Abstract
The tryptophan metabolite kynurenine increases with aging and inflammation, and appears to contribute directly to the development and progression of several age-related conditions. Kynurenine is now known to signal through the aryl hydrocarbon receptor (Ahr) to modulate levels of reactive oxygen species (ROS). The Ahr promoter region contains several sites for NF-kB binding, indicating that inflammation is a key factor modulating Ahr expression. Furthermore, kynurenine activation of Ahr is observed to stimulate expression of the enzyme IDO1, which generates kynurenine by degrading tryptophan, representing a positive feedback loop that may link inflammation with ROS production. On the other hand, the antioxidant Nrf2 can be stimulated by Ahr, and Nrf2 can itself activate Ahr expression. The balance between pro- and antioxidant functions of Ahr mediated by kynurenine may therefore regulate healthy versus unhealthy aging in different tissues and organ systems. Potential therapeutic approaches to target this pathway include exercise to alter kynurenine production or molecules such as metformin or resveratrol that may suppress Ahr activity.
Keywords: Reactive oxygen species, inflammaging, Nrf2, SLC7A5
INTRODUCTION
According to data from the United Nations approximately 1 in 6 people around the world will be over the age of 65 by the year 2050. This large segment of the global population represented by older adults means that diseases associated with older age such as Alzheimer’s disease, osteoarthritis, and osteoporosis will become a greater burden on healthcare systems worldwide. Research has begun to tackle the challenge of lengthening the healthspan of humans, so that the later years of life are relatively disease-free and older adults can maintain independence. The “fountain of youth” in the classic sense remains elusive, though recently it has become apparent that local and systemic factors that increase with age may contribute to age-related declines in cell and tissue function (Hühne et al., 2014). The tryptophan metabolite kynurenine has been gaining interest in the aging research community as an important factor that increases with age and is now implicated in a number of age-related diseases. Kynurenine inhibition in C. elegans can extend lifespan and suppress α-synuclein toxicity (Van Der Goot et al., 2012) and inhibiting kynurenine production in D. melanogaster can increase mean lifespan by more than 25% (Oxenkrug, 2011). Furthermore, a rodent model of tryptophan depletion has been observed to extend life span (Segall and Timiras, 1976), and increased kynurenine levels have been associated with increased mortality in human subjects (Pertovaara et al., 2006; Yu et al., 2017; Zuo et al., 2016)
Kynurenine is now recognized as an agonist of the aryl hydrocarbon receptor (Ahr; Opitz et al., 2011; Bessede et al., 2014; Yamamoto et al., 2019). Importantly, Ahr has recently been highlighted as playing a key role in regulating organismal aging and lifespan. Eckers et al. (2016) suggested that Ahr activation promoted aging by increasing vascular stiffness in mice, which was further supported by data in human subjects showing a positive association between Ahr expression and vascular stiffness. These authors also documented increased lifespan in C. elegans worms lacking functional Ahr. On the other hand, Bravo-Ferrer and colleagues (2019) observed that Ahr expression decreased significantly with age in the mouse brain, and that mice lacking Ahr showed elevated circulating levels of inflammatory cytokines and reduced lifespan. Their work is consistent with the data from studies on hematopoietic stem cells by Singh et al. (2011, 2014) and Bennett et al. (2015) who found that aged Ahr knockout mice showed decreased survival, splenomegaly, and increased circulating white blood cells. These findings are also in general agreement with earlier research documenting liver fibrosis and cardiac hypertrophy in Ahr null animals (Fernandez-Salguero et al., 1995; Vasquez et al., 2003).
These somewhat contradictory findings regarding Ahr function and organismal aging are further complicated by studies showing that, while ligands such as kynurenine and dioxin may have pathological effects via Ahr, other Ahr ligands have been identified such as quercetin that are well-documented to have anti-aging functions (Dietrich, 2016; Table 1). Thus, ligand-specific effects may exist such that downstream effectors of Ahr signaling such as cytochrome P450 1A1 enzyme (CYP1A) may be activated with particular ligands but other ligands may activate different pathways such as the antioxidant nuclear factor erythroid 2–related factor 2 (Nrf2; Nuti et al., 2014; Dietrich, 2016; Larigot et al., 2018). In addition, Ahr ligand specificity and affinity can differ between mice and humans (Murray et al., 2014), further complicating observed relationships among Ahr activation, inhibition, and disease processes. Here we provide an overview of Ahr signaling with special reference to the kynurenine pathway, and its potential role in modulating healthspan with aging.
Table 1.
Exogenous and endogenous ligands of the aryl hydrocarbon receptor and their potential impact on age-related diseases.
| Molecule | Function | Potential impact on aging |
|---|---|---|
| Endogenous | ||
| Kynurenine | Ahr agonist | Muscle atrophy, increased bone resorption, reduced bone formation1–3 |
| Indoxyl sulfate | Ahr agonist | Muscle atrophy, reduced bone formation4, 5 |
| Exogenous-Diet | ||
| Quercetin | Ahr agonist | Senolytic when combined with dasatinib, also an antioxidant6, 7 |
| Galangin | Ahr agonist/antagonist | Anti-inflammatory, Inhibits CYP1A1 activation8 |
| Apigenin | Ahr agonist/antagonist | Inhibits CYP1A1 activation9 |
| Resveratrol | Ahr antagonist | Stimulates mitochondrial biogenesis, increases autophagy10 |
ARYL HYDROCARBON RECEPTOR SIGNALING MEDIATED BY KYNURENINE
The aryl hydrocarbon receptor is highly expressed in peripheral tissues, with the highest levels of expression in peripheral organs such as the lung, liver and adipose tissue and lower levels in the brain (Fig. 1A). Ahr resides in the cell cytoplasm in an inactive state, bound to the molecular chaperone heat-shock protein 90 (Hsp90). The complex translocates to the nucleus upon ligand binding, where Ahr dissociates from Hsp90 and then dimerizes with the aryl hydrocarbon receptor nuclear translocator (Arnt). The heterodimer binds specific DNA sequences located in the promoter region’s many potential target genes (Fig. 1B). The most well-known Ahr agonist, dioxin (TCDD), stimulates Ahr translocation and dimerization which binds xenobiotic response elements to activate targets such as members of the cytochrome P450 family. Ahr activation by dioxin induces a number of factors contributing to organ-level dysfunction including inflammatory factors such as interleukin-6 (IL-6; Hollingshead et al, 2009) and factors that can increase oxidative stress and fibrosis such as CYP1A1, CYP1B1 and transforming growth factor beta 1 (TGF beta 1; Nuti et al., 2014; Enoki et al., 2016; Roman et al., 2018; Sas et al., 2018). The Ahr promoter region contains binding regions for the pro-inflammatory mediator nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB; Vogel et al., 2014), further supporting a link between Ahr signaling and inflammatory responses (Fig. 1). It has also been shown that the antioxidant Nrf2 is not only activated by Ahr (see below) but can itself induce Ahr expression (Shin et al. 2007).
Figure 1.
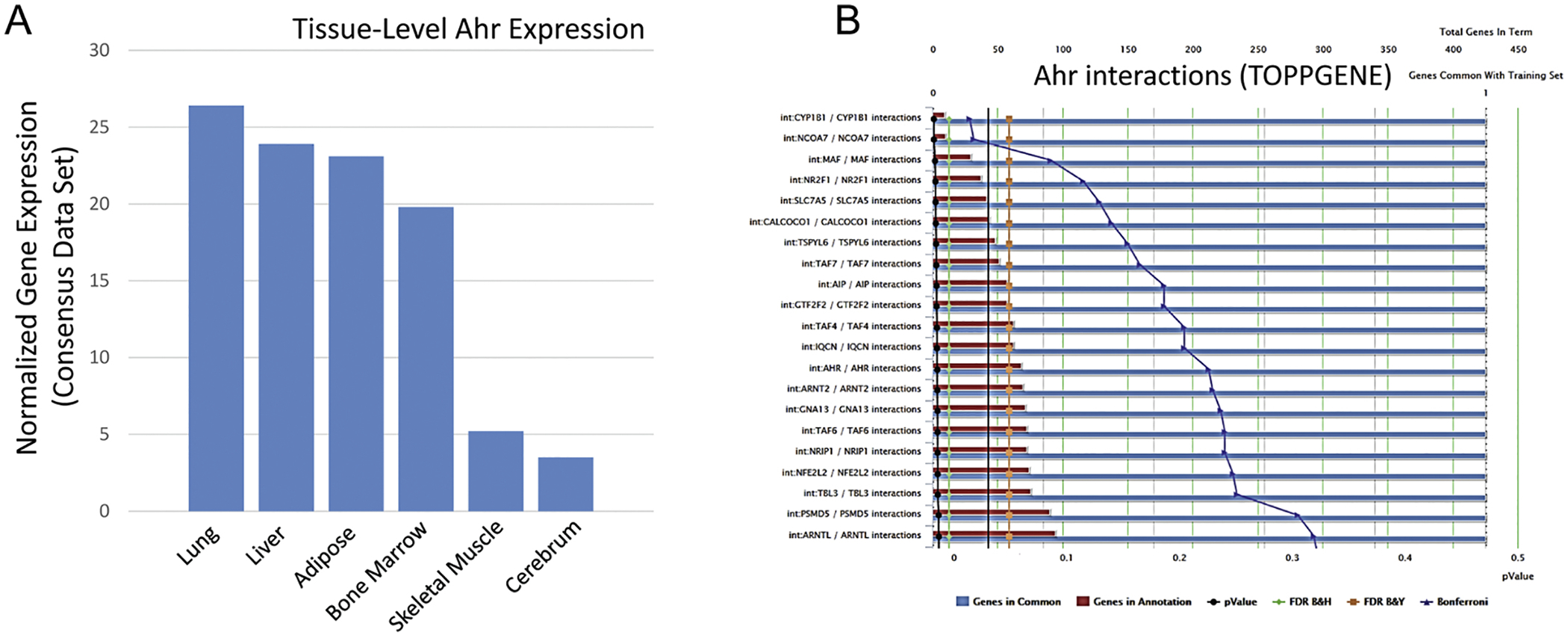
A. Tissue-specific gene expression of the human aryl hydrocarbon receptor gene. Relative expression is based on the consensus data set compiled from GTEx, HPA, and FANTOM 5, data sets in proteinatlas.org. B. Interactions of the human aryl receptor with different genes summarized using TOPPGENE functional enrichment. Key interactions referenced in this paper include interactions with the antioxidant Nrf2 and the kynurenine transporter SLC7A5.
While toxic xenobiotic agents such as dioxin are well-established agonists of Ahr, the tryptophan metabolite kynurenine was the first endogenous Ahr ligand to be discovered. Endogenous kynurenine was found to be upregulated in brain tumors, suppressing the normal immunological response thereby promoting tumor growth and malignant progression (Optiz et al., 2011). Tryptophan and its metabolite kynurenine both enter the cell via the same transporter, solute carrier family 7 member 5 (SLC7A5, or LAT1; Sinclair et al., 2018). Once inside the cell kynurenine is able to stimulate Ahr translocation to the nucleus (Opitz et al., 2011; Yamamoto et al., 2019). Ahr binding and transcriptional activation by kynurenine have previously been shown to stimulate the expression of both TGF beta 1 and indoleamine 2, 3-dioxygenase 1 (IDO1; Vogel et al., 2008; Nuti et al., 2014). The induction of IDO1 by Ahr is significant, since IDO1 generates kynurenine by degrading tryptophan, thus forming a positive feedback loop (Fig. 2). In addition, Ahr has also been observed to positively regulate SLC7A5 expression (Kim et al., 2005; Brauze et al., 2017; Tomblin et al., 2018). The increase in SLC7A5 with Ahr activation by kynurenine presumably facilitates further kynurenine entry into the cell, representing yet another “feed forward” mechanism of Ahr pathway activation in the setting of inflammation (Fig. 2).
Figure 2.
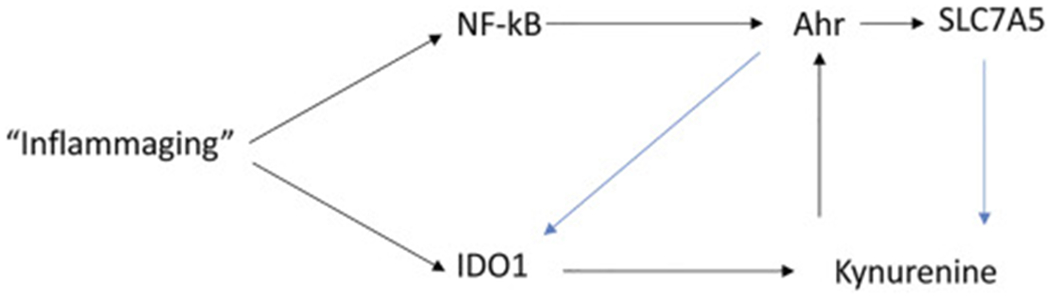
Factors regulating Ahr gene expression. The Ahr promoter region contains multiple binding regions for NK kappa B (NF-kB). Kynurenine, a ligand of Ahr, is produces by the degradation of tryoptophan by the enzyme IDO1, which is itself induced by inflammatory stimuli and interferon gamma expression. The aryl hydrocarbon receptor activates expression of the amino acid transporter SLC7A5, which transports both kynurenine and tryptophan into the cell. Positive feedback mechanisms are indicated in blue, and include Ahr upregulation of IDO1, further increasing kynurenine concentrations, and SLC7A5 expression increasing kynurenine transport to further activate Ahr nuclear translocation.
While the bulk of literature on IDO1-kynurenine-Ahr signaling reveals mechanisms by which this pathway is involved in inflammatory responses and oxidative stress in the settings of cancer and dioxin exposure (e.g., Dalton et al., 2002), it is also clear that Ahr can serve a protective function in certain contexts. This is most obvious from the phenotype of mice lacking Ahr, which show a number of abnormalities noted above including liver and lung dysfunction. Ahr can modulate ROS accumulation by activating CYP1A1, which increases ROS (Senft et al., 2002), but this is balanced by the direct and indirect activation of Nrf2 (Dietrich, 2016). Certain exogenous ligands of Ahr, such as the flavonoid quercetin (Table 1; Ashida et al., 2000; Larigo et et al., 2018) may mediate beneficial effects of Ahr by not only increasing Nrf2 but also increasing expression of the Ahr repressor, thus promoting a negative feedback mechanism (Niestroy et al., 2011). The very elegant summary of Ahr function by Perdew presented in Esser et al. (2018) describes three settings of Ahr activity: i) absence of Ahr, which provides no protective effect on cell and tissue function and thus may have detrimental effects, ii) moderate levels of Ahr activation, which have beneficial effects by modulating ROS and other potentially harmful stimuli, and iii) high levels of Ahr activation, which have significant adverse effects. This model is similar to that described by Dietrich (2016), in which detrimental effects of Ahr are observed when the normal balance of pro- and antioxidant functions of Ahr activation are disrupted.
FUTURE DIRECTIONS: BALANCING KYNURENINE AND AHR IN HEALTHY AGING
A growing body of work now suggests that elevated kynurenine, and thus increased Ahr activation, with aging is detrimental to healthspan. Pertovaara et al. (2006) presented evidence from older adults showing that increased circulating kynurenine and an increase in the kynurenine/tryptophan ratio was significantly associated with all-cause mortality. Kim et al. (2019) recently found that kynurenine increased with age in serum, and that bone-derived levels of kynurenine were significantly elevated in patients with fragility hip fracture. Data from the Hordaland Health study showed that increased plasma kynurenine and an increased kynurenine/tryptophan ratio were associated with hip fractures (Apalset et al, 2014) and also all-cause mortality, particularly from cardiovascular disease (CVD; Zuo et al., 2016). Consistent with the Hordaland data, Yu et al. (2017) recently found that low kynurenic acid and high tryptophan concentrations at baseline were correlated with lower risk of myocardial infarction after one year, and if tryptophan concentrations were increased there was a strong association with lower risk of CVD. There is also evidence that kynurenine may be involved in age-related loss of muscle mass, or sarcopenia. Lustgarten and Fielding (2017) found that an increase in the kynurenine/tryptophan ratio is associated with poor muscle composition and reduced density (Lustgarten and Fielding, 2017). This group also examined associations between interleukin-6 (IL-6) levels and circulating metabolites, and found positive correlations between IL-6 and circulating kynurenine and between IL-6 and the kynurenine/tryptophan ratio (Lustgarten and Fielding, 2016). Together these studies suggest that increasing kynurenine is associated with many syndromes linked to “inflammaging” such as sarcopenia, hip fracture, and cardiovascular disease.
Data from animal models provide additional insights into kynurenine, Ahr and healthspan. We have recently found that kynurenine can increase muscle atrophy in young mice and ROS production in cultured muscle cells, whereas muscle atrophy in aged mice can be attenuated with IDO inhibition (Kaiser et al., 2019). Kynurenine can also induce bone loss directly in mice (El Refaey et al., 2017). Our findings are similar to those presented by Enoki et al. (2016) for the endogenous Ahr ligand indoxyl sulfate, which increases inflammation and muscle wasting. Blocking Ahr activation also appears to have beneficial effects in other organ systems. In an experimental model of stroke recovery is enhanced by loss of Ahr function, indicating that Ahr deficiency is neuroprotective in this model (Cuartero et al., 2014). Interestingly Ahr inhibition also appears to prevent the development of obesity in animals on a high-fat diet. This has been observed in mice lacking Ahr (Xu et al., 2015), in mice treated with an Ahr inhibitor (Moyer et al., 2016), and in mice with liver-specific loss of Ahr function (Girer et al., 2019). Importantly the loss of liver-specific Ahr increased expression of fibroblast growth factor 21, which plays a key role in stimulating thermogenesis in brown adipose tissue. These animal studies may in part explain the increased risk of cardiovascular disease with elevated kynurenine levels noted above.
The detrimental effects of elevated kynurenine and increased Ahr activation on healthspan raise the question of how to prevent these processes with aging. Resistance exercise, which is known to slow the development and progression of sarcopenia with aging, has been shown to downregulate Ahr and Ahr-pathway genes (Phillips et al., 2013). In addition to downregulating Ahr, exercise increases Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC1 alpha) expression in skeletal muscle, which in turn increases kynurenine aminotransferase (KAT) activity (Schlittler et al., 2016; Agudelo et al., 2018; Allison et al., 2019). The activation of KAT in muscle converts kynurenine, which can cross the blood-brain barrier, to kynurenic acid, which cannot reach the brain. Kynurenic acid is a ligand for a novel G protein-coupled receptor GPR35, which is known to activate metabolic and anti-inflammatory genes (Agudelo et al., 2018), and the exercise-induced conversion of kynurenine to kynurenic acid also increased glycolysis (Agudelo et al., 2019). In another study, endurance exercise increased kynurenic acid concentrations one hour after exercise (Schlittler et al., 2016). The polyphenol resveratrol, which is well-recognized in the aging literature as a Sirt1 activator, can inhibit the DNA nuclear binding of Ahr and thus function as an Ahr antagonist (Table 1; Ciolino et al., 1998). Finally, the drug metformin, which is currently being explored in clinical trials for its potential anti-aging effects, can suppress Ahr activity in a number of different cell types (Do et al., 2014; Maayah, et al., 2015; Dean et al., 2016). These data suggest that there may be several pharmacological and behavioral strategies for targeting kynurenine-Ahr interactions with aging that may promote healthspan (Fig. 3)
Figure 3.
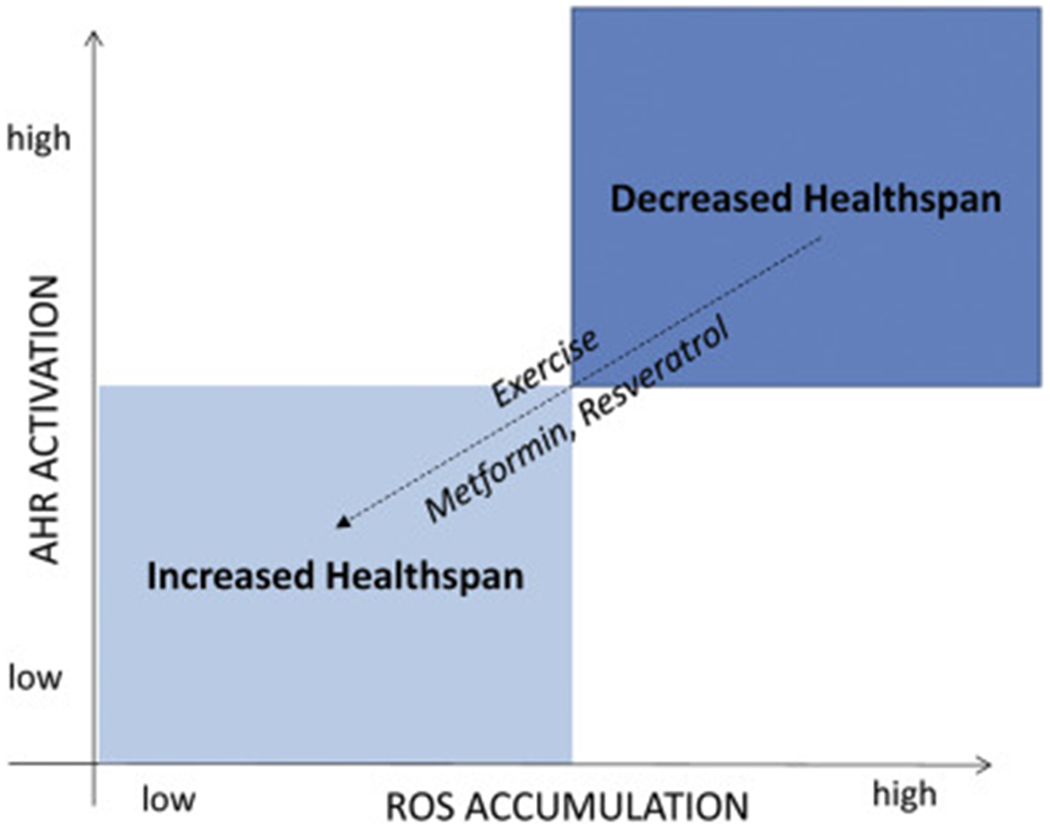
Overall balance of oxidant and antioxidant functions of Ahr balancing healthspan. Healthspan is potentially decreased with chronic elevation of Ahr activity and ROS accumulation. This may be partially attenuated by exercise, which can suppress Ahr expression in muscle and increase kynurenine aminotransferase expression. Small molecules such as metformin and resveratrol may also improve healthspan by decreasing Ahr expression and antagonizing Ahr activity.
Acknowledgments
Funding for this research was provided by the National Institute on Aging, US National Institutes of Health (AG 036675).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudelo LZ, Ferreira DM, Cervenka I et al. 2018. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metabolism 27, 378–92. [DOI] [PubMed] [Google Scholar]
- Agudelo LZ, Ferreira DM, Dadvar S et al. 2019. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nature Commun. 10, 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DJ, Nederveen JP, Snijders T et al. Exercise training impacts skeletal muscle gene expression related to the kynurenine pathway. Am J Phys-Cell Physiol. 316, C444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apalset EM, Gjesdal CG, Ueland PM et al. 2014. Interferon gamma (IFN-γ)-mediated inflammation and the kynurenine pathway in relation to risk of hip fractures: the Hordaland Health Study. Osteopor. Intl. 25, 2067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida H, Fukuda I, Yamashita T et al. 2000. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Letters 476,213–217. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Singh KP, Unnisa Z et al. 2015. Deficiency in Aryl Hydrocarbon Receptor (AHR) Expression throughout Aging Alters Gene Expression Profiles in Murine Long-Term Hematopoietic Stem Cells. PLoS One 10, e0133791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessede A, Gargaro M, Pallotta M et al. 2014. Aryl hydrocarbon receptor control of a disease tolerance pathway. Nature 511, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauze D, Zawierucha P, Kiwerska K et al. 2017. Induction of expression of aryl hydrocarbon receptor-dependent genes in human HepaRG cell line modified by shRNA and treated with β-naphthoflavone. Mol. Cell Biochem. 425, 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Ferrer I, Cuartero M, Medina V et al. 2019. Lack of the aryl hydrocarbon receptor accelerates aging in mice. FASEB Journal doi: 10.1096/fj.201901333R. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC 1999. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 79, 1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC 1998. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 58, 5707–12. [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, de la Parra J et al. 2014. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 130, 2040–51. [DOI] [PubMed] [Google Scholar]
- Dalton T, Puga A, Shertzer H 2002. Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chemico-Biol Interactions 141, 77–95. [DOI] [PubMed] [Google Scholar]
- Dean A, Nilsen M, Loughlin L et al. 2016. Metformin Reverses Development of Pulmonary Hypertension via Aromatase Inhibition. Hypertension. 68, 446–54. [DOI] [PubMed] [Google Scholar]
- Dietrich C 2016. Antioxidant functions of the aryl hydrocarbon receptor. Stem Cells Int. 7943495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Kim HG, Tran TT et al. 2014. Metformin suppresses CYP1A1 and CYP1B1 expression in breast cancer cells by down-regulating aryl hydrocarbon receptor expression. Toxicol. Appl. Pharmacol. 280, 138–48. [DOI] [PubMed] [Google Scholar]
- Eckers A, Jakob S, Heiss C et al. 2016The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 6, 19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Refaey M, McGee-Lawrence ME, Fulzele S et al. 2017. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J. Bone Miner. Res. 32, 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki Y, Watanabe H, Arake R et al. 2016. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 6, 32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Lawrence BP, Sherr D et al. 2018. Old receptor, new tricks—the ever-expanding universe of aryl hydrocarbon receptor functions. Int. J. Mol. Sci. 19, 3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM et al. 1995. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268, 722–726 [DOI] [PubMed] [Google Scholar]
- Girer N, Carter D, Bhattarai N et al. 2019. Inducible loss of the aryl hydrocarbon receptor activates perigonadal white fat respiration and brown fat thermogenesis via fibroblast growth factor 21. Int. J. Mol. Sci. 20, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead B, Beischlag T, DiNatale B et al. 2008. Inflammatory Signaling and Aryl Hydrocarbon Receptor Mediate Synergistic Induction of Interleukin 6 in MCF-7 Cells. Cancer Res. 68, 3609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hühne R, Thalheim T, Sühnel J 2014. AgeFactDB - the JenAge Ageing Factor Database - Towards data integration in ageing research. Nucleic Acids Res. 42, D892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Park H, Li X et al. 2018. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicol. Sci. 164, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H, Fulzele S, Mendhe B et al. 2019. Kynurenine, a tryptophan metabolite that increases with age, induces muscle atrophy and lipid peroxidation. Oxid. Med Cell. Longev. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dere E, Burgoon LD et al. 2009. Comparative analysis of AhR-mediated TCDD-elicited gene expression in human liver adult stem cells. Toxicol. Sci. 112, 229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Hamrick MW, Yoo HJ et al. 2019The Detrimental Effects of Kynurenine, a Tryptophan Metabolite, on Human Bone Metabolism. The Journal of Clinical Endocrinology & Metabolism. 2019 February 1;104(6):2334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T 2017. Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larigot L, Juricek L, Dairou J, Coumoul X 2018. AhR signaling pathways and regulatory functions Biochimie Open 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten MS, Fielding RA 2016. Metabolites associated with circulating interleukin-6 in older adults. J Gerontol. Series A: Biomed Sic. Med Sci. 72, 1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten MS, Fielding RA 2017. Metabolites related to renal function, immune activation, and carbamylation are associated with muscle composition in older adults. Exp. Gerontol. 100, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayah ZH, Ghebeh H, Alhaider AA et al. 2015. Metformin inhibits 7,12-dimethylbenz[a]anthracene-induced breast carcinogenesis and adduct formation in human breast cells by inhibiting the cytochrome P4501A1/aryl hydrocarbon receptor signaling pathway. Toxicol. Appl. Pharmacol. 284, 217–26. [DOI] [PubMed] [Google Scholar]
- Moyer B, Rojas I, Kerley-Hamilton J et al. 2016. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for Ahr activation by kynurenine via oxidized-LDL, TLR2/4, TGF beta, and IDO1. Toxicol. Appl. Pharmacol. 300,13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Patterson AD, Perdew GH 2014. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer 14, 801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niestroy J, Barbara A, Herbst K et al. 2011. Single and concerted effects of benzo[a]pyrene and flavonoids on the AhR and Nrf2-pathway in the human colon carcinoma cell line Caco-2. Toxicol. In Vitro 25, 671–83. [DOI] [PubMed] [Google Scholar]
- Nuti R, Garagaro M, Matino D et al. 2014. Ligand binding and functional selectivity of L-Tryptophan metabolites at the mouse aryl hydrocarbon receptor (mAhr). J. Chem. Inform. Model. 54, 3373–83. [DOI] [PubMed] [Google Scholar]
- Optiz CA, Litzenburger UM, Sahm F et al. 2011. An endogenous tumour-producing ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF, Navrotskaya V, Voroboyva L, Summergrad P 2011. Extension of life span of Drosophila melanogaster by the inhibitors of tryptophan-kynurenine metabolism. Fly 5, 307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara M, Raitala A, Lehtimäki T, Karhunen P et al. 2006. Indoleamine 2, 3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech. Aging Dev. 127, 497–9. [DOI] [PubMed] [Google Scholar]
- Phillips BE, Williams JP, Gustafsson T et al. 2013. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet. 9, e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman A, Carvajal-Gonzalez J, Merino J et al. 2018. The aryl hydrocarbon receptor in the crossroad of signaling networks with therapeutic value. Pharmacol. Therapeutics 185, 50–63. [DOI] [PubMed] [Google Scholar]
- Sas K, Szabó E, Vécsei L 2018. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 23, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlittler M, Goiny M, Agudelo LZ et al. 2016. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol.-Cell Physiol. 310, C836–40. [DOI] [PubMed] [Google Scholar]
- Segall PE, Timiras PS 1976. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev. 5, 109–24. [DOI] [PubMed] [Google Scholar]
- Senft A, Dalton T, Nebert D et al. 2002. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic. Biol. Med. 33, 1268–78. [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V et al. 2007. Nrf2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 27, 7188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Neyens D, Ramsay G et al. 2018. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 9, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Garrett RW, Casado FL, Gasiewicz TA 2011. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev. 20, 769–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KP, Bennett JA, Casado FL et al. 2014. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem Cells Dev. 23, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JK, Arthur S, Primerano DA .et al. 2016. Aryl hydrocarbon receptor (AHR) regulation of L-Type Amino Acid Transporter 1 (LAT-1) expression in MCF-7 and MDA-MB-231 breast cancer cells. Biochem. Pharmacol. 106, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez A, Atallah-Yunes N, Smith F et al. 2003. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by Ahr knockout mice. Cardiovasc. Toxiciol. 3, 153–63. [DOI] [PubMed] [Google Scholar]
- Van Der Goot AT, Zhu W, Vázquez-Manrique RP et al. 2012. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc. Natl. Acad. Sci. USA 109, 14912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B et al. 2008. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2, 3-dioxygenase. Biochem. Biophys. Res. Commun. 375, 331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Khan E, Leung P et al. 2014. Cross-talk between aryl hydrocarbon receptor and the inflammatory response. J. Biol. Chem. 289, 1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tominari T, Hirata M et al. 2017. Indoxyl sulfate, a uremic toxin in chronic kidney disease, suppresses both bone formation and bone resorption. FEBS Open Bio. 7, 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang C, Zhang Z et al. 2015. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int. J. Obes. 39, 1300–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hatabayashi K, Arita M et al. 2019. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 12, 587. [DOI] [PubMed] [Google Scholar]
- Yu E, Ruiz-Canela M, Guasch-Ferré M et al. 2017. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J. Nutr. 147, 314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Ueland P, Ulvik A et al. 2016. Plasma Biomarkers of Inflammation, the Kynurenine Pathway, and Risks of All-Cause, Cancer, and Cardiovascular Disease Mortality: The Hordaland Health Study. Am. J. Epidemiol. 183, 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


