Abstract
Background and Objectives
Medication exposure is a potential risk factor for falls and subsequent death and functional decline among older adults. However, controversy remains on the best way to assess medication exposure and which approach best predicts falls. The objective of the current study was to examine the association between different measures of medication exposure and falls risk among community-dwelling older adults.
Research Design and Methods
This retrospective cohort study was conducted using Falls Free PA program data and a linked prescription claims data from Pennsylvania’s Pharmaceutical Assistance Contract for the Elderly program. Participants were community-dwelling older adults living in Pennsylvania, United States. Three measures of medication exposure were assessed: (a) total number of regular medications (polypharmacy); (b) counts of potentially inappropriate medications derived from current prescription guidance tools (Fall Risk-Increasing Drugs [FRIDs], Beers Criteria); and (c) medication burden indices based on pharmacologic mechanisms (Anticholinergic Cognitive Burden, Drug Burden Index) all derived from claims data. The associations between the different medication risk measures and self-reported falls incidence were examined with univariate and multivariable negative binomial regression models to estimate incidence rate ratios (IRRs).
Results
Overall 343 older adults were included and there were 236 months with falls during 2,316 activity-adjusted person-months (10.2 falls per 100 activity-adjusted person-months). Of the 6 measures of medication risk assessed in multivariate models, only the use of 2 or more FRIDs (adjusted IRR 1.67 [95% CI: 1.04–2.68]) independently predicted falls risk. Among the 13 FRID drug classes, the only FRID class associated with an increased fall risk was antidepressants.
Discussion and Implications
The presence of multiple FRIDs in a prescription is an independent risk factor for falls, even in older adults with few medications. Further investigation is required to examine whether deprescribing focused on FRIDs effectively prevents falls among this population.
Keywords: Falls risk, Medication exposure, Pharmacoepidemiology, Prescription guidance
Translational Significance: The current study examined different medication exposure measures to see which best predicts falls among community-dwelling older adults. The study found that the presence of multiple Fall Risk-Increasing Drugs (FRIDs) in a prescription is an independent risk factor for falls, even in people with few medications. This result has important clinical implications for future deprescribing strategies, where focusing on FRIDs would be more effective than simply reducing the number of medications.
Background and Objectives
The frequency of falls increases as people age. According to the Behavioral Risk Factor Surveillance System survey, approximately 29% of people aged 65 and older experienced an accidental fall in any given year (1). Accidental falls and fall-related injuries among this population have been shown to result in significant morbidity and mortality (2–4). Thus, along with the increasing aging population, falls prevention has been a major public health priority.
Falls occur as a result of a complex interaction of numerous risk factors: age, sex, comorbidities, previous falls, functional dependency, and medication burden are previously described potential risk factors (5,6). Among potential risk factors for falls, several risk factors such as medication exposure, excess alcohol use, and sedentary lifestyle are potentially modifiable. It is important to further elucidate modifiable fall risk factors among older adults so as to inform evidence-based public health interventions.
Risks from medication exposure can be defined in a number of ways. One is simply a count of medications, or polypharmacy. Although no standard definition has been set, polypharmacy is often defined as regular use of five or more medications. Several previous studies showed that polypharmacy was associated with falls risk (7–9) and impaired balance (10) among older populations. On the contrary, other authors reported that polypharmacy itself was not an independent risk factor for accidental falls after controlling for other variables such as age, sex, and comorbidities (11,12). A recent systematic review regarding health outcomes associated with polypharmacy reported that 19 out of 23 studies found at least one positive association between polypharmacy and either falls or fall-related outcomes. However, the authors questioned whether the number of medications prescribed itself is an independent risk factor for falls or whether the association could be explained by the fact that the exposure to Fall Risk-Increasing Drugs (FRIDs) is likely to be present as a result of polypharmacy (13). In addition, polypharmacy can be appropriate, especially in the management of older patients with multimorbidity.
A second approach defines medication exposure by a count of potentially inappropriate medications (PIMs), which have been specified in prescribing guidance tools. These tools include the Beers (14) and the STOPP/START criteria (15). More recently, the Swedish National Board of Health and Welfare developed prescribing guidance for FRIDs (16). The list includes five drug classes under “drugs that cause high risk of falling” (e.g., opioids and antipsychotics) and eight drug classes under “drugs that cause orthostatism/hypotension” (e.g., diuretics and beta-blockers). Each of these prescribing guidelines identifies PIMs, and a count of these medications is sometimes used as a measure of medication exposure risk (6,17).
Finally, explicit medication burden measures have been proposed to assess the cumulative effect of anticholinergic and sedative properties. These have proven useful in the field of pharmacoepidemiology. The Drug Burden Index (DBI) measures overall exposure to medications with anticholinergic (DBI-Ach) and sedative properties (DBI-Se) (18). Another widely used tool, the Anticholinergic Cognitive Burden (ACB) (19), focuses on cognitive effects of medications with anticholinergic activity.
To date, controversy remains regarding which approach is best for determining falls risk among older adults. In this research we examined (a) polypharmacy, (b) counts of PIMs specified by the Beers Criteria and Swedish National Board of Health and Welfare FRIDs prescription guidance tool, and (c) medication burden measures as alternative predictors of falls risk in a community sample.
We hypothesized that explicit medication burden measures such as ACB and DBI, which incorporate cumulative burden of medication exposure, would have better predictive capacity compared to the simple count of regular medications or prescription guidance based on PIMs. The objectives of the current study were to examine the association between different medication exposure measures and falls and to determine which best predicts falls among community-dwelling older adults.
Research Design and Methods
Study Design and Setting
This retrospective cohort study examined the risk of falls according to medication exposure among community-dwelling older adults in Pennsylvania, United States. Falls Free PA program data were linked to prescription claims data from Pennsylvania’s Pharmaceutical Assistance Contract for the Elderly (PACE) program. Falls Free PA is a previously conducted cohort study comparing falls incidence between participants of Pennsylvania’s Healthy Steps for Older Adults program and a control group (20).
Study Sample
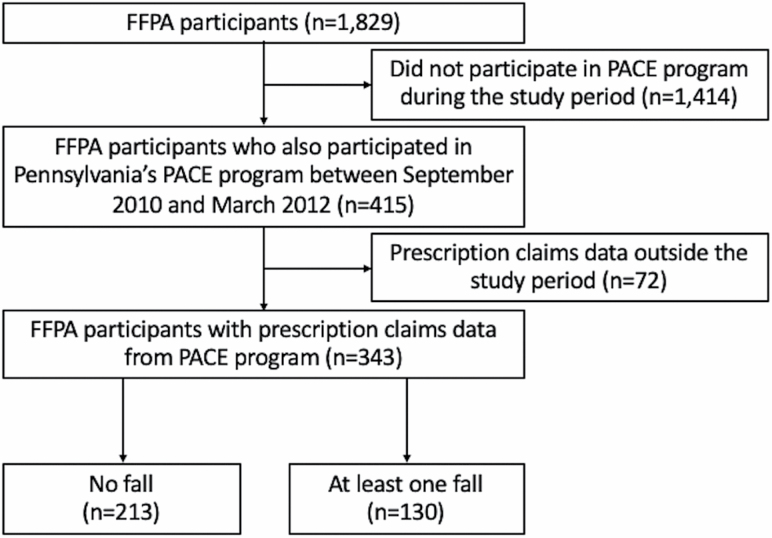
The original Falls Free PA included a total of 1,829 older adults who had participated in senior center activities across 19 Pennsylvania counties from 2010 to 2011. Among the Falls Free PA participants, our analysis included those who also participated in Pennsylvania’s PACE program between September 2010 and March 2012. PACE eligibility criteria (21) include (a) adults aged 65 years or older; (b) Pennsylvania residency for at least 90 days; (c) not being enrolled in the Medicaid prescription benefit; and (d) a total annual income of $14,500 or less (for a married couple combined total annual income must be $17,700 or less). Exclusion criteria were language use other than English or Spanish, and inability to participate in telephone follow-up calls (20). The patient selection flow diagram is shown in Figure 1.
Figure 1.
Flow diagram of participant selection. Notes: FFPA = Falls Free PA; PACE = Pennsylvania’s Pharmaceutical Assistance Contract for the Elderly.
Outcome Measurements
The primary outcome was self-reported falls during one of the monthly telephone interviews that occurred during the 12-month Falls Free PA cohort study. The incidence rate of fall-months was calculated as self-reported months in which participants reported at least one fall, per 100 person-months of follow-up, adjusted for participants’ activity levels. Interviews with Falls Free PA program participants who had fallen revealed that 89% of fall-months involved a single fall. Accordingly, we used fall-months per 100 person-months to indicate incidence. We adjusted follow-up time using the number of days participants reported physical activity during follow-up. This decision was based on evidence that older people with mobility limitations may reduce their activities to minimize the fall risk (22). We defined “physically active” as participating in moderate or vigorous activities for at least 30 min on a day (20).
Medication Exposure Variables
We used study participants’ PACE prescription claims data between the baseline and the 12-month follow-up to measure risks from medication exposure. The medication data gathered from prescription claims included drug name, daily dose, form, and days supply. All forms of oral, suppository, and transdermal medications were included in the current analysis. The FRIDs list proposed by the Swedish National Board of Health and Welfare (16) was used as the primary exposure of interest, utilizing the Anatomical Therapeutic Chemical classification system (Supplementary Table 1). Medications were considered to be “regular” for an individual participant when claims for a specific medication for the participant were provided 30 days or longer at least twice during the study period. For the current study, the number of regular medications was categorized into 0, 1–4, and 5 or more medications, with people with no medications serving as the reference group. We defined “polypharmacy” as the prescription of five or more “regular medications.” Likewise, categories were established based on a number of regular FRIDs of 0, 1, and 2 or more. Patients who took no FRIDs served as the reference group. The number of medications recommended to be avoided for older adults in table 2 of the Beers Criteria (23) was also counted and dichotomized into 0 and 1 or more for medications in the Beers Criteria. Drugs that require prescription indications (e.g., opioids: avoid, excludes pain management due to recent fractures or joint replacement) in the Beers Criteria were not measured because of data limitations. Other exposure variables to quantify the medication burden included the ACB (19) and the DBI (18). The drug class overlap between FRIDs, ACB, DBI, and the Beers Criteria are summarized in Supplementary Table 2. The cumulative medication burden of ACB, Drug Burden Index—sedative property (DBI-Se), and DBI-Ach was quantified based on the following equations (24,25) and split into categories of 0, low, and high burden, with cutoff values between low and high burden being the median values among those with scores above 0:
i: subject; a: drug name listed in ACB list.
s: drug name listed in DBI-Se list; D: daily dose of the drug; δ: minimum effective dose of the drug.
b: drug name listed in DBI-ACh list.
Covariates
At baseline, other variables measured included age, gender, race, living status, education level, income level, falls in the preceding year, self-rated mobility, medical conditions, the EuroQoL five-dimension three-level (EQ-5D-3L) summary index (26), and memory performance (Memory Impairment Screen—Telephone [MIS] (27)).
Statistical Analyses
A univariate negative binomial regression model was used to estimate incidence rate and 95% confidence interval (CI) for fall-months stratified by the medication exposure measures and to assess potential associations between each measure and falls risk. The associations between each medication exposure and falls incidence were examined with separate multivariable negative binomial regression models, adjusting for age, gender, baseline MIS score, baseline self-rated mobility, and falls in the preceding year. Activity-adjusted follow-up was used as an offset variable to give incidence rate ratios (IRRs). Multicollinearity was tested based on the variance inflation factor, with a cutoff at 10. Subgroup analyses of FRIDs on the risk of falls by FRID classes were evaluated adjusting for age, gender, and total number of regular medications. We used a significance level of .05 for hypothesis testing. All statistical analyses were performed using STATA/SE 15.0 (StataCorp LLC, College Station, TX).
Results
Among 415 older adults included in the Falls Free PA and Pennsylvania’s PACE program with fall incidence data, 72 were excluded from the current analysis due to missing data regarding prescription claims data, leaving 343 older adults. Their mean (SD) age was 78.3 (6.6) years, 35 (10.2%) were men, 257 (74.8%) lived alone, 104 (30.3%) used an assistive device for walking, and 108 (31.6%) reported that they had fallen at least once within the year prior to the research. The mean (SD) number of comorbidities among the 17 indicator chronic diseases was 3.8 (1.8), and the most common comorbidity was hypertension (n = 259, 75.5%) followed by arthritis (n = 248, 72.3%). The number with one or more medications identified with each specific medication exposure measure was 254 (74.1%) for FRIDs, 102 (29.7%) for ACB, 168 (49.0%) for DBI-total, 126 (36.7%) for DBI-Se, 82 (23.9%) for DBI-ACh, and 66 (19.2%) for the Beers Criteria. The mean (SD) number of prescribed regular medications were 3.99 (3.07) among those who did not fall during the follow-up and 4.33 (2.87) among fallers. Compared to nonfallers, participants with one or more falls were more likely to have fallen in the year before study participation and had lower baseline memory score and EQ-5D score (Table 1).
Table 1.
Baseline Characteristics of Study Participants (N = 343)
| Characteristics | Participants without fall-month (n = 213) | Participants with ≧1 fall-months (n = 130) | p Valuea |
|---|---|---|---|
| Age—mean (SD) | 77.9 (6.7) | 79.0 (6.4) | .13 |
| Gender (male, %) | 19 (8.9%) | 16 (12.3%) | .32 |
| Race (%) | .02 | ||
| White | 201 (94.8%) | 113 (86.9%) | |
| Black | 11 (5.2%) | 15 (11.5%) | |
| Others | 0 (0%) | 2 (1.6%) | |
| Live with someone (yes, %) | 51 (24.1%) | 35 (27.1%) | .53 |
| Education (college or more, %) | 44 (20.7%) | 40 (30.8%) | .04 |
| Income (sufficient for daily living, %) | 164 (80.0%) | 101 (80.8%) | .86 |
| Fall in the previous year (yes, %) | 49 (23.1%) | 59 (45.4%) | <.01 |
| Use of assistive devices (yes, %) | 59 (27.7%) | 45 (34.6%) | .18 |
| Number of comorbidity (SD) | 3.7 (1.9) | 4.0 (1.7) | .10 |
| Memory score (SD) (range 0–8) | 6.4 (1.4) | 6.1 (1.6) | .04 |
| Self-rated mobility (SD) (range 1–5)b | 2.6 (0.9) | 2.9 (1.0) | .01 |
| EQ-5D index (SD) (range 0–1) | 0.84 (0.01) | 0.80 (0.01) | .01 |
| Medication-related variables | |||
| Number of regular medications (SD)c | 3.99 (3.07) | 4.33 (2.87) | .31 |
| Number of regular FRIDs (SD)c | 1.54 (1.42) | 1.65 (1.28) | .47 |
| Number of Beers Criteria medications (SD)c | 0.23 (0.55) | 0.24 (0.49) | .95 |
| Cumulative ACB score (SD) | 0.55 (0.91) | 0.57 (0.81) | .84 |
| Cumulative DBI-Se score (SD) | 0.10 (0.22) | 0.15 (0.27) | .04 |
| Cumulative DBI-Ach score (SD) | 0.05 (0.15) | 0.06 (0.13) | .61 |
Notes: ACB = Anticholinergic Cognitive Burden; DBI-Ach = Drug Burden Index—anticholinergic property; DBI-Se = Drug Burden Index—sedative property; EQ-5D = EuroQOL 5 dimensions; FRIDs = Fall Risk-Increasing Drugs.
a p Values are for the comparison between fallers and nonfallers.
b1 = excellent; 5 = poor.
cNumber of drugs prescribed at least 30 days for at least twice during the study period.
Of the 343 participants, 152 (44.3%) reported at least one fall-month during the study period. There were 236 months with falls during 2,316 activity-adjusted person-months, which is equivalent to 10.2 fall-months per 100 person-months (95% CI: 10.07–10.33). The fall risk per 100 activity-adjusted person-months based on the different medication risk measure strata are shown in Table 2.
Table 2.
Medication Exposure and the Fall Risk Per 100 Activity-Adjusted Person-Months
| Medication exposure | N | Number of falls (months) | Person-months adjusted for activity | Fall-months incidence rate per 100 person-months of activity-adjusted follow-up |
|---|---|---|---|---|
| Number of regular medications | ||||
| 0 | 40 | 15 | 268.09 | 5.60 (3.13–9.23) |
| 1–4 | 166 | 115 | 1,153.93 | 9.97 (8.23–11.96) |
| 5+ | 137 | 106 | 893.64 | 11.86 (9.71–14.35) |
| Number of FRIDs | ||||
| 0 | 89 | 42 | 592.59 | 7.09 (5.11–9.58) |
| 1 | 90 | 64 | 640.21 | 10.00 (7.70–12.77) |
| 2+ | 164 | 130 | 1,082.86 | 12.01 (10.03–14.26) |
| Number of Beers Criteria medications | ||||
| 0 | 277 | 191 | 1,862.56 | 10.25 (8.85–10.40) |
| ≥1 | 66 | 45 | 453.1 | 9.93 (7.24–13.29) |
| Cumulative ACB scorea | ||||
| 0 | 141 | 88 | 967.12 | 9.10 (7.30–11.21) |
| Low ACB score | 100 | 60 | 665.58 | 9.01 (6.88–11.60) |
| High ACB score | 102 | 88 | 682.96 | 12.86 (10.33–15.87) |
| Cumulative DBI—sedative property scorea | ||||
| 0 | 217 | 136 | 1,500.74 | 9.06 (7.60–10.72) |
| Low DBI-Se score | 61 | 44 | 364.81 | 12.06 (8.76–16.19) |
| High DBI-Se score | 65 | 56 | 450.11 | 12.44 (9.40–16.16) |
| Cumulative DBI—anticholinergic property scorea | ||||
| 0 | 261 | 176 | 1,798.5 | 9.79 (8.39–11.34) |
| Low DBI-Ach score | 41 | 19 | 256.78 | 7.40 (4.45–11.55) |
| High DBI-Ach score | 41 | 41 | 260.38 | 15.75 (11.30–21.36) |
| Total | 343 | 236 | 2,315.66 | 10.20 (8.93–11.58) |
Notes: ACB = Anticholinergic Cognitive Burden; DBI-Ach = Drug Burden Index—anticholinergic property; DBI-Se = Drug Burden Index—sedative property; FRIDs = Fall Risk-Increasing Drugs; IRR = incidence rate ratio.
aThe cutoff values between the low and high scores were determined to be median values among those higher than 0 (ACB: 0.655, DBI-Se: 0.233, DBI-Ach: 0.150).
Of the six medication risk measures, only FRIDs showed a statistically significant dose effect in models adjusting for age, gender, and baseline mobility score. The adjusted IRR (95% CI) for 2+ FRIDs was 1.67 (1.04–2.68; Table 3). Polypharmacy (five or more regular medications) was marginally significant as a falls risk factor after adjusting for these baseline potential confounders (adjusted IRR 1.92 [95% CI: 0.94–3.92]). Other cumulative medication burden scales of ACB (19) and DBI (18), as well as the Beers Criteria (23), did not predict falls after adjusting for covariates. To assess the relative role of polypharmacy and FRIDs for falls risk, we conducted additional stratified analyses according to the presence or absence of two or more FRIDs in the medication list. Taking two or more FRIDs at baseline revealed a nonsignificant increase in the risk of falls among older adults with 0–4 regular medications, whereas FRIDs were no longer a falls risk among those with five or more regular medications (Table 4).
Table 3.
The Association of Medication Exposure and Falls Incidence Among Community-Dwelling Older Adults
| Medication exposure | Unadjusted incidence rate ratio for fall-months | Adjusted incidence rate ratio for fall-monthsa |
|---|---|---|
| Number of regular medications | ||
| 0 | Ref | Ref |
| 1–4 | 1.69 (0.84–3.42) | 1.65 (0.81–3.34) |
| 5+ | 2.08 (1.02–4.25)* | 1.92 (0.94–3.92) |
| Number of FRIDs | ||
| 0 | Ref | Ref |
| 1 | 1.37 (0.80–2.36) | 1.44 (0.84–2.45) |
| 2+ | 1.69 (1.04–2.74)* | 1.67 (1.04–2.68)* |
| Number of Beers Criteria medications | ||
| 0 | Ref | Ref |
| ≥1 | 1.01 (0.62–1.64) | 1.15 (0.72–1.84) |
| Cumulative ACB score | ||
| 0 | Ref | Ref |
| 0 < ACB ≦ 0.655b | 0.96 (0.60–1.54) | 1.00 (0.63–1.57) |
| 0.655 < | 1.41 (0.90–2.20) | 1.24 (0.80–1.92) |
| Cumulative DBI—sedative property score | ||
| 0 | Ref | Ref |
| 0 < DBI-Se ≦ 0.233b | 1.45 (0.87–2.42) | 1.32 (0.80–2.18) |
| 0.233 < | 1.40 (0.87–2.26) | 1.30 (0.82–2.06) |
| Cumulative DBI—anticholinergic property score | ||
| 0 | Ref | Ref |
| 0 < DBI-Ach ≦ 0.15b | 0.70 (0.37–1.33) | 0.52 (0.27–1.01) |
| 0.15 < | 1.76 (1.02–3.04)‡ | 1.51 (0.88–2.58) |
Notes: ACB = Anticholinergic Cognitive Burden; DBI = Drug Burden Index; FRIDs = Fall Risk-Increasing Drugs.
aAdjusted for age, gender, falls in the preceding year, baseline memory score, and baseline mobility score.
bThe cutoff values were determined to be median values among those higher than 0.
*p < .05.
Table 4.
Adjusted Incidence Rate Ratio of Falls Stratified by Number of Regular Medications and FRID
| Medication exposure | n | Number of fall-months | Person-months adjusted for activity | Fall-months incidence rate per 100 person-months of activity-adjusted follow-up | Adjusted incidence rate ratio for fall-monthsa |
|---|---|---|---|---|---|
| Number of regular medications 0–4 (n = 206) | |||||
| 0–1 FRID | 156 | 90 | 1,090.87 | 8.25 (6.63–10.14) | Ref |
| 2+ FRIDs | 50 | 40 | 331.15 | 12.08 (8.63–16.45) | 1.43 (0.84–2.43) |
| Number of regular medications 5+ (n = 137) | |||||
| 0–1 FRID | 23 | 16 | 141.93 | 11.27 (6.44–18.31) | Ref |
| 2+ FRIDs | 114 | 90 | 751.71 | 11.97 (9.63–14.72) | 0.96 (0.44–2.11) |
Notes: FRIDs = Fall Risk-Increasing Drugs.
aAdjusted for age, gender, falls in the preceding year, baseline memory score, and baseline mobility score.
The five most common regular FRID classes were agents acting on the renin–angiotensin system (42.9%), beta-blockers (28.9%), diuretics (23.9%), calcium channel blockers (23.0%), and antidepressants (16.3%). Among the 13 FRID classes, the only FRID class associated with an increased fall risk was antidepressants. This association remained significant after adjusting for age, gender, and total number of regular medications (adjusted IRR 1.71 [95% CI: 1.05–2.78]). Other FRID classes including cardiovascular medications and central nervous system medications revealed no significant association with falls (Table 5).
Table 5.
Incidence Rate Ratio (IRR) of Falls for the 13 FRID Classes Among Older Adults (Exploratory Analysis)
| FRIDs drug class | Fallers (N = 130) | Nonfallers (N = 213) | Crude IRR | Adjusted IRRa |
|---|---|---|---|---|
| Drugs that cause high risk of falling | ||||
| Opioids (%) | 1 (0.77) | 6 (2.8) | 0.24 (0.03–2.25) | 0.19 (0.02–1.80) |
| Antipsychotics (%) | 0 (0) | 3 (1.4) | — | — |
| Anxiolytics (%) | 11 (8.5) | 21 (9.9) | 0.75 (0.38–1.51) | 0.69 (0.34–1.37) |
| Hypnotics and sedatives (%) | 4 (3.1) | 7 (3.3) | 0.56 (0.16–2.01) | 0.49 (0.14–1.75) |
| Antidepressants (%) | 32 (24.6) | 24 (11.3) | 1.85 (1.15–2.96)* | 1.71 (1.05–2.78)* |
| Drugs that cause orthostatism/hypotension | ||||
| Vasodilators used in cardiac diseases (%) | 3 (2.3) | 6 (2.8) | 1.40 (0.45–4.32) | 1.22 (0.40–3.70) |
| Diuretics (%) | 30 (23.1) | 52 (24.4) | 1.27 (0.82–1.97) | 1.03 (0.63–1.68) |
| Beta-blockers (%) | 40 (30.8) | 59 (27.7) | 1.32 (0.87–1.98) | 1.10 (0.71–1.72) |
| Calcium channel blockers (%) | 31 (23.8) | 48 (22.5) | 1.38 (0.89–2.14) | 1.16 (0.73–1.84) |
| Agents acting on the renin–angiotensin system (%) | 52 (40.0) | 95 (44.6) | 0.97 (0.66–1.42) | 0.81 (0.53–1.22) |
| Alpha-adrenoreceptor antagonists (%) | 3 (2.3) | 2 (0.9) | 1.39 (0.30–6.37) | 0.99 (0.20–4.85) |
| Other antihypertensives (%) | 3 (2.3) | 3 (1.4) | 0.96 (0.23–4.08) | 0.77 (0.18–3.37) |
| Anti-Parkinson drugs (%) | 5 (3.8) | 3 (1.4) | 1.91 (0.66–5.55) | 1.56 (0.53–4.58) |
Notes: FRIDs = Fall Risk-Increasing Drugs.
aAdjusted for age, gender, and number of regular medications.
*p < .05.
Discussion and Implications
In this retrospective cohort study, the incidence of falls among low-income community-dwelling older adults increased with greater medication exposure across all three types of exposure. However, in multivariable models that adjusted for other falls risk factors, only the use of two or more FRIDs remained a significant predictor. Polypharmacy (five or more regular medications) was only marginally associated with falls risk after adjusting for age, gender, baseline MIS score, baseline self-rated mobility, and falls in the preceding year. Similar to our results, Zia et al. have reported that the use of two or more FRIDs independently predicted recurrent and injurious falls (6). Likewise, Bennett et al. revealed an association between the number of FRIDs on discharge and a greater risk of recurrent falls (17). It has been argued that the harm of polypharmacy may not consistently outweigh its benefit, depending on the appropriateness of medications prescribed (28). At the same time, polypharmacy is known to be associated with inappropriate prescribing (13). Thus, it is possible that polypharmacy increases the risk of FRIDs exposure that poses an increased risk of falling. Our results imply that the effect of polypharmacy on falls risk is likely mediated by the presence of FRIDs. The inconsistent association of polypharmacy with falls in previous studies may result from not taking into account the number of FRIDs in counts of medications.
As stated before, we hypothesized that state-of-the-art medication burden indices, such as the cumulative ACB and cumulative DBI, would be superior to polypharmacy and PIMS based on prescribing guidance, such as the FRIDs list and Beers Criteria. Although evidence is limited regarding such head-to-head comparisons, the association between medication burden scales, such as higher DBI scores and decreases in physical function among older adults, suggests such an association (29,30). However, previous research has yielded conflicting evidence regarding the effect of DBI on falls risk. A study conducted by Wilson et al. revealed a significant association between DBI and falls in older adults living in residential aged care facilities (31). More recently, Cardwell et al. reported that a higher DBI score was associated with a greater risk of mortality but not with an increased rate of falls among older participants of a cohort study in New Zealand (32). Our results imply that the simple count of FRIDs, as well as the number of regular medications, could be at least as effective as the more complex medication burden measurements in measuring falls risk among older adults. More evidence is required to confirm the utility of FRIDs for screening and intervention of older adults at risk for falls.
Based on our stratified analyses, polypharmacy was not a significant falls risk in models that stratified for the presence of two or more FRIDs. Thus, we infer that exposure to FRIDs is important for the polypharmacy–falls relationship. This inference is supported by a large-scale cross-sectional study conducted in the Netherlands that revealed an association between polypharmacy and falls only when falls risk-increasing medications were included in prescriptions (33). Further investigations with larger sample sizes are required to confirm whether the number of medications prescribed itself has some effect on falls risk when FRIDs are prescribed.
When examining individual FRID classes and falls incidence, antidepressants was the only FRID class with an increased falls risk. Our finding is consistent with findings by Kuschel et al., who reported that central nervous system drugs including antidepressants showed an increased risk of falls (34). In our analysis, other central nervous system drugs were not significant for falls risk, potentially due to the relatively small number of prescriptions involving these drugs. This may result from the fact that the PACE program generally does not encourage clinicians to prescribe high-risk medications. In addition, our analysis examined all falls rather than injurious falls or recurrent falls, which is often the outcome for research on falls. It is noteworthy that another study of community-dwelling older adults found antidepressants to be associated with recurrent falls in community-dwelling older adults (adjusted odds ratio: 1.48; 95% CI = 1.12–1.96) (35).
Several limitations of our study need to be considered. Our data did not include over-the-counter medications, which could also contain sedative and anticholinergic properties. Assuming that multimorbid older adults with polypharmacy are more likely to take over-the-counter medications, our results may overestimate the effect of prescribed FRIDs. In addition, our data set did not include information regarding medication adherence, which is a crucial aspect of pharmacoepidemiology studies. Secondly, we found increased effect sizes for all of the medication measures, except for the Beer’s Criteria, which were not significant possibly due to the small sample. Thus, we are unable to rule out that some of these other measures are also related to falls. Finally, PACE program features (e.g., eligibility based on low income) require caution in generalizing our findings to the population of community-dwelling older adults.
The major finding of our results is that the presence of multiple FRIDs in a prescription is an independent risk factor for falls, even in people with few medications. Despite the growing body of evidence regarding the association between medication exposure and falls, there is limited evidence available for the effect of reducing FRIDs. A few studies have demonstrated the potential benefit of deprescribing on falls prevention (36,37), while other randomized controlled trials have failed to demonstrate clinical benefit (38,39). Thus, future studies with sufficient statistical power will be required to confirm whether deprescribing focused on FRIDs, either overall or for specific FRID classes, effectively prevents falls and subsequent adverse health outcomes among older adults.
Supplementary Material
Funding
This research was supported by Cooperative Agreement Number DP002657 from the Centers for Disease Control and Prevention, Prevention Research Centers program, National Institute on Aging Grant K01 AG044433, U.S. National Library of Medicine Grant R01 LM011838, and National Institutes of Health AG024827, Pittsburgh Older Americans Independence Center.
Conflict of Interest
None declared.
References
- 1. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults ages over 65 years. Morb Mortal Wkly Rep. 2016;65(37):993–998. doi: 10.1093/geront/gnw074 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO Global Report on Falls Prevention in Older Age. World Health Organization. doi: 10.1353/jowh.2004.0010 [DOI] [Google Scholar]
- 3. Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. doi: 10.1001/jama.1995.03520410042024 [DOI] [PubMed] [Google Scholar]
- 4. Stevens J, Ryan G, Kresnow M. Fatalities and injuries from falls among older adults—United States, 1993–2003 and 2001–2005. JAMA. 2007;297(1):32–33. doi: 10.1001/jama.297.1.32 [DOI] [Google Scholar]
- 5. Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43(11):1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x [DOI] [PubMed] [Google Scholar]
- 6. Zia A, Kamaruzzaman SB, Tan MP. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr Gerontol Int. 2017;17(3):463–470. doi: 10.1111/ggi.12741 [DOI] [PubMed] [Google Scholar]
- 7. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people. J Am Geriatr Soc. 1999;47:40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x [DOI] [PubMed] [Google Scholar]
- 8. Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KMMC. Meta-analysis of the impact of 9 medication classes on falls in elderly persons (Archives of Internal Medicine (2009) 169, 21 (1952–1960)). Arch Intern Med. 2010;170(5):477. doi: 10.1001/archinternmed.2009.510 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Zhu LL, Zhou Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Ther Clin Risk Manag. 2014;10(1):437–448. doi: 10.2147/TCRM.S63756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agostini JV, Han L, Tinetti ME. The relationship between number of medications and weight loss or impaired balance in older adults. J Am Geriatr Soc. 2004;52(10):1719–1723. doi: 10.1111/j.1532-5415.2004.52467.x [DOI] [PubMed] [Google Scholar]
- 11. Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43(8):912–918. doi: 10.1111/imj.12203 [DOI] [PubMed] [Google Scholar]
- 12. Lim LM, McStea M, Chung WW, et al. Prevalence, risk factors and health outcomes associated with polypharmacy among urban community-dwelling older adults in multiethnic Malaysia. PLoS One. 2017;12(3):1–18. doi: 10.1371/journal.pone.0173466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried TR, Goldstein MK, Trentalange M, Martin DK, O’Leary J, Towle V. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi: 10.1111/jgs.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 15. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Swedish National Board of Health and Welfare. Indikatorer för god Läkemedelsterapi hos Äldre 2010. https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18085/2010-6-29.pdf.
- 17. Bennett A, Gnjidic D, Gillett M, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug–drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs and Aging. 2014;31(3):225–232. doi: 10.1007/s40266-013-0151-3 [DOI] [PubMed] [Google Scholar]
- 18. Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–787. doi: 10.1001/archinte.167.8.781 [DOI] [PubMed] [Google Scholar]
- 19. Boustani MA, Campbell N, Munger S, Maidment I, Chris F. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. http://www.futuremedicine.com/doi/pdf/10.2217/1745509X.4.3.311%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed8&NEWS=N&AN=2008361540. [Google Scholar]
- 20. Albert SM, King J, Boudreau R, Prasad T, Lin CJ, Newman AB. Primary prevention of falls: effectiveness of a statewide program. Am J Public Health. 2014;104(5):77–84. doi: 10.2105/AJPH.2013.301829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aging PD of Prescriptions. Commonwealth of Pennsylvania http://www.aging.pa.gov/aging-services/prescriptions/Pages/default.aspx. Published 2017.
- 22. Wijlhuizen GJ, de Jong R, Hopman-Rock M. Older persons afraid of falling reduce physical activity to prevent outdoor falls. Prev Med (Baltim). 2007;44(3):260–264. doi: 10.1016/j.ypmed.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 23. Samuel MJ American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 24. Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85–90. doi: 10.1111/jgs.13206 [DOI] [PubMed] [Google Scholar]
- 25. Ie K, Chou E, Boyce RD, Albert SM. Potentially harmful medication use and decline in health-related quality of life among community-dwelling older adults. Drugs Real World Outcomes. 2017;4(4):257–264. doi: 10.1007/s40801-017-0123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Group E EuroQol—a new facility for the measurement of health-related quality of life. Health Policy (New York). 1990;16(3):199–208. https://www.ncbi.nlm.nih.gov/pubmed/?term=10109801. [DOI] [PubMed] [Google Scholar]
- 27. Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x [DOI] [PubMed] [Google Scholar]
- 28. Gnjidic D, Tinetti M, Allore HG. Assessing medication burden and polypharmacy: finding the perfect measure. Expert Rev Clin Pharmacol. 2017;10(4):345–347. doi: 10.1080/17512433.2017.1301206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gnjidic D, Cumming RG, Le Couteur DG, et al. Drug burden index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68(1):97–105. doi: 10.1111/j.1365-2125.2009.03411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12):1142–1149.e1-2. doi: 10.1016/j.amjmed.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59(5):875–880. doi: 10.1111/j.1532-5415.2011.03386.x [DOI] [PubMed] [Google Scholar]
- 32. Cardwell K, Kerse N, Ryan C, et al. The association between drug burden index (DBI) and health-related outcomes: a longitudinal study of the “Oldest Old” (LiLACS NZ). Drugs and Aging. 2020;37(3):205–213. doi: 10.1007/s40266-019-00735-z [DOI] [PubMed] [Google Scholar]
- 33. Ziere G, Dieleman JP, Hofman A, Pols HAP, Van Der Cammen TJM, Stricker BHC. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61(2):218–223. doi: 10.1111/j.1365-2125.2005.02543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuschel BM, Laflamme L, Möller J. The risk of fall injury in relation to commonly prescribed medications among older people—a Swedish case-control study. Eur J Public Health. 2015;25(3):527–532. doi: 10.1093/eurpub/cku120 [DOI] [PubMed] [Google Scholar]
- 35. Marcum ZA, Perera S, Thorpe JM, et al. Antidepressant use and recurrent falls in community-dwelling older adults: findings from the health ABC study. Ann Pharmacother. 2016;50(7):525–533. doi: 10.1177/1060028016644466.Antidepressant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of “Fit fOR The Aged” (FORTA) on pharmacotherapy and clinical endpoints—a pilot randomized controlled study. Eur J Clin Pharmacol. 2014;70(10):1261–1267. doi: 10.1007/s00228-014-1731-9 [DOI] [PubMed] [Google Scholar]
- 37. Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A Quality Use of Medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187(1):23–30. http://www.ncbi.nlm.nih.gov/pubmed/17605699. [DOI] [PubMed] [Google Scholar]
- 38. Sjöberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture—a randomized controlled study. J Am Geriatr Soc. 2013;61(9):1464–1472. doi: 10.1111/jgs.12412 [DOI] [PubMed] [Google Scholar]
- 39. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–854. doi: 10.1038/clpt.2011.44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.