihood ratio χ2 = 16.18, df = 2, P < .001; Hosmer-Lemeshow goodness-of-fit χ2 = 17.99, df = 4, P < .01) (Supplementary Figure 1). The calibration slope, however, showed no evidence of overfitting, and the effects of the predictors were therefore similar in the development and validation cohorts.
Clinical Decision Support Tool
Performance of the CDST in the derivation cohort is described in Supplementary Tables 4–6. Among the ITT population of the GEMINI 1 trial, the difference in clinical remission rates between VDZ and placebo at week 6 was incrementally higher according to stratification into low probability (≤26 points; VDZ 8.5% vs placebo 3.3%; difference 5.2%), intermediate probability (>26 to 32 points; VDZ 16% vs placebo 4.7%; difference 11.3%), and high probability (>32 points) of response to VDZ (VDZ 25.4% vs placebo 8.8%; difference 16.6%). Using baseline week 0 values for CDST calculation in rerandomized week 6 responders, a similar incremental benefit in treatment effect size was seen for CSFREM at week 52 between the low probability (VDZ 28.2% vs placebo 10.5%; difference 17.7%), intermediate probability (VDZ 35.7% vs placebo 15.2%; difference 20.5%), and high probability (VDZ 55.4% vs placebo 17.1%; difference 38.3%) groups.
In the VICTORY cohort, a score of 26 had a high sensitivity (93%; 95% CI, 79%–98%) and a good negative likelihood ratio (0.50; 95% CI, 0.16–1.61) for identifying patients less likely to achieve CSFREM with VDZ (Figure 1, Table 3). Poor discriminative performance for the VDZ-CDST was observed in the TNF antagonist–treated patients from the VICTORY cohort (Figure 1, Supplementary Table 7). Rates of CSFREM were higher for VDZ-CDST–predicted high-probability VDZ-treated patients (32%) than for the VDZ-CDST–predicted high-probability TNF antagonist–treated patients (23%). Rates of CSFREM were lower for the VDZ-CDST–predicted low-probability VDZ-treated patients (12%) than for the VDZ-CDST–predicted low-probability TNF antagonist–treated patients (21%).
Figure 1.
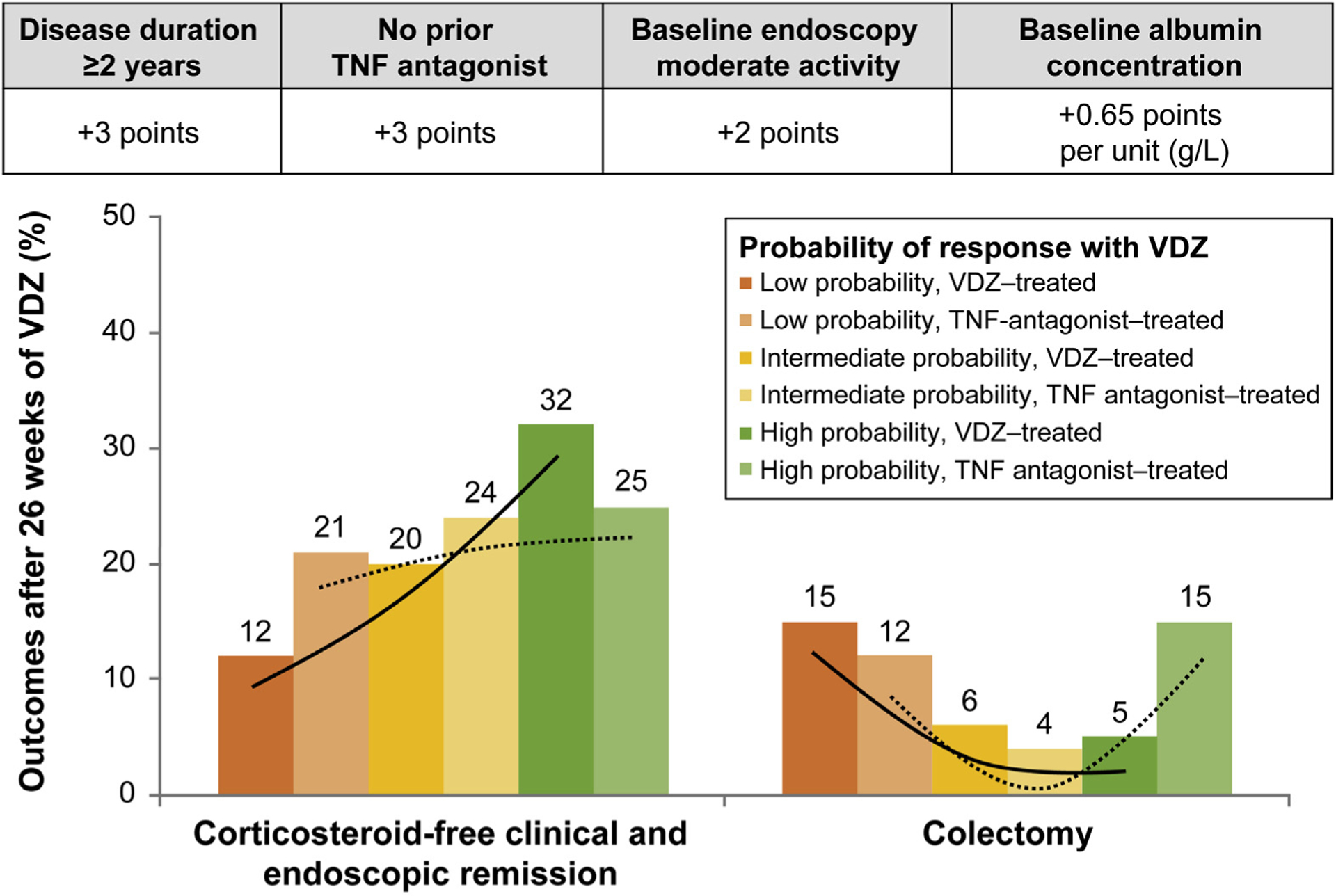
Prognostic CDST with stratified treatment outcomes in the VICTORY cohort.
Table 3.
Diagnostic Performance of Clinical Decision Support Tool in the VICTORY Cohort Among Vedolizumab-Treated Patients
| Sensitivity (95% Cl) (%) | Specificity (95% Cl) (%) | Positive likelihood ratio (95% Cl) | Negative likelihood ratio (95% Cl) | |
|---|---|---|---|---|
| 26 points | ||||
| Corticosteroid-free remissiona at 26 wk | 93 (79–98) | 15 (10–21) | 1.08 (0.97–1.21) | 0.50 (0.16–1.61) |
| Colectomy-free at 26 wk | 88 (83–92) | 29 (8–58) | 1.23 (0.88–1.73) | 0.42 (0.17–1.04) |
| 32 points | ||||
| Corticosteroid-free remissiona at 26 wk | 51 (35–67) | 68 (60–75) | 1.59 (1.09–2.31) | 0.72 (0.52–1.00) |
| Colectomy-free at 26 wk | 37 (30–44) | 71 (42–92) | 1.29 (0.55–3.01) | 0.89 (0.62–1.26) |
CI, confidence interval; VICTORY, Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases.
Remission defined as full Mayo score of ≤2 points with no subscore >1 point and being off steroids.
VDZ Drug Exposure-Efficacy Relationships
A statistically significant linear trend was observed for VDZ concentrations within the GEMINI 1 trial derivation cohort when stratified by the CDST (Figure 2, Supplementary Table 4). The percent reduction in fecal calprotectin at week 6 was 20% in the low-probability group compared with 49% and 56% in the intermediate- and high-probability groups. By week 30, patients in the low-probability group had achieved a 55% reduction in fecal calprotectin compared with baseline values. There were also statistically significant differences in change from baseline of partial Mayo score across 3 probability groups in the GEMINI 1 trial at all visits from week 2 to week 42, and week 52, based on closed test procedure (Figure 3, Supplementary Table 5).
Figure 2.
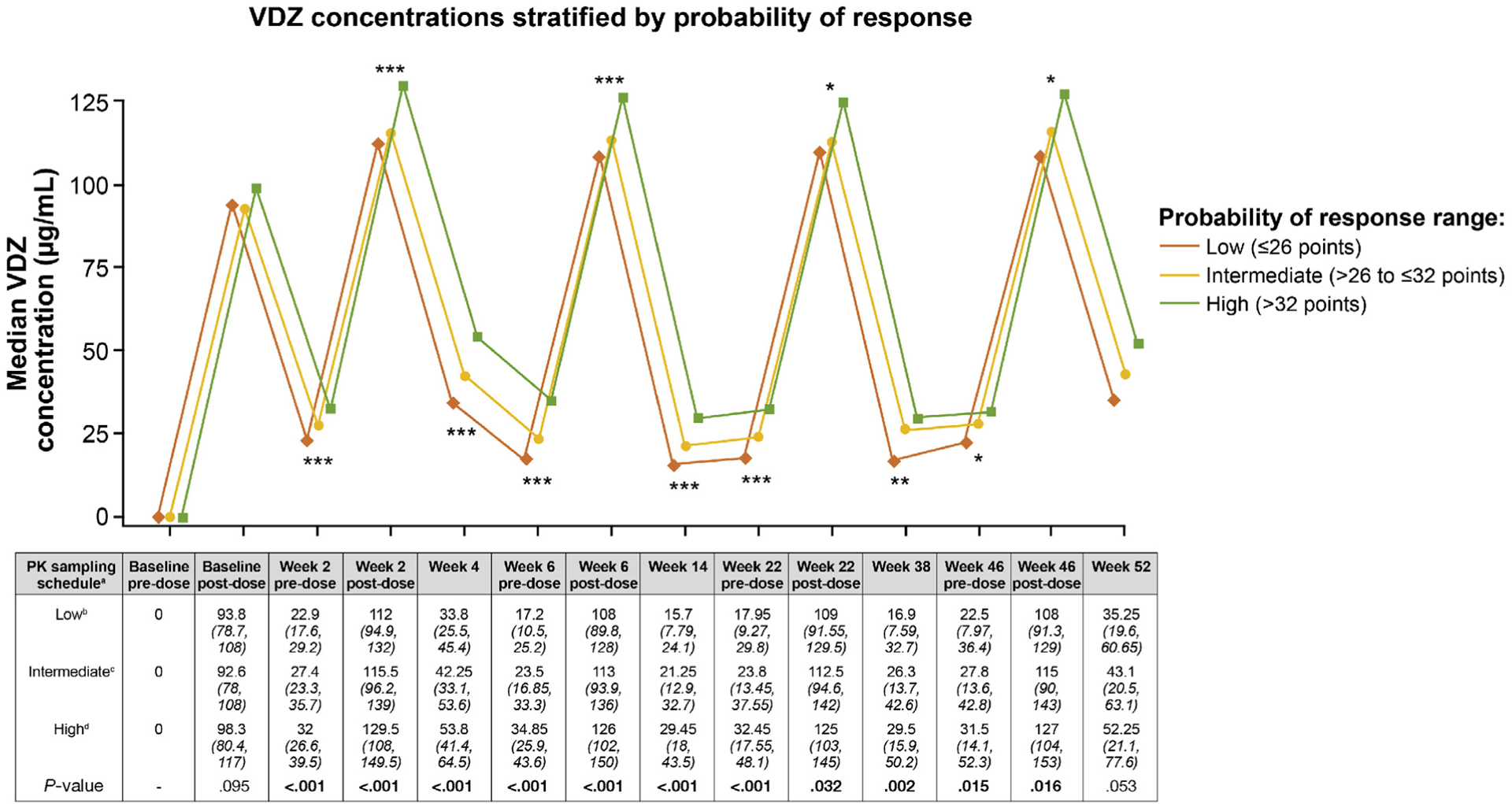
Prognostic CDST with stratified VDZ concentrations in the GEMINI 1 trial. Three-group statistical comparisons at each time point done using nonparametric testing (Kruskal-Wallis). *P < .05, **P < .01, ***P < .001. aAll values in the table are median VDZ concentration (interquartile range) (μg/mL); postdose concentration was measured 2 hours after dosing. bLow probability; ≤26 points in the CDST model at baseline. cIntermediate probability; >26 to ≤32 points in the CDST model at baseline. dHigh probability; >32 points in the CDST model at baseline. PK, pharmacokinetics.
Figure 3.
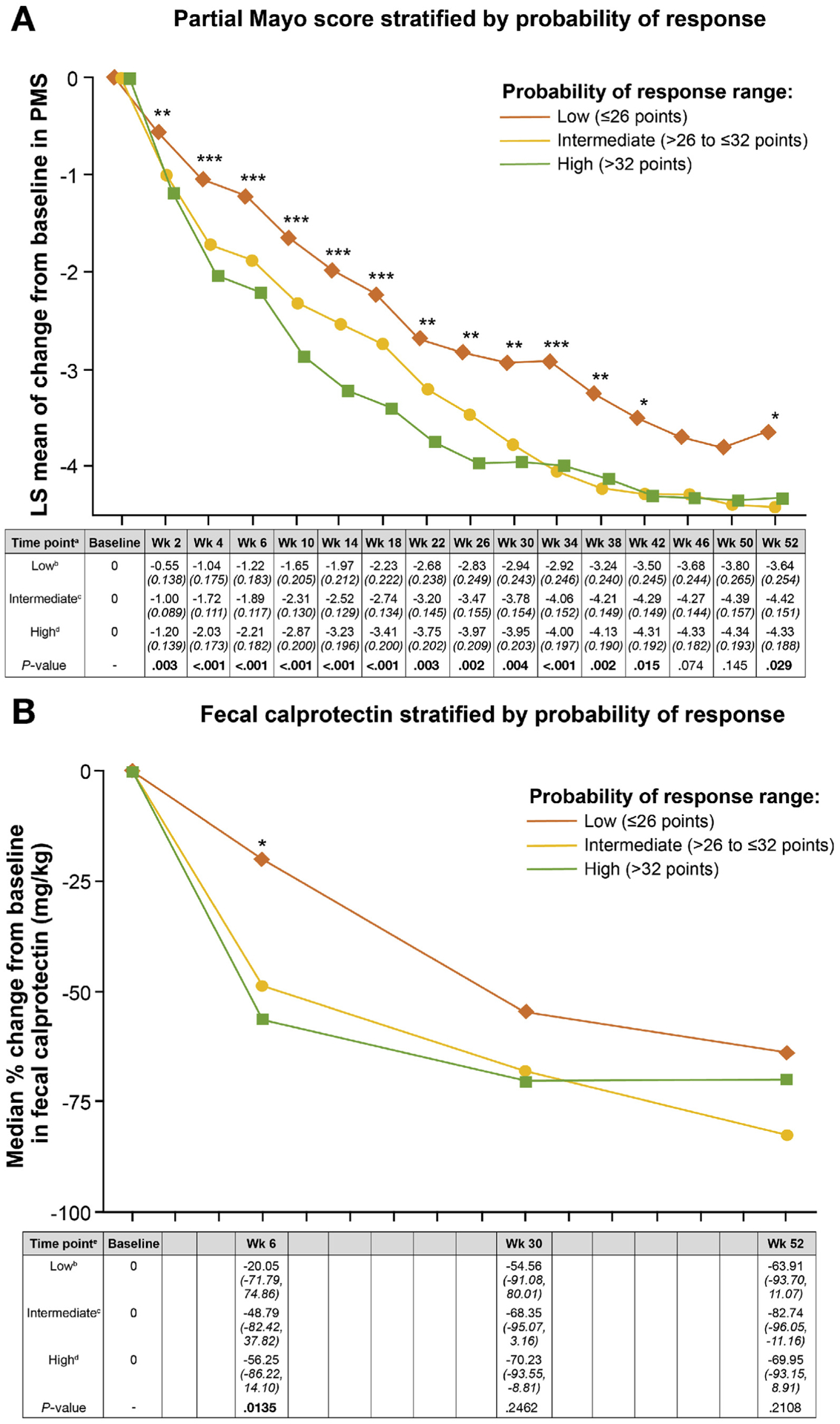
Changes in (A) partial Mayo score and (B) fecal calprotectin in the GEMINI 1 trial cohort stratified by CDST. Statistical comparisons at each time point for partial Mayo score was done by ANOVA with type I error controlled based on a closed test procedure; fecal calprotectin statistical analysis was done using nonparametric testing (Kruskal-Wallis). *P < .05, **P < .01, ***P < .001 for both. aAll values in the table are least-squares (LS) mean partial Mayo score (PMS) (with standard error in parentheses). bLow probability; ≤26 points in the CDST model at baseline. cIntermediate probability; >26 to ≤32 points in the CDST model at baseline. dHigh probability; >32 points in the CDST model at baseline. eAll values in the table are median percent change in fecal calprotectin (interquartile range).
In the VICTORY cohort, a clinical response (>50% reduction in symptom activity) to VDZ interval shortening was seen in 46% (n = 10 of 22) of patients classified as low or intermediate probability of response using the CDST. However, among patients undergoing VDZ interval shortening classified as high probability using the CDST (n = 6), none achieved a clinical response to VDZ interval shortening (P = .024).
Discussion
We derived and validated a VDZ-specific multivariable prediction model and CDST capable of predicting differences in measured VDZ drug exposure, onset of action, and VDZ treatment effectiveness, as well as identifying patients potentially most likely to benefit from VDZ interval shortening to optimize response. At a cutoff of 26 points the tool is sensitive for identifying patients who will not respond to VDZ. With increasing score there is increased confidence in expectation of achieving remission with VDZ in UC, with the greatest confidence achieved at a cutoff of 32 points. When applied to a TNF antagonist–treated observational cohort in a routine clinical practice setting, the VDZ-CDST was not able to predict differences in treatment effectiveness, confirming the drug-specific prediction of this VDZ-CDST.
Four predictors for CSFREM with VDZ were identified (1) previous TNF antagonist exposure, (2) baseline endoscopic activity, (3) baseline albumin, and (4) disease duration. Previous TNF antagonist exposure and severe disease have been shown to be consistent predictors of reduced effectiveness for VDZ in clinical practice across multiple cohorts.3 Albumin is the main determinant of VDZ clearance, and a correlation between VDZ exposure and efficacy has been observed in post hoc analyses of the GEMINI 1 trial.9,10 A novel observation was that longer disease duration was associated with improved effectiveness of VDZ. In the GEMINI 1 trial derivation cohort, patients with longer disease duration more often had prior exposure to TNF antagonists. It may have been anticipated that patients with longer disease duration would therefore be less likely to respond to VDZ; however, the opposite was seen. The biological rationale for this is unclear, although it could be speculated that chronic inflammation in those with longer disease duration results in continuous inflammatory signaling causing cytokine-based signaling pathways to become refractory to further stimuli, or that resident proinflammatory T cells are exhausted from chronic stimuli. In other chronic autoimmune conditions, T cell exhaustion has been associated with a good prognosis.14 This finding does not imply that clinicians should wait until a patient has longer disease duration to start VDZ, but rather that among patients with a chronic course, VDZ may have improved effectiveness.
We observed an exposure-efficacy relationship across model-derived prognostic groupings that may be related to differences in drug disposition. An exposure-efficacy relationship for VDZ induction has been observed in UC,10 and post hoc analyses of the GEMINI 1 trial have indicated that patients with higher VDZ trough concentrations had higher deep remission rates at 52 weeks.15 Despite these associations, clinicians are unable to predict at baseline who may benefit from early therapeutic drug monitoring with attempts at dose optimization through interval shortening. We observed a significant trend in increasing exposure-efficacy relationships across the low-, intermediate-, and high-probability groups with the VDZ-CDST. Furthermore, a clinical response to VDZ interval shortening was only observed in the low- to intermediate-probability group within the VICTORY cohort, presumably because these patients had lower trough concentrations than patients in the high-probability group. Although trough VDZ concentration testing was not routinely performed in the VICTORY cohort, these data help support the potential use of the VDZ-CDST to identify at baseline which patients are likely to have lower VDZ trough concentrations and are thus potentially most likely to benefit from early proactive therapeutic drug monitoring with VDZ interval shortening or upfront dose optimization strategies. The ongoing Vedolizumab Intravenous (IV) Dose Optimization in Ulcerative Colitis (ENTERPRET) trial (NCT03029143) will evaluate higher doses vs standard doses of VDZ and will help inform our understanding of the role of dose optimization in UC.
One of the main limitations with prior prediction model work is that it remains unclear whether identified predictors in those models are specific to the drug being assessed or are global markers of improved responsiveness to all biologics. In our study, we addressed this gap and observed that the VDZ-CDST was not able to predict treatment effectiveness with TNF antagonist therapy for UC patients in routine clinical practice. Among patients deemed to have low probability of response based on the VDZ-CDST, we observed a CSFREM rate of 21% among those treated with TNF antagonist therapy, compared with 12% for patients treated with VDZ therapy. In contrast, among patients deemed to have high probability of response based on the VDZ-CDST, we observed a CSFREM rate of 25% among those treated with TNF antagonist therapy, compared with 32% for patients treated with VDZ therapy. This would suggest that patients with a low probability of response might be more appropriately treated with TNF antagonist therapy, and those with a high probability are the best candidates for VDZ therapy. Among patients classified as having intermediate probability of response, the rates of CSFREM and colectomy were comparable between those who received TNF antagonist or VDZ therapy. For these patients, a careful discussion is warranted that should take into consideration the broader literature for comparative effectiveness and comparative safety when determining optimal treatment selection.
Our study has several strengths, including external validation in an independent, real-world dataset derived from multiple sites, ease of use in routine clinical practice, the ability to screen for patients who are less likely to achieve key outcomes (CSFREM) and more likely to require colectomy, and the drug-specific prediction of our CDST. There also are several limitations to our study. The lower bound of the confidence interval for the performance reached 0.5, suggesting that model discrimination may not be ideal. Further validation will therefore be needed to understand external validity on additional cohorts. Prospective validation will also be needed for the observation regarding interval-shortening benefits being limited to the low-probability cohort, ideally in a randomized, controlled trial setting. Caution should be taken when interpreting comparisons of subgroups to placebo recipients within the ITT population. The negative likelihood ratio of the VDZ-CDST predicts an approximate 15% reduction in effectiveness and posttest odds of achieving the outcome (CSFREM) in the low-probability group,16 but this is likely to be further modified by the ability of clinicians to achieve these outcomes (through enhanced monitoring and care pathways), irrespective of treatment assignment. Further work will need to be done to understand how care pathways integrated with therapeutic CDSTs affect overall probabilities of achieving key outcomes.
In conclusion, we have derived and externally validated a prediction model and CDST for achieving CSFREM with VDZ in UC. The VDZ-CDST was observed to have a high sensitivity for identifying patients with a latency of onset for response, who were less likely to achieve CSFREM with VDZ and were more likely to require colectomy while on VDZ. Furthermore, the CDST was observed to predict treatment effectiveness with VDZ but not TNF antagonist therapy, confirming its drug-specific use. We have made several key novel observations regarding VDZ exposure-efficacy relationships, and the use of this VDZ-CDST in the clinical setting will likely help to better guide the decision-making process for choosing VDZ as a therapeutic option and monitoring or adjusting therapy over time. To aid in the integration of this tool in clinical practice, an online tool is available to providers at: https://rme.arche.services/curriculum/a26dcdf0-00c3-4209-a67c-b4d4abe02f32. This learning health platform will allow the user to gain Continuing Medical Education credits for navigating through a search and learn educational platform which includes the CDST presented here. We anticipate this educational platform will help to streamline the integration of guidelines, evidence-based best practices, and all decision support tools as they become available over time.
Supplementary Material
What You Need to Know.
Background
Studies have identified factors that might be used to predict responses of patients with ulcerative colitis to treatment with vedolizumab, but these have not been systematically analyzed.
Findings
The authors developed and validated a tool, based on clinical and laboratory values, to identify patients most likely to enter corticosteroid-free remission during vedolizumab therapy. Scores associated with patient drug exposure, time until onset of action, and achievement of corticosteroid-free remission for vedolizumab but not tumor necrosis factor antagonist therapy.
Implications for patient care
This study has generated and validated an easy to use clinical decision support tool specific to vedolizumab to help clinicians optimize the treatment of individual patients with ulcerative colitis.
Funding
Parambir S. Dulai, and the work outlined in this manuscript, was supported by an American Gastroenterology Association Research Scholar Award.
Abbreviations used in this paper:
- CDST
clinical decision support tool
- CI
confidence interval
- CSFREM
corticosteroid-free remission
- ITT
intention to treat
- OR
odds ratio
- TNF
tumor necrosis factor
- UC
ulcerative colitis
- VDZ
vedolizumab
Footnotes
Conflicts of interest
These authors disclose the following: Parambir S. Dulai and the University of California, San Diego hold a provisional patent for the prediction modeling outlined herein. Parambir S. Dulai reports consulting for Takeda, Janssen, Pfizer, and AbbVie; travel support from Takeda and Janssen; and grant support from Takeda, Pfizer, Janssen, and AbbVie. Siddharth Singh reports research support from AbbVie and Pfizer; consulting for Pfizer, AbbVie, Takeda, and AMAG Pharmaceuticals; and career development awards from the American College of Gastroenterology and Crohn’s and Colitis Foundation. Niels Vande Casteele reports consulting for Takeda and Janssen. Joseph Meserve reports travel support from Takeda. Jenna L. Koliani-Pace reports travel support from Takeda. Brigid S. Boland reports research support from Takeda and Janssen; and consulting for AbbVie and Prometheus. John T. Chang reports grant support from Takeda. David Faleck reports travel support from Takeda. Robert Hirten reports speaking or advisory boards for Takeda and Janssen. Ryan Ungaro reports service as a consultant or advisory board member for Takeda, Pfizer, and Janssen. Dana Lukin reports consulting for AbbVie, Janssen, and Salix. Keith Sultan reports speaking engagement for AbbVie; research support for Takeda, Celgene, Hoffmann-La Roche, Gilead, and Pfizer. David Hudesman reports consulting for AbbVie, Takeda, Janssen, and Pfizer. Shannon Chang reports consulting for Oshi. Eugenia Shmidt reports travel support from Takeda. Arun Swaminath reports fellowship support from Janssen, AbbVie, and Pfizer; and grant support from Pfizer. Maria Rosario reports being an employee of Takeda Pharmaceuticals U.S.A., Inc. Vipul Jairath reports consulting fees from AbbVie, Janssen, Takeda, Sandoz, Ferring, Pfizer, GlaxoSmithKline, Robarts Clinical Trials, Eli Lilly, and Arena; and speaker fees from Takeda, Ferring, Janssen, and Shire. Leonardo Guizzetti reports being an employee of Robarts Clinical Trials. Brian G. Feagan reports grant support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Roche, Genentech, Johnson & Johnson, Janssen, Millennium, Pfizer, Receptos, Tillotts, and UCB; service as a consultant or advisory board member for AbbVie, ActoGeniX, Akros, Albireo, Amgen, AstraZeneca, Avaxia Biologics, Avir Pharma, Baxter Healthcare Corp, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, enGene, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, GiCare Pharma, Gilead, Given Imaging, GlaxoSmithKline, Inception IBD Inc, Ironwood Pharmaceuticals, Johnson & Johnson, Janssen, Japan Tobacco, Kyowa Hakko Kirin Co Ltd, Lexicon, Lilly, Lycera Biotech, Merck, Mesoblast Ltd, Millennium, Nektar, Nestlé, Novartis, Novo Nordisk, Pfizer, Prometheus Therapeutics & Diagnostics, Protagonist, Receptos, Salix, Shire, Sigmoid Pharma, Synergy Pharmaceuticals Inc, Takeda, Teva Pharmaceutical Industries Ltd, TiGenix, Tillotts, UCB, Vertex Pharmaceuticals, VHsquared Ltd, Warner Chilcott, Wyeth, Zealand Pharma, and Zyngenia. Corey A. Siegel reports service as a consultant or advisory board member for AbbVie, Amgen, Celgene, Lilly, Janssen, Sandoz, Pfizer, Prometheus, Sebela, and Takeda; speaker for AbbVie, Janssen, Pfizer, and Takeda; grant support from the Crohn’s and Colitis Foundation, AHRQ (1R01HS021747–01), AbbVie, Janssen, Pfizer, and Takeda; intellectual property for MiTest Health, LLC, and ColonaryConcepts, LLC; and equity interest for MiTest Health and Colonary Concepts. Bo Shen reports consulting for Janssen, Salix, AbbVie, Takeda, Theravance, and Robarts Clinical Trials. Sunanda Kane reports consulting for AbbVie, Merck, Spherix Health, Seres Pharmaceuticals, and Samsung Bioepis. Edward V. Loftus Jr reports consulting for Janssen, Takeda, AbbVie, UCB, Amgen, Pfizer, Salix, Mesoblast, Eli Lilly, Celgene, and CVS Caremark; and research support from Janssen, Takeda, AbbVie, UCB, Amgen, Pfizer, Genentech, Gilead, Receptos, Celgene, MedImmune, Seres Therapeutics, and Robarts Clinical Trials. William J. Sandborn reports personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Novo Nordisk, Mesoblast Inc, Shire, Ardelyx Inc, Actavis, Seattle Genetics, MedImmune (AstraZeneca), ActoGeniX NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc, Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adheron Therapeutics, Immune Pharmaceuticals, Celgene, and Arena Pharmaceuticals; personal fees from Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, and University of Western Ontario (owner of Robarts Clinical Trials); grants and personal fees from Prometheus Laboratories, AbbVie, Gilead Sciences, Boehringer Ingelheim, Amgen, Takeda, Atlantic Pharmaceuticals, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Pfizer, Nutrition Science Partners, Receptos, and Amgen; grants, personal fees, and nonfinancial support from Janssen; and grants from the Broad Foundation, American College of Gastroenterology, and Exact Sciences. Bruce E. Sands reports consulting/advisory board or honoraria from 4D Pharma, AbbVie, Allergan Sales, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Capella BioScience, Celgene, EnGene, Ferring, Gilead, Janssen, Lilly, Lyndra, MedImmune, Oppilan Pharma, Otsuka, Palatin Technologies, Pfizer, Progenity, Rheos Pharmaceuticals, Seres Therapeutics, Synergy Pharmaceuticals, Takeda, Target PharmaSolutions, Theravance Biopharma R&D, Inc., TiGenix, Vivelix Pharmaceuticals, and WebMD. Jean-Frederic Colombel reports service as a consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co, Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc, Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag; speaker for AbbVie, Ferring, Takeda, and Celgene Corporation; stock options for Intestinal Biotech Development and Genefit; and research grants from AbbVie, Takeda, Janssen, and Janssen. Karen Lasch and Charlie Cao report being employees of Takeda Pharmaceuticals USA, Inc. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.02.010.
References
- 1.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 2.Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD-lessons from real-world experience: a systematic review and pooled analysis. J Crohns Colitis 2018;12:245–257. [DOI] [PubMed] [Google Scholar]
- 3.Barre A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:896–905. [DOI] [PubMed] [Google Scholar]
- 4.Waljee AK, Liu B, Sauder K, et al. Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther 2018;47:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55–63. [DOI] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY Consortium. Am J Gastroenterol 2016;111:1147–1155. [DOI] [PubMed] [Google Scholar]
- 8.Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY Consortium. Am J Gastroenterol 2018;113:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis 2017; 11:921–929. [DOI] [PubMed] [Google Scholar]
- 11.Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology 2009;136:1206–1214. [DOI] [PubMed] [Google Scholar]
- 12.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet 2017;56:1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinney EF, Lee JC, Jayne DR, et al. T-cell exhaustion, costimulation and clinical outcome in autoimmunity and infection. Nature 2015;523:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandborn W, Colombel JF, Panaccione R, et al. P-008 relationship between vedolizumab concentrations and deep remission in patients with moderately-to-severely active ulcerative colitis: a GEMINI 1 post hoc analysis. Am J Gastroenterol 2018;113:S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee S Simplifying likelihood ratios. J Gen Intern Med 2002; 17:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


