Abstract
Background:
Hnrnph1 is a validated quantitative trait gene for methamphetamine behavioral sensitivity that encodes for heterogeneous nuclear ribonucleoprotein H1 (hnRNP H1). This RNA-binding protein is involved in all stages of RNA metabolism that impacts mesocorticolimbic dopamine neurotransmission to influence addiction-related behavior.
Methods:
We characterized the alcohol behavioral phenotypes of mice heterozygous for a deletion in the first coding exon of Hnrnph1 (Hnrnph1+/−). We examined alcohol intake under both continuous- and limited-access procedures, as well as alcohol-induced place-conditioning. Follow-up studies examined genotypic differences in the psychomotor-activating and sedative-hypnotic effects of acute and repeated alcohol, and a behavioral test battery was employed to determine the effects of Hnrnph1 deletion on the manifestation of negative affect during alcohol withdrawal.
Results:
Relative to wild-type (WT) controls, Hnrnph1+/− males exhibited blunted intake of high alcohol concentrations under both drinking procedures. Hnrnph1 deletion did not impact the conditioned rewarding properties of low-dose alcohol, but reversed the conditioned place-aversion elicited by higher alcohol doses (2 and 4 g/kg), with more robust effects in male versus female mice. No genotypic differences were observed for alcohol-induced locomotor activity. Hnrnph1+/− mice exhibited a modest increase in sensitivity to alcohol’s sedative-hypnotic effects, but did not differ from WT mice with regard to tolerance to alcohol’s sedative-hypnotic effects or alcohol metabolism, Inconsistent effects of Hnrnph1 deletion were observed in models for withdrawal-induced negative affect.
Conclusions:
These data identify Hnrnph1 as a novel, male-selective, driver of alcohol consumption and high-dose alcohol aversion that is potentially relevant to the neurobiology of alcohol abuse and alcoholism.
Keywords: hnRNP H1, binge-drinking, place-preference, intoxication, negative affect, ethanol, dysphoria
1. INTRODUCTION
hnRNP H1 (heterogenous nuclear ribonucleoprotein H1) is an RNA-binding protein (RBP) that is ubiquitously expressed in brain (Lein et al., 2007) and can regulate all aspects of RNA metabolism, including pre-mRNA splicing through binding at specific intron sites, mRNA stability, and translational regulation via 5’UTR and 3’UTR binding and poly-adenylation control (e.g., Han et al., 2010; Dreyfuss et al., 2002; Schaub et al., 2007). hnRNP H family proteins (including hnRNP H1) are considered critical regulators of neuron and oligodendrocyte differentiation (Aranburu et al., 2006; Tiruchiinapalli et al., 2008). Congenic mice harboring Hnrnph1 polymorphisms associated with decreased methamphetamine sensitivity express a set of down-regulated genes involved in neurodevelopment, including a 1.5-fold decrease in the transcription factor Nurr1/Nr4a2 (Yazdani et al., 2015). While RBPs, including hnRNP H1, are localized to the nucleus, exposure to extracellular stimuli (e.g., stressors, neuronal activity, drugs) can cause their translocation to cytoplasm where they can be positioned to regulate local translation underlying activity-dependent synaptic plasticity (Fukuda et al., 2009; Guil et al., 2006; Markmiller et al., 2018; Wall et al., 2020; Zhang et al., 2012).
As reviewed elsewhere (Bryant and Yazdani, 2016), there is a growing appreciation that RBPs play a pivotal role in addiction-related synaptic plasticity. Using an unbiased, forward genetic and fine mapping approach, we positionally cloned and validated Hnrnph1 as a quantitative trait gene underlying sensitivity to the locomotor stimulant response to methamphetamine (Yazdani et al., 2015) that we subsequently showed is likely mediated by a set of four, 5’ UTR variants that cause decreased 5’ UTR usage and decreased hnRNP H protein expression (Ruan et al., 2020b). Homozygous deletion of Hnrnph1 is lethal (Yazdani et al., 2015), however, we subsequently showed that a heterozygous mutation in the first coding exon of Hnrnph1 also decreased the rewarding and reinforcing properties of methamphetamine (Ruan et al., 2020a). Hnrnph1 contributes to post-transcriptional processing of OPRM1, including translational repression (Song et al., 2012) and splicing (Xu et al., 2014). OPRM1 encodes the mu opioid receptor, which is the primary molecular target underlying the addictive and analgesic properties of opioid drugs (e.g., Matthes et al., 1996). Supporting a potential role for Hnrnph1 in substance use disorders, an intronic variant in HNRNPH1 was associated with the severity of heroin dependence and differential splicing of OPRM1 in humans (Xu et al., 2014). Further, mice with a small frameshift deletion within the first coding exon of Hnrnph1 (Hnrnph1+/) (Yazdani et al., 2015) self-administer less fentanyl than their wild-type (WT) counterparts, independent of any observable effects of gene deletion on fentanyl-induced antinociception or physiological dependence (Bryant et al., 2020).
Decades of evidence from both human and laboratory animal studies implicate OPRM1 polymorphisms in the etiology and treatment prognosis of alcoholism (see Berrentini 2016 for review). Moreover, a survey of 17 proteomic studies indicate an association between alcohol exposure and an increase in Hnrnph expression in the brains of laboratory rodents (Wang et al. 2011). To the best of our knowledge, the functional relevance of hnRNP H1 in alcohol drinking and dependence is unexplored. Thus, the present study characterized the effect of a heterozygous Hnrnph1 deletion on AUD-related behaviors. Both female and male Hnrnph1+/− mice showed reduced sensitivity to the locomotor stimulant response to methamphetamine (Yazdani et al., 2015), while only Hnrnph1+/− females showed reduced fentanyl-induced locomotion (Bryant et al., 2020). Thus, we compared Hnrnpnh1+/− versus +/+ mice of both sexes for alcohol-induced locomotor activity and sedative-hypnotic effects. Prior co-administration studies indicated common neural adaptations contribute to both methamphetamine and alcohol intake and conditioned reward (Fultz et al., 2017; Fultz and Szumlinski, 2019; Sern et al., 2020). Thus, we also tested for genotypic differences in alcohol consumption and conditioned reward. Finally, as the severity of alcohol withdrawal correlates with the motivation to drink, we compared Hnrnpnh1+/− versus +/+ mice in behavioral models for alcohol withdrawal-induced negative affect. These results identify select, sometimes sexually dimorphic, alcohol behavioral phenotypes that were modified by acute and repeated alcohol exposure in Hnrnph1 mutant mice.
2. MATERIALS AND METHODS
2.1. Subjects.
Hnrnph1+/− and their wild-type (WT; +/+) littermates were originally generated on an isogenic C57BL/6J background using TALENs targeting the first coding exon which induced a small deletion and frameshift mutation resulting in a premature stop codon (Yazdani et al., 2015). Hnrnph1+/− mice were maintained at UCSB by mating Hnrnph1+/− males from a colony established at UC Santa Barbara with C57BL/6J females purchased from The Jackson Laboratory (Sacramento, CA). At weaning, offspring were housed with same-sex littermates (a minimum of 2 mice per cage) and genotyped as detailed below. Behavioral testing commenced no earlier than PND 50, and the mice ranged in age between PND50 and PND100, with a vast majority of mice aged PND56–70 at the start of testing. At least one week prior to commencement of experimental testing, mice involved in the place-conditioning or withdrawal-induced anxiety studies were relocated to a colony room maintained on a 12:12 h light–dark cycle (lights on at 0700 h), while those involved in the alcohol-drinking studies were relocated to a colony room maintained on a 12:12 h reverse light cycle (lights off: 1000 h). Food and water were available ad libitum in the home cage. All procedures were approved by the UC Santa Barbara Animal Care and Use Committee and were conducted in strict accordance with National Institute of Health guidelines for the care and use of laboratory animals.
2.2. Genotyping.
Genomic DNA was extracted from tail clips obtained upon weaning and used in a PCR reaction with primers amplifying approximately 100 base pairs upstream and downstream of the TALENs binding domains, as detailed in prior reports (Bryant et al., 2020; Ruan et al., 2018,2020; Yazdani et al., 2015). After PCR, samples were mixed with a restriction enzyme cocktail overnight (BstNI), run on a 2% ethidium bromide Tris-borate-EDTA gel for 1.2 hrs, and imaged with ultraviolet light. TALENs-edited mice were identified by bands that were uncut by the restriction enzyme due to the loss of the restriction enzyme binding site (Yazdani et al., 2015).
2.3. Alcohol Drinking Procedures:
Female and male (n=8/genotype) mice were single-housed under a 12-h reverse cycle (lights off: 1000 h) for at least 7 days prior to commencing alcohol drinking procedures, which began with an examination for genotypic differences in alcohol intake under continuous-access, followed by limited-access, conditions in the same mice. For the continuous-access procedure, mice were presented in the home cage with 4 sipper tubes containing 0, 5, 10 or 20% alcohol (v/v) for 14 consecutive days. This continuous-access procedure was employed by our group previously to generate a within-subjects dose-response function for alcohol intake and preferences (e.g., Lominac et al., 2006). Bottles were weighed daily at the same time each day and the volume consumed from each sipper tube was calculated to determine intake (expressed as a function of body weight, determined weekly). Then, following a 3-day respite, the same mice underwent testing for alcohol intake under limited-access conditions. For the limited-access procedure, we employed our 4-bottle-choice version of the Drinking-in-the-Dark (DID) paradigm (e.g., Cozzoli et al., 2014; Lee et al., 2015, 2016), in which mice were presented with 4 sipper tubes containing 5, 10, 20 and 40% alcohol (v/v) for 2 h/day, beginning at 3 h into the dark phase of the circadian cycle. Limited-access drinking procedures were conducted for 10 consecutive days. Alcohol intake was determined immediately at the end of each 2-h session as described for the continuous-access procedures.
2.4. Alcohol-induced place-conditioning:
A separate cohort of experimentally naïve female and male mice (n>8/genotype) underwent alcohol-induced place-conditioning procedures to determine how Hnrnph1 deletion impacts the motivational valence of alcohol. The apparatus and procedures employed were similar to those described previously (e.g., Ary et al., 2012; Szumlinski et al., 2008). In brief, an unbiased place-conditioning procedure involving 8 pairings of alcohol (0.5, 1.0, 1.5, 2 or 4 g/kg) was conducted, with one compartment of a 2-compartment apparatus that differed in wall pattern (marbled vs. wood-paneled) and floor texture (rough vs. smooth). Random counterbalancing of the alcohol-paired side assignment was employed, irrespective of initial side preference. Conditioning commenced with a pre-conditioning test (PreTest) in which mice were allowed to explore both compartments for 15 min. This PreTest was conducted mid-day (around 1200 h) and mice were returned to the colony room. Then, in the morning (between 0900–1100 h), mice were injected IP with saline (vol=0.2 ml/10 g) and confined to one of the compartments for 15 min, with animals randomly assigned to the saline-conditioned compartment. In the afternoon (between 1630–1830 h), mice were injected IP with their assigned dose of alcohol and confined to the opposite compartment. Following 8 conditioning days, mice were tested for preference for the alcohol-paired compartment in the absence of any injection and the total time spent in the alcohol- versus saline-paired side (CPP Score) served to index the motivational valence of alcohol (Post-Test). Similar to the PreTest, the Post-Test was conducted mid-day, approximately 18 h following the last alcohol- conditioning session. The locomotor activity of the mice was recorded during each of the alcohol-conditioning sessions to index drug-induced psychomotor activity and changes in psychomotor activity with repeated alcohol treatment. Both the time spent in the two compartments and the distance traveled during conditioning were tracked using AnyMaze™ tracking software (Stoelting Co., Wood Dale, IL, USA).
2.5. Alcohol withdrawal-induced negative affect:
To examine the possibility that genotypic differences in the direction of the alcohol-conditioned response under high-dose alcohol place-conditioning procedures might reflect differential sensitivity to withdrawal-induced anxiety, another separate cohort of experimentally naïve female and male mice were injected IP, once daily, with 4 g/kg alcohol for a total of 8 days of injections (to mimic the place-conditioning injection regimen). Alcohol was injected in this study to control for the precise amount of alcohol exposure and to avoid the interpretational confounds associated with genotypic differences in alcohol drinking (see Results). The day following the last injection, mice were then tested for alcohol withdrawal-induced anxiety using a behavioral test battery consisting of light-dark shuttle-box, marble-burying and forced swim tests. These paradigms were selected as they are pharmacologically-validated models for negative affect and are consistently sensitive to the negative affective state produced by withdrawal from alcohol drinking in C57BL/6J mice (e.g., Lee et al., 2016, 2017a,b; 2018a,b). Recently, we observed inconsistent effects of Hnrnph1 deletion on indices of anxiety-like behavior expressed by male mice only (Bryant et al., 2020; but see Ruan et al., 2020). Thus, a subset of alcohol-naïve Hnrnph1+/+ and Hnrnph1−/− mice were included to further examine the potential genotype by sex interaction in basal affective state.
The light/dark shuttle box test indexes anxiety-like behaviors (Bourin & Hascoet, 2003; Crawley, 1985) and involves placing mice into a polycarbonate box (46cm long×24cm high×22cm wide), which is equally subdivided into a white, uncovered compartment and a black, covered compartment, separated by a central divider with an opening. Testing began with the mice on the dark side and the latency to enter the light side, number of light-side entries, and total time spent in the light side of the shuttle box were recorded during the 15-min trial using Any-maze™ tracking software (Stoelting Co., Wood Dale, IL).
The marble-burying test provides an additional index for anxiety-like behavior (Nicolas et al., 2006). Here, we placed 10 square glass pieces (2.5 cm2 × 1.25 cm tall) in the animals’ home cage, 5 at each end. The total number of marbles buried by at least 75% (i.e., at least ¾ of the marble was covered by bedding) at the end of the 20-min trial was recorded and video-recordings during the 20-min session were scored for the latency to begin burying and the total time spent burying by experimenters, who were blinded to the treatment of the mice.
Behavioral testing ended in the Porsolt forced swim test, in which each mouse was placed into an 11-cm diameter cylindrical container and the latency to first exhibit immobility (defined as no horizontal or vertical displacement of the animal’s center of gravity for ≥ 5 s), total time spent immobile, and the numbers of immobile episodes were monitored throughout the entire 6-min trial period using AnyMaze™ tracking software. All testing for negative affect was conducted during the animals’ circadian light phase.
2.6. Alcohol-induced intoxication and sedation.
In another cohort of alcohol-naïve mice, genotypic differences in the intoxicating and sedative properties of alcohol were assayed, respectively, using rotarod and regain of righting reflex procedures. The rotarod procedures were similar to those employed previously to examine genotypic differences in basal motor coordination (Ruan et al., 2020a) and commenced with successive training of mice to walk on a fixed speed (10 rpm) rotarod for 2 min. The following day, mice were tested for baseline rotarod performance over a 3-min period and then were injected with either 2 or 3 g/kg alcohol (the doses were reported previously by our group to induce motor in-coordination in mice; see Quadir et al., 2016, 2017) and, 15 min later, the average time to fall from the rotarod was determined in 3 successive 3-min tests. To examine the development of tolerance to alcohol’s intoxicating effects, mice were injected with their assigned dose of alcohol once daily (~1100 h) for 8 injections (i.e., the same number of injections as those employed in the place-conditioning study). Then, mice were assayed again for alcohol-induced changes in rotarod performance using procedures identical to the test for alcohol’s acute intoxicating effects. For the righting reflex study, a distinct cohort of alcohol-naïve female and male mice (n=7–9/sex/genotype) were injected acutely (~1100 h) with 4 g/kg and placed in an empty home cage. Upon observing the loss of righting reflex (defined as the inability to turn over and place all 4 paws on the floor of the cage; occurred within 1–2 min post-injection), mice were placed in a supine position and the latency to right themselves was determined using a stop-watch by an observer who was blinded to the genotype of the mice. The mice tested for righting reflex were then injected once daily (~1100 h), with 4 g/kg alcohol, followed by testing for withdrawal-induced anxiety as described earlier.
2.7. Alcohol pharmacokinetics:
To test for the potential relationship between genotypic differences in behavior and alcohol metabolism, mice were injected IP with 1.5 g/kg and blood was sampled from the submandibular vein at 5, 15, 30 and 60 min post-injection. Samples were analyzed by gas chromatography due to its effectiveness and accuracy in determining ethanol levels in various substances, including blood (Tiscione et al., 2011). Blood alcohol concentrations (BACs) were determined using a Shimadzu GC-2014 gas chromatography system (Shimadzu, Columbia, MD) and GC Solutions 2.10.00 software. Samples were diluted at 1:9 with non-bacteriostatic saline (50 μl of sample). Acetone and dichloromethane were used as the pre-solvents due to their lower boiling point versus ethanol. Each sample was tested within 1-week of blood collection to reduce the potential for alcohol evaporation during storage. The determination of ethanol concentration from each sample was derived using the standard curve equation determined prior to analyses of the samples. A new standard curve was formulated for each cohort of blood samples to ensure maximal accuracy. After the ethanol peak area was determined, the peak area was used to determine the ethanol concentration and subsequently the percent of ethanol in the blood (Campbell et al., 2019; Jimenez-Chavez et al., 2020).
2.8. Statistical Analyses:
The data were analyzing using multi-factorial ANOVAs, with sex and genotype included as between-subjects factors for all initial analyses. Failure to detect sex effects or interactions prompted removal of the factor and data re-analysis. Significant interactions were deconstructed along the relevant factor(s), followed by t-tests (when fewer than 3 comparisons were conducted), tests for simple main effects or LSD post-hoc tests, when appropriate. Alpha = 0.05 for all analyses.
3. RESULTS
3.1. Alcohol intake under continuous-access.
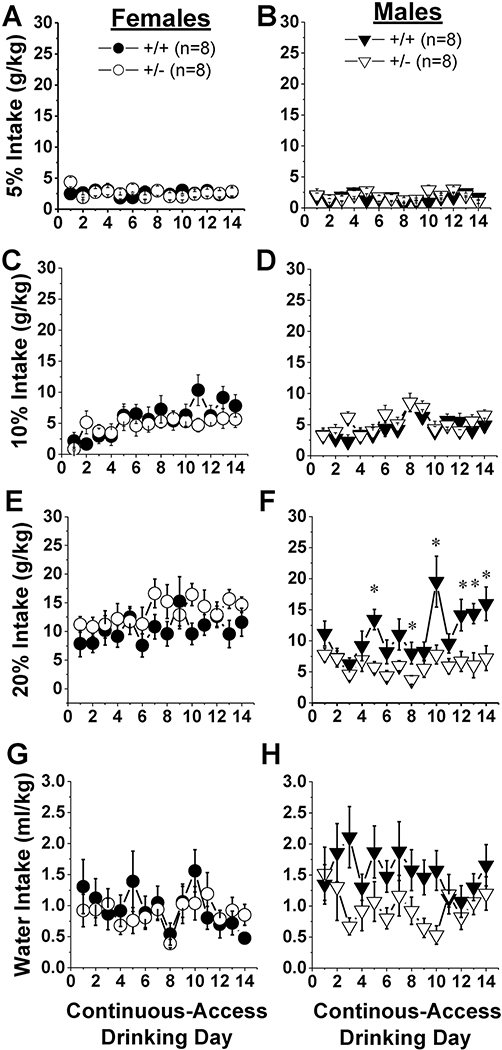
The effect of Hnrnph1 deletion on consumption of 5, 10 and 20% alcohol (v/v) under free-access conditions was sex-dependent (Fig.1) [Sex X Genotype X Concentration X Day: F(26,728)=1.81, p=0.008]. Interestingly, Hnrnph1 deletion did not produce any detectable effect on intake of alcohol at any concentration in female mice (Fig.1, left) [Dose effect: F(2,364)=29.36, p<0.0001; Dose X Day: p=0.08; Genotype effect and interactions: all p’s>0.20]. In contrast, the effect of gene deletion in males varied as a function of alcohol concentration (Fig.1, right) [Genotype X Dose X Day: F(26,364)=1.87, p=0.007]. Specifically, gene deletion did not influence the intake of 5 or 10% alcohol in male mice (Fig.1B,D) [for 5% alcohol, Genotype X Day ANOVA: all p’s>0.06; for 10% alcohol, Day effect: F(13,182)=5.92, p<0.0001; Genotype effect and interaction, p’s>0.25]. However, Hnrnph1 +/− males exhibited lower intake of the 20% solution, particularly during the 2nd week of testing (Fig.1F) [Genotype X Day: F(13,182)=2.45, p=0.004; post-hoc tests for simple main effects]. In contrast to alcohol intake, water intake declined in all groups over the course of testing [Day effect: F(13,364)=1.91, p=0.03], but we failed to observe any overt sex or genotypic difference in this regard (Fig.1G,H; Genotype or Sex effects/interactions: all p’s>0.07)
Figure 1: Male Hnrnph1+/− mice consume less high-concentration alcohol under continuous-access procedures.
Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) were offered 24-h, concurrent, access to 0, 5, 10 and 20% alcohol (v/v) in the home-cage over the course of a 14-day period. No genotypic difference was detected for water intake in female (A) or male (B) mice. Likewise, no genotypic difference was detected for intake of 5% alcohol in either sex (C,D). Furthermore, for females, there was no genotypic difference in the intake of 10% (E) or 20% (G) alcohol. In contrast, male +/− mice exhibited blunted intake of both 10% (F) and 20% alcohol (H). Data represent the means ± SEMs of the number of mice for each genotype (n) indicated in Panels A and B. *p<0.05 vs. +/+.
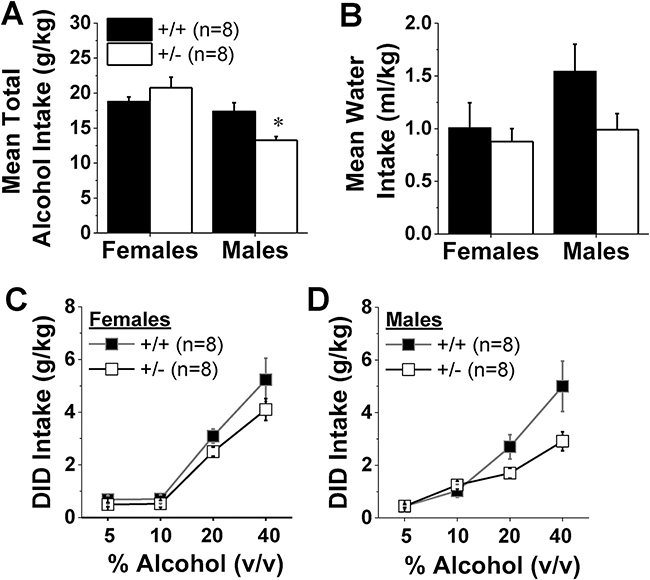
The sex difference in the effect of Hnrnph1 deletion on alcohol drinking was also apparent with respect to the total alcohol intake exhibited by the mice drinking under continuous-access conditions (Fig.2A) [Sex X Genotype interaction: F(1,31)=8.11, p=0.008]. Male Hnrnph1+/− mice exhibited lower alcohol intake than their male Hnrnph1+/+ counterparts [t(14)=2.99, p=0.01], while no genotypic difference was apparent in females (t-test: p=0.25). Although the average water intake exhibited by male Hnrnph1+/− mice was also lower than their respective male controls (Fig.2B), this difference was not statistically reliable (Sex X Genotype ANOVA: all p’s>0.10). Taken together, these data implicate Hnrnph1 in regulating alcohol intake under continuous-access procedures, with the Hnrnph1 mutation reducing alcohol consumption only in males.
Figure 2: Male Hnrnph1+/− mice consume less high-concentration alcohol under limited-access procedures.
(A) The data from Fig. 1 are expressed as the average total alcohol intake over the 14-day course of drinking under continuous-access procedures and highlight the male-selective effect of gene deletion on alcohol-drinking. (B) No significant genotypic difference was observed for the average water intake during continuous-access procedures in either female or male mice. In assessing alcohol intake under Drinking-in-the-Dark (DID) procedures (concurrent access to 10, 20 and 40% alcohol v/v for 2 h/day), no genotypic difference was detected in female mice (C), while male Hnrnph1+/− mice consumed less high-concentration alcohol than their +/+ counterparts (D). The data represent the means ± SEMs of the number of mice indicated in Panel A. *p<0.05 vs. +/+.
3.2. Binge Alcohol intake under limited-access.
As reported previously (e.g., Lee et al., 2016), alcohol intake under limited-access procedures was stable across the 14 days of testing [Day effect and interactions, F(13,364)<1.2, p’s>0.26] and mice of both genotypes consumed amounts of alcohol that are predicted to result in BAC’s in excess of the 0.08 g/dL criterion for binge-drinking (e.g., Rhodes et al. 2005; Lee et al., 2016). Although we did not detect a significant Genotype X Sex X Dose interaction (p=0.53) for alcohol intake under DID procedures, Hnrnph1 deletion reduced alcohol intake in this paradigm [Genotype effect: F(1,28)=7.24, p=0.01; Genotype X Dose interaction [F(3,84)=4.18, p=0.008], with a non-significant trend for a Sex X Dose interaction [F(3,84)=2.38, p=0.07]. Given the sex-specific effect of Hnrnph1 deletion on alcohol intake under continuous-access procedures (Fig.1; Fig.2A), we deconstructed the data for binge-intake along the sex factor for re-analysis of potential sex-specific effects and confirmed no effect of Hnrnph1 deletion on the dose-intake function for female mice drinking under DID procedures (Fig.2C) [Dose effect: F(3,42)=68.34, p<0.0001; Genotype effect: p=0.10; interaction: p=0.48]. In contrast, male Hnrnph1+/− mice tended to binge-drink less 20% and 40% alcohol than their WT counterparts, but the genotypic differences were statistically unreliable (Fig.2D) [Genotype X Dose: F(3,42)=3.67, p=0.02; post-hoc tests for simple main effects, p’s>0.05]. Thus, while not as robust as the results observed for alcohol intake under continuous-access procedures, these data nonetheless are consistent with a sex-specific effect of Hnrnph1 deletion also on binge alcohol-drinking.
3.3. Alcohol-induced locomotor activity.
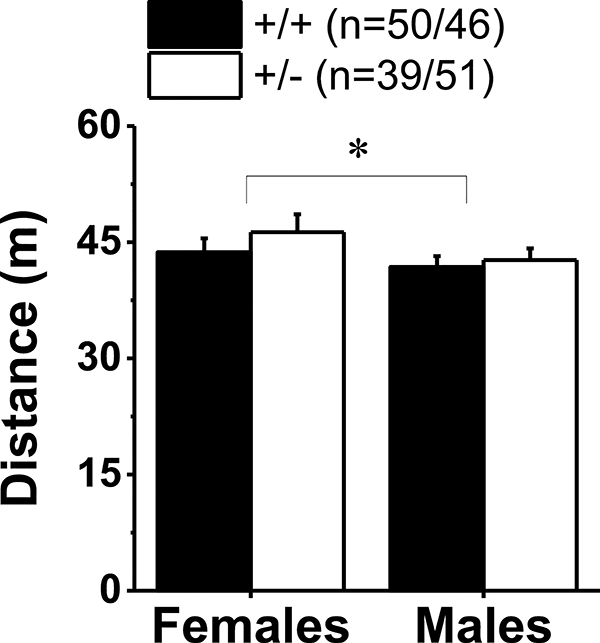
No genotypic difference was apparent with respect to distance traveled during the Pre-Test, when mice had access to both compartments of the place-conditioning apparatus, although females tended to locomote more than males (Fig.3) [Sex effect: F(1,185)=3.54, p=0.06; Genotype effect: F(1,185)=2.75, p=0.09; interaction, p=0.61]. However, no sex or genotype differences were apparent with respect to the locomotor response to an acute saline injection (Genotype X Sex ANOVA, p’s>0.10; data not shown).
Figure 3:
Hnrnph1 deletion does not alter spontaneous locomotion. Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) were allowed to habituate to the place-conditioning apparatus for 15 min. Females locomoted more than males, irrespective of genotype with no effect of gene deletion detected. The data represent the means ± SEMs of the number of mice indicated. *p < 0.05 vs. males.
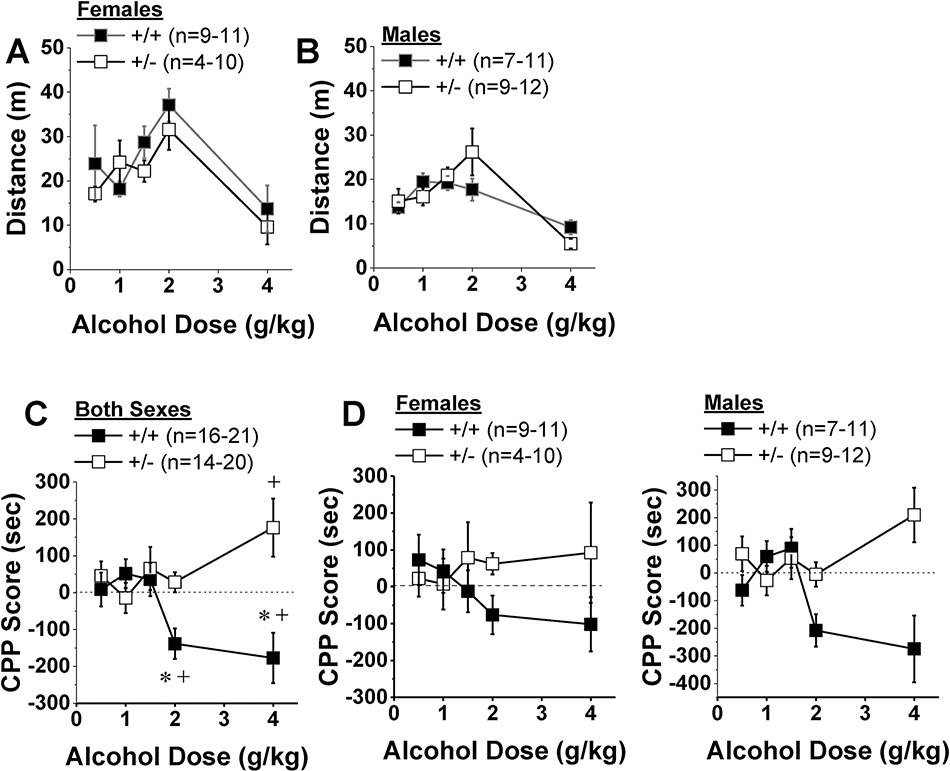
Analysis of the dose-response function for acute alcohol-induced locomotion (0.5, 1, 1.5, 2 and 4 g/kg) indicated a shift upwards in females versus males (Fig.4A,B) [Dose effect: F(4,185)=12.16, p<0.0001; Sex effect: F(1,185)=14.16, p<0.0001], but no genotypic difference (Genotype X Sex X Dose ANOVA, all other p’s>0.15). As no genotypic difference was noted for the acute locomotor response to alcohol, we next examined the effect of the Hnrnph1 mutation on the change in alcohol-induced locomotion during the course of place-conditioning by subtracting the distance traveled on Injection 1 from that on Injection 8. While the analysis of this dose-response function revealed a significant Genotype X Sex X Dose interaction [F(4,185)=2.89, p=0.02], deconstruction of the interaction along the sex factor failed to detect any dose or genotype effect in females (data not shown; Genotype X Dose ANOVA, p’s>0.10), and only a statistical trend for lower responding in male Hnrnph1 mutants [data not shown; Dose effect: F(1,96)=3.10, p=0.02; Genotype effect: p=0.08; interaction, p>0.20]. Thus, in contrast to both methamphetamine (Yazdani et al., 2015; Ruan et al., 2020) and fentanyl (Bryant et al. 2020), Hnrnph1 deletion does not significantly affect the acute or sensitized locomotor response to alcohol.
Figure 4:
Hnrnph1+/− mice do not exhibit alcohol-induced place-aversion. Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) underwent an alcohol-induced place-conditioning procedure involving 8 pairings of alcohol (0.5–4 g/kg) with a distinct compartment of a 2-compartment apparatus. While female mice (A) locomoted more than males (B) in response to alcohol injection during the first conditioning session, no genotypic differences were detected in the shape of the dose-response function for acute alcohol-induced locomotion. (C) When allowed free-access to both compartments following conditioning, we detected no sex difference in alcohol-induced place-conditioning. Thus, the data were collapsed across sexes to illustrate the large genotypic difference in the direction of the conditioned response between +/+ (aversion) and +/− mice (preference). The data represent the means ± SEMs of the number of mice indicated in Panels A and B. *p < 0.05 vs. +/+; +p < 0.05 vs. unpaired side (place-conditioning)
3.4. Alcohol-induced place-conditioning.
In contrast to sex-dependent genotypic differences in drinking, a robust genotypic difference was detected with respect to the dose-response function for alcohol-induced place-conditioning, irrespective of Sex [Genotype X Side X Dose: F(4,166)=4.45, p=0.002; 4-way interaction, p=0.24], with Hnrnph1+/+ mice exhibiting a strong alcohol-conditioned place-aversion at the two highest doses tested, whereas Hnrnph1+/− mice did not show any significant aversion at either dose of alcohol (Fig.4C) [for 2 g/kg, Side X Genotype: F(1,37)=11.63, p=0.002; for 4 g/kg, Side X Genotype: F(1,28)=11.60, p=0.002; for other doses, Side X Genotype ANOVAs, all p’s>0.17]. Further, direct comparisons of the time spent in the alcohol- versus saline-paired side during the conditioning test confirmed a place-aversion in Hnrnph1+/+ controls at both the 2 g/kg [F(1,18)=11.21, p=0.004] and 4 g/kg doses [F(1,15)=6.77, p=0.02]. In contrast, Hnrnph1+/−mutants were place-ambivalent at the 2 g/kg dose (p=0.31) and instead, exhibited a significant place-preference at the 4 g/kg dose [F(1,13)=4.96, p=0.04]. While the results of the ANOVA failed to indicate any sex effect or interactions, the prior Sex by Genotype interactions that we observed for alcohol-drinking prompted a comparison of the dose-response functions for alcohol-induced place-conditioning between female versus male mice. As illustrated in Fig.4D, no signs of high-dose alcohol-conditioned place-aversion were apparent in either Hnrnph1+/− male or female mice. However, the large genotypic difference in CPP scores observed when the data are collapsed across sex (Fig.4C) is driven primarily by the larger, less variable genotypic differences in conditioning of the male mice, including a more robust preference in +/− males and a more robust aversion in +/+ males (Fig.4D). These data indicate that Hnrnrph1 deletion reduces sensitivity to the aversive effects of high-dose alcohol, without impacting the rewarding properties of lower alcohol doses, which is a finding in line with our previous study indicating greater high-dose methamphetamine CPP compared to wild-types (Ruan et al., 2020a). However, in contrast to our previous methamphetamine study, the effect of Hnrnph1 deletion on alcohol’s motivational valence is more pronounced in males.
3.5. Alcohol Intoxication and Sedation.
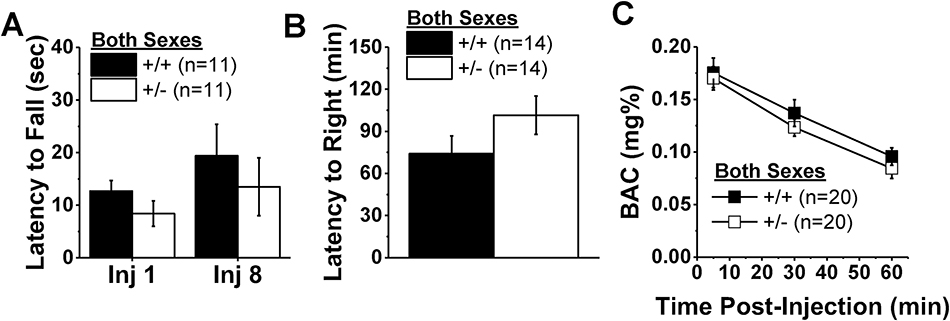
No genotype or sex differences were noted in the number of trials required for alcohol-naïve mice to remain on the fixed speed rotarod for 2 min during training (Hnrnph1+/+: 3.0 ± 0.0 trials, n=11; Hnrnph1+/−: 3.1 ± 0.1 trials, n=11; Genotype X Sex ANOVA, all p’s>0.30) and both alcohol-naïve Hnrnph1+/+ and +/− mice remained on the rotarod for the entire 3-min period prior to alcohol injection. Overall, the latency to fall from the rotarod appeared to be longer in mice injected repeatedly with 3 g/kg alcohol, with Hnrnrph+/+ mice exhibiting better rotarod performance than Hnrnph1+/− mice (Fig.5A). However, an analysis of these data failed to support the development of tolerance to alcohol’s intoxicating effects (no Injection effect or interactions, p’s>0.20), nor did it indicate any overall effects of, or interaction between, the Genotype and Sex factors (all p’s>0.20). Likewise, Hnrnph1+/+ controls tended to right themselves in a shorter period of time than Hnrnph1+/− mice following an acute injection with 4 g/kg alcohol (Fig.5B), but no significant genotype or sex differences were detected for this variable (Genotype X Sex ANOVA, all p’s>0.20). Thus, Hnrnph1 deletion does not reliably alter the intoxicating or sedative effects of higher alcohol doses.
Figure 5:
Hnrnph1 deletion does not alter alcohol intoxication, sedation or metabolism. (A) Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) did not differ with regard to time spent on a fixed speed rotarod following their first and eighth injection of 3 g/kg alcohol. (B) Similarly, no genotypic difference was detected for the time taken to right themselves following an acute injection of 4 g/kg alcohol. (C) No genotypic difference in plasma alcohol levels were detect over the course of a 1-h period following injection with 3 g/kg alcohol. The data represent the means ± SEMs of the number of mice indicated in each panel. *p < 0.05 vs. +/+.
3.6. Blood Alcohol Levels.
We next tested for the relationship between genotypic differences in alcohol intake and alcohol aversion to alcohol metabolism. As expected, BACs declined over time following injection with 1.5 g/kg alcohol [Time effect: F(2,32)=50.23, p<0.0001], but there were no genotype or sex differences in this regard (Fig.5C; Genotype X Sex X Time ANOVA, other p’s>0.40). These BAC data are consistent with no overt effect of gene deletion on the locomotor, intoxicating, and sedative properties of alcohol.
3.7. Alcohol Withdrawal-Induced Anxiety.
3.7.1. Light-Dark Box.
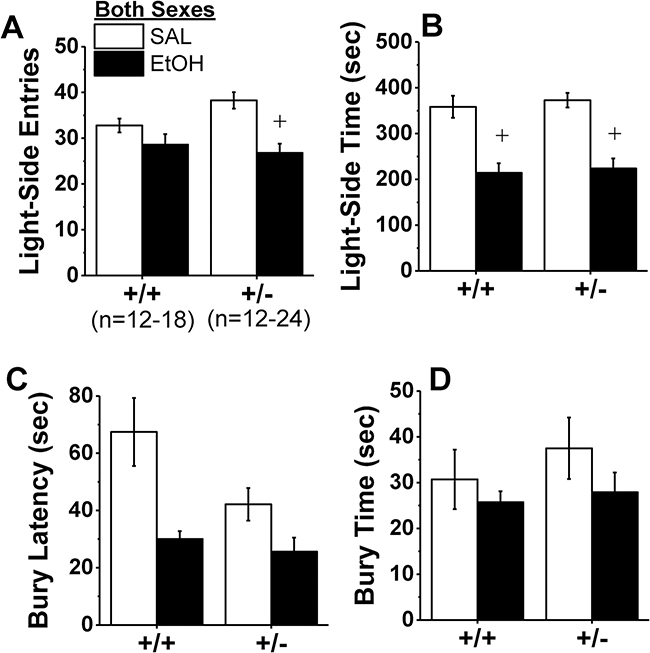
No group differences were observed in the latency to first enter the light side (data not shown; Genotype X Sex X Treatment ANOVA, all p’s>0.16). Overall, alcohol withdrawal reduced the number of light-side entries (Fig.6A) [Treatment effect: F(1,65)=16.14, p<0.0001], indicative of anxiety-like behavior. However, this alcohol withdrawal effect was more pronounced in Hnrnph1−/− mice as indicated by a significant Genotype X Treatment interaction [F(1,65)=3.96, p=0.05] and the results of within-genotype comparisons between alcohol- and saline-experienced mice [for +/+: t(28)=1.61, p=0.12; for +/−: t(34)=4.01, p<0.0001]. Alcohol withdrawal also reduced the time spent in the light side [Treatment effect: F(1,65)=44.46, p<0.0001]; however, the magnitude of this effect did not vary significantly with Genotype (Fig.6B; Genotype effects and interactions, p’s>0.50) or with Sex (Sex effects and interactions, p’s>0.08).
Figure 6:
Hnrnph1+/− deletion does not consistently alter baseline, or alcohol withdrawal-induced increases in anxiety-like behavior. Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) were injected repeatedly with 4 g/kg alcohol (EtOH) or saline (SAL) and then assayed for negative affect using a test battery including the light-dark shuttle-box and marble-burying tests. (A) +/− mice but not +/+ mice showed a significant withdrawal-induced decrease in the latency to enter the light side of a light-dark shuttle-box compared to their +/− control counterparts while (B) no genotypic difference was detected for the withdrawalinduced reduction in the time spent in the light side. In the marble-burying assay, alcohol withdrawal produced a nonsignificant reduction in the latency to begin burying marbles (C) and the time spent burying (D), but no genotypic differences were detected for either variable. The data represent the means ± SEMs of the number of mice indicated in Panel A. +p < 0.05 vs. SAL (alcohol withdrawal effect).
3.7.2. Marble-burying.
Compared to alcohol-naïve controls, mice in alcohol withdrawal exhibited a significantly shorter latency to begin marble-burying (Fig.6C) [Treatment effect: F(1,65)=10.22, p=0.002], and buried more marbles than alcohol-naïve controls (Fig.6D) [Treatment effect: F(1,65)=191.56, p<0.0001]. While, Hnrnph1−/− mice tended to exhibit a shorter latency to bury overall (Fig.6C; Genotype effect, p=0.07), neither Genotype nor Sex significantly interacted with the Treatment factor for this variable (Genotype effect, p=0.07; other p’s>0.20) or the number of marbles buried (Genotype X Sex X Treatment ANOVA, other p’s>0.16]. In contrast, no group differences were observed regarding the time spent burying (data not shown; Genotype X Sex X Treatment ANOVA, all p’s>0.30). Thus, while alcohol withdrawal-induced anxiety was also observed in the marble-burying test, the intensity of this state was not affected by Hnrnph1 deletion.
3.7.3. Forced Swim.
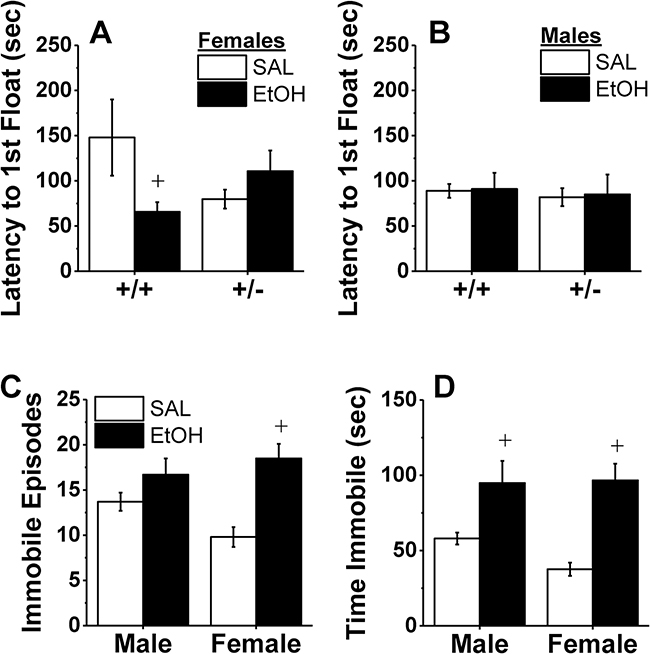
Analysis of the latency to first float in the forced swim test revealed a modest Genotype X Sex X Treatment interaction [F(1,65)=3.94, p=0.05]. Deconstruction of this interaction along the Sex factor indicated a significant Genotype X Treatment interaction only in female mice (Fig.7A) [F(1,30)=4.83, p=0.037], but not for males (Fig.7B) [p’s>0.65]. As illustrated in Fig. 7A, the interaction in females reflected a shorter latency to float in alcohol-withdrawn Hnrnph1+/+ mice versus their alcohol-naïve controls [t(17)=1.81, p=0.08], while no alcohol withdrawal effect was apparent in the mutant females (t-test, p=0.15). While it appeared that alcohol-naïve female +/− mice also exhibited a shorter latency to float than their +/+ counterparts, follow-up analyses failed to indicate any significant genotypic differences in either alcohol-naïve or -experienced females (t-tests, all p’s>0.10).
Figure 7.
Hnrnph1+/− deletion does not consistently alter baseline, or alcohol withdrawal-induced increases in depressivelike behavior. Wild-type mice (+/+) and their littermates with a heterozygous deletion of Hnrnph1 (+/−) were also assayed for genotypic differences in behavior in the forced swim test during early alcohol withdrawal. (A) A withdrawalinduced decrease in the latency to first float was detected in female +/+, but not female +/− mice, while no genotypic difference in float latency was observed in males (B). Alcohol withdrawal increased (C) the incidences of floating moreso in female versus male mice, while a withdrawal-induced increase in the time spent floating was comparable in male and female mice. No genotypic differences were detected for the number of floats or time spent floating, thus the data in Panels C and D are collapsed across genotype. The data represent the means ± SEMs of the number of mice indicated in Fig. 5, Panel A. +p < 0.05 vs. SAL (alcohol withdrawal effect).
A sex difference was detected for the withdrawal-induced increase in the number of immobile episodes [Sex X Treatment: F(1,65)=5.35, p=0.02], but there was no effect of Genotype (all p’s>0.07). Alcohol withdrawal doubled the number of immobile episodes exhibited by female mice [t(29)=4.79, p<0.0001], but had no effect on immobile episodes in males (Fig.7C; t-test, p=0.13). Alcohol-withdrawal also increased the time spent immobile (Fig.7D) [Treatment effect: F(1,65)=36.31, p<0.0001], but this effect did not vary with sex or genotype (all other p’s>0.13).
Taken altogether, these data for withdrawal-induced negative affect provide little evidence that hnRNP H1 plays a key role in regulating the basal affective state or alcohol withdrawal-induced changes therein.
4. Discussion
The present study sought to characterize the alcohol-related behavioral phenotype of mice with a heterozygous deletion of Hnrnph1. When allowed 24-h concurrent access to water and alcohol (10, 20 and 40%, v/v), male Hnrnph1+/− mice consumed less alcohol than WT controls, while no effect of gene deletion on drinking was apparent in female mice, with a similar pattern of results being observed under limited-access drinking procedures. The larger genotypic difference observed in male drinking in the DID versus continuous access procedure likely reflects the timing of alcohol presentation in DID, which coincides with the time of peak fluid intake during the circadian cycle (Gill et al., 1996; Rhodes et al. 2005). However, the fact that Hnrnph1 heterozygous males exhibited lower alcohol intake under two distinct drinking paradigms is consistent with the results of meta-analysis indicating a correlation between continuous-access alcohol drinking (when water is freely available) and DID drinking, both in WT and mutant mice (see Blednov et al., 2012) and suggests that Hnrnph1 deletion impacts a common underlying psychobiological mechanism to curb alcohol intake in males.
Interestingly, while no overt Sex by Genotype interaction was observed with respect to the effects of Hnrnph1 deletion on oral methamphetamine intake (Ruan et al., 2020b), for intake with the mu opioid receptor agonist fentanyl, only male Hnrnph1+/− mice exhibited lower operant self-administration (Bryant et al., 2020). Further, as reported previously for fentanyl intake (Bryant et al., 2020), the genotypic difference in alcohol intake observed herein was only observed at the higher alcohol concentrations tested. This result suggests that heterozygous hnrnpn1 deletion induces a sex-specific shift in both alcohol and opioid sensitivity. Although the mechanism by which male Hnrnph1+/− mice exhibit reduced alcohol or fentanyl consumption is unclear, a gene homolog, Hnrnph2, is located on the X chromosome in both rodents and humans. Mutations in both Hnrnph1 and Hnrpnh2 are linked to a rare, x-linked neurodevelopmental disorder in females (Bain et al. 2016; Pilch et al. 2018; Harmsen et al. 2019). If Hnrnph2 undergoes variable X-inactivation, heterozygous deletion of Hnrnph1 could induce sex-dependent changes in hnrnph2 expression to influence the self-administration of certain drugs of abuse by males. While cocaine (Reynolds et al., 2011) and opioids (Suder et al., 2009) are reported to alter hnRNP H2 expression in rodent brain, it remains to be determined if alcohol can also regulate hnRNPH 1/2 mRNA or protein expression. Alternatively, sex hormones are well-characterized to influence alcohol intake in both humans and laboratory rodents (for recent reviews, Finn, 2020; Verplaetse et al., 2020) and may contribute to sex differences in the effect of Hnrnph1 deletion on alcohol drinking. While the molecular mechanisms by which Hnrnph1 deletion exerts sex-specific effects on alcohol (and fentanyl) intake are unknown, the present findings provide novel evidence that Hnrnph1 function is necessary for alcohol drinking behavior in males.
We employed alcohol-induced place-conditioning procedures to relate genotypic differences in alcohol intake to the affective/motivational valence of alcohol, with the hypothesis that reduced alcohol intake by Hnrnph1+/− males would reflect either less sensitivity to the conditioned rewarding properties of alcohol (as reported for both low-dose methamphetamine- and low-dose fentanyl-induced place-conditioning; Bryant et al., 2020; Ruan et al., 2020a) and/or greater sensitivity to the conditioned aversive properties of the drug. While the results of the statistical analyses failed to indicate a significant sex difference in the effect of Hnrnph1 deletion on the dose-response function for alcohol-induced place-conditioning, a comparison across sexes suggests that the marked genotypic difference in the direction of the conditioned response to high-dose alcohol was driven, in large part, by male subjects. While the direction of the observed Hnrnph1+/− effect on place-conditioning is opposite our original hypothesis, these data nevertheless provide additional support for a male-selective effect of Hrnrnph1 deletion on measures of alcohol reward and argue instead that the low alcohol intake exhibited by male Hnrnph1+/− mice might reflect a compensation for their increased sensitivity to alcohol’s positive interoceptive effects.
It is interesting to note that, akin to the present findings for alcohol, Hnrnph1+/− mice also exhibited blunted sensitivity to the conditioned aversive properties of 2 mg/kg methamphetamine, as indicated by a greater conditioned place-preference in mutant mice, relative to WT controls at this dose (Ruan et al., 2020a). A similar trend was also observed with fentanyl (Bryant et al., 2020) which together, raises the intriguing possibility that Hnrnph1+/− blocks the negative affective/motivational valence of a variety of drugs of abuse. At least in the case of methamphetamine, the attenuated aversion exhibited by Hnrnph1+/− mice cannot be readily explained by an effect of gene deletion on nucleus accumbens dopamine, as no genotypic difference is observed for basal dopamine content (Ruan et al., 2020a). Moreover, Hnrnph1+/− blunted the capacity of acute methamphetamine to elevate nucleus accumbens extracellular dopamine levels (Ruan et al., 2020a) and blunted dopamine release within the nucleus accumbens is reported to promote, rather than prevent, a methamphetamine-conditioned place-aversion (Lominac et al., 2014). Likewise, we know from prior our work that methamphetamine-induced place-conditioning is bidirectionally regulated by nucleus accumbens glutamate levels (Szumlinski et al., 2016); however, Hnrnph1+/− did not alter either basal, or acute methamphetamine-induced changes in, extracellular glutamate within the nucleus accumbens (Ruan et al., 2020a). Of relevance to the manifestation of place-preference/aversion, we have yet to determine the effects of Hnrnph1+/− on drug-induced neurotransmitter levels within the nucleus accumbens of mice following repeated drug exposure, nor do we know how gene deletion alters neurotransmitter levels following acute or repeated alcohol. Alcohol-induced place-aversion is linked to anomalies in glutamate plasticity within both the nucleus accumbens (Szumlinski et al., 2005) and bed nucleus of the stria terminalis (Campbell et al., 2019), implicating the extended amygdala as at least one potential neurocircuit affected by Hnrnph1 deletion.
Under taste-conditioning procedures, binge alcohol-drinking inversely correlated with magnitude of a conditioned taste aversion in WT mice across various genetic backgrounds (Blednov et al., 2012; Rhodes et al., 2007) and this relationship was disrupted in a number of different transgenic mutations (see Blednov et al., 2012). While we did not assay Hnrnph1+/− mice for alcohol-conditioned taste-aversion, our place-conditioning data indicate that heterozygous Hnrnph1 deletion not only blocked, but reversed the negative affective/motivational valence of high-dose alcohol. As C57BL/6J mice are reported to exhibit weak alcohol-induced place-preference (Cunningham, 2014; Cunningham et al., 1992), the failure of alcohol to elicit a place-preference in our WT mice likely reflects their genetic background rather than the alcohol doses selected and it remains to be determined whether or not the Hnrnph1+/− mutation would exert a similar effect on alcohol place-conditioning or any of our other measures in mice of a different genetic background more prone to exhibit place-preference or less likely to consume alcohol (e.g., DBA/2J). Nevertheless, the incongruency in results between our drinking and place-conditioning studies indicates that heterozygous Hnrnph1 deletion blurs the inverse relationship between the conditioned aversive properties of alcohol and alcohol intake. This “blurring” is consistent with the results of alcohol-conditioned taste aversion studies of other mutant mouse lines (Blednov et al., 2012). and highlights the importance of conducting multiple assays of drug reward when phenotyping mice of both sexes.
In humans, the perception of alcohol’s interoceptive effects as aversive or appetitive typically relates to individual variation in sensitivity to alcohol-induced intoxication (e.g., Krystal et al., 2003; Schuckit and Smith, 2000) or the severity of alcohol withdrawal (e.g., Anton and Becker, 1995; Schuckit et al., 1998), as well as individual differences in alcohol metabolism (c.f., Cederbaum, 2012). However, a number of results from the present study argue against these psychopharmacological factors as contributing to the alcohol reward phenotype of Hnrnph1+/− mice. For one, we did not detect any consistent effect of Hnrnph1 deletion on acute alcohol-induced locomotor activity, locomotor sensitization, intoxication or sedation, nor did we detect differences in alcohol pharmacokinetcs. Thus, the alcohol reward phenotype of Hnrnph1 mutants is unrelated to changes in sensitivity to any of alcohol’s effects on motor behavior or alcohol metabolism. Our findings for alcohol-induced locomotor activity contrast sharply with our prior results for both methamphetamine- (Yazdani et al., 2015; Ruan et al., 2020a, 2020b) and fentanyl-induced locomotion (Bryant et al. 2020), suggesting that hnRNP H1 does not play a universal role in regulating all drug-induced psychomotor activity. Alternatively, our lack of genotypic differences in alcohol-induced locomotion may reflect procedural differences related to the duration of locomotor testing as genotypic differences in methamphetamine-induced locomotion were most robust when saline and drug trials were conducted over a 1-h period compared to a 30-min period (Yazdani et al., 2015). This being said, we have successfully detected large genotypic differences in alcohol-induced locomotion and/or sensitization using our place-conditioning procedures (e.g., Ary et al., 2012; Campbell et al., 2019; Szumlinski et al., 2005; 2008). Unfortunately, the limited number of alcohol-naïve mice available at the time of study precluded further investigation of this procedural issue.
Notably, we also failed to detect consistent effects of Hnrnph1 deletion on negative affect-like measures in alcohol-naïve mice – a finding replicating our results indicating that Hnrnph1+/− does not affect baseline emotionality in mice (Bryant et al., 2020; Ruan et al., 2020a). We have shown repeatedly that early withdrawal from a history of binge-drinking induces a negative affective state in mice (e.g., Jimenez Chavez et al., 2020; Lee et al., 2015, 2016, 2017a,b, 2018a,b; Szumlinski et al., 2019), raising the possibility that the marked genotypic differences in alcohol-induced place-conditioning could reflect reduced sensitivity to a withdrawal-induced negative affective state. Given group differences in place-conditioning, we opted to inject mice repeatedly with high-dose alcohol (4 g/kg) in a manner consistent with the regimen employed during place-conditioning procedures and showed that this injection regimen was sufficient to increase anxiety- and depression-like behaviors when assessed during early alcohol withdrawal. However, as reported for fentanyl withdrawal (Bryant et al., 2020), we did not detect a consistent Genotype effect or Sex by Genotype interactions in the alcohol withdrawal-induced negative affective state. Thus, there does not appear to be a relationship between either reduced alcohol intake or an absence of alcohol-conditioned place-aversion and the severity of alcohol withdrawal in Hnrnph1+/− mice.
In conclusion, heterozygous deletion of Hnrnph1 reduced high-concentration alcohol intake under two distinct drinking paradigms in male mice only. Hnrnph1+/− profoundly reversed the negative affective/motivational valence of high-dose alcohol – an effect that was more pronounced and less variable in males. The effects of Hnrnph1 deletion on these measures of alcohol reward were unrelated to changes in alcohol pharmacokinetics, sensitivity to the psychomotor-activating, intoxicating or sedative properties of the drug, or the severity of alcohol withdrawal. These findings further support a general and surprisingly selective role for Hnrnph1 function specifically following exposure to multiple addictive substances, although the underlying mechanisms regarding the effect of Hnrnph1+/− on behavior are likely to differ among drug classes and sex.
Highlights.
Heterozygous deletion of Hnrnph1 (+/−) reduces alcohol intake by mice under continuous- and limited-access procedures.
Hnrnph1+/− mice are resistant to the conditioned aversive properties of high-dose alcohol.
The effects of hnrnph1 deletion on alcohol reward are male-selective.
Hnrnph1 deletion does not alter alcohol metabolism, withdrawal-induced anxiety, or its sedative-hypnotic effects.
Hnrnph1 is a novel regulator of alcohol reward.
Acknowledgements
This project was funded by NIH grants DA039168 (CDB), U01DA050243 (CDB), and AA024044 (KKS).
Role of Funding Source
Nothing declared
Footnotes
Conflict of Interest
Nothing declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Becker HC, 1995. Pharmacotherapy and pathophysiology of alcohol withdrawal In: Kranzler HR, (Ed.). The Pharmacology of Alcohol Abuse. Berlin: Springer-Verlag; 1995 pp. 315–367. [Google Scholar]
- Aranburu A, Liberg D, Honorø B, Leanderson T, 2006. CArG box-binding factor—a interacts with multiple motifs in immunoglobulin promoters and has a regulated subcellular distribution, Eur. J. Immunol. 36, 2192–2202. [DOI] [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK, 2012. Ethanol up-regulates nucleus accumbens neuronal activity-dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol 46, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, 2016. Alcohol addiction and the mu-opioid receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 65, 228–233. [DOI] [PubMed] [Google Scholar]
- Bain JM, Cho MT, Telegrafi A, Wilson A, Brooks S, Botti C, Gowans G, Autullo LA, Krishnamurthy V, Willing MC, Toler TL, Ben-Zev B, Elpeleg O, Shen Y, Retterer K, Monaghan KG & Chung WK, 2016. Variants in HNRNPH2 on the X Chromosome Are Associated with a Neurodevelopmental Disorder in Females. Am. J. Hum. Genet. 99, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, and Hascoet M, 2003. The mouse light/dark box test. European Journal of Pharmacology, 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Brabant C, Guarnieri DJ, Quertemont E, 2014. Stimulant and motivational effects of alcohol: lessons from rodent and primate models. Pharmacol. Biochem. Behav, 122, 37–52. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Healy AF, Ruan QT, Coehlo MA, Lustig E, Yazdani N, Luttik KP, Tran T, Swancy I, Brewin LW, Chen MM, Szumlinski KK, 2020. Sex-dependent effects of an Hnrnph1 mutation on fentanyl addiction-relevant behaviors but not antinociception in mice. Genes Brain Behav, 3:e12711. doi: 10.1111/gbb.12711. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bryant CD and Yazdani N, 2016. RNA-binding proteins, neural development and the addictions. Genes Brain Behav, 15, 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RR, Domingo RD, Williams AR, Wroten MG, McGregor HA, Waltermire RS, Greentree DI, Goulding SP, Thompson AB, Lee KM, Quadir SG, Jimenez Chavez CL, Coelho MA, Gould AT, von Joquieres G, Klugmann M, Worley PF, Kippin TE, Szumlinski KK, 2019. Increased alcohol-drinking induced by manipulations of mGlu5 phosphorylation within the bed nucleus of the stria terminalis. J. Neuroscience, 39, 2745–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI, 2012. Alcohol metabolism. Clin Liver Dis, 16, 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ, 2016. Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol. Biochem. Behav, 148, 99–105. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK, 2014. Binge alcohol drinking by mice requires intact Group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology, 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF, 2006. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol, 11, 195–269. [DOI] [PubMed] [Google Scholar]
- Crawley JN (1985). Exploratory behavior models of anxiety in mice. Neurosci. Biobehavi. Reviews, 9, 37–44. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, 2014. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav. Neurosci, 128, 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK, 1992. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl), 107, 385–393. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Kim VN, Kataoka N, 2002. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell. Biol, 3, 195–205. [DOI] [PubMed] [Google Scholar]
- Finn DA, 2020. The Endocrine System and Alcohol Drinking in Females. Alcohol Res, 40: 02. doi: 10.35946/arcr.v40.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Nakadai T, Shimada M, Hisatake K, 2009. Heterogeneous nuclear ribonucleoprotein R enhances transcription from the naturally configured c-fos promoter in vitro. J. Biol. Chem, 284, 23472–23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz EK, Martin DL, Hudson CN, Kippin TE, Szumlinski KK, 2017. Methamphetamine-alcohol interactions in murine models of oral drug-taking. Drug Alcohol Dep, 177, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz EK, Szumlinski KK, 2018. Prior binge-drinking history promotes methamphetamine-preference in mice. Drug Alcohol Dep, 183, 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill K, France C, Amit Z, 1986. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin. Exp. Res, 10, 457–462. [DOI] [PubMed] [Google Scholar]
- Guil S, Long JC, Cáceres JF, 2006. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell. Biol, 26, 5744–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SP, Tang YH, Smith R, 2010. Functional diversity of the hnRNPs: past, present and perspectives. Biochem. J, 430, 379–392. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Gründer G, Hirth N, Noori HR, Spanagel R, Sommer WH, 2019. Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci. Biobehav. Rev, 106, 141–164. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Buchert R, Mayatepek E, Haack TB, Distelmaier F, 2019. Bain type of X-linked syndromic mental retardation in boys. Clin. Genet, 95, 734–735. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, Dohmen BM, Braus DF, Schumann G, Machulla HJ, Bares R, Mann K, 2005. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch. Gen. Psychiatry, 62, 57–64. [DOI] [PubMed] [Google Scholar]
- Jimenez Chavez CL, Coelho MA, Brewin LW, Swancy I, Tran T, Albanese T, Laguna A, Garbriela I, Szumlinski KK, 2020. Incubation of negative affect during protracted alcohol withdrawal is age-, but not sex-selective. Brain Sciences, 10, 405. doi: 10.3390/brainsci10060405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park A, 2018. A systematic review: Candidate gene and environment interaction on alcohol use and misuse among adolescents and young adults. Am. J. Addict, 10: 10.1111/ajad.12755.. doi: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan D’Souza DC, 2003. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment and vulnerability. Pharmacol. Ther, 99, 79–94. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Szumlinski KK, 2018a. mGlu5-dependent modulation of anxiety during withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Dep, 68, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK, 2016. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Frontiers Cell. Neurosci. 10: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho M, McGregor HA, Waltermire RS, Szumlinski KK, 2015. Binge Alcohol Drinking Elicits a Persistent Negative Affective State in Mice. Behav. Brain Res, 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, Szumlinski KK, 2017a. Anxiolytic effects of buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress 1:2470547017712985. doi: 10.1177/2470547017712985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Szumlinski KK, 2018b. Homer2 within the central nucleus of the amygdala gates withdrawal-induced anxiety in a mouse model of binge-drinking. Neuropharmacology, 128, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo MA, Solton NR, Szumlinski KK, 2017b. Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not, adult mice. Front Psychology, 8, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. , 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature, 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK (2006) Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Dep, 85, 142–156. [DOI] [PubMed] [Google Scholar]
- Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Phillips TJ, Szumlinski KK, 2014. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front. Systems Neurosci, 8, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmiller S, Soltanieh S, Server KL, Mak R, Jin WJ, Fang MY, Luo E-C, Krach F, Yang D, Sen A, et al. , 2018. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell, 172, 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP & Kieffer BL, 1996. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature, 383, 819–823. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EP, 2006. A combined marble burying locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol, 547, 106–115. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL, 1991. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav, 38, 63–67. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH (1996) Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–82. [DOI] [PubMed] [Google Scholar]
- Pilch J, Koppolu AA, Walczak A, Murcia Pienkowski VA, Biernacka A, Skiba P, Machnik-Broncel J, Gasperowicz P, Kosińska J, Rydzanicz M, Emich-Widera E & Płoski R (2018) Evidence for HNRNPH1 being another gene for Bain type syndromic mental retardation. Clin Genet 94, 381–385. [DOI] [PubMed] [Google Scholar]
- Quadir SG, Guzelian E, Palmer MA, Martin DL, Kim J, Szumlinski KK, 2019. Complex interactions between the subject factors of biological sex and prior histories of binge-drinking and unpredictable stress influence behavioral sensitivity to alcohol and alcohol intake. Physiol. Behav, 203, 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK, 2016. Homer2 regulates alcohol and stress cross-sensitization. Addiction Biol, 21, 613–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF, 2012. The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: a critical review. Alcohol Clin. Exp. Res, 36, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Bindukumar B, Sykes D, Schwartz SA, Nair MP, 2006. Proteomic analysis of the effects of cocaine 284 Mol Neurobiol (2011) 44:269–286 on the enhancement of HIV-1 replication in normal human astrocytes (NHA). Brain Res, 1123, 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, and Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr, Crabbe JC, 2007. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav, 6, 1–18. [DOI] [PubMed] [Google Scholar]
- Ruan QT, Yazdani N, Beierle JA, Hixson KM, Hokenson KE, Apicco DJ, Luttik KP, Zheng K, Maziuk BF, Ash PEA, Szumlinski KK, Russek SJ, Wolozin B, Bryant CD, 2018. Changes in neuronal immunofluorescence in the C- versus N-terminal domains of hnRNP H following D1 dopamine receptor activation. Neurosci. Lett, 684, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan QT, Yazdani N, Blum BC, Beierle JA, Lin W, Coelho MA, Fultz EK, Healy AF, Shahin JR, Kandola AK, Luttik KP, Zheng K, Smith NJ, Cheung J, Mortazavi F, Apicco DJ, Ragu Varman D, Ramamoorthy S, Ash PEA, Rosene DL, Emili A, Wolozin B, Szumlinski KK, Bryant CD, 2020. A Mutation in Hnrnph1 That Decreases Methamphetamine-Induced Reinforcement, Reward, and Dopamine Release and Increases Synaptosomal hnRNP H and Mitochondrial Proteins. J. Neurosci, 40, 107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub MC, Lopez SR, Caputi M 2007. Members of the heterogeneous nuclear ribonucleoprotein H family activate splicing of an HIV-1 splicing substrate by promoting formation of ATP-dependent spliceosomal complexes, J. Biol. Chem, 282, 13617–13626. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, 2000. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J. Stud. Alcohol, 61 827–835. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Daeppen JB, Eng M, Li TK, Hesselbrock VM, Nurnberger JI Jr, Bucholz KK, 1998. Clinical relevance of the distinction between alcohol dependence with and without a physiological component. Am. J. Psychiatry, 155, 733–740. [DOI] [PubMed] [Google Scholar]
- Sern KR, Fultz EK, Coelho MA, Bryant CD, Szumlinski KK, 2020. A prior history of binge-drinking increases sensitivity to the motivational valence of methamphetamine in female C57BL/6J mice. Subst Abuse, 20, 14:1178221819897073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KY, Choi HS, Law PY, Wei LN, Loh HH, 2012. Post-transcriptional regulation of mu-opioid receptor: role of the RNA-binding proteins heterogeneous nuclear ribonucleoprotein H1 and F. Cell. Mol. Life Sci, 69, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suder P, Bodzon-Kulakowska A, Mak P, Bierczynska-Krzysik A, Daszykowski M, Walczak B, Lubec G, Kotlinska JH, Silberring J, 2009. The proteomic analysis of primary cortical astrocyte cell culture after morphine administration. J. Proteome Res, 8, 4633–4640 [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE, 2008. Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology, 33, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugamann M, Phillips TH, Kippin TE, 2017. Methamphetamine addiction vulnerability: The glutamate, the bad and the ugly. Biol. Psychiat, 81, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During MT, Worley PF, Middaugh LD, Kalivas PW, 2005. Homer2 is necessary for ethanol-induced neuroplasticity. J. Neurosci., 25, 7054–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Ehlers MD, Keene JD, 2008. Activity-dependent expression of RNA binding protein HuD and its association with mRNAs in neurons. RNA Biol, 5, 157–168. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Cosgrove KP, Tanabe J, McKee SA, 2020. Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. J. Neurosci. Res, 24: 10.1002/jnr.24625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ML, Bera A, Wong FK, Lewis SM, 2020. Cellular stress orchestrates the localization of hnRNP H to stress granules. Exp. Cell Res, 394, 112111. [DOI] [PubMed] [Google Scholar]
- Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X, Liu H, Ding S, Hurd YL, Pasternak GW, Klein RJ, Cartegni L, Zhou W, Pan YX, 2014. A heroin addiction severity-associated intronic single nucleotide polymorphism modulates alternative pre-mRNA splicing of the mu opioid receptor gene OPRM1 via hnRNPH interactions. J. Neurosci, 34, 11048–11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani N, Parker CC, Shen Y, Reed ER, Guido MA, Kole LA, Kirkpatrick SL, Lim JE, Sokoloff G, Cheng R, Johnson WE, Palmer AA & Bryant CD (2015) Hnrnph1 Is A Quantitative Trait Gene for Methamphetamine Sensitivity. PLoS Genet 11, e1005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaso MJ, Maisto SA, Glatt SJ, Belote JM, Park A, 2017. Interaction Between the μ-Opioid Receptor Gene and the Number of Heavy-Drinking Peers on Alcohol Use. Alcohol Clin. Exp. Res. 41, 2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Neubert TA, Jordan BA, 2012. RNA binding proteins accumulate at the postsynaptic density with synaptic activity. J. Neurosci, 32, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]