Summary
Intra-operative hypotension is a known predictor of adverse events and poor outcomes following major surgery. Hypotension often occurs on induction of anaesthesia, typically attributed to hypovolaemia and the haemodynamic effects of anaesthetic agents. We assessed the efficacy of fluid optimisation for reducing the incidence of hypotension upon anaesthesia induction. This prospective protocol enrolled 283 patients undergoing radical cystectomy and randomised them to goal-directed fluid therapy (n = 142) or standard fluid therapy (n = 141). Goal-directed fluid therapy patients received fluid optimisation based on stroke volume response to passive leg raise before induction; those with positive passive leg raise received intravenous crystalloid fluid boluses until stroke volume was optimised. Baseline mean arterial pressure was measured on the morning of surgery and upon operating room arrival. This post-hoc analysis defined haemodynamic instability as either a > 30% relative drop in mean arterial pressure compared to baseline or absolute mean arterial pressure < 55 mmHg, within 15 minutes of induction. Forty-two (30%) goal-directed fluid therapy patients underwent fluid optimisation after finding an intravascular fluid deficit via passive leg raise testing; 106 (75%) goal-directed fluid therapy and 112 (79%) standard fluid therapy patients met criteria for haemodynamic instability. There was no significant difference in haemodynamic instability incidence between the goal-directed fluid therapy and standard fluid therapy groups using absolute mean arterial pressure drop below 55 mmHg (p=0.58) or using pre-surgical testing or pre-surgical mean arterial pressure values as baseline (p = 0.21, p = 0.89); however, the difference in haemodynamic instability incidence was significant using the operating room baseline mean arterial pressure (p = 0.004). We conclude that fluid optimisation before induction of general anaesthesia did not significantly impact haemodynamic instability.
Keywords: fluid optimisation, haemodynamic instability, hypovolaemia, intra-operative hypotension, passive leg raise
Studies report an association between intra-operative hypotension and mortality far beyond the peri-operative period, with significant associations for 30-day and 1-year mortality [1–4]. However, methods to prevent or mitigate the impact of intra-operative hypotension still require further investigation.
Haemodynamic instability can occur during induction of anaesthesia that historically has been attributed to hypovolaemia secondary to pre-operative fasting and to the vasodilatory and negative inotropic effects of certain induction agents [5]. For example, it has been taught that prevention of propofol-induced hypotension could be achieved by intravenous fluid preloading [6]. Although there are conflicting data regarding the efficacy of fluid preloading, the general consensus remains that patients entering the operating room require fluids to make up for reduced pre-operative intake. As normovolaemia is not a well-defined clinical concept, an established method of assessing fluid status is to evaluate stroke volume response to intravenous infusion of a fluid bolus [7]. In the critical care setting, to avoid administering fluid to individuals who may already be normovolaemic, the passive leg raise has been shown to have good prognostic benefit as an alternative to fluid infusion [8]. While this is a validated method of assessing fluid status in the critical care setting [9], the impact of fluid optimisation on post-induction hypotension has not been well studied.
This post-hoc analysis of prospectively collected data from a randomised, controlled trial assessed if pre-induction optimisation of patient fluid status was associated with a reduction in hypotension during induction of anaesthesia. Patients in the intervention arm had their fluid status assessed through a passive leg raise manoeuvre; those shown to be fluid responsive underwent fluid optimisation, under the assumption that normovolaemic patients should be more haemodynamically stable.
Methods
This analysis addresses a secondary set of objectives using prospectively collected data from a single-centre, single-blinded, prospective, randomised controlled trial approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. All patients gave written, informed consent. The trial’s primary objective was to examine the incidence of postoperative ileus in the goal-directed fluid therapy (GDFT) vs. standard fluid therapy (SFT) arms, but the stroke volume data from the study provided the opportunity to assess the effect of fluid optimisation on hypotension following induction. All eligible patients scheduled to undergo open radical cystectomy with urinary diversion for urothelial carcinoma of the bladder at Memorial Sloan Kettering Cancer Center were approached and enrolled by their urologic surgeon. Exclusion criteria included age < 21 y, active atrial fibrillation, and BMI > 45 (the last included due to the limitations of stroke volume variation reading). Patients were randomly assigned 1:1 to the GDFT or SFT arm using randomly sized permuted blocks with the Memorial Sloan Kettering Clinical Research Database. Patients were blinded as to their study arm; physicians were not blinded.
Patients were required to fast after midnight prior to their procedure with no pre-operative carbohydrate drink and fluid administration restricted in pre-operative holding. Additionally, all cases entered the Operating Room by 08.00, consequently fasting time was uniform across the cohort. Prior to induction of anaesthesia, an EV1000™ haemodynamic monitor using FloTrac™ sensors (Edwards Lifesciences, Irvine, CA, USA) was placed in all patients to allow pre-induction recording of stroke volume and cardiac output. The SFT arm received standard induction without optimisation of fluid status. The GDFT arm was optimised based on stroke volume response to a passive leg raise: patients with a ≥ 10% increase in stroke volume were deemed to have a positive passive leg raise result and to be, therefore, hypovolaemic. After being placed supine, these patients were given intravenous crystalloid boluses (Normosol-R, ICU Medical Inc., Lake Forest, IL, USA) in 250 ml increments until the stroke volume was considered optimised, which we defined as a change of less than 10% following fluid bolus. Once optimised, GDFT patients proceeded with induction as normal. The SFT cohort did not receive any fluids before induction of anaesthesia.
All anaesthesia was induced with propofol, fentanyl and rocuronium. Propofol and fentanyl were administered manually as an intravenous bolus titrated to effect. All patients underwent general anaesthesia with tracheal intubation. Phenylephrine and ephedrine were given to treat hypotension at the discretion of the practitioner, based on absolute or relative hypotension and medical status of the patient.
The lowest mean arterial pressure (MAP) recorded within the first 15 min of induction was, for the purposes of this analysis, compared to three different baseline MAP values: the MAP calculated from blood pressure readings (1) taken with upper limb automated oscillotonometry during the pre-surgical testing clinic visit or (2) in the pre-surgical centre on the morning of the procedure, and (3) the first MAP measured after arterial line placement prior to anaesthesia induction in the operating room. Stroke volume was also recorded immediately before and 5 min after induction. Dosages of induction medication and vasoactive medications given within 15 min of induction were recorded.
The primary endpoint of this analysis was haemodynamic instability on induction, which we defined as occurring in patients whose minimum MAP 15 min after induction was below 55 mmHg or decreased by more than 30% from baseline [2, 4, 10, 11]. These definitions of hypotension have previously been associated with adverse postoperative outcomes, even in transient intra-operative episodes. Induction time for both arms was considered the time of propofol injection. Patients were analysed in the arms into which they were randomised. All analyses were performed in a modified intention-to-treat population, which included all patients who had undergone randomisation and anaesthesia with EV1000 monitoring for eligible surgery. Change in stroke volume was assessed before and after induction among all patients and by arm, focusing on patients whose stroke volume decreased by at least 10% from pre-induction to post-induction and whether these patients were also haemodynamically unstable at each time-point. A decrease in stroke volume of at least 10% is a known proxy of fluid status; stroke volume values before and after induction were compared to assess if volume status and induction-associated hypotension were correlated. Continuous variables are described using median and interquartile range; Wilcoxon rank sum test was used to test for a difference between groups. Categorical variables are described using frequency and percent; Fisher’s exact test was used to test for a difference between groups. Analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA). All tests were two-sided and p < 0.05 was considered significant.
Results
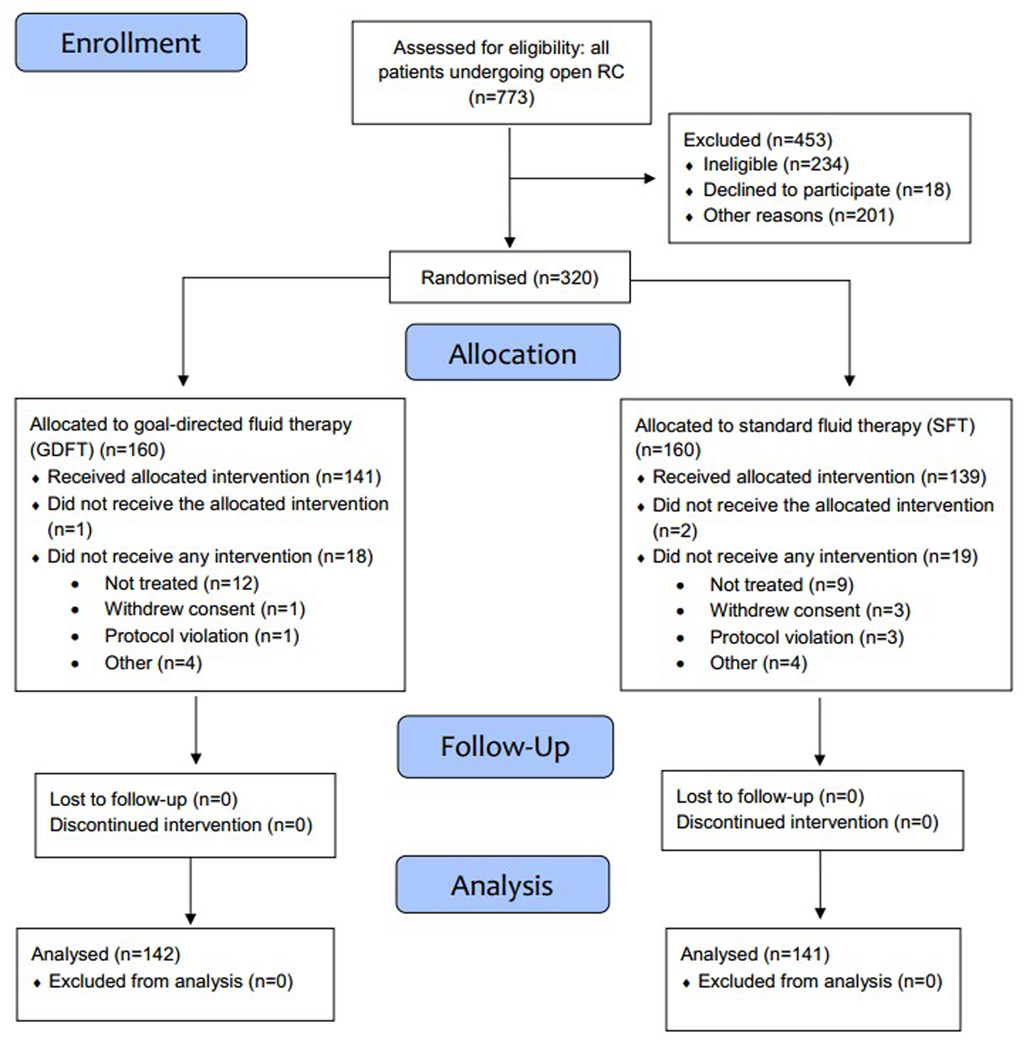
A total of 320 patients were randomised (Fig. 1). Twenty-nine were taken off protocol due to a change in surgical plan or unavailability of participating anaesthetists, 4 withdrew consent, and 4 were ineligible after consent due to atrial fibrillation on pre-operative ECG. The protocol’s planned goal of 283 evaluable patients was met (141 in the SFT arm and 142 in the GDFT arm), enrolled between 5 August 2014 and 9 April 2018. Table 1a describes the baseline characteristics of the cohort and study arms. There were no significant differences between the treatment groups in intra-operative characteristics, including propofol dosage or MAP (Table 1b). Forty-two (29.6%) patients in the GDFT group were found to be hypovolaemic, i.e. had an intravascular volume deficit on passive leg raise testing and were optimised as described in the Methods.
Figure 1. CONSORT flow diagram of patient inclusion.
GDFT, goal-directed fluid therapy; RC, radical cystectomy; SFT, standard fluid therapy.
Table 1a.
Baseline characteristics of 283 patients undergoing radical cystectomy randomly allocated to goal-directed fluid therapy (GDFT) or standard fluid therapy (SFT). Values are mean (SD), number (proportion) or median (IQR [range]).
| Total n=283 | GDFT n=142 | SFT n=141 | |
|---|---|---|---|
| Baseline/pre-operative characteristics | |||
| Age, y | 68.9 (9.83) | 69.0 (10.2) | 68.7 (9.49) |
| Sex; female | 62 (21.9%) | 30 (21.1%) | 32 (22.7%) |
| Anaesthesia category (highest value between pre-surgical testing and pre-operative day) | |||
| ASA 1* or 2 | 85 (30.0%) | 44 (31.0%) | 41 (29.1%) |
| ASA 3 or 4 | 198 (70.0%) | 98 (69.0%) | 100 (70.9%) |
| BMI; kg.m−2 | 28.8 (5.1%) | 28.9 (5.5%) | 28.8 (4.7%) |
| BMI categories | |||
| Underweight | 2 (0.7%) | 2 (1.4%) | 0 |
| Normal | 63 (22.3%) | 33 (23.2%) | 30 (21.3%) |
| Overweight | 107 (37.8%) | 50 (35.2%) | 57 (40.4%) |
| Obese | 103 (36.4%) | 52 (36.6%) | 51 (36.2%) |
| Morbid | 8 (2.8%) | 5 (3.5%) | 3 (2.1%) |
| Neoadjuvant chemotherapy | 124 (43.8%) | 65 (45.8%) | 59 (41.8%) |
| Baseline MAP values, mmHg | |||
| Pre-surgical testing | 96 (88–104 [61–128]) | 98 (91–105 [66–127]) | 94 (86–102 [61–128]) |
| Pre-surgical centre | 96 (89–105 [54–123]) | 98 (92–106 [54–122]) | 95 (87–103 [74–123]) |
| Operating room | 84 (73–94 [45–127]) | 79 (72–92 [45–127]) | 85 (74–95 [52–113]) |
No patient had an ASA physical status of 1.
GDFT, goal-directed fluid therapy; SFT, standard fluid therapy; ASA, American Society of Anesthesiologists physical status; MAP, mean arterial pressure.
Table 1b.
Intra-operative characteristics of 283 patients undergoing radical cystectomy randomly allocated to goal-directed fluid therapy (GDFT) or standard fluid therapy (SFT). Values are number (proportion) or median (IQR [range])
| Intra-operative characteristics | Total n=283 | GDFT n=142 | SFT n=141 | p value |
|---|---|---|---|---|
| Propofol dose, mg.kg−1* | 1.7 (1.3–2.1 [0.0–4.2]) | 1.8 (1.4–2.1 [0.0–3.0]) | 1.7 (1.3–2.1 [0.6–4.2]) | 0.532 |
| Induction MAP, mmHg | 58 (51–64 [36–95]) | 59 (52–65 [37–95]) | 57 (51–64 [36–88]) | 0.353 |
| Vasopressors required within 15 min of induction | 85 (30.0%) | 41 (28.9%) | 44 (31.2%) | 0.699 |
1 patient in the GDFT group did not receive propofol
Table 2 shows the proportion of the cohort determined to be “haemodynamically unstable”: those whose MAP on induction was < 55 mmHg or who experienced a > 30% decrease from baseline, using the three baseline MAP time-points described in the Methods. The most objective measure of haemodynamic instability, induction MAP < 55 mmHg, had a similar rate for the GDFT and SFT arms: 37% vs. 40%, respectively, p = 0.626. Rates of relative haemodynamic instability (> 30% drop from baseline) were higher overall but still similar between arms when comparing induction MAP to pre-surgical testing baseline MAP (75% vs. 81%, p = 0.255) or comparing induction MAP to pre-surgical centre baseline MAP (79% vs. 78%, p = 0.773). Using either pre-surgical testing or pre-surgical centre baseline values, three-quarters of patients in both arms were haemodynamically unstable. However, comparing induction MAP to the operating room baseline MAP did show a significantly lower proportion of haemodynamically unstable patients in the GDFT arm vs. the SFT arm (39% vs. 57%, p = 0.004). When using this study’s definition of haemodynamically unstable as patients who met either the absolute or relative criterion, data were similar to those for relative alone, including a borderline significant difference when comparing induction MAP to operating room baseline MAP (52% for GDFT vs. 65% for SFT, p = 0.040).
Table 2.
Incidence of haemodynamic instability and mean arterial pressure (MAP) decrease from baseline, using Values are number (proportion) or proportion (95%CI).
| Total n=283 | GDFT n=142 | SFT n=141 | p value | |
|---|---|---|---|---|
| Haemodynamic instability | ||||
| Absolute (MAP < 55 mmHg) | 108 (38.2%) | 52 (36.6%) | 56 (39.7%) | 0.626 |
| Relative (>30% MAP drop from baseline) | ||||
| Using PST baseline value | 215 (76.0%) | 104 (73.2%) | 111 (78.7%) | 0.207 |
| Using PSC baseline value | 220 (77.7%) | 111 (78.2%) | 109 (77.3%) | 0.886 |
| Using OR baseline value | 136 (48.1%) | 56 (39.4%) | 80 (56.7%) | 0.004 |
| Met criteria for either absolute or relative haemodynamic instability | ||||
| Using PST baseline value | 218 (77.0%) | 106 (74.6%) | 112 (79.4%) | 0.255 |
| Using PSC baseline value | 221 (78.1%) | 112 (78.9%) | 109 (77.3%) | 0.773 |
| Using OR baseline value | 165 (58.3%) | 74 (52.1%) | 91 (64.5%) | 0.040 |
| Change from baseline MAP | ||||
| Using PST baseline value | −39.9% (−47.2 to −30.7%) | −39.9% (−47.7 to −28.8) | −39.8% (−46.1 to −32.7) | 0.968 |
| Using PSC baseline value | −39.6% (−47.3 to −31.7%) | −39.4% (−47.6 to −31.9%) | −39.9% (−47.3 to −31.6%) | 0.972 |
| Using OR baseline value | −29.6% (−39.8 to −18.3%) | −27.3% (−37.1 to −13.9%) | −32.4% (−40.5 to −20.9%) | 0.005 |
GDFT, goal-directed fluid therapy; SFT, standard fluid therapy; PST, pre-surgical testing; PSC, pre-surgical centre; OR, operating room.
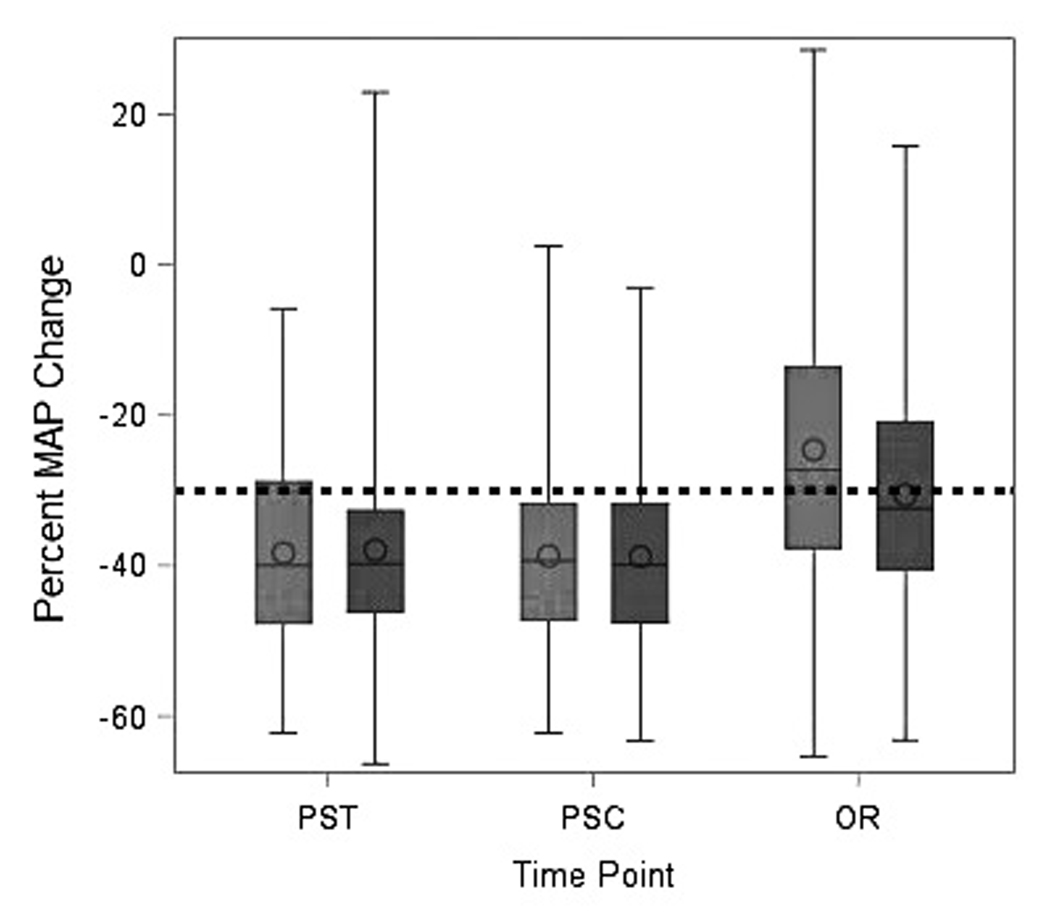
The degree of change in blood pressure upon induction, measured as percent change from baseline, was not significantly different between GDFT and SFT groups when using pre-surgical testing or pre-surgical centre baseline blood pressure (both p > 0.95). Using operating room MAP baseline values, the median percent decrease was significantly smaller in both arms and significantly different between arms (−27% for GDFT vs. −32% for SFT; p = 0.005) (Table 2). These findings are illustrated in Figure 2, showing the median percent decrease from pre-surgical testing and pre-surgical centre values being >30%.
Figure 2. Box plots of percent change from baseline MAP to induction MAP minimum, by study arm.
Light grey bars represent the GDFT arm and dark grey bars represent the SFT arm. Dashed line represents the unstable threshold, defined as MAP change of at least −30%. GDFT, goal-directed therapy; MAP, mean arterial pressure; OR, operating room; PSC, pre-surgical centre; PST, pre-surgical testing.
Stroke volumes before and after induction were measured using EV1000 data. Stroke volume reductions from baseline of at least 10% were compared to the incidence of haemodynamic instability across all patients. Table 3 summarises, for each of the three baseline time-points, the proportion of stable to unstable patients, dividing the cohort by < 10% vs. ≥ 10% stroke volume decrease. None of the differences were statistically significant and these data did not display any correlation between stroke volume change on induction and haemodynamic stability.
Table 3.
Incidence of haemodynamic instability by stroke volume decrease on induction (< 10% vs. ≥ 10%) and using different mean arterial pressure (MAP) baseline values. Values are number (proportion) and stratified by time-point of baseline MAP determination. Haemodynamic instability was defined as either induction MAP < 55 mmHg or a > 30% decrease from baseline.
| Total | < 10% stroke volume decrease | ≥ 10% stroke volume decrease | p value | |
|---|---|---|---|---|
| Pre-surgical testing (n = 265*) | ||||
| Stable | 59 (22.3%) | 39 (14.7%) | 20 (7.5%) | 0.751 |
| Unstable | 206 (77.7%) | 142 (53.6%) | 64 (24.2%) | |
| Pre-surgical centre (n = 265*) | ||||
| Stable | 55 (20.8%) | 33 (12.5%) | 22 (8.3%) | 0.142 |
| Unstable | 210 (79.2%) | 149 (56.2%) | 61 (23%) | |
| Operating room (n = 267*) | ||||
| Stable | 107 (40.1%) | 71 (26.6%) | 36 (13.5%) | 0.688 |
| Unstable | 160 (59.9%) | 111 (41.6%) | 49 (18.4%) | |
Of the total 283 patients in the study, 16 were missing stroke volume or induction MAP data and thus could not be included in this analysis. An additional two patients were missing pre-surgical testing data and two were missing pre-surgical centre data.
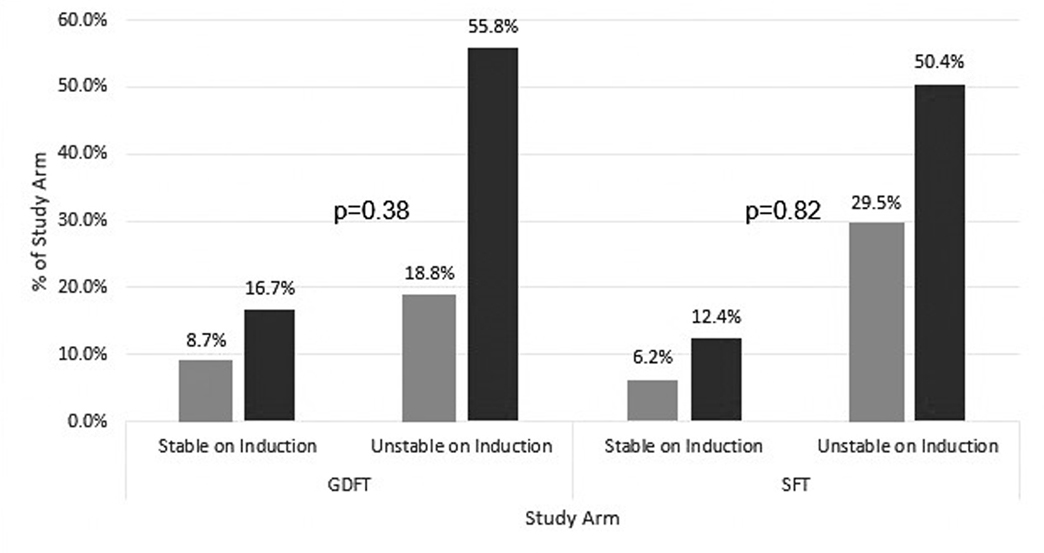
There was no difference between arms in the percent of patients whose stroke volume decrease was smaller than 10% (GDFT: 100/138 [72.5%] vs. SFT: 82/129 [63.6%]; p = 0.148). We also found no statistically significant correlation between a clinically significant change in stroke volume on induction (defined as a decrease of at least 10%) and subsequent haemodynamic instability. These findings are summarised in Table 3, Supplementary Table S1 and illustrated in Fig. 3, which portrays the relative proportion of each patient subgroup that met these criteria. The stratified analysis by arm in Supplementary Table S1 was done only for the pre-surgical testing time-point since this time-point was likely the most accurate representation of each patient’s true baseline.
Figure 3. Clustered column graph comparing incidence of haemodynamic instability in patients with and without clinically significant stroke volume decrease on induction (defined as a ≥ 10% decrease), for the fluid optimisation (GDFT) and standard (SFT) arms.
Light grey bars represent patients with at least a 10% decrease in SV, dark grey bars represent patients who had less than a 10% decrease in SV. Haemodynamic instability was defined as either an induction MAP < 55 mmHg or a > 30% decrease from baseline. Baseline MAP was defined as the pre-surgical testing MAP baseline for each patient, as this time-point was closest to the pre-induction stroke volumes used. Proportions of patients in each subgroup are indicated above each bar. No significant association was found in either group between incidence of haemodynamic instability and stroke volume change (p = 0.38, GDFT; p = 0.82, SFT). P-values listed for each study arm as comparison was done within each arm between the patient subgroups. GDFT, goal-directed fluid therapy; MAP, mean arterial pressure; SFT, standard fluid therapy; SV, stroke volume.
Discussion
Our analysis of prospective study data showed that fluid optimisation before induction of general anaesthesia did not significantly affect the occurrence or degree of haemodynamic instability during induction. This has a number of potential implications. Primarily, this result likely points to the poor viability of prophylactic or GDFT as a method of pre-induction haemodynamic optimisation. Given the lack of difference in induction-related hypotension between theoretically optimised patients and those without any intervention, we believe this indicates that the optimisation of patient fluid status in elective surgical patients has no impact on hypotension upon induction [4]. Taking into account recent data questioning the commonly held notion that pre-operative fasting induces hypovolaemia and subsequent hypotension on induction [12], our data confirm that fluid optimisation has no impact.
Interestingly, it should also be noted that stroke volume decrease from before to after induction was not significantly different between groups. This, in addition to the lack of correlation between stroke volume change and subsequent hypotension on induction, further illustrates the disconnect between propofol-induced hypotension and volume status. No significant correlation was found between stroke volume changes on induction and haemodynamic stability, in terms of overall number of patients or the relative proportions of patients; this also lends weight to the disconnect between volume status and response to anaesthesia induction. Given that propofol has minimal effect on cardiac output [5], this finding is in agreement with our understanding of the cardiac effects of anaesthesia induction. Propofol maintains a net neutral effect on stroke volume change and, therefore, on cardiac output by the extent of afterload reduction via decreased arterial tone and a preload reduction secondary to its venodilatory effects. This is further supported by our findings, as any benefit from optimisation of patient volume status would be negated in a vasodilatory state. Since stratification of patients based on study arm also failed to show any significant influence of GDFT on the haemodynamic impact of induction, any hypotension associated with induction will best be mitigated by use of vasopressor medications rather than fluid loading. This treatment strategy would target the true source of blood pressure variation, which is due to changes in systemic vascular resistance and is not preload-dependent. Further studies should be conducted to assess the efficacy of running low-dose vasoactive agents during induction of anaesthesia to confirm this theory.
A strength of our study is that despite our population undergoing radical cystectomy being elderly with comorbidities, mainly ASA 3 and 4 patients, the results can be extrapolated to the general elderly population undergoing abdominal surgery and can be applied to induction of any general anaesthesia because peripheral vascular tone seems to be more important than fluid status in maintaining stability. Additionally, this study provides a particularly in-depth analysis of the effects of fluid optimisation on induction since all arterial lines were placed prior to induction. Even if some negative passive leg raise results could be attributed to patients with poor myocardial contractility, this circumstance further strengthens our theory that fluid optimisation has, at most, minimal benefit for prevention of induction-associated hypotension.
One limitation is that we did not perform passive leg raise in our SFT arm. Therefore, we cannot draw any conclusions about the specific haemodynamic behaviour upon induction of patients who had a positive passive leg raise result but did not subsequently receive fluid optimisation. Despite this limitation, we believe the stroke volume data collected before and after induction serve as a satisfactory proxy for the effects of patient volume status when compared to haemodynamic stability. Nonetheless, subsequent studies should include passive leg raise for both study arms, and specific systemic vascular resistance measurements during induction. In addition, although comparisons between the GDFT and SFT arms using the operating room baseline MAP value were found to be statistically significant, the greater degree of variability in patient blood pressures within each group was viewed more as a statistical anomaly than evidence of treatment efficacy, as evident in Figure 2. Additionally, due to the workflow in the operating room, baseline operating room MAP was recorded after sedation was administered for the placement of the arterial line. For this reason, we believe that the use of operating room MAP to determine stability on induction is inherently confounded, amounting to a MAP that is not truly baseline. On the other hand, pre-surgical testing and pre-surgical centre baselines are a more accurate picture of the baseline MAP for each patient. Regardless, subsequent stratification and comparison still showed no significant correlation between stroke volume changes and haemodynamic instability. Another notable corollary of our study was the indication that, despite the large variation between patient blood pressures when comparing MAP values across the three time-points, there was no significant difference between pre-surgical testing and pre-surgical centre when used as a baseline to determine haemodynamic stability on induction. These findings are in contrast to traditional views on pre-operative “white coat hypertension” or patient’s pre-operative mood causing a significant enough difference in blood pressure from their “real” baseline that these blood pressures cannot be used to make comparisons. As such, it may be of benefit for subsequent studies to determine the true amount of clinically significant variation between these values, as it may allow or even encourage physicians to trust pre-operative blood pressure values even on the morning of surgery.
Taking into account recent research indicating a lack of efficacy of prophylactic intravascular fluid loading and its lack of impact on induction hypotension [13], it stands to reason that the relation between patient fluid status and haemodynamic stability on induction is not as straightforward as has been thought in the past. Even in a patient population predominantly ASA 3 or higher, prophylactic boluses of fluid did not reduce the degree of haemodynamic impact from induction agents. While some studies have shown that patients arrive for elective surgery with a functional intravascular deficit [13], and we identified such a deficit in 30% of the GDFT patients, our findings indicate there is no clinical benefit to reducing that deficit before anaesthesia induction. Our results instead support the adoption of vasopressor medications early in the induction period.
Supplementary Material
Acknowledgments
This research was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and funded in part by the Cancer Center Support Grant (Grant no. P30 CA008748) from the National Institutes of Health/National Cancer Institute to Memorial Sloan Kettering Cancer Center, and by the Gary Gladstein Family. Registered on ClinicalTrials.gov (NCT02145871).
Footnotes
No conflicts of interests declared.
References
- 1.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007; 107: 213–20. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology 2015; 123: 307–19. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesthesia and Analgesia 2005; 100: 4–10. [DOI] [PubMed] [Google Scholar]
- 4.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126: 47–65. [DOI] [PubMed] [Google Scholar]
- 5.de Wit F, van Vliet AL, de Wilde RB, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. British Journal of Anaesthesia 2016; 116: 784–9. [DOI] [PubMed] [Google Scholar]
- 6.el-Beheiry H, Kim J, Milne B, Seegobin R. Prophylaxis against the systemic hypotension induced by propofol during rapid-sequence intubation. Canadian Journal of Anaesthesia 1995; 42: 875–8. [DOI] [PubMed] [Google Scholar]
- 7.Minto G, Scott MJ, Miller TE. Monitoring needs and goal-directed fluid therapy within an enhanced recovery program. Anesthesiology Clinics 2015; 33: 35–49. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey GE, Dubrey SW, Handy JM. A prospective observational study of stroke volume responsiveness to a passive leg raise manoeuvre in healthy non-starved volunteers as assessed by transthoracic echocardiography. Anaesthesia 2014; 69: 306–13. [DOI] [PubMed] [Google Scholar]
- 9.Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Medicine 2010; 36: 1475–83. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. British Journal of Anaesthesia 2015; 115: 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119: 507–15. [DOI] [PubMed] [Google Scholar]
- 12.Iwayama S, Tatara T, Osugi T, Hirose M. Preoperative oral rehydration solution intake volume does not affect relative change of mean arterial blood pressure and crystalloid redistribution during general anesthesia in low-risk patients: an observational cohort study. Journal of Anesthesia 2014; 28: 132–5. [DOI] [PubMed] [Google Scholar]
- 13.Bundgaard-Nielsen M, Jørgensen CC, Secher NH, Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiologica Scandinavica 2010; 54: 464–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.