Leucostasis, a life-threatening complication of acute leukaemia, occurs when leukaemia cells accumulate in and damage the microvasculature of vital organs, with the brain and lungs being the most commonly affected. Neurological signs and symptoms range from mild visual disturbances to coma, while pulmonary manifestations range from cough and dyspnea to respiratory failure (Porcu et al, 2002). Although leucostasis predominantly occurs in patients with hyperleucocytosis and with acute myeloid leukaemia rather than acute lymphoblastic leukaemia (ALL), no reliable clinical predictor currently exists.
The pathophysiology of leucostasis is poorly understood but is thought to involve the biophysical properties of leukaemia cells and their interactions with the microvascular environment. Previous work has shown that leukaemic blasts are stiffer than their more mature counterparts and have suggested that cell deformability may play a significant role in leucostasis (Lichtman, 1973).
Atomic force microscopy (AFM) is a sensitive tool for measuring cellular mechanical properties on the nanometer scale and has been used extensively in cell biology to measure single cell deformability (Radmacher, 2007). Although several groups have measured the stiffness of different leukaemia cell types using other techniques (Lichtman, 1973; Sharma, 1993), no comparisons have been reported between acute leukaemia patients with and without leucostasis. Furthermore, as leucostasis is especially rare in paediatric ALL, more research is required to determine which specific patients are at risk and should receive preventive measures (i.e. leucaphaeresis). To determine if cell stiffness is associated with leucostasis, we used AFM to measure the stiffness of individual leukaemia cells taken from patients with and without symptoms consistent with leucostasis.
Blood was obtained, with informed consent, from 15 paediatric ALL patients with >10% blasts in their peripheral blood. Leukaemic blasts were then immediately isolated via density-gradient centrifugation and measured with AFM. Measurements were performed with the methodology previously described using a Hertzian mechanics model (Rosenbluth et al, 2006) (Fig 1A and B). Cells were immobilized in microfabricated wells to prevent movement during AFM measurements. Media was constantly perfused into and out of the sample chamber, and measurements were taken at 37°C using a heated stage.
Fig 1.
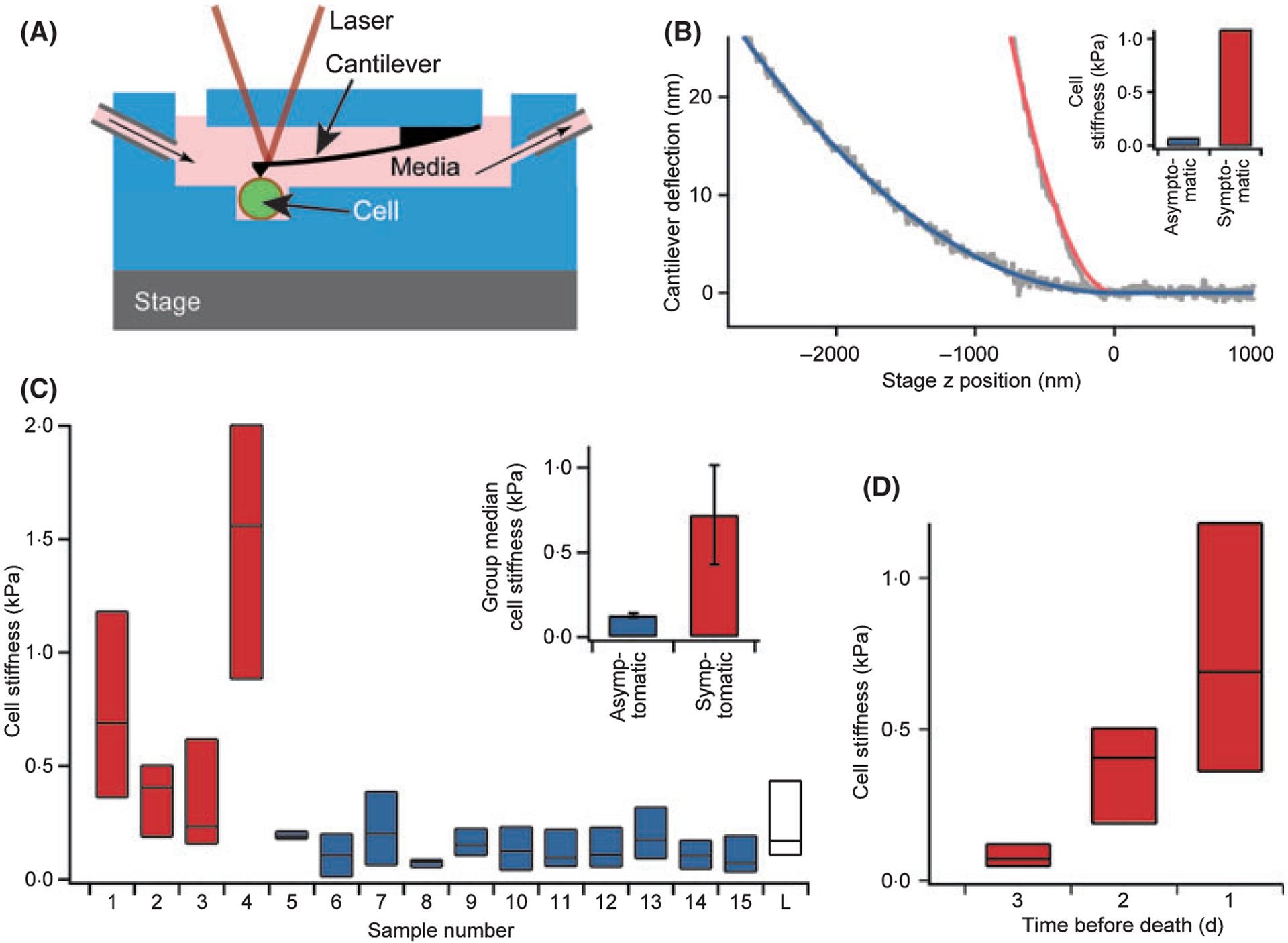
Leukaemia cell stiffness, as measured with atomic force microscopy (AFM), is higher in paediatric acute lymphoblastic leukaemia (ALL) patients with leucostasis symptoms than in asymptomatic patients. (A) Suspensions of ALL cells were pipetted into a flow chamber for AFM measurements. All stiffness measurements were taken on a heated stage held at 37°C. Cells were immobilized in microfabricated wells to prevent movement during experiments, and media was perfused into and out of the chamber. Illustration not to scale. n ≥ 15 cells for all populations. (B) Typical AFM cantilever deflection vs. distance curves (raw data in grey). The cell stiffness can be determined using a Hertzian mechanics model (fits are coloured lines). Shown here are the curves of an ALL cell with a stiffness of 1·1 kPa taken from a patient with leucostasis symptoms (red, samples 1–4) and an ALL cell with a stiffness of 0·06 kPa taken from an asymptomatic patient (blue, samples 5–15). (C) Stiffness of leukaemia cell populations taken from the peripheral blood of patients with symptoms of leucostasis (red) and asymptomatic patients (blue). For each sample, 75 percentile, median, and 25 percentile stiffness values are represented by the top, middle, and bottom lines, respectively, of each bar. A population of normal lymphocytes (white, labelled ‘L’) taken from a healthy individual via the same isolation protocol were used as controls for comparison. All reported stiffness values represent the average of five consecutive AFM measurements. Insert: Group median stiffness of the symptomatic (red) and asymptomatic (blue) patients were 0·72 ± 0·29 kPa and 0·13 ± 0·01 kPa, respectively (P = 0·001, errors represent standard error of the mean) (D) Serial cell stiffness measurements, represented by 75 percentile, median, and 25 percentile values, taken daily from a 15-year-old boy with relapsed, refractory ALL (patient 1) receiving only palliative care during his last 3 d of life. Median leukaemia cell stiffness significantly increased during that time from 0·07 to 0·41 kPa (P < 0·0001) to 0·69 kPa (P < 0·01). Cell stiffness values taken at 1 d prior to patient 1’s death are used in (C) for this patient.
As the central nervous system (CNS) and lungs are the major leucostasis target organs (Porcu et al, 2002), symptoms and signs of neurological and pulmonary dysfunction were considered evidence of leucostasis only when the onset occurred at presentation and no other aetiology could be identified, similar to previous studies on leucostasis (Novotny et al, 2005, 2006). Out of the 15 patients with paediatric ALL and peripheral blasts, four had symptoms and/or abnormalities on physical examination or diagnostic imaging consistent with CNS or pulmonary leucostasis (Table I). All patients were newly diagnosed, with the exception of patient 1, who had multiply relapsed refractory disease and was receiving only end-of-life palliative care at the time of this study. This patient ultimately died of respiratory failure, and leukaemia cell stiffness was measured daily during his last 3 d of life. Leucostasis symptoms resolved for the other three patients during induction chemotherapy.
Table I.
Patient demographic and clinical information.
| Patients with symptoms consistent with leucostasis* | Asymptomatic patients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Age, years | 15 | 5 months | 8 | 13 | 5 | 7 | 8 | 3 | 7 | 16 | 18 | 3 | 14 | 6 months | 2 |
| Imaging | NA | Brain MR - CSF prominence | Brain MR - thalamic infarct | Brain MR - normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | CXR - large anterior mass | Normal | Normal |
| Symptoms and physical examination findings | Haemoptysis, respiratory failure** | Bilateral papilledema, chloroma of right orbit | Dyspnea, cough, tachnypnea | Blurry vision in left eye with decreased visual acuity, bilateral papilledema | Normal | Normal | Splenomegaly | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Sex, M/F | Male | Female | Male | Female | Male | Male | Male | Female | Male | Female | Female | Female | Male | Female | Female |
| ALL subtype | Pre-B (refractory, relapsed) | Pre-B (CALLA negative) | T-cell precursor | Pre-B (CALLA negative) | Pre-B | Pre-B | T-cell precursor | Pre-B | T-cell precursor | Pre-B | Pre-B | Pre-B | T-cell precursor | Pre-B | Pre-B |
| Cytogenetics | t(9;22) (q34;qll) | t(4;11) (q21;q23) | Normal | t(4;11) (q21;q23) | Normal | Normal | t(11;14) (pl3;ql) | Normal | -Y, −9p | Normal | Normal | Hyperploidy | XYY, t(2;13) | t(4;ll) (q21;q23) | Hyperploidy |
| WBC × 109/l | 33·3 | 420·5 | 453·0 | 169·0 | 142·4 | 256·3 | 476·3 | 189·4 | 221·0 | 2·1 | 55·0 | 51·8 | 225.0 | 14.4 | 94.1 |
| Blasts × 109/l | 32·0 | 395·3 | 317·1 | 150·4 | 110·7 | 230·2 | 433·2 | 158·6 | 179·01 | 0·4 | 52·8 | 36·77 | 209·25 | 11·52 | 81·87 |
| Hb (g/l) | 93 | 67 | 113 | 40 | 56 | 52 | 97 | 82 | 98 | 85 | 86 | 81 | 53 | 107 | 97 |
| Platelets × 109/l | 26 | 21 | 9 | 7 | 125 | 11 | 45 | 65 | 44 | 78·0 | 39 | 37 | 37 | 50 | 37 |
| Uric acid (μmol/l) | 53·5 | 374·7 | 398·5 | 339·0 | 463·9 | 190·3 | 398·5 | 410·4 | 547·2 | 422·3 | 446·1 | 130·9 | 606·7 | 172·5 | 565·1 |
| Lactate Dehydrogenase (IU/l) | NA | 1619 | >2700 | 1266 | 825 | 1225 | 2544 | 543 | 7000 | 434 | 1069 | 407 | 707 | 317 | 1250 |
NA, not available; MR, magnetic resonance; CSF, cerebrospinal fluid; ALL, acute lymphoblastic leukaemia; WBC, white blood cells.
Although leucostasis may occur in other organ systems as well, only haematological, neurological, and/or respiratory dysfunction were observed in our patient sample. In addition, no evidence of serious bacterial infection/sepsis (all patients were afebrile at the time of the blood draw) or renal dysfunction due to uric acid nephropathy and tumor lysis syndrome was seen in any of the patients studied.
Of note, patient 1 was diagnosed with aspergillous pneumonia (diagnosed via broncho-alveolar lavage) 6 months prior to his death. He was successfully treated with caspofungin and voriconazole, which he received continuously until his death. During the last 2 weeks of his life, he did not experience any respiratory symptoms or fever until the day of his death. Serial chest radiographs revealed no abnormality during that time. The only detectable change in his clinical status, aside from increase in leukaemia cell stiffness, was an increasing number of circulating leukaemia cells during his last week (leukaemia cell concentration during his last 3 d of life: 10·92, 15·57, 32·0 × 109 cells/l respectively). On the day he died, he remained afebrile but developed progressively worsening respiratory failure over several hours and haemoptysis in the last hour of life. The correlation between cell concentration and cell stiffness was not statistically significant (P = 0.23), and the leukaemia cell concentration did not reach levels consistent with hyperleucocytosis.
Leukaemia cell stiffness values were calculated for each patient sample (Fig 1C). Mann–Whitney analysis showed that the median stiffness values were higher in the four patients with symptoms consistent with CNS and respiratory leucostasis (group median: 0·72 ± 0·29 kPa) than in the 11 asymptomatic patients (group median: 0·13 ± 0·01 kPa, P = 0·001, effect size = −0·74, errors represent standard errors of the mean). That such a considerable statistical significance and effect size, which indicates the magnitude of difference between groups, were obtained with a relatively small patient sample demonstrated that a substantial difference exists between these two patient populations. Furthermore, the 10, 25, 75, and 90 percentile cell stiffness values were higher in the symptomatic than asymptomatic patients (P range: 0·005–0·01), indicating that the observed differences in stiffness were consistent throughout entire cell populations. Finally, the cell stiffness variance was more prominent in symptomatic patients, raising the possibility that a small proportion of stiff cells within the population may be sufficient to trigger leucostasis.
Although leucostasis tends to occur with hyperleucocytosis (Lowe et al, 2005), the exact relationship between leucostasis risk and white blood cell (WBC) count in paediatric ALL remains unclear. Our results showed no significant difference in WBC count between symptomatic and asymptomatic patients (P = 0·40) and leukaemia cell stiffness (R2 = 0·004, P = 0·84) did not correlate with WBC count, suggesting that increased cell stiffness may be an additional independent leucostasis risk factor.
Cell stiffness measurements taken daily during patient 1’s last 3 d of life showed that the median leukaemia cell stiffness significantly increased from 0·07 to 0·41 kPa (P < 0·0001) to 0·69 kPa (P < 0·01). This increase preceded the development of respiratory failure, indicating that cell stiffness trends may possibly be used to predict the onset of leucostasis (Fig 1D, Table I).
However, the underlying cause for the observed cellular stiffness increase is unclear. We have recently shown that chemotherapy-induced cell death increases leukaemia cell stiffness (Lam et al, 2007). However, no patient in this study received chemotherapy around the time the sample was drawn. Elevated cytokines levels (i.e. tumour necrosis factor-α), which increase neutrophil stiffness in sepsis leading to microvascular occlusion (Skoutelis et al, 2000), may alter cell stiffness in acute leukaemia, but further studies are required to elucidate the aetiology of leukaemia cell stiffness and the exact role it plays in leucostasis pathophysiology.
Our results suggest that increased leukaemia cell stiffness is associated with leucostasis in paediatric ALL. This is consistent with recent data showing that leucostasis-positive leukaemia cells have longer transit times through capillary-sized channels than cells from asymptomatic leukaemia patients (Rosenbluth et al, 2008). Though leucostasis is rare in paediatric ALL (Lowe et al, 2005), it is associated with high mortality, and a reliable clinical predictor would enable physicians to identify high-risk patients before irreversible damage occurs. Further research is required to determine whether increased leukaemia cell stiffness, as well as other factors, such as adhesion or transmigration, can be used as independent risk and/or prognostic factors for leucostasis. In addition, more patients, especially those with autopsy-confirmed leucostasis, must be enroled to definitively determine the correlation, if any, between immunophenotype, cytogenetics and cell stiffness.
Acknowledgements
The authors would like to thank Mignon Loh, Katherine Matthay, Suzanne Henderson, Leia Cruz, Sharon Lee and the rest of the Pediatric Hematology/Oncology Division at UCSF for assistance in obtaining patient samples.
Funding sources
This work was supported by a NIH NRSA and the Hammond Research Fellowship of the National Childhood Cancer Foundation/Children’s Oncology Group to Wilbur A. Lam, a NSF Graduate Research Fellowship to Michael J. Rosenbluth, and a NSF CAREER Award and support from the University of California Cancer Research Coordinating Committee and Lawrence Livermore National Laboratory to Daniel A. Fletcher.
References
- Lam WA, Rosenbluth MJ & Fletcher DA (2007) Chemotherapy exposure increases leukemia cell stiffness. Blood, 109, 3505–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman MA (1973) Rheology of leukocytes, leukocyte suspensions, and blood in leukemia. Possible relationship to clinical manifestations.. Journal of Clinical Investigation, 52, 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EJ, Pui CH, Hancock ML, Geiger TL, Khan RB & Sandlund JT (2005) Early complications in children with acute lymphoblastic leukemia presenting with hyperleukocytosis. Pediatric Blood & Cancer, 45, 10–15. [DOI] [PubMed] [Google Scholar]
- Novotny JR, Muller-Beissenhirtz H, Herget-Rosenthal S, Kribben A & Duhrsen U (2005) Grading of symptoms in hyperleukocytic leukaemia: a clinical model for the role of different blast types and promyelocytes in the development of leukostasis syndrome. European Journal of Haematology, 74, 501–510. [DOI] [PubMed] [Google Scholar]
- Novotny JR, Nuckel H & Duhrsen U (2006) Correlation between expression of CD56/NCAM and severe leukostasis in hyperleukocytic acute myelomonocytic leukaemia. European Journal of Haematology, 76, 299–308. [DOI] [PubMed] [Google Scholar]
- Porcu P, Farag S, Marcucci G, Cataland SR, Kennedy MS & Bissell M (2002) Leukocytoreduction for acute leukemia. Therapeutic Apheresis, 6, 15–23. [DOI] [PubMed] [Google Scholar]
- Radmacher M (2007) Studying the mechanics of cellular processes by atomic force microscopy. Methods in Cell Biology, 83, 347–372. [DOI] [PubMed] [Google Scholar]
- Rosenbluth MJ, Lam WA & Fletcher DA (2006) Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophysical Journal, 90, 2994–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth MJ, Lam WA & Fletcher DA (2008) Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab on a Chip, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K (1993) Cellular deformability studies in leukemia. Physiological Chemistry and Physics and Medical NMR, 25, 293–297. [PubMed] [Google Scholar]
- Skoutelis AT, Kaleridis V, Athanassiou GM, Kokkinis KI, Missirlis YF & Bassaris HP (2000) Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Critical Care Medicine, 28, 2355–2359. [DOI] [PubMed] [Google Scholar]


