Abstract
The pancreatic islet is a dense cellular network comprised of several cell types with endocrine function vital in the control of glucose homeostasis, metabolism, and feeding behaviour. Within the islet, endocrine hormones also form an intricate paracrine network with supportive cells (endothelial, neuronal, immune) and secondary signaling molecules regulating cellular function and survival. Modulation of these signals has potential consequences for diabetes development, progression, and therapeutic intervention.
Beta cell loss, reduced endogenous insulin secretion, and dysregulated glucagon secretion are hallmark features of both type 1 and 2 diabetes that impact systemic regulation of glucose, but also contribute to the function and survival of cells within the islet. Advancing research and technology has revealed new islet biology (cellular identity and transcriptomes) and identified previously unrecognized paracrine signals and mechanisms (somatostatin and ghrelin paracrine actions), while shifting prior views of intra-islet communication. This review will summarize the paracrine signals regulating islet endocrine function and survival, the disruption and dysfunction that occurs in diabetes, and potential therapeutic targets to preserve beta cell mass and function.
Keywords: paracrine signaling, islet function, survival, islet biology, diabetes
Architecture of islet paracrine signaling
Pancreatic islets are endocrine organs comprised of several cell types responsible for glucose control, metabolism, and feeding behaviour. Dysfunction or loss of any one cell type contributes to development of congenital hyperinsulinism, neonatal diabetes, type 1 and 2 diabetes mellitus. Islet beta cells secrete insulin in the post-prandial state to suppress hepatic glucose production while stimulating glucose uptake in skeletal muscle and white adipose tissue. Insulin action is opposed by the primary alpha cell secretory product glucagon, which mobilizes energy stores, mainly in the liver, to increase blood glucose during periods of fasting. The delta cell is the third main islet cell type, which secretes the inhibitory hormone somatostatin for regulation of distal endocrine functions, such as growth hormone and thyroid stimulating hormone release. Ghrelin producing epsilon cells and pancreatic polypeptide (PP) cells comprise a minor proportion of islets and exhibit opposing actions on feeding behavior and gastric secretions. While islet hormones maintain systemic homeostasis, they also critically regulate cells within the islet through paracrine signaling mechanisms to fine-tune cell function and survival.
The densely packed nature of islet endocrine cells and blood vessels facilitates a paracrine signaling network mediated by proximity, cell contact via gap junctions, and local blood flow (Figure 1). Rodent beta cells dominate the islet core surrounded mainly by alpha cells in the mantle; in contrast, human islets are a heterogenous mixture of endocrine cells at varying proportions between islets [1–3]. Islet endocrine cells are therefore well-positioned to strongly influence nearby cells through paracrine signaling factors, including islet hormones, peptides, neurotransmitters, metabolites, and extracellular vesicles. Dysregulation of these factors contribute substantially to impaired glucose regulation, insulin resistance, and diabetes [4–9].
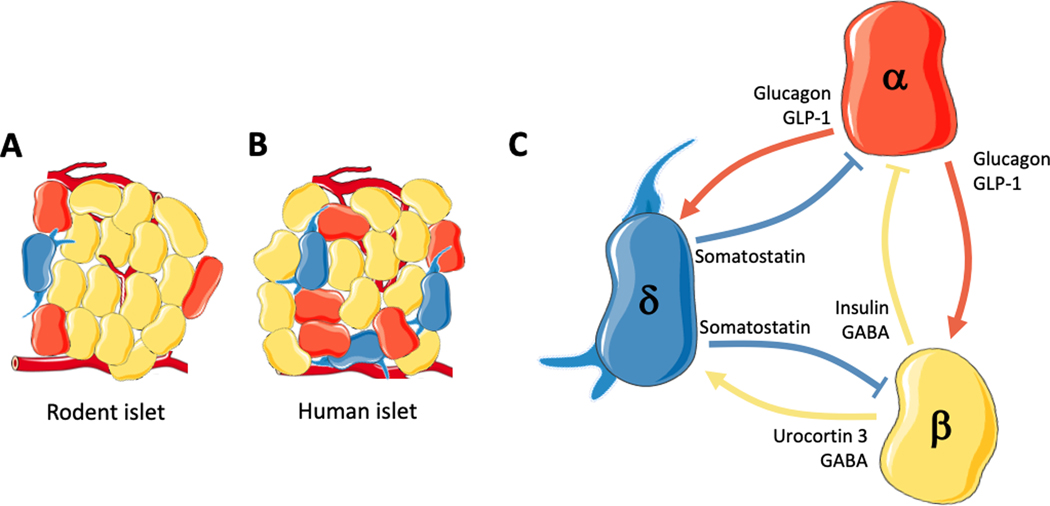
Figure 1. The islet paracrine signaling network.
The three primary islet endocrine cells, alpha (red), beta (yellow), and delta (blue) regulate the function and survival of nearby cells within the islet. A) Rodent and B) human islet cells are densely packed and surrounded by blood vessels to facilitate a paracrine signaling network mediated by proximity, cell contact via gap junctions, and local blood flow. C) Primarily, in response to high glucose alpha cells secrete glucagon to combat systemic hypoglycemia, as well as initiate a negative feedback loop through stimulation of somatostatin release from delta cells. Additionally, during high glucose small glucagon paracrine effects have been observed to stimulate insulin release. Alpha cells also secrete GLP-1 under some conditions, which stimulates insulin and somatostatin secretion from beta and delta cells, respectively. During hyperglycemia, somatostatin and insulin negatively feedback on alpha cells to decrease glucagon secretion. Beta cells also secrete Urocortin 3 to stimulate somatostatin secretion from delta cells, which negatively feeds back on beta cells and GABA that stimulates somatostatin secretion while inhibiting glucagon secretion. GABA and GLP-1 promote beta cell proliferation and survival, while somatostatin inhibits beta cell proliferation.
Insulin
Paracrine insulin action modulates the function and survival of islet endocrine cells (Figure 2–3). Insulin acts on nearby alpha cells to decrease glucagon secretion [10–12, 4] to maintain an anabolic state after feeding. The pulsatile release of insulin that controls glucagon secretion is best described by the “switch off hypothesis”, whereby switching off insulin during rat pancreatic infusion turns on glucagon secretion in response to hypoglycemia [5, 13, 14]. Accordingly, infusion of insulin secretagogues to elevate intraislet insulin turns off hypoglycemia-induced glucagon secretion in humans [4].
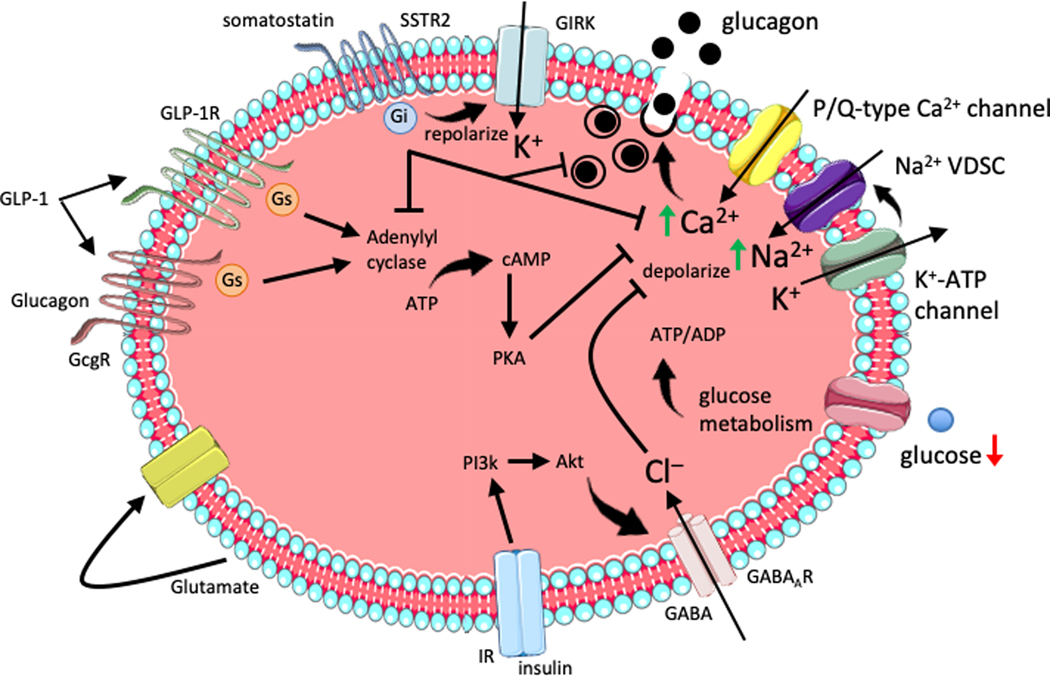
Figure 2. Islet paracrine regulation of alpha cell glucagon secretion.
During hypoglycemia, reduced glucose metabolism lowers the ATP/ADP ratio reducing inhibition of the K+-ATP channel. Moderate depolarization by intracellular K+ accumulation stimulates the voltage-dependent sodium channel (VDSC) leading to action potential firing and activation of P/Q-type Ca2+ channels. The rise in intracellular calcium stimulates glucagon exocytosis. Insulin secretion in response to high glucose activates the insulin receptor (IR) on alpha cells to induce PI3k-Akt recruitment of the GABAA receptor to the membrane. Subsequent GABA stimulation induces Cl– influx leading to hyperpolarization and inhibition of actional potential firing and glucagon exocytosis. GLP-1 signaling in alpha cells can occur through either the GLP-1R or glucagon receptor (GcgR) to activate adenylyl cyclase, elevation of intracellular cAMP and PKA-dependent inhibition of Q/P-type calcium channels, prevent glucagon exocytosis. Glucagon auto-/paracrine effects on alpha cells are suggested to occur similar to GLP-1 with weak activation of adenylyl cyclase. Somatostatin inhibits glucagon secretion through somatostatin receptor (SSTR) 2 activation of inhibitory Gi protein which decreases adenylyl cyclase and voltage-gated calcium channel activity, as well as inducing membrane repolarization and inhibition of action potentials via the G protein-activated inward rectifier K+ (GIRK) channel. Glutamate released by alpha cells may act in an auto-/paracrine manner to stimulate glucagon secretion.
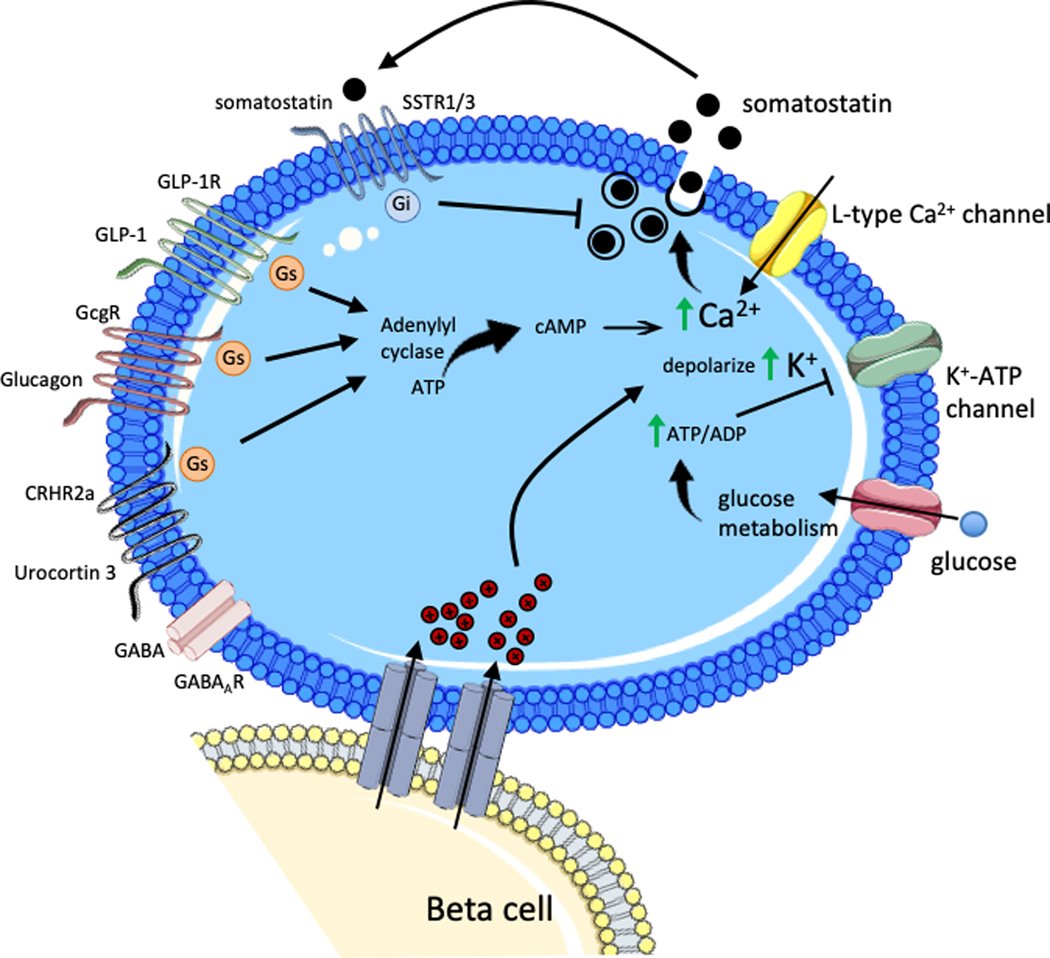
Figure 3. Islet paracrine regulation of delta cell somatostatin secretion.
During hyperglycemia, increasing glucose is taken up by the delta cell and metabolized to generate ATP, which increases the ATP/ADP ratio causing closure of K+-ATP channel. The resulting depolarization by intracellular K+ accumulation stimulates the L-type calcium channel leading to action potential firing and a rise in intracellular calcium that stimulates somatostatin exocytosis. Gap junctions connecting beta cells and delta cells allows transmission of electrical signals from beta cells induce depolarization and somatostatin secretion in adjoining delta cells. Beta cell secretory molecules Urocortin 3 and GABA, packaged with insulin granules, mediate beta cell action on delta cells. Urocortin 3 acts through the corticotropin-releasing hormone receptor (CRHR) 2a activating the stimulatory Gs protein, which induces adenylyl cyclase. Subsequent elevation of intracellular cAMP and rise in intracellular calcium levels leads to somatostatin exocytosis. GLP-1 and glucagon similarly act through Gs activation of adenylyl cyclase to stimulate somatostatin secretion. Somatostatin auto-/paracrine signaling in delta cells occurs through somatostatin receptor (SSTR) 1/3 activation of inhibitory Gi protein.
To clarify insulin-specific effects on alpha cells, Kawamori et al. generated glucagon-Cre driven insulin receptor (IR) knockout (KO) mice [6]. During the fed state, alpha cell IRKO mice develop hyperglycemia and hyperglucagonemia. In contrast, the glucagon response to fasting induced hypoglycemia was blunted in alpha cell IRKO, consistent with the switch off hypothesis. In the absence of IR signaling, insulin depletion via beta cell destruction did not permit a further increase in glucagon secretion, supporting a role for insulin regulation of glucagon secretion. Mechanistically, insulin receptor signaling induces alpha cell membrane hyperpolarization through ATP-regulated K+ channel activation and recruitment of GABAA receptor inward Cl− channels to lower intracellular calcium and glucagon release [15–17] (Figure 2).
Dysregulated glucagon in insulin deficient diabetes further underscores the significance of paracrine insulin action on alpha cells [18–20]. During hyperglycemia, glucagon secretion is inappropriately high in the absence of insulin suppression, which further propagates hyperglycemia in insulin deficient diabetes [21–24]. Iatrogenic hypoglycemia and loss of glucagon regulation is a serious concern for patients with T1D and to a lesser extent T2D [25–27]. The counterregulatory measures to hypoglycemia progressively decline during diabetes, failing to activate glucagon secretion, which is mainly attributed to the deterioration of endogenous insulin production [4, 28]. Supporting these observations, reducing insulin through beta cell destruction in Wistar rats or pigs abrogates the glucagon response to hypoglycemia, while pancreatic infusion of pulsatile insulin restored glucagon regulation in STZ-diabetic rats [5, 13]. T1D alpha cells are associated with reduced expression of primary alpha cell transcription factors (ARX, MAFB) and increased expression of beta cell markers (Pdx-1, Nkx6.1), which might account for decreased glucagon secretion [29, 30]. Alpha cells might also secrete insulin or convert into beta cells in insulin deficient diabetes [30–32]. Interestingly, alpha cell IRKO mice have a decreased alpha to beta cell mass ratio in aging [6], potentially supporting conversion of alpha to beta cells. Thus, insulin signaling in alpha cells rather than hypoglycemia may be important for regulation of alpha cell function and identity.
Of note, reports from rat studies suggest that secreted zinc bound to insulin inhibits glucagon secretion through activation of ATP-sensitive K+ channels [15, 33, 34]. Rat pancreatic artery infusions of insulin or zinc-chloride alone during hypoglycemia improved the glucagon response, while switching off zinc-free insulin infusions had no effect on glucagon release [33]. In contrast, studies from humans and immortalized alpha cell lines suggest zinc has no effect on glucagon secretion [35, 36]. Additionally, glucagon secretion was normal in mouse islets lacking the primary beta cell Zn2+ transporter ZnT8 (Slc30a8) [37]. While these divergent observations may reflect species specific differences or experimental context, insulin suppressive effects on glucagon might involve molecules packaged in insulin secretory granules.
Insulin action on delta cells and somatostatin secretion has evolved over the years. Initial studies from the 1980s demonstrated insulin inhibits glucose induced somatostatin secretion in the perfused rat and chicken pancreas [38, 39]. More recently, work with isolated mouse islets suggested insulin had no effect on somatostatin release during basal or elevated glucose stimulation [40]. Additional reports implicate urocortin 3 and gamma-aminobutyric acid (GABA) co-released from insulin secretory granules as likely mediators of the beta cell stimulatory effects on somatostatin [41, 42] (Figure 3). Furthermore, beta cells are coupled to delta cells through gap junctions allowing the flow of electrical activity between cells to stimulate somatostatin secretion, which in turn suppresses alpha cell function [43]. These recent observations suggest insulin may not have direct effects on delta cells, although it remains to be determined if insulin has any impact on delta cell growth and survival. Interestingly, delta cells convert to beta cells in young diabetic mice following near total ablation of beta cells [44]. These effects reflect the loss of sufficient insulin signaling in delta cells, but otherwise may result from a compensatory response to changes in systemic glucose or other factors.
Significant efforts have been put towards understanding beta cell specific effects of insulin autocrine or paracrine actions. Insulin receptor overexpression in βTC6-F7 beta cells increased insulin gene transcription and insulin content, while mutant kinase-dead insulin receptor expression modestly affected insulin secretion [45]. Insulin stimulation of insulin gene transcription involved signaling through IRS-2, PI-3k, p70S6K, and CaM kinase in rat islets and HIT-T15 cells, suggesting an autocrine mechanism for insulin action on beta cells [46]. Similarly, glucose-induced proliferation and survival of MIN6 beta cells required insulin and PI-3k signaling [47]. These in vitro studies support an autocrine mechanism of insulin action on beta cells to replenish insulin stores and promote growth.
Initial in vivo studies attempted to characterize loss of insulin receptor signaling in beta cells. [48]. Beta cell insulin receptor knockout (BIRKO) mice exhibited glucose intolerance, reduced insulin secretion, and reduced beta cell mass [48, 49]. Surprisingly, only 25% of BIRKO mice were diabetic at 7–8 months of age [49]. Further examination of insulin effects on beta cells challenged the interpretation of the BIRKO model. First, insulin also signals through the IGF-1 receptor [50–52] and thus defining the distinct contribution of insulin and IGF-1 receptor signaling is critical. Beta cell loss of IGF-1R impairs glucose-stimulated insulin secretion with no effect on beta cell mass [51, 53]. Beta cell double knockouts of IR and IGF-1R enhanced apoptosis accompanied by reduced beta cell mass, hyperglycemia, and glucose intolerance [53]. Despite these findings implicating insulin and IGF-1 receptor signaling in beta cell function and survival, the distinct contribution of each hormone in vivo remains unclear. Perhaps studying double null IGF-1, IGF-1R mice [54], other related conditional deletion models, or pancreas insulin perfusions in IGF-1R KO mice will better inform insulin receptor signaling specific beta cell effects.
The second concern regarding mouse models of beta cell specific IR signaling was raised by Wicksteed et al. who demonstrated widespread Cre recombination in the brain of multiple RIP-Cre mouse lines [55]. Importantly, insulin signaling through IR and IGF-1R in the brain modulates hepatic glucose output, hypoglycemic responses, appetite, white fat mass, reproductive function, and body temperature (reviewed in detail by Kleinridders et al [56]), which could impact interpretation of RIP-Cre deletion of IR. Lastly, the RIP-Cre and MIP-Cre promoter constructs contain a human growth hormone (hGH) minigene associated with hGH protein biosynthesis and unintended beta cell and off target endocrine effects [57–59]. The last two concerns of beta cell specificity and transgene activity were addressed by the development of a beta cell specific Ins1-Cre knockin mouse [60]. Female Ins1-Cre;InsRfl/fl mice with beta cell specific deletion of IR exhibit improved glucose tolerance through increased insulin secretion, indicating a negative feedback role for insulin secretion [61, 62]. Thus, improved mouse models (Table 1) will advance our understanding of insulin action and reveal new islet biology.
Table 1.
Genetic models used in islet paracrine signaling studies.
| Model | Target | Cre driver | Goal | Caveats | Reference |
|---|---|---|---|---|---|
| bTC6-F7 cell IR overexpression | insulin receptor | N/A | gain of function | [45] | |
| bTC6-F7 cell IR kinase dead | insulin receptor | N/A | loss of function | [45] | |
| beta cell specific IR KO | insulin receptor | Insulin I Cre knockin | loss of function | [60–62] | |
| BIRKO | insulin receptor | Rat insulin II promoter | loss of function | promoter contains hGF minigene deletion in brain | [48, 49] |
| beta IGF-1R KO | IGF-1 receptor | Rat insulin II promoter | loss of function | promoter contains hGF minigene deletion in brain | [51, 53] |
| beta double IR IGFR KO | IR and IGF-1R | Rat insulin II promoter | loss of function | promoter contains hGF minigene deletion in brain | [53] |
| alpha cell IR KO | insulin receptor | glucagon promoter | loss of function | [6] | |
| delta cell IR KO | insulin receptor | SST-Cre | loss of function | not specific to islet delta cells | [119] |
| GcgR null mice | GcgR | N/A | loss of function | [73, 74] | |
| beta cell GcgR KO | GcgR | Mouse insulin I promoter | loss of function | promoter contains hGF minigene | [83] |
| beta cell GcgR overexpression | GcgR | Rat insulin II promoter | gain of function | promoter contains hGF minigene deletion in brain | [66] |
| GLP-1R null mice | GLP-1R | N/A | loss of function | [83] | |
| SSTR2 null mice | SSTR2 | N/A | loss of function | [116] | |
| SSTR5 null mice | SSTR5 | N/A | loss of function | [126] | |
| SST-Cre;R26-DTA | Somatostatin+ cells | SST-Cre | loss of function | [118] | |
| Ghrelin null mice | Ghrl | N/A | loss of function | [130] | |
| Ghrelin receptor null mice | Ghsr | N/A | loss of function | [130] | |
| ZnT8 null mice | Slc30a8 | N/A | loss of function | [37] |
Glucagon
Glucagon was originally proposed to be a pancreatic contaminant with glucose mobilizing properties and later identified as the secretion product of islet alpha cells [63, 64]. It is now well-established glucagon is secreted in response to hypoglycemia and inhibited during hyperglycemia, acting as the key counter-regulatory hormone to insulin [65]. As such, glucagon stimulates glycogenolysis and gluconeogenesis by the liver to maintain glucose levels during fasting. Additionally, glucagon serves an important paracrine role to regulate function and survival of islet endocrine cells (Figure 1–4).
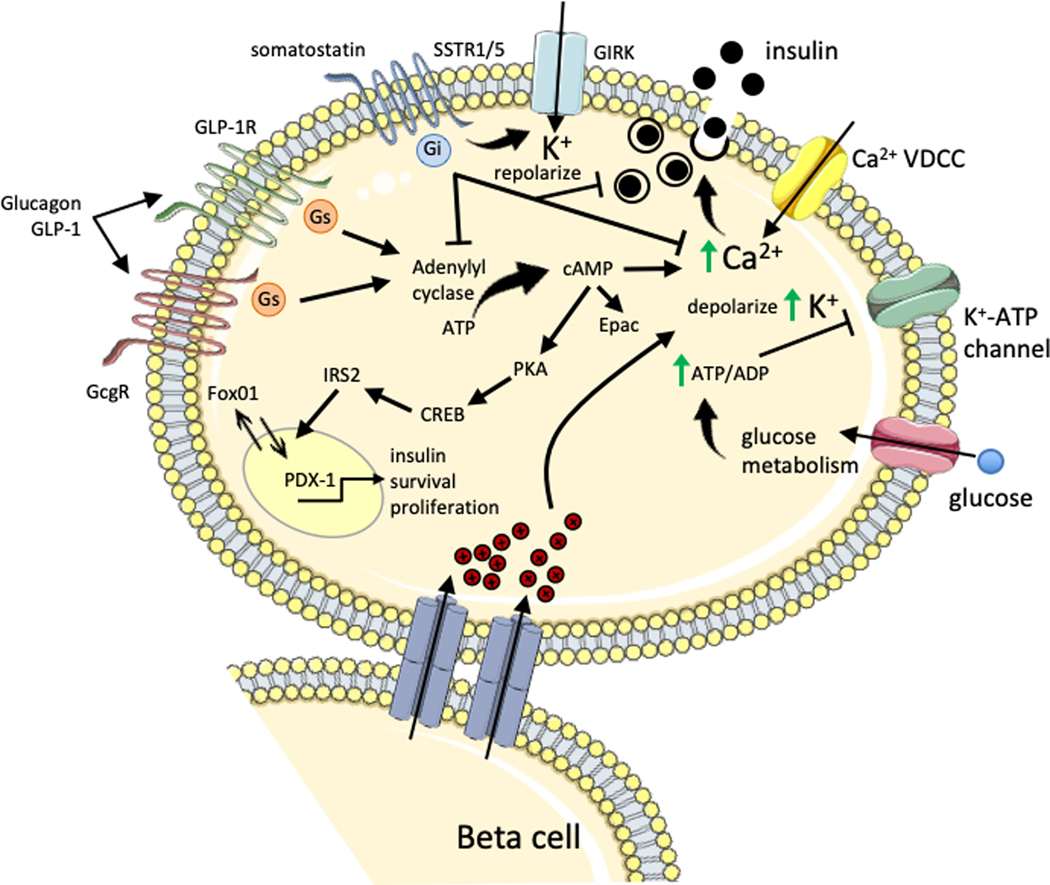
Figure 4. Islet paracrine regulation of beta cell insulin secretion.
During hyperglycemia, increasing glucose is taken up by the beta cell and metabolized to generate ATP, which increases the ATP/ADP ratio causing closure of K+-ATP channel. The resulting depolarization by intracellular K+ accumulation stimulates the voltage-dependent calcium channel (VDCC) leading to action potential firing and a rise in intracellular calcium that stimulates insulin exocytosis. Gap junctions connecting beta cells allows transmission of electrical signals to induce depolarization and insulin secretion in adjoining beta cells. GLP-1 signaling in beta cells can occur through either the GLP-1R or glucagon receptor (GcgR) coupled to the stimulatory Gs protein that activates adenylyl cyclase. Subsequent elevation of intracellular cAMP and rise in intracellular calcium levels leads to insulin exocytosis. These signals are potentiated by Epac activation of L-type voltage dependent calcium channels and ER ryanodine receptor calcium channels. GLP-1 induced PKA activation also stimulates CREB-IRS2 nuclear localization of Pdx-1 which activates transcription of the insulin gene, as well as positive regulators of survival and proliferation. Paracrine release of glucagon during elevated glucose is suggested to induce insulin secretion through activation of GcgR or GLP-1R on beta cells. Somatostatin inhibits insulin secretion through somatostatin receptor (SSTR) 1/5 activation of inhibitory Gi protein which decreases adenylyl cyclase and voltage-gated calcium channel activity, as well as inducing membrane repolarization and inhibition of action potentials via the G protein-activated inward rectifier K+ (GIRK) channel. Somatostatin also inhibits beta cell proliferation, while GLP-1 promotes beta cell proliferation and survival.
The glucagon receptor (GcgR) is expressed on both beta and delta cells [66–68]. As early as 1965, Glucagon was described as an insulin secretagogue [69] capable of potentiation of glucose stimulated insulin secretion from isolated rat beta cells and human islets [70–72]. Further evidence comes from beta cell glucagon receptor (GcgR) overexpressing mice, which exhibit increased insulin secretion, improved glucose control, and a small increase in beta cell volume [66]. Reciprocal studies examining loss of glucagon receptor signaling do not sufficiently address beta cell specific glucagon signaling. Decreased serum insulin in GcgR null or antagonist-treated mice is likely due to lower glucose levels (via decreased hepatic glucose production) and improved insulin sensitivity [73–78]. Regardless, there is plenty of evidence to support a role for glucagon as an insulin secretagogue, including several isolated pancreas perfusion studies.
Glucagon effects on insulin secretion were proposed to be mediated only through the GcgR [79] (Figure 4). However, glucagon may also bind the GLP-1 receptor (GLP-1R) on beta cells to induce insulin release (Figure 4), although with lower binding affinity than the GcgR [71, 80–82]. Using purified rat beta cells, glucagon-induced insulin secretion was inhibited by the GLP-1R antagonist exendin 9–39 [71]. However, in situ experiments with the isolated perfused rat pancreas suggested glucagon-induced insulin secretion at basal or high glucose could be inhibited by a GcgR antagonist but not with the GLP-1R antagonist exendin 9–39 [79, 82]. A more recent study used multiple genetic mouse models and pharmacological antagonists to define the mechanisms of glucagon action on insulin release [83]. Isolated pancreas perfusions performed in GcgR or GLP-1R null mice both exhibited blunted insulin secretion following exogenous glucagon administration compared to wild-type mice. Similarly, exendin 9–39 administration reduced glucagon-induced insulin release. The combination of GcgR null mice and exendin 9–39 abolished glucagon-induced insulin secretion. These observations strongly support a paracrine effect of glucagon to stimulate insulin release through activation of either GcgR or GLP-1R, which occurred only at high glucose levels [83]. Caicedo and colleagues suggest the insulinotropic effects of glucagon outside of hypoglycemia are due to a second regulatory circuit wherein activation of glucagon secretion reaches concentrations large enough to amplify insulin secretion from beta cells, but unlikely to impact systemic plasma glucagon levels [84]. This would be consistent with the idea from Rorsman and colleagues that small amounts of hormone significantly increase local concentration, with the release of one insulin granule increasing interstitial insulin concentration between islet cells to >100-fold higher than circulating levels of insulin [7, 85]. In contrast, under hypoglycemic conditions, the glucagon response cannot stimulate beta cells because glucose levels are no longer permissive for insulin secretion [84]. These studies emphasize the dynamic nature of paracrine signaling and regulation of hormone secretion that occur in response to changes in ambient glucose.
Delta cells also express low levels of GcgR, which transduce glucagon signals to increase somatostatin secretion [67, 86] (Figure 3). Somatostatin is a potent inhibitor of glucagon release, and thus, glucagon stimulated somatostatin secretion reflects a negative feedback loop to turn off glucagon release [43, 86, 87]. Conversely, glucagon may suppress delta cell expansion based on studies from GcgR null mice [73]. The opposing effects of glucagon to stimulate somatostatin release while inhibiting delta cell growth indicate the importance of maintaining a balance of the inhibitory effects of somatostatin.
Glucagon might also regulate its own secretion (Figure 2). One study suggested glucagon weakly increased exocytosis through weak induction of cAMP in isolated mouse and rat alpha cells resembling the inhibitory effects of GLP-1 on glucagon secretion [88]. Accordingly, GcgR null mice develop hyperglucagonemia and alpha cell hyperplasia [73–75], suggesting loss of glucagon sensing by the alpha cell results in compensatory function and growth responses. Isolated pancreas perfusion of GcgR null mice exhibit elevated glucagon secretion, independent of beta cell glucagon receptor signaling [83]. These data provide support that glucagon may negatively feedback on alpha cells, in addition to glucagon-induced somatostatin suppression of glucagon secretion. Notably, the recent discovery from several groups of a conserved endocrine loop between the liver and alpha cells in vivo [76, 89–91] might suggest the autocrine effects of glucagon on alpha cell secretion are more important locally to temper islet paracrine signaling rather than regulating systemic glucagon action.
One implication of the revelations of paracrine signaling is the clinical use of glucagon receptors antagonists (GRAs). Systemically, GRAs attenuate hepatic glucose production to alleviate hyperglycemia in T2D. GcgR deletion or antagonism protects against hyperglycemia, which is attributed to reduced hepatic glucose production. Clinical trials have shown promising glucose lowering effects with GRAs [92], similar to the beneficial effects observed in mouse models of diabetes (STZ beta cell depletion, HFD-induced; db/db) [74, 93, 94], and has led to development of GRAs with fewer and/or reversible side effects [92, 95, 96]. Importantly, observations that glucagon activation of GLP-1R in beta cells, at least partially, may provide beneficially effects that contribute to improved glycemia through stimulation of beta cell function [83, 93].
Somatostatin
Somatostatin is the primary endocrine hormone produced by pancreatic delta cells. While mouse delta cells comprise only ~6% of mouse islets and localize to the mantle, human delta cells are more abundant (up to 22%) and distributed across the islet [1] (Figure 1a–b). Delta cells possess an atypical shape with long neurite-like processes, opposed to the rounded or rhomboid shape of other islet endocrine cells, for increased sympathetic tone and extension of the delta cell paracrine signaling network [7, 97]. Somatostatin is secreted in response to increasing glucose concentrations, similar to insulin [98, 99], and can also be stimulated by amino acids leucine and arginine [39, 86, 100]. Somatostatin is very short-lived in circulation [101] and islet delta cells contribute only ~5% of somatostatin in circulation, with the remaining derived from the GI tract and hypothalamus [102]. These data highlight the importance of delta cell derived somatostatin in islet paracrine signaling.
Within the islet, somatostatin inhibits the secretion of glucagon and insulin from alpha and beta cells, respectively [103–105, 12, 17, 43, 87] (Figure1–4). Somatostatin signaling through somatostatin receptor (SSTR) subtypes in alpha and beta cells decreases hormone exocytosis through inhibition of adenylyl cyclase and voltage-gated calcium channels, as well as inducing membrane repolarization and inhibition of electrical activity via activation of G protein-activated inward rectifier K+ channel [106–108] (Figure 4). However, the SSTR subtypes that mediates these effects have been more challenging to resolve. Several reports describe divergent expression patterns for somatostatin receptor (SSTR) subtypes in alpha and beta cells that elicit different responses to the two somatostatin isoforms (SS-14, SS-28) [103, 109–115]. Contemporary views suggest SS-14 is the primary isoform secreted by islet delta cells [7] acting through SSTR2 on alpha cells and SSTR1/5 on beta cells to inhibit glucagon and insulin secretion, respectively [104, 113, 116, 117]. Accordingly, cell type SSTR specificity was demonstrated in vitro with SSTR2 KO mouse islets lacking somatostatin suppression of glucagon, without impacting glucose stimulated insulin secretion [116]. Given the suggested predominance of SS-14 within the islet [7, 109] coupled with earlier reports of preferential SS-28 binding to beta cells [111, 112] and differential cell SSTR subtype expression [113, 117], it is interesting to speculate that in vivo somatostatin primarily regulates glucagon secretion. However, a role for somatostatin suppression of insulin was evident by depleting somatostatin expressing cells (SST-Cre;R26-DTA), including islet delta cells, resulting in increased pancreatic insulin content, plasma insulin, and lower glucose levels in neonatal mice [118].
Beta cells are electrically coupled to delta cells via gap junctions, suggesting somatostatin regulation of glucagon may, in part, occur through beta cell gap junction-dependent activation of somatostatin release [43] (Figure 3). Similarly, somatostatin suppression of glucagon was dependent on paracrine insulin signaling as demonstrated in somatostatin-secreting delta cell insulin receptor KO mice [119]. The redundant actions of insulin signaling and beta cell electrical coupling to induce somatostatin secretion might indicate a safety mechanism to ensure glucagon suppression and prevent of hyperglycemia. Additionally, mouse delta cells express SSTR1 and SSTR3 as part of negative autocrine feedback loop evidenced by increased somatostatin secretion during SSTR antagonism [68, 120, 121]. Insulinopenia or somatostatin dysregulation in diabetes might therefore account for hyperglucagonemia.
In addition to functional regulation, somatostatin is also a potent regulator of cell growth [122]. Somatostatin inhibits proliferation of MIN6 cells through suppression of c-fos and Mapk expression [123]. Inhibition of beta cell proliferation in mouse and human islets likely occurs through inhibitory G-protein couple receptor repression of cAMP [124, 125]. Hence, SSTR5 deletion increased PDX-1, beta cell function, and expression of cell cycle regulators associated with proliferation [126]. Somatostatin regulation of PDX-1 expression occurred through ubiquitination. However, deletion of somatostatin has no effect on beta cell mass [127], although another study observed increased beta cell apoptosis [128]. Under extreme conditions of beta cell loss, delta to beta cell conversion occurs [44], likely due to the shared common lineage with overlapping transcriptomes in maturity [120, 129]. T1D pancreata have a few somatostatin positive cells that contain low levels of insulin immunoreactivity [30]. The functional contribution of low-insulin expressing delta cells to systemic insulin levels and whether delta cells are capable of complete cell fate conversion in humans is unclear, but a therapeutically attractive hypothesis.
Ghrelin & Pancreatic Polypeptide
Ghrelin and PP constitute the remaining major islet hormones, produced by epsilon and PP-cells, respectively. Islet epsilon cells produce ghrelin, a known hunger hormone, that suppresses insulin and glucagon secretion through paracrine stimulation of somatostatin release [68]. Transcriptomics revealed the ghrelin receptor was highly enriched in delta cells and ghrelin activation increased cytosolic calcium and somatostatin release [68, 120]. Consequently, ghrelin treatment robustly suppressed insulin and glucagon in a SSTR dependent manner [120]. These observations from isolated islets and perfused pancreata only occurred at high glucose [68, 120], whereas in vivo ghrelin levels are highest during fasting [130, 131]. Therefore, the significance of these observations may be limited to paracrine effects as a feedback mechanism to fine tune behavior of a few nearby cells, similar to the paracrine glucagon response to higher glucose [83, 84]. In a recent study, ghrelin or ghrelin receptor deficient mice exhibited normal glucose tolerance and no effect on insulin after a 6 hour fast [130], suggesting paracrine ghrelin signaling may be dispensable for regulation of insulin secretion. Perhaps ghrelin turns off insulin in the transition from the post-prandial period to fasting state or possibly maintain low insulin levels during periods of fasting. Additionally, ghrelin reduces islet blood flow, particularly during fasting, which may have indirect consequences for insulin secretion [131]. Alternatively, islet paracrine ghrelin signaling may play a bigger role in development when epsilon cells are in greatest abundance [130, 132] or during obesity to reduce hyperinsulinemia [133, 134], although this possibility has not been uncoupled from the central effects of ghrelin in obesity.
Several biological functions have been ascribed to PP, including satiety and regulation of hepatic glucose production, in response to parasympathetic activity [135]. One study by Aragon et al. demonstrated glucose stimulates PP secretion, which then activates its receptor (PPYR1) exclusively expressed on alpha cells within the islet to reduce glucagon secretion [136]. Another report suggests PP inhibits glucose-induced insulin secretion from rat and human beta cells, although no effect on glucose tolerance was observed during in vivo mouse studies [137]. Additionally, PP islet immunoreactive area was increased in STZ treated mice, possibly through protective effects of PP against STZ-induced DNA beta cell damage [32, 137]. In summary, ghrelin and PP appear to serve as additional paracrine signals to fine-tune islet endocrine function that may have further roles to promote beta cell survival.
Other islet paracrine signals
GLP-1 is an alternatively processed product of the pre-proglucagon peptide generated by prohormone convertase (PC) 1/3. GLP-1 is primarily produced and secreted into circulation by intestinal L cells, although some studies suggest alpha cells, which typically express PC2 for the production of glucagon, might be capable of producing GLP-1 under certain conditions [83, 138–140]. GLP-1 is well known to potentiate glucose stimulated insulin secretion [141] and several drugs are widely used for T2D patients to activate the GLP-1R (semaglutide, liraglutide, exenatide) or prevent GLP-1 degradation by inhibition of DPP4 (sitagliptin, saxaglipitin, linagliptin). GLP-1R stimulation activates adenylyl cyclase, increasing cAMP which elevates intracellular calcium through PKA and Epac activation of L-type voltage dependent calcium channels and ER ryanodine receptor calcium channels, respectively [142–145] (Figure 4). PKA further activates voltage-dependent calcium influx through closure of KATP and voltage gated K+ channels, delaying repolarization [142, 145]. GLP-1 also inhibits alpha cell function, evidenced by administration of DPP4 inhibitors that promote glucagon secretion [146, 147]. In the perfused rodent pancreas, the inhibitory effects of GLP-1 on glucagon are eliminated by SSTR2 blockade, indicating the requirement of paracrine somatostatin signaling [121, 148]. Although isolated mouse islet studies suggest SSTR2 antagonism does not fully inhibit GLP-1 suppression of glucagon secretion [149]. GLP-1 signaling in alpha cells occurred through adenylyl cyclase, elevation of intracellular cAMP and PKA-dependent inhibition of P/Q-type calcium channels signaling, preventing glucagon exocytosis [149, 150] (Figure 2). Redundant signaling may also occur via the GcgR as evidenced by GLP-1 suppression of glucagon secretion in GLP-1R −/− islets [150].
GLP-1 also critically promotes beta cell survival and blocks stress or toxin induced beta cell apoptosis in various mouse and in vitro models [151–156] (Figure 5). Briefly, these actions involve upregulation of Pdx-1 and pro-survival factors (Bcl-2, Bcl-xL), while modulating pro-apoptotic caspase induction. Durable GLP-1 stimulation of rodent and human beta cell proliferation has been demonstrated [156–159], but is unlikely to contribute meaningfully to beta cell mass expansion [157]. One critical question, however, is whether islet GLP-1 paracrine activity contributes substantially to these observations. In support of locally derived GLP-1, Svendson et al. show increased GLP-1 secretion in pancreas perfused GcgR null mice, suggesting that during hyperglucagonemia and increased secretory activity, increased PC1 may drive bioactive local GLP-1 [83]. In contrast, GLP-1 measured from perfused pancreata or pancreatic extracts from control mice is undetectable, suggesting islet-derived GLP-1 may only be important for adaptation to metabolic stress and increased secretory demands [83, 160].
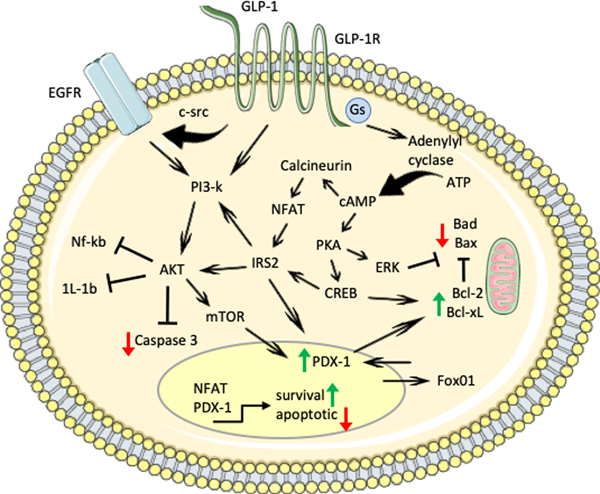
Figure 5. Beta cell survival and apoptotic signaling pathways.
Pro-survival factors (GLP-1, EGFR ligands, and others) signal through different pathways (PI3k/Akt, calineurin/NFAT, PKA/MAPK/ERK) to increase expression and activity of pro-survival factors (i.e. Bcl-1, Bcl-xL) and decrease expression and inhibit activity of pro-apoptotic factors (i.e. Bad, Bid, Bax). PI3k or IRS2 activation of Akt inhibits pro-inflammatory pathways and Caspase-3 cleavage, while stimulating mTOR activity and increasing PDX-1 expression and nuclear localization. GLP-1R signaling increases Gs activity, which induces adenylyl cyclase conversion of ATP to cAMP. Increased cAMP signals through calcineurin and NFAT to increase IRS2 or through PKA, CREB and MAPK/ERK1/2 to phosphorylate and inhibit pro-apoptotic factors and stimulate pro-survival factors. NFAT and PDX-1 transcriptional activity also regulate expression of genes to increase survival.
The major excitatory neurotransmitter glutamate is secreted by alpha cells, acting in an auto-/paracrine manner to increase glucagon exocytosis without effecting insulin [161] (Figure 2). In contrast, the inhibitory neurotransmitter GABA is co-secreted with insulin and contributes to inhibition of glucagon secretion [16, 162, 163] (Figure 2). GABA promotes survival and proliferation of human and mouse beta cells through activation of Akt and PKA induced p-CREB, which was protective in various mouse models of diabetes [164–167]. In mice, beta cell expansion via sustained GABA treatment is unlikely to involve mature alpha cell transdifferentiation to beta cells [168, 169]; nonetheless, GABA has notable effects on human beta cell survival and expansion that might also involve beta cell neogenesis and warrants further investigation [167, 170]. Acetylcholine increases insulin secretion during the pre- and post-absorptive state of mice [171, 172] relevant to centrally regulated parasympathetic outflow rather than local paracrine signaling; however, in human islets acetylcholine released from alpha cells can recruit delta cells to reduce beta cell insulin secretion [173, 174].
Several other paracrine signaling mechanisms have also been described. Urocortin 3 is released by alpha (non-human primates) and beta cells (rodents, humans) to promote somatostatin secretion, acting as a negative feedback loop to attenuate glucagon and insulin secretion [42, 175] (Figure 3). Urocortin 3 also defines beta cell maturation, marked by an increased glucose threshold for insulin secretion [176, 177]. Additional paracrine signals for beta cell growth and maturation derive from paracrine interactions with blood vessels through the VEGF – HGF axis [178–181]. Beta cells secrete VEGF to stimulate blood vessel growth in response to hypoxia and increasing nutrient demands during beta cell growth, while blood vessels in turn secrete the beta cell growth factor HGF. Parallel growth of beta cells and blood vessels is particularly important during islet development and rodent beta cell regeneration [178, 179]. Moreover, islet perfusion is controlled by paracrine signals involving contractile pericytes covering capillaries [182]. Sympathetic input activates islet pericyte contraction, restricting local blood flow thereby limiting islet cell nutrient and hormone access, while adenosine likely derived from ATP co-secreted with insulin relaxes pericytes leading to dilation of islet capillaries. Notably, loss of islet pericytes observed in T2D likely contributes to impaired islet blood flow and function, furthering hyperglycemia [182]. Within the islet, blood flow largely moves from the beta cell core to the mantle containing alpha and delta cells, with a small portion of the afferent arterioles entering the islet at the mantle without ever passing through the beta cell core, while other capillary vessels form an insuloacinar portal system, passing through the beta cell core to the surrounding acinar cells without passing through non-beta cell regions [183, 184]. More recent in vivo studies in mouse islets support islet blood flow from core to mantle, in addition to a second pattern of blood flow from top to bottom, across the islet without bias towards cell type [185]. These collective findings suggest that beta cell secretory products primarily flow downstream toward non-beta cells in rodent islets, while only some islets (depending on size [183]) or regions within an islet exhibit blood carrying somatostatin or glucagon towards beta cells [185]. Complementary studies are needed for human islet blood flow where the core-mantle structure is absent.
Lastly, there is also mounting evidence that islet endocrine cells communicate through release of small extracellular vesicles or exosomes. These cargo carrying microparticles deliver metabolites, peptides, and miRNAs to impact function and survival of nearby and distal cells. miRNAs are of particular interest for their extensive roles in beta cell development, function, and survival (reviewed in detail [186]). Members of the miR-200 family are elevated in models of diabetes and promote beta cell apoptosis, mediated partially through Trp53 and the apoptotic factor Bax [187, 188]. Human islets exposed to inflammatory and hypoxic stress differentially expressed 190 exosomal-miRNAs [189], which might act to perpetuate stress or apoptotic signals, or possibly trigger compensatory functional responses. Therefore, paracrine actions of exosomes carrying miRNAs and other cargo, might strongly influence islet cell function and survival.
In summary, paracrine signaling within the pancreatic islet involves a diverse set of signals that exert important regulatory effects on cell function and survival. The primary islet endocrine hormones (insulin, glucagon, and somatostatin) are involved in feedback loops to control hormone secretion (Figure 1c) and in some cases, cell survival and proliferation. Secondary paracrine mediators, such as ghrelin, PP, Urocortin 3, GABA and others fill supportive roles to fine-tune the primary systemic or local hormone release (Figure 2–4). In some cases, these secondary paracrine signals have significant biological effects, best exemplified by the VEGF-HGF loop between beta cells and the islet vasculature. Disruption of islet paracrine signaling has significant consequences for local cell function, proliferation, and survival, as well as systemic glucose regulation, potentially leading to diabetes development and progression. Advances in islet biology and paracrine signaling continue to identify new interactions and potential therapies (GLP-1, GRAs), as well as potential side effects (GRAs, SGLT2 inhibitors) that will continue to be evaluated in an effort to strengthen beta cell function and survival in diabetes.
Perspectives
Pancreatic islets were originally described as a composition of 3 distinct islet cell types, but recent advances demonstrate the islet is comprised of many cell types, some of which may exist in different states or phenotypes. The resulting diversity within islets has significant implications for paracrine signaling, function, and growth. Single-cell RNA-seq technologies have revealed cellular heterogeneity within islet endocrine cell types that constitute diverse cell functions (reviewed in detail by Carrano et al. [190], Nasteka and Hodson [191]). Beta cells may exist in 4–6 different states [192–195]. These beta cell states reflect insulin biosynthesis, stress level, and proliferative capacity. Beta cells with higher levels of insulin biosynthesis respond to stress and then transition to low insulin and elevated stress responses associated with increased proliferation [192, 193, 196]. Consequently, functional heterogeneity may uncover previously unrecognized paracrine signaling pathways. Interestingly, <10% of beta cells containing low insulin and immature beta cell markers, denoted as “hub cells”, may coordinate islet insulin secretion in a pacemaker like fashion [197]. Herrera and colleagues demonstrate insulin and smoothened signaling maintain alpha cell fate, wherein beta cell ablation or IR antagonism coupled with smoothened inactivation induced insulin expression in 2–5% of alpha cells [198]. Consequently, loss of islet endocrine cell heterogeneity may contribute to decreased functional and cellular plasticity leading to diabetes [195, 197], with some cell states potentially reflecting compensatory or protective responses to increased metabolic demand and immune cell-mediated beta cell apoptosis [30, 199, 200].
Islet paracrine signals, as discussed above, have profound effects on beta cell function and survival. Coupling stress reduction [201] with stimulation of pro-survival pathways is an intriguing approach to diabetes treatment. Activation of GLP-1R signaling through receptor agonists and DPP4 inhibitors are known to dampen pro-apoptotic pathways and increase beta cell survival, in addition to their primary clinical use to stimulate insulin secretion [141]. SGLT2 inhibitors reduce ambient glucose levels, decreasing the functional demand on beta cells, although evaluation of direct and paracrine effects of SGLT2 inhibitors on islets is on-going [119, 202]. Targeting immune cells and inflammation to reduce beta cell stress has shown promise to preserve function, and presumably mass. Recent clinical trials suggest T cell targeting delayed progression to T1D in high-risk patients and also partially preserves beta cell function in new onset T1D cases [203, 204]. In T2D, metformin and thiazolidinediones are prescribed to decrease hepatic glucose production and improve insulin sensitivity, respectively, but also exert anti-inflammatory effects and inhibit reactive oxygen species (ROS) production [205, 206]. Continued investigation should focus on combined approaches to reduce beta stressors and stress response pathways along with mediators of beta cell survival, such as GLP-1. One final consideration is the increasing evidence that diabetes pathogenesis may arise through diverse or overlapping mechanisms in contrast to the standard definitions of T1D and T2D, such as ketosis prone diabetes [207]. Going forward precision medicine will become more integral for patient specific diabetes treatments to preserve beta cell mass and function [208, 209].
Acknowledgements
This work was supported by American Diabetes Association #1–18-IBS-105 and NIH R01DK114356. This work was also supported in part by an award from the Baylor College of Medicine Nutrition and Obesity Pilot and Feasibility Fund.
Abbreviations
- BIRKO
beta cell insulin receptor knockout
- DPP4
dipeptidyl peptidase-4
- ER
endoplasmic reticulum
- GABA
gamma-aminobutyric acid
- GcgR
glucagon receptor
- GLP-1
glucagon-like peptide-1
- GRA
glucagon receptor antagonist
- HGF
hepatocyte growth factor
- hGH
human growth hormone
- HIP
hybrid insulin peptides
- IGF-1
insulin-like growth factor-1
- IR
insulin receptor
- IRS2
insulin receptor substrate-2
- JNK
c-Jun N-terminal kinase
- KO
knockout
- MAPK
mitogen-activated protein kinase
- miRNA
microRNA
- mTOR
mammalian target of rapamycin
- Nf-kB
nuclear factor-kB
- PC
prohormone convertase
- PP
pancreatic polypeptide
- ROS
reactive oxygen species
- SGLT2
sodium/glucose cotransporter 2
- SSTR
somatostatin receptor
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Brissova M, Fowler MJ, Nicholson WE, et al. (2005) Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem Off J Histochem Soc 53:1087–1097. 10.1369/jhc.5C6684.2005 [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Misawa R, Zielinski MC, et al. (2013) Regional differences in islet distribution in the human pancreas--preferential beta-cell loss in the head region in patients with type 2 diabetes. PloS One 8:e67454 10.1371/journal.pone.0067454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dybala MP, Hara M (2019) Heterogeneity of the Human Pancreatic Islet. Diabetes 68:1230–1239. 10.2337/db19-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banarer S, McGregor VP, Cryer PE (2002) Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 51:958–965. 10.2337/diabetes.51.4.958 [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Kjems LL, Veldhuis JD, et al. (2006) Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 55:1051–1056. 10.2337/diabetes.55.04.06.db05-1449 [DOI] [PubMed] [Google Scholar]
- 6.Kawamori D, Kurpad AJ, Hu J, et al. (2009) Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 9:350–361. 10.1016/j.cmet.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rorsman P, Huising MO (2018) The somatostatin-secreting pancreatic δ-cell in health and disease. Nat Rev Endocrinol 14:404–414. 10.1038/s41574-018-0020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gromada J, Chabosseau P, Rutter GA (2018) The α-cell in diabetes mellitus. Nat Rev Endocrinol 14:694–704. 10.1038/s41574-018-0097-y [DOI] [PubMed] [Google Scholar]
- 9.Brereton MF, Vergari E, Zhang Q, Clark A (2015) Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J Histochem Cytochem Off J Histochem Soc 63:575–591. 10.1369/0022155415583535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir GC, Knowlton SD, Atkins RF, et al. (1976) Glucagon secretion from the perfused pancreas of streptozotocin-treated rats. Diabetes 25:275–282. 10.2337/diab.25.4.275 [DOI] [PubMed] [Google Scholar]
- 11.Maruyama H, Hisatomi A, Orci L, et al. (1984) Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74:2296–2299. 10.1172/JCI111658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samols E, Bonner-Weir S, Weir GC (1986) Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab 15:33–58 [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Tran POT, Yang S, et al. (2004) Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 53:1482–1487. 10.2337/diabetes.53.6.1482 [DOI] [PubMed] [Google Scholar]
- 14.Hope KM, Tran POT, Zhou H, et al. (2004) Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes 53:1488–1495. 10.2337/diabetes.53.6.1488 [DOI] [PubMed] [Google Scholar]
- 15.Franklin I, Gromada J, Gjinovci A, et al. (2005) Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815. 10.2337/diabetes.54.6.1808 [DOI] [PubMed] [Google Scholar]
- 16.Xu E, Kumar M, Zhang Y, et al. (2006) Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3:47–58. 10.1016/j.cmet.2005.11.015 [DOI] [PubMed] [Google Scholar]
- 17.Elliott AD, Ustione A, Piston DW (2015) Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Am J Physiol Endocrinol Metab 308:E130–143. 10.1152/ajpendo.00344.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerich JE, Langlois M, Noacco C, et al. (1973) Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 182:171–173. 10.1126/science.182.4108.171 [DOI] [PubMed] [Google Scholar]
- 19.Bolli G, Feo PD, Compagnucci P, et al. (1983) Abnormal Glucose Counterregulation in Insulin-dependent Diabetes Mellitus: Interaction of Anti-Insulin Antibodies and Impaired Glucagon and Epinephrine Secretion. Diabetes 32:134–141. 10.2337/diab.32.2.134 [DOI] [PubMed] [Google Scholar]
- 20.Sherr J, Tsalikian E, Fox L, et al. (2014) Evolution of Abnormal Plasma Glucagon Responses to Mixed-Meal Feedings in Youth With Type 1 Diabetes During the First 2 Years After Diagnosis. Diabetes Care 37:1741–1744. 10.2337/dc13-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerich JE, Lorenzi M, Karam JH, et al. (1975) Abnormal pancreatic glucagon secretion and postprandial hyperglycemia in diabetes mellitus. JAMA 234:159–155 [PubMed] [Google Scholar]
- 22.Gerich JE, Lorenzi M, Bier DM, et al. (1975) Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med 292:985–989. 10.1056/NEJM197505082921901 [DOI] [PubMed] [Google Scholar]
- 23.Shah P, Vella A, Basu A, et al. (2000) Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85:4053–4059. 10.1210/jcem.85.11.6993 [DOI] [PubMed] [Google Scholar]
- 24.Rizza RA (2010) Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59:2697–2707. 10.2337/db10-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laing SP, Swerdlow AJ, Slater SD, et al. (1999) The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med J Br Diabet Assoc 16:466–471 [DOI] [PubMed] [Google Scholar]
- 26.(1995) U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group; Diabetes 44:1249–1258 [PubMed] [Google Scholar]
- 27.Cryer PE, Davis SN, Shamoon H (2003) Hypoglycemia in diabetes. Diabetes Care 26:1902–1912. 10.2337/diacare.26.6.1902 [DOI] [PubMed] [Google Scholar]
- 28.Fukuda M, Tanaka A, Tahara Y, et al. (1988) Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes 37:81–88. 10.2337/diab.37.1.81 [DOI] [PubMed] [Google Scholar]
- 29.Brissova M, Haliyur R, Saunders D, et al. (2018) α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Rep 22:2667–2676. 10.1016/j.celrep.2018.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam CJ, Chatterjee A, Shen E, et al. (2019) Low-Level Insulin Content Within Abundant Non-β Islet Endocrine Cells in Long-standing Type 1 Diabetes. Diabetes 68:598–608. 10.2337/db18-0305 [DOI] [PubMed] [Google Scholar]
- 31.Thorel F, Népote V, Avril I, et al. (2010) Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464:1149–1154. 10.1038/nature08894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuyama K, Chera S, van Gurp L, et al. (2019) Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 567:43–48. 10.1038/s41586-019-0942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H, Zhang T, Harmon JS, et al. (2007) Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes 56:1107–1112. 10.2337/db06-1454 [DOI] [PubMed] [Google Scholar]
- 34.Bloc A, Cens T, Cruz H, Dunant Y (2000) Zinc-induced changes in ionic currents of clonal rat pancreatic -cells: activation of ATP-sensitive K+ channels. J Physiol 529 Pt 3:723–734. 10.1111/j.1469-7793.2000.00723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravier MA, Rutter GA (2005) Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 54:1789–1797. 10.2337/diabetes.54.6.1789 [DOI] [PubMed] [Google Scholar]
- 36.Cooperberg BA, Cryer PE (2010) Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59:2936–2940. 10.2337/db10-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolson TJ, Bellomo EA, Wijesekara N, et al. (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083. 10.2337/db09-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honey RN, Fallon MB, Weir GC (1980) Effects of exogenous insulin, glucagon, and somatostatin on islet hormone secretion in the perfused chicken pancreas. Metabolism 29:1242–1246. 10.1016/0026-0495(80)90152-3 [DOI] [PubMed] [Google Scholar]
- 39.Gerber PP, Trimble ER, Wollheim CB, Renold AE (1981) Effect of insulin on glucose- and arginine-stimulated somatostatin secretion from the isolated perfused rat pancreas. Endocrinology 109:279–283. 10.1210/endo-109-1-279 [DOI] [PubMed] [Google Scholar]
- 40.Hauge-Evans AC, Anderson RL, Persaud SJ, Jones PM (2012) Delta cell secretory responses to insulin secretagogues are not mediated indirectly by insulin. Diabetologia 55:1995–2004. 10.1007/s00125-012-2546-9 [DOI] [PubMed] [Google Scholar]
- 41.Braun M, Ramracheya R, Bengtsson M, et al. (2010) Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 59:1694–1701. 10.2337/db09-0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Meulen T, Donaldson CJ, Cáceres E, et al. (2015) Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med 21:769–776. 10.1038/nm.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briant LJB, Reinbothe TM, Spiliotis I, et al. (2018) δ-cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J Physiol 596:197–215. 10.1113/JP274581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chera S, Baronnier D, Ghila L, et al. (2014) Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514:503–507. 10.1038/nature13633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu GG, Rothenberg PL (1998) Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine beta-cell regulation. Diabetes 47:1243–1252. 10.2337/diab.47.8.1243 [DOI] [PubMed] [Google Scholar]
- 46.Leibiger IB, Leibiger B, Moede T, Berggren PO (1998) Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol Cell 1:933–938 [DOI] [PubMed] [Google Scholar]
- 47.Muller D, Jones PM, Persaud SJ (2006) Autocrine anti-apoptotic and proliferative effects of insulin in pancreatic beta-cells. FEBS Lett 580:6977–6980. 10.1016/j.febslet.2006.11.066 [DOI] [PubMed] [Google Scholar]
- 48.Kulkarni RN, Brüning JC, Winnay JN, et al. (1999) Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339. 10.1016/s0092-8674(00)80546-2 [DOI] [PubMed] [Google Scholar]
- 49.Otani K, Kulkarni RN, Baldwin AC, et al. (2004) Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am J Physiol Endocrinol Metab 286:E41–49. 10.1152/ajpendo.00533.2001 [DOI] [PubMed] [Google Scholar]
- 50.Xuan S, Kitamura T, Nakae J, et al. (2002) Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 110:1011–1019. 10.1172/JCI15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulkarni RN, Holzenberger M, Shih DQ, et al. (2002) beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet 31:111–115. 10.1038/ng872 [DOI] [PubMed] [Google Scholar]
- 52.Slaaby R, Schäffer L, Lautrup-Larsen I, et al. (2006) Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem 281:25869–25874. 10.1074/jbc.M605189200 [DOI] [PubMed] [Google Scholar]
- 53.Ueki K, Okada T, Hu J, et al. (2006) Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 38:583–588. 10.1038/ng1787 [DOI] [PubMed] [Google Scholar]
- 54.Liu JP, Baker J, Perkins AS, et al. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- 55.Wicksteed B, Brissova M, Yan W, et al. (2010) Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59:3090–3098. 10.2337/db10-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleinridders A, Ferris HA, Cai W, Kahn CR (2014) Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63:2232–2243. 10.2337/db14-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brouwers B, de Faudeur G, Osipovich AB, et al. (2014) Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab 20:979–990. 10.1016/j.cmet.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baan M, Kibbe CR, Bushkofsky JR, et al. (2015) Transgenic expression of the human growth hormone minigene promotes pancreatic β-cell proliferation. Am J Physiol Regul Integr Comp Physiol 309:R788–794. 10.1152/ajpregu.00244.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estall JL, Screaton RA (2015) To Be(ta Cell) or Not to Be(ta cell): New Mouse Models for Studying Gene Function in the Pancreatic β-Cell. Endocrinology 156:2365–2367. 10.1210/en.2015-1418 [DOI] [PubMed] [Google Scholar]
- 60.Thorens B, Tarussio D, Maestro MA, et al. (2015) Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia 58:558–565. 10.1007/s00125-014-3468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skovsø S, Elghazi L, Dionne DA, et al. (2016) Insulin Receptor Knockout Using Two Beta-Cell-Specific Cre Mouse Lines Transiently Improves Glucose Homeostasis. Diabetes 65:A458 [Google Scholar]
- 62.Skovsø S, Dionne DA, Panzhinskiy E, et al. (2018) Glucose homeostasis, insulin secretion and beta cell transcriptomics of mice with beta cell specific insulin resistance. Diabetologia 61:S96 [Google Scholar]
- 63.Murlin JR, Clough HD, Gibbs CBF, Stokes AM (1923) Aqueous extracts of the pancreas. Influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem 56:253–296 [Google Scholar]
- 64.Sutherland EW, De Duve C (1948) Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem 175:663–674 [PubMed] [Google Scholar]
- 65.Gromada J, Franklin I, Wollheim CB (2007) Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116. 10.1210/er.2006-0007 [DOI] [PubMed] [Google Scholar]
- 66.Gelling RW, Vuguin PM, Du XQ, et al. (2009) Pancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and mass. Am J Physiol Endocrinol Metab 297:E695–707. 10.1152/ajpendo.00082.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weir GC, Samols E, Day JA, Patel YC (1978) Glucose and glucagon stimulate the secretion of somatostatin from the perfused canine pancreas. Metabolism 27:1223–1226. 10.1016/0026-0495(78)90047-1 [DOI] [PubMed] [Google Scholar]
- 68.Adriaenssens AE, Svendsen B, Lam BYH, et al. (2016) Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59:2156–2165. 10.1007/s00125-016-4033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samols E, Marri G, Marks V (1965) Promotion of insulin secretion by glucagon. Lancet Lond Engl 2:415–416. 10.1016/s0140-6736(65)90761-0 [DOI] [PubMed] [Google Scholar]
- 70.Pipeleers DG, Schuit FC, in’t Veld PA, et al. (1985) Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology 117:824–833. 10.1210/endo-117-3-824 [DOI] [PubMed] [Google Scholar]
- 71.Moens K, Flamez D, Van Schravendijk C, et al. (1998) Dual glucagon recognition by pancreatic beta-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 47:66–72. 10.2337/diab.47.1.66 [DOI] [PubMed] [Google Scholar]
- 72.Huypens P, Ling Z, Pipeleers D, Schuit F (2000) Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43:1012–1019. 10.1007/s001250051484 [DOI] [PubMed] [Google Scholar]
- 73.Gelling RW, Du XQ, Dichmann DS, et al. (2003) Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 100:1438–1443. 10.1073/pnas.0237106100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conarello SL, Jiang G, Mu J, et al. (2007) Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50:142–150. 10.1007/s00125-006-0481-3 [DOI] [PubMed] [Google Scholar]
- 75.Parker JC, Andrews KM, Allen MR, et al. (2002) Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 290:839–843. 10.1006/bbrc.2001.6265 [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Okamoto H, Huang Z, et al. (2017) Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab 25:1348–1361.e8. 10.1016/j.cmet.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ben-Zvi D, Barrandon O, Hadley S, et al. (2015) Angptl4 links α-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci U S A 112:15498–15503. 10.1073/pnas.1513872112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okamoto H, Cavino K, Na E, et al. (2017) Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc Natl Acad Sci U S A 114:2753–2758. 10.1073/pnas.1621069114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawai K, Yokota C, Ohashi S, et al. (1995) Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia 38:274–276 [DOI] [PubMed] [Google Scholar]
- 80.Thorens B (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A 89:8641–8645. 10.1073/pnas.89.18.8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marie JC, Boissard C, Skoglund G, et al. (1996) Glucagon acts through its own receptors in the presence of functional glucagon-like peptide-1 receptors on hamster insulinoma. Endocrinology 137:4108–4114. 10.1210/endo.137.10.8828464 [DOI] [PubMed] [Google Scholar]
- 82.Moens K, Berger V, Ahn J-M, et al. (2002) Assessment of the role of interstitial glucagon in the acute glucose secretory responsiveness of in situ pancreatic beta-cells. Diabetes 51:669–675. 10.2337/diabetes.51.3.669 [DOI] [PubMed] [Google Scholar]
- 83.Svendsen B, Larsen O, Gabe MBN, et al. (2018) Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Rep 25:1127–1134.e2. 10.1016/j.celrep.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez-Diaz R, Tamayo A, Hara M, Caicedo A (2019) The Local Paracrine Actions of the Pancreatic Alpha Cell. Diabetes. 10.2337/dbi19-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rorsman P, Ashcroft FM (2018) Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev 98:117–214. 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patton GS, Ipp E, Dobbs RE, et al. (1977) Pancreatic immunoreactive somatostatin release. Proc Natl Acad Sci U S A 74:2140–2143. 10.1073/pnas.74.5.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Starke A, Imamura T, Unger RH (1987) Relationship of glucagon suppression by insulin and somatostatin to the ambient glucose concentration. J Clin Invest 79:20–24. 10.1172/JCI112784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma X, Zhang Y, Gromada J, et al. (2005) Glucagon stimulates exocytosis in mouse and rat pancreatic alpha-cells by binding to glucagon receptors. Mol Endocrinol Baltim Md 19:198–212. 10.1210/me.2004-0059 [DOI] [PubMed] [Google Scholar]
- 89.Longuet C, Robledo AM, Dean ED, et al. (2013) Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 62:1196–1205. 10.2337/db11-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solloway MJ, Madjidi A, Gu C, et al. (2015) Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of α-Cell Mass. Cell Rep 12:495–510. 10.1016/j.celrep.2015.06.034 [DOI] [PubMed] [Google Scholar]
- 91.Dean ED, Li M, Prasad N, et al. (2017) Interrupted Glucagon Signaling Reveals Hepatic α Cell Axis and Role for L-Glutamine in α Cell Proliferation. Cell Metab 25:1362–1373.e5. 10.1016/j.cmet.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kazda CM, Ding Y, Kelly RP, et al. (2016) Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY2409021 in Patients With Type 2 Diabetes: 12- and 24-Week Phase 2 Studies. Diabetes Care 39:1241–1249. 10.2337/dc15-1643 [DOI] [PubMed] [Google Scholar]
- 93.Rivero-Gutierrez B, Haller A, Holland J, et al. (2018) Deletion of the glucagon receptor gene before and after experimental diabetes reveals differential protection from hyperglycemia. Mol Metab 17:28–38. 10.1016/j.molmet.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Z, Kim W, Chen Z, et al. (2011) Insulin and glucagon regulate pancreatic α-cell proliferation. PloS One 6:e16096 10.1371/journal.pone.0016096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pearson MJ, Unger RH, Holland WL (2016) Clinical Trials, Triumphs, and Tribulations of Glucagon Receptor Antagonists. Diabetes Care 39:1075–1077. 10.2337/dci15-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watanabe C, Seino Y, Miyahira H, et al. (2012) Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61:74–84. 10.2337/db11-0739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hauge-Evans AC, King AJ, Fairhall K, et al. (2010) A role for islet somatostatin in mediating sympathetic regulation of glucagon secretion. Islets 2:341–344. 10.4161/isl.2.6.13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berts A, Ball A, Dryselius G, et al. (1996) Glucose stimulation of somatostatin-producing islet cells involves oscillatory Ca2+ signaling. Endocrinology 137:693–697. 10.1210/endo.137.2.8593819 [DOI] [PubMed] [Google Scholar]
- 99.Nadal A, Quesada I, Soria B (1999) Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. J Physiol 517 ( Pt 1):85–93. 10.1111/j.1469-7793.1999.0085z.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ipp E, Dobbs RE, Arimura A, et al. (1977) Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. J Clin Invest 60:760–765. 10.1172/JCI108829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel YC, Wheatley T (1983) In vivo and in vitro plasma disappearance and metabolism of somatostatin-28 and somatostatin-14 in the rat. Endocrinology 112:220–225. 10.1210/endo-112-1-220 [DOI] [PubMed] [Google Scholar]
- 102.Martinez V, Tache Y (2004) Somatostatin In: Johnson L (ed) Encyclopedia of Gastroenterology, 1st ed. Elsevier, pp 426–433 [Google Scholar]
- 103.Taborsky GJ, Smith PH, Porte D (1978) Interaction of somatostatin with the A and B cells of the endocrine pancreas. Metabolism 27:1299–1302. 10.1016/0026-0495(78)90062-8 [DOI] [PubMed] [Google Scholar]
- 104.Klaff LJ, Taborsky GJ (1987) Pancreatic somatostatin is a mediator of glucagon inhibition by hyperglycemia. Diabetes 36:592–596. 10.2337/diab.36.5.592 [DOI] [PubMed] [Google Scholar]
- 105.Koerker DJ, Ruch W, Chideckel E, et al. (1974) Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science 184:482–484. 10.1126/science.184.4135.482 [DOI] [PubMed] [Google Scholar]
- 106.Braun M (2014) The somatostatin receptor in human pancreatic β-cells. Vitam Horm 95:165–193. 10.1016/B978-0-12-800174-5.00007-7 [DOI] [PubMed] [Google Scholar]
- 107.Kailey B, van de Bunt M, Cheley S, et al. (2012) SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β- and α-cells. Am J Physiol Endocrinol Metab 303:E1107–1116. 10.1152/ajpendo.00207.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Renström E, Ding WG, Bokvist K, Rorsman P (1996) Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron 17:513–522. 10.1016/s0896-6273(00)80183-x [DOI] [PubMed] [Google Scholar]
- 109.Benoit R, Böhlen P, Brazeau P, et al. (1980) Isolation and characterization of rat pancreatic somatostatin. Endocrinology 107:2127–2129. 10.1210/endo-107-6-2127 [DOI] [PubMed] [Google Scholar]
- 110.Ravazzola M, Benoit R, Ling N, et al. (1983) Immunocytochemical localization of prosomatostatin fragments in maturing and mature secretory granules of pancreatic and gastrointestinal D cells. Proc Natl Acad Sci U S A 80:215–218. 10.1073/pnas.80.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amherdt M, Patel YC, Orci L (1987) Selective binding of somatostatin-14 and somatostatin-28 to islet cells revealed by quantitative electron microscopic autoradiography. J Clin Invest 80:1455–1458. 10.1172/JCI113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuit FC, Derde MP, Pipeleers DG (1989) Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia 32:207–212 [DOI] [PubMed] [Google Scholar]
- 113.Kumar U, Sasi R, Suresh S, et al. (1999) Subtype-selective expression of the five somatostatin receptors (hSSTR1–5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48:77–85. 10.2337/diabetes.48.1.77 [DOI] [PubMed] [Google Scholar]
- 114.Ludvigsen E, Olsson R, Stridsberg M, et al. (2004) Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem Off J Histochem Soc 52:391–400. 10.1177/002215540405200310 [DOI] [PubMed] [Google Scholar]
- 115.Patel YC, Greenwood MT, Panetta R, et al. (1995) The somatostatin receptor family. Life Sci 57:1249–1265. 10.1016/0024-3205(95)02082-t [DOI] [PubMed] [Google Scholar]
- 116.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM (2000) Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141:111–117. 10.1210/endo.141.1.7263 [DOI] [PubMed] [Google Scholar]
- 117.Cejvan K, Coy DH, Efendic S (2003) Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52:1176–1181. 10.2337/diabetes.52.5.1176 [DOI] [PubMed] [Google Scholar]
- 118.Li N, Yang Z, Li Q, et al. (2018) Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents. Cell Death Dis 9:682 10.1038/s41419-018-0741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vergari E, Knudsen JG, Ramracheya R, et al. (2019) Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 10:139 10.1038/s41467-018-08193-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DiGruccio MR, Mawla AM, Donaldson CJ, et al. (2016) Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab 5:449–458. 10.1016/j.molmet.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Heer J, Rasmussen C, Coy DH, Holst JJ (2008) Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51:2263–2270. 10.1007/s00125-008-1149-y [DOI] [PubMed] [Google Scholar]
- 122.Reubi JC, Schonbrunn A (2013) Illuminating somatostatin analog action at neuroendocrine tumor receptors. Trends Pharmacol Sci 34:676–688. 10.1016/j.tips.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshitomi H, Fujii Y, Miyazaki M, et al. (1997) Involvement of MAP kinase and c-fos signaling in the inhibition of cell growth by somatostatin. Am J Physiol 272:E769–774. 10.1152/ajpendo.1997.272.5.E769 [DOI] [PubMed] [Google Scholar]
- 124.Berger M, Scheel DW, Macias H, et al. (2015) Gαi/o-coupled receptor signaling restricts pancreatic β-cell expansion. Proc Natl Acad Sci U S A 112:2888–2893. 10.1073/pnas.1319378112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vivot K, Moullé VS, Zarrouki B, et al. (2016) The regulator of G-protein signaling RGS16 promotes insulin secretion and β-cell proliferation in rodent and human islets. Mol Metab 5:988–996. 10.1016/j.molmet.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou G, Liu S-H, Shahi KM, et al. (2012) Negative regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5. Mol Endocrinol Baltim Md 26:1225–1234. 10.1210/me.2012-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hauge-Evans AC, King AJ, Carmignac D, et al. (2009) Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58:403–411. 10.2337/db08-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Richardson CC, To K, Foot VL, et al. (2015) Increased perinatal remodelling of the pancreas in somatostatin-deficient mice: potential role of transforming growth factor-beta signalling in regulating beta cell growth in early life. Horm Metab Res Horm Stoffwechselforschung Horm Metab 47:56–63. 10.1055/s-0034-1390427 [DOI] [PubMed] [Google Scholar]
- 129.Schaffer AE, Taylor BL, Benthuysen JR, et al. (2013) Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet 9:e1003274. 10.1371/journal.pgen.1003274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gray SM, Niu J, Zhang A, et al. (2019) Intraislet Ghrelin Signaling Does Not Regulate Insulin Secretion From Adult Mice. Diabetes 68:1795–1805. 10.2337/db19-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drott CJ, Franzén P, Carlsson P-O (2019) Ghrelin in rat pancreatic islets decreases islet blood flow. Am J Physiol Endocrinol Metab 317:E139–E146. 10.1152/ajpendo.00004.2019 [DOI] [PubMed] [Google Scholar]
- 132.Wierup N, Svensson H, Mulder H, Sundler F (2002) The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 107:63–69. 10.1016/s0167-0115(02)00067-8 [DOI] [PubMed] [Google Scholar]
- 133.Sun Y, Asnicar M, Saha PK, et al. (2006) Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386. 10.1016/j.cmet.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 134.Dezaki K, Sone H, Koizumi M, et al. (2006) Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 55:3486–3493. 10.2337/db06-0878 [DOI] [PubMed] [Google Scholar]
- 135.Ahrén B, Wierup N, Sundler F (2006) Neuropeptides and the Regulation of Islet Function. Diabetes 55:S98–S107. 10.2337/db06-S013 [DOI] [Google Scholar]
- 136.Aragón F, Karaca M, Novials A, et al. (2015) Pancreatic polypeptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochim Biophys Acta 1850:343–351. 10.1016/j.bbagen.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 137.Khan D, Vasu S, Moffett RC, et al. (2017) Influence of neuropeptide Y and pancreatic polypeptide on islet function and beta-cell survival. Biochim Biophys Acta Gen Subj 1861:749–758. 10.1016/j.bbagen.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 138.Thyssen S, Arany E, Hill DJ (2006) Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology 147:2346–2356. 10.1210/en.2005-0396 [DOI] [PubMed] [Google Scholar]
- 139.Marchetti P, Lupi R, Bugliani M, et al. (2012) A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55:3262–3272. 10.1007/s00125-012-2716-9 [DOI] [PubMed] [Google Scholar]
- 140.Taylor SW, Nikoulina SE, Andon NL, Lowe C (2013) Peptidomic profiling of secreted products from pancreatic islet culture results in a higher yield of full-length peptide hormones than found using cell lysis procedures. J Proteome Res 12:3610–3619. 10.1021/pr400115q [DOI] [PubMed] [Google Scholar]
- 141.Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17:819–837. 10.1016/j.cmet.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 142.MacDonald PE, Wang X, Xia F, et al. (2003) Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 278:52446–52453. 10.1074/jbc.M307612200 [DOI] [PubMed] [Google Scholar]
- 143.Holz GG (2004) Epac: A New cAMP-Binding Protein in Support of Glucagon-Like Peptide-1 Receptor-Mediated Signal Transduction in the Pancreatic β-Cell. Diabetes 53:5–13. 10.2337/diabetes.53.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Doyle ME, Egan JM (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113:546–593. 10.1016/j.pharmthera.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Müller TD, Finan B, Bloom SR, et al. (2019) Glucagon-like peptide 1 (GLP-1). Mol Metab 30:72–130. 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Müller TD, Finan B, Clemmensen C, et al. (2017) The New Biology and Pharmacology of Glucagon. Physiol Rev 97:721–766. 10.1152/physrev.00025.2016 [DOI] [PubMed] [Google Scholar]
- 147.Sandoval DA, D’Alessio DA (2015) Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 95:513–548. 10.1152/physrev.00013.2014 [DOI] [PubMed] [Google Scholar]
- 148.Ørgaard A, Holst JJ (2017) The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia 60:1731–1739. 10.1007/s00125-017-4315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Marinis YZ, Salehi A, Ward CE, et al. (2010) GLP-1 Inhibits and Adrenaline Stimulates Glucagon Release by Differential Modulation of N- and L-Type Ca2+ Channel-Dependent Exocytosis. Cell Metab 11:543–553. 10.1016/j.cmet.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guida C, Miranda C, Asterholm IW, et al. (2019) Promiscuous receptor activation mediates glucagonostatic effects of GLP-1(9–36) and GLP-1(7–36). bioRxiv 785667. 10.1101/785667 [DOI] [Google Scholar]
- 151.Van de Velde S, Hogan MF, Montminy M (2011) mTOR links incretin signaling to HIF induction in pancreatic beta cells. Proc Natl Acad Sci U S A 108:16876–16882. 10.1073/pnas.1114228108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buteau J, El-Assaad W, Rhodes CJ, et al. (2004) Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 47:806–815. 10.1007/s00125-004-1379-6 [DOI] [PubMed] [Google Scholar]
- 153.Yusta B, Baggio LL, Estall JL, et al. (2006) GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406. 10.1016/j.cmet.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 154.Li Y, Hansotia T, Yusta B, et al. (2003) Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 278:471–478. 10.1074/jbc.M209423200 [DOI] [PubMed] [Google Scholar]
- 155.Wang Q, Li L, Xu E, et al. (2004) Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 47:478–487. 10.1007/s00125-004-1327-5 [DOI] [PubMed] [Google Scholar]
- 156.Brubaker PL, Drucker DJ (2004) Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 145:2653–2659. 10.1210/en.2004-0015 [DOI] [PubMed] [Google Scholar]
- 157.Cox AR, Lam CJ, Rankin MM, et al. (2017) Incretin Therapies Do Not Expand β-Cell Mass or Alter Pancreatic Histology in Young Male Mice. Endocrinology 158:1701–1714. 10.1210/en.2017-00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Y, Cao X, Li L-X, et al. (2005) β-Cell Pdx1 Expression Is Essential for the Glucoregulatory, Proliferative, and Cytoprotective Actions of Glucagon-Like Peptide-1. Diabetes 54:482–491. 10.2337/diabetes.54.2.482 [DOI] [PubMed] [Google Scholar]
- 159.Buteau J, Spatz ML, Accili D (2006) Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes 55:1190–1196. 10.2337/db05-0825 [DOI] [PubMed] [Google Scholar]
- 160.Traub S, Meier DT, Schulze F, et al. (2017) Pancreatic α Cell-Derived Glucagon-Related Peptides Are Required for β Cell Adaptation and Glucose Homeostasis. Cell Rep 18:3192–3203. 10.1016/j.celrep.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 161.Cabrera O, Jacques-Silva MC, Speier S, et al. (2008) Glutamate is a positive autocrine signal for glucagon release. Cell Metab 7:545–554. 10.1016/j.cmet.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rorsman P, Berggren PO, Bokvist K, et al. (1989) Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341:233–236. 10.1038/341233a0 [DOI] [PubMed] [Google Scholar]
- 163.Bailey SJ, Ravier MA, Rutter GA (2007) Glucose-dependent regulation of gamma-aminobutyric acid (GABA A) receptor expression in mouse pancreatic islet alpha-cells. Diabetes 56:320–327. 10.2337/db06-0712 [DOI] [PubMed] [Google Scholar]
- 164.Soltani N, Qiu H, Aleksic M, et al. (2011) GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A 108:11692–11697. 10.1073/pnas.1102715108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Purwana I, Zheng J, Li X, et al. (2014) GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 63:4197–4205. 10.2337/db14-0153 [DOI] [PubMed] [Google Scholar]
- 166.Untereiner A, Abdo S, Bhattacharjee A, et al. (2019) GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J Off Publ Fed Am Soc Exp Biol 33:3968–3984. 10.1096/fj.201801397R [DOI] [PubMed] [Google Scholar]
- 167.Ben-Othman N, Vieira A, Courtney M, et al. (2017) Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 168:73–85.e11. 10.1016/j.cell.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 168.van der Meulen T, Lee S, Noordeloos E, et al. (2018) Artemether Does Not Turn α Cells into β Cells. Cell Metab 27:218–225.e4. 10.1016/j.cmet.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ackermann AM, Moss NG, Kaestner KH (2018) GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Cell Metab 28:787–792.e3. 10.1016/j.cmet.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tian J, Dang H, Karashchuk N, et al. (2019) A Clinically Applicable Positive Allosteric Modulator of GABA Receptors Promotes Human β-Cell Replication and Survival as well as GABA’s Ability to Inhibit Inflammatory T Cells. J Diabetes Res 2019:5783545. 10.1155/2019/5783545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gautam D, Han S-J, Hamdan FF, et al. (2006) A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3:449–461. 10.1016/j.cmet.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 172.Ahrén B (2000) Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia 43:393–410. 10.1007/s001250051322 [DOI] [PubMed] [Google Scholar]
- 173.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. (2011) Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 17:888–892. 10.1038/nm.2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Molina J, Rodriguez-Diaz R, Fachado A, et al. (2014) Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 63:2714–2726. 10.2337/db13-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li C, Chen P, Vaughan J, et al. (2003) Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology 144:3216–3224. 10.1210/en.2002-0087 [DOI] [PubMed] [Google Scholar]
- 176.van der Meulen T, Mawla AM, DiGruccio MR, et al. (2017) Virgin Beta Cells Persist throughout Life at a Neogenic Niche within Pancreatic Islets. Cell Metab 25:911–926.e6. 10.1016/j.cmet.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Blum B, Hrvatin S, Schuetz C, et al. (2012) Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 30:261–264. 10.1038/nbt.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nikolova G, Lammert E (2003) Interdependent development of blood vessels and organs. Cell Tissue Res 314:33–42. 10.1007/s00441-003-0739-8 [DOI] [PubMed] [Google Scholar]
- 179.Nicholson JM, Arany EJ, Hill DJ (2010) Changes in islet microvasculature following streptozotocin-induced beta-cell loss and subsequent replacement in the neonatal rat. Exp Biol Med Maywood NJ 235:189–198. 10.1258/ebm.2009.009316 [DOI] [PubMed] [Google Scholar]
- 180.Reinert RB, Brissova M, Shostak A, et al. (2013) Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 62:4154–4164. 10.2337/db13-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alvarez-Perez JC, Ernst S, Demirci C, et al. (2014) Hepatocyte growth factor/c-Met signaling is required for β-cell regeneration. Diabetes 63:216–223. 10.2337/db13-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Almaça J, Weitz J, Rodriguez-Diaz R, et al. (2018) The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab 27:630–644.e4. 10.1016/j.cmet.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bonner-Weir S, Orci L (1982) New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883–889. 10.2337/diab.31.10.883 [DOI] [PubMed] [Google Scholar]
- 184.Samols E, Stagner JI, Ewart RB, Marks V (1988) The order of islet microvascular cellular perfusion is B----A----D in the perfused rat pancreas. J Clin Invest 82:350–353. 10.1172/JCI113593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nyman LR, Wells KS, Head WS, et al. (2008) Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 118:3790–3797. 10.1172/JCI36209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.LaPierre MP, Stoffel M (2017) MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol Metab 6:1010–1023. 10.1016/j.molmet.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Belgardt B-F, Ahmed K, Spranger M, et al. (2015) The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med 21:619–627. 10.1038/nm.3862 [DOI] [PubMed] [Google Scholar]
- 188.Filios SR, Xu G, Chen J, et al. (2014) MicroRNA-200 Is Induced by Thioredoxin-interacting Protein and Regulates Zeb1 Protein Signaling and Beta Cell Apoptosis. J Biol Chem 289:36275–36283. 10.1074/jbc.M114.592360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saravanan PB, Vasu S, Yoshimatsu G, et al. (2019) Differential expression and release of exosomal miRNAs by human islets under inflammatory and hypoxic stress. Diabetologia. 10.1007/s00125-019-4950-x [DOI] [PubMed] [Google Scholar]
- 190.Carrano AC, Mulas F, Zeng C, Sander M (2017) Interrogating islets in health and disease with single-cell technologies. Mol Metab 6:991–1001. 10.1016/j.molmet.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nasteska D, Hodson DJ (2018) The role of beta cell heterogeneity in islet function and insulin release. J Mol Endocrinol 61:R43–R60. 10.1530/JME-18-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xin Y, Dominguez Gutierrez G, Okamoto H, et al. (2018) Pseudotime Ordering of Single Human β-Cells Reveals States of Insulin Production and Unfolded Protein Response. Diabetes 67:1783–1794. 10.2337/db18-0365 [DOI] [PubMed] [Google Scholar]
- 193.Zeng C, Mulas F, Sui Y, et al. (2017) Pseudotemporal Ordering of Single Cells Reveals Metabolic Control of Postnatal β Cell Proliferation. Cell Metab 25:1160–1175.e11. 10.1016/j.cmet.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baron M, Veres A, Wolock SL, et al. (2016) A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst 3:346–360.e4. 10.1016/j.cels.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Segerstolpe Å, Palasantza A, Eliasson P, et al. (2016) Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab 24:593–607. 10.1016/j.cmet.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Szabat M, Page MM, Panzhinskiy E, et al. (2016) Reduced Insulin Production Relieves Endoplasmic Reticulum Stress and Induces β Cell Proliferation . Cell Metab 23:179–193. 10.1016/j.cmet.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 197.Johnston NR, Mitchell RK, Haythorne E, et al. (2016) Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab 24:389–401. 10.1016/j.cmet.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cigliola V, Ghila L, Thorel F, et al. (2018) Pancreatic islet-autonomous insulin and smoothened-mediated signalling modulate identity changes of glucagon+ α-cells. Nat Cell Biol 20:1267–1277. 10.1038/s41556-018-0216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Oram RA, McDonald TJ, Shields BM, et al. (2015) Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 38:323–328. 10.2337/dc14-0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rui J, Deng S, Arazi A, et al. (2017) β Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell Metab 25:727–738. 10.1016/j.cmet.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma RB, O’Donnell AC, Stamateris RE, et al. (2015) Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest 125:3831–3846. 10.1172/JCI79264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bonner C, Kerr-Conte J, Gmyr V, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21:512–517. 10.1038/nm.3828 [DOI] [PubMed] [Google Scholar]
- 203.Haller MJ, Long SA, Blanchfield JL, et al. (2019) Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes 68:1267–1276. 10.2337/db19-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Herold KC, Bundy BN, Long SA, et al. (2019) An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 10.1056/NEJMoa1902226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Isoda K, Young JL, Zirlik A, et al. (2006) Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26:611–617. 10.1161/01.ATV.0000201938.78044.75 [DOI] [PubMed] [Google Scholar]
- 206.Soccio RE, Chen ER, Lazar MA (2014) Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 20:573–591. 10.1016/j.cmet.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M (2008) Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 29:292–302. 10.1210/er.2007-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gloyn AL, Drucker DJ (2018) Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol 6:891–900. 10.1016/S2213-8587(18)30052-4 [DOI] [PubMed] [Google Scholar]
- 209.Merino J, Florez JC (2018) Precision medicine in diabetes: an opportunity for clinical translation. Ann N Y Acad Sci 1411:140–152. 10.1111/nyas.13588 [DOI] [PMC free article] [PubMed] [Google Scholar]