Abstract
Hippocampus and entorhinal cortex form cognitive maps that represent relations among memories within a multidimensional space. While these relational maps have long been proposed to contribute to episodic memory, recent work suggests that they also support concept formation by representing relevant features for discriminating among related concepts. Cognitive maps may be refined by medial prefrontal cortex, which selects dimensions to represent based on their behavioral relevance. Hippocampal pattern completion, which is critical for retrieval of episodic memories, may also contribute to generalization of existing concepts to new exemplars. Navigation within hippocampal cognitive maps, which is guided by grid coding in entorhinal cortex, may contribute to imagination through recombination of event elements or concept features.
Keywords: cognitive map, episodic memory, category learning, imagination
Introduction
Reasoning about the world is greatly facilitated by knowledge of concepts, which represent combinations of features shared by things of the same kind and allow generalization from limited experience to novel exemplars. Distinctions between concepts are drawn based on differences in their component features and on the behavioral relevance of those features. For example, when shopping for a vehicle, prior knowledge about vehicle categories—such as sedans or trucks and their associated features such as shape, size, and cargo capacity—guides decision making about new vehicles one has not encountered before. While concepts may relate to perceptual categories, concepts may also be abstract; for instance, social cognition may be supported by knowledge of common personality types. Distinguishing between distinct concepts often requires simultaneous consideration of multiple features. For example, determining someone’s personality type might require considering both extraversion and conscientiousness. Recent work suggests that concept learning is supported by multidimensional cognitive maps within hippocampus, entorhinal cortex, and medial prefrontal cortex (mPFC) [1–4]. Cognitive maps encode multiple relationships within a common representational space [5], forming a simplified model of stimulus relationships and rewards that can be used to flexibly guide behavior [6,7]. We review recent evidence suggesting how computational processes of cognitive map formation, pattern completion, and dimensionality reduction contribute to concept learning. We propose that similar processes are involved in both concept learning and episodic memory, contributing to retrieval, generalization, and imagination in both domains.
Hippocampus, entorhinal cortex, and medial prefrontal cortex represent conceptual cognitive maps
Recent work demonstrates that the hippocampus and entorhinal cortex, which are thought to have critical roles in mapping spatial environments [8–10], also form cognitive maps of concepts that facilitate generalization of relationships [2,11,12]. Conceptual cognitive maps represent features that distinguish different concepts [11]. A simple example of how hippocampus supports one-dimensional representations that code the relationships among items comes from transitive inference paradigms, in which participants are presented with pairs of items that are arranged in a hierarchy (e.g., A<B<C<D<E) and must learn to select the correct item from each pair. Rats with lesions that disconnect inputs to hippocampus are able to correctly respond to the individual adjacent pairs that were learned explicitly (e.g., B<C, D<E), but fail to correctly infer relationships that have not been directly experienced (e.g., B<D) [13]. These findings indicate that hippocampus forms one-dimensional cognitive maps, which represent the relational distance between items and allow inference beyond direct experience. Similar to decisions about memory [14], hippocampal representations that code the relational distance between items are thought to guide decisions about concepts [11].
However, real-world concepts often cannot be captured by a single feature dimension; rather, multiple features contribute to defining concept boundaries. Hippocampus forms non-spatial cognitive maps that represent multiple dimensions simultaneously [12,15–17], suggesting that hippocampus might contribute to learning of multidimensional concepts. In a recent study, participants learned about social rankings of fictional people along two dimensions, popularity and competence (Figure 1A) [18]. After learning, activation patterns in hippocampus and entorhinal cortex during retrieval of individual people reflected the distance between them in a 2-dimensional space combining both dimensions. Although the individual dimensions were learned separately, they were represented within a unified cognitive map [18], suggesting that the hippocampal-entorhinal system forms multidimensional maps to organize information about related stimuli.
Figure 1.
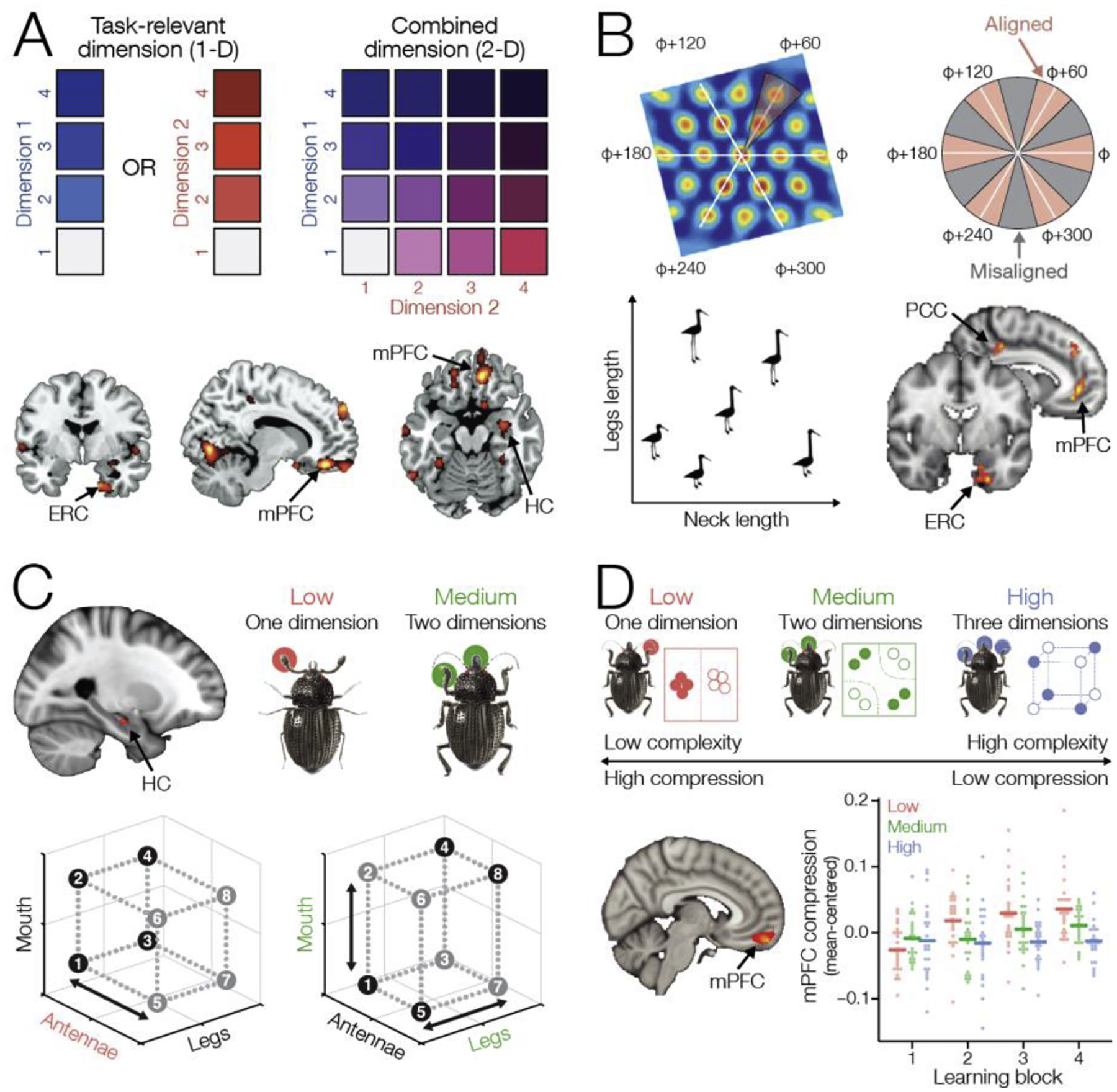
(A) Participants learned about separate hierarchies of fictional people along two dimensions, popularity and competence. After learning, although these two dimensions were never relevant at the same time, neural representations in hippocampus (HC), entorhinal cortex (ERC), and mPFC reflected the two-dimensional Euclidean distance between items, suggesting that the individual item dimensions were represented within a unified space. Adapted from [18]. (B) Grid cells, which are thought to provide a metric for navigating cognitive maps, fire at points along a hexagonal grid within a space. Grid cell responses are predicted to result in greater activity during movement along preferred angles. Participants learned to navigate a two-dimensional feature space to move to goal locations. Grid-like activity was observed in entorhinal cortex, mPFC, and posterior cingulate cortex (PCC). Adapted from [2]. (C) Participants learned a series of different categorization problems using the same stimuli that required taking into account one stimulus dimension (low complexity) or two dimensions (medium complexity). Item representations in hippocampus reflect attentional weighting of relevant dimensions of different concept learning problems. In a one-dimensional problem, the relevant stimulus dimension is weighted more heavily. In a two-dimensional problem, both relevant dimensions are weighted highly relative to an irrelevant dimension. Adapted from [3]. (D) Through learning, activity patterns in mPFC formed lower-dimensional, compressed representations which facilitated focusing on only the relevant dimension(s) for each problem. The mPFC representations formed during learning of low- and medium-complexity problems showed greater evidence of compression compared to the high-complexity problem that requires combining information from three dimensions. Adapted from [43].
Multidimensional spaces in hippocampus may contribute to concept learning by representing combinations of features that are diagnostic of category membership [3,19,20]. Consistent with this hypothesis, a recent study found that hippocampal representations did not reflect all features within a 3-D stimulus space; instead, hippocampus represented only the two dimensions that were important for a categorization decision [12]. Furthermore, another study found that hippocampal concept representations are dynamic, emphasizing the feature dimensions that are relevant for the current task [3]. The same stimuli elicited distinct representations in hippocampus during different concept learning problems that required attending to different feature combinations (Figure 1C) [3], suggesting that hippocampus may represent distinctions between concepts rather than perceptual feature dimensions per se. These observations, which suggest that hippocampal concept representations are context-dependent, raise the possibility that hippocampus may represent only concept features that are currently relevant for behavior. However, there is also evidence that hippocampus represents associations between well-learned concepts during passive viewing, suggesting a more general role for hippocampus in forming stable concept representations [21,22]. An important direction for future research will be to characterize the conditions under which hippocampal concept representations are invariant or task dependent.
Regardless of the behavioral task, flexible access to concept representations requires that different exemplars of a concept, including exemplars that have never been seen before, activate the same concept representation. The episodes-to-concepts (EpCon) model proposes that such generalization is supported by a process of integration whereby different items come to elicit the same representation [16]. The hippocampus has been implicated in integrating events with overlapping features [23,24] to connect related events and in integrating item representations to form clusters of items that predict similar behavioral outcomes [11,16,25]. The EpCon model proposes that these clusters support flexible concept learning, which may facilitate categorization based on complex concept boundaries that require considering multiple dimensions simultaneously [16]. The EpCon model successfully predicts the representational geometry of hippocampal representations after learning of different categorization problems (Figure 1C) [3]. An open question, however, is how different scales of categories are represented within hippocampus; for example, a vehicle could be described broadly as a car, more specifically as a coupe, or very specifically as a 1967 Camaro. The scale of hippocampal representations varies along its anterior/posterior axis, raising the possibility that different areas represent different conceptual scales [11]. Consistent with this proposal, one study found evidence of an anterior/posterior gradient in how extended narratives are represented in hippocampus [26]; however, it remains unclear whether concepts are coded in a similar manner.
A related facet of hippocampal representation that may be advantageous for concept learning is its ability to encode information hierarchically. A study in rats found that population firing activity in hippocampus reflected a hierarchy of multiple dimensions, with behaviorally distinct contexts represented in opposing neural codes [27,28]. Hippocampus represented both superordinate distinctions between stimuli (the different behavioral contexts) and subordinate distinctions (e.g., the correct object to select within a given context). Similarly, a recent fMRI study found that events with a common associative structure are represented in a hierarchical cognitive map [29]. Participants studied pairs of novel objects which included initial (AB) pairs followed by overlapping (BC) pairs (Figure 2A). After learning, item representations in hippocampus, parahippocampal cortex, and mPFC exhibited a consistent geometry that reflected the conceptual triad relationship, i.e., that all triads shared the same structure regardless of their individual features (Figure 2B). The common neural geometry formed a hierarchy that represented both the general conceptual structure while also distinguishing between triads of associated items. The representation of the conceptual dimension predicted later performance in retrieving individual associations between triads, suggesting that hierarchical concept representations may facilitate retrieval of individual memories (Figure 2C). While yet to be tested directly, hierarchical representations within hippocampus may similarly facilitate different types of concept decisions that rely on superordinate and subordinate discriminations.
Figure 2.
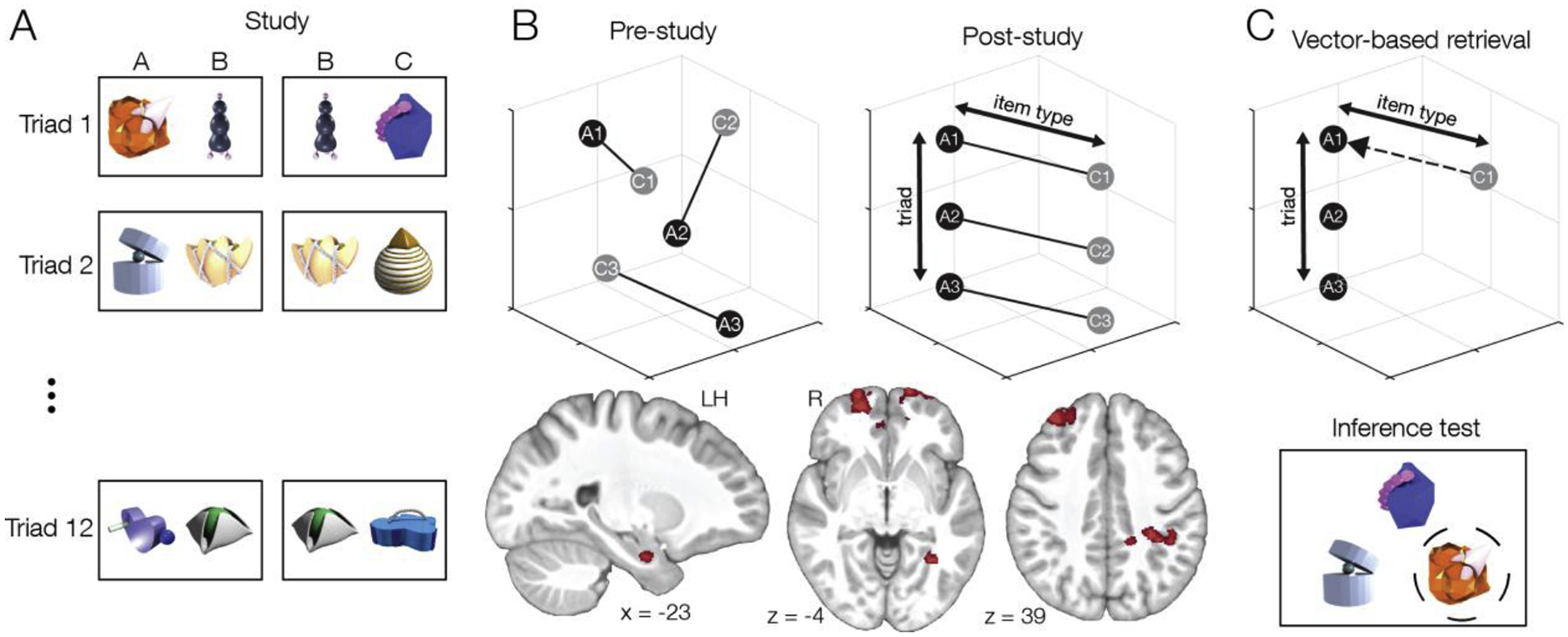
Cognitive maps form organized representations of conceptually similar events. (A) Participants studied initial (AB) and overlapping (BC) pairs that shared a common object and were instructed to learn each pair. They studied 12 triads with the same structure. (B) Brain activity elicited by each item was measured using fMRI before and after the study phase. There was no particular organization before the study phase, but after study neural representations in medial temporal lobe and frontoparietal areas reflected the common structure across events in the form of consistent representational geometry of the distinction between A and C items. (C) The reliability of neural organization across triads in parahippocampal cortex, lateral parietal cortex, and hippocampus predicted performance on an inference test where participants had to select the correct A item that had been indirectly associated with a cue C item. These results suggest that retrieval may be guided by navigation within an organized cognitive map using a vector-based retrieval mechanism. Adapted from [29].
Entorhinal representations, along with those in hippocampus discussed above, may provide additional capacity for multidimensional organization that is beneficial for concept learning. Entorhinal grid cells, which fire at regularly spaced intervals within a spatial environment [30], are thought to provide a consistent spatial metric that supports navigation within cognitive maps [31]. A recent study found that grid-like activity in entorhinal cortex, measured using fMRI, may also provide a metric within conceptual cognitive maps [2]. When participants navigated through a “bird space” composed of combinations of leg and neck length of cartoon birds, entorhinal cortex exhibited grid-like activation, suggesting that entorhinal cortex has a general role in navigating both spatial and non-spatial cognitive maps (Figure 1B) [2]. While the involvement of grid cells in representing 2-D spaces is well-established, representing the relevant features of a concept may require higher-dimensional spaces [3]. Simulations of grid cell modules in entorhinal cortex suggest that it may be able to flexibly represent higher-dimensional spaces [32], potentially facilitating navigation of higher-dimensional cognitive maps of concept features.
While the hippocampal-entorhinal system may represent multidimensional cognitive maps with Euclidean geometry [18,31], recent work suggests that Euclidean cognitive maps may result from a more general capacity of this system to learn graph structures [33–36]. Learning of different graph structures that define relationships between concepts may support formation of cognitive maps of both continuous spaces (e.g., the popularity and competence of different people) and other relational structures (e.g., relationships between people in a family tree) [33].
When learning real-world concepts (e.g., types of vehicles, personality types), a key challenge is to determine what features are relevant for distinguishing between concepts [37]. The mPFC has been proposed to play a critical role in determining the behavioral relevance of stimuli based on current goals [38], a process critical for concept learning. The mPFC receives input from the hippocampus, which is thought to provide information about the current context [39,40]. The mPFC represents a cognitive map of stimulus-outcome relationships and is thought to shape hippocampal representations to select relevant dimensions based on the current context [41,42]. Cognitive maps in mPFC are flexible, forming efficient representations that reflect the behavioral relevance of features such as changes in context [4] or stimulus dimensions [18]. A recent concept learning study showed that mPFC performs goal-directed dimensionality reduction to form efficient representations, in which lower-dimensional representations are formed for less complex concepts. Importantly, mPFC representations were learned, with concept representation being higher-dimensional early in learning and then compressed over learning to only represent relevant dimensions (Figure 1D) [43]. Hippocampus is functionally coupled with mPFC during concept learning, suggesting that mPFC input helps refine hippocampal representation to form efficient concept spaces that are tuned based on goal relevance (Figure 1C) [16].
Parallel computations support decisions about concepts and episodic memory
Real-world experiences include both conceptual knowledge and episodic details [16]. For example, a memory of seeing your neighbor’s new car may include multiple levels of information, including the context (your neighborhood), items and entities within that context (your neighbor and their new sportscar), and relevant concepts (e.g., knowledge about the features of cars in general and sportscars in particular). Multidimensional content encoded within hippocampal representations, together with a common set of computations, may support retrieval of both episodic detail and conceptual knowledge (Figure 3) [16]. In this section, we review how the mechanisms of pattern completion and recombination drive expression of both episodic memory and concepts in different cognitive tasks.
Figure 3.
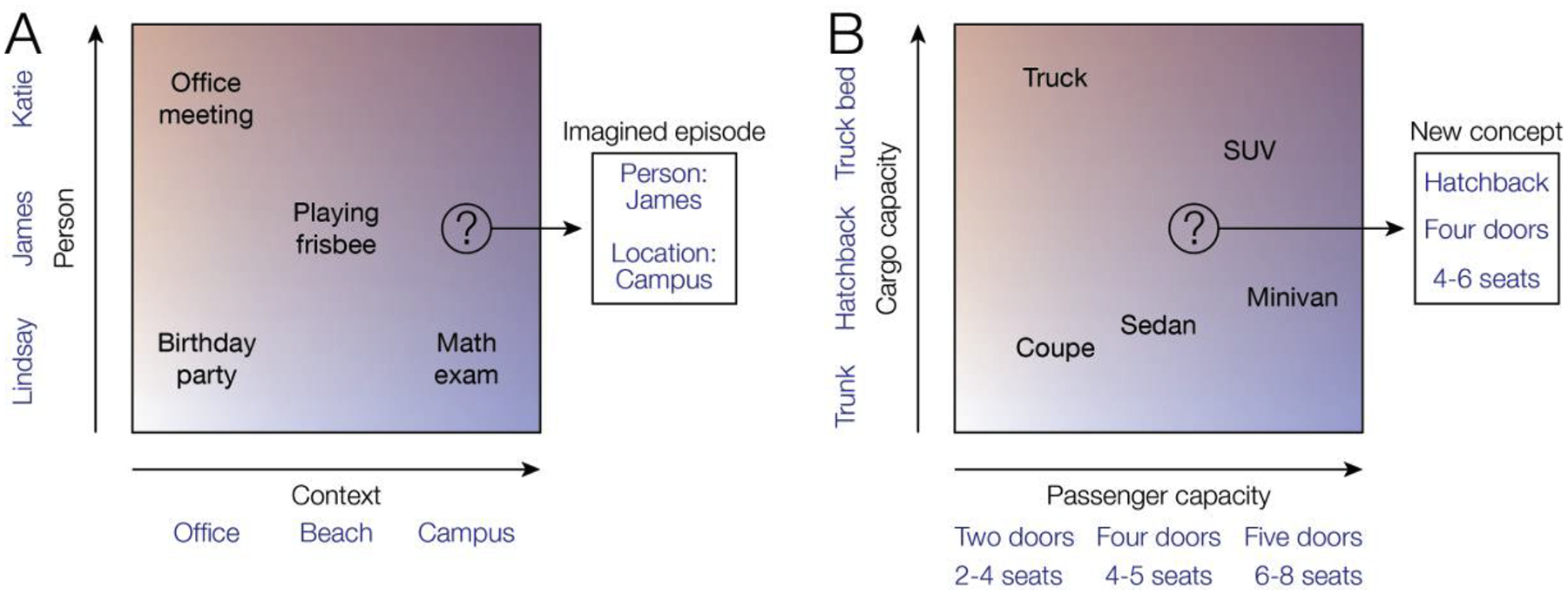
(A) Hippocampus is thought to bring together information about contexts (e.g., an office, a beach, and a college campus) and information about items such as people. Recent evidence suggests this factorization of situations into combinations of items and context facilitates flexible recombination of elements to facilitate imagination or reconstruction of specific episodes (here, imagining a new episode involving James at the college campus). (B) Within a context, such as the context of selecting a new vehicle, hippocampus may represent relevant item dimensions such as cargo capacity and passenger capacity. Representation of relevant feature dimensions may support a number of operations, including categorization (e.g., identifying a new vehicle as a truck) and reconstruction of features of a specific remembered item (e.g., if the vehicle was a truck it must have had a truck bed). Multidimensional spaces in hippocampus may also support exploration of new concepts through flexible recombination of features; for example, a potential vehicle located at the point between sedans and SUVs could be predicted to have a different set of properties from known categories.
Recurrent connections within hippocampus form attractor states, whereby patterns near to a learned attractor will settle into the learned state [44]. In the context of episodic memory, attractor states allow pattern completion of stored episodes, which are conjunctions of context and item information [45]. For example, seeing your neighbor’s car again might cue retrieval of the context in which you saw it earlier. Recent work suggests that hippocampal pattern completion might also be involved in retrieval of individual concepts [46]. Participants were asked to generate features related to a concept, such as “book” or “grapefruit.” Participants with hippocampal damage generally produced features that were semantically related to the prompt, while healthy controls produced valid features that were more distantly related [46]. These results suggest that hippocampus may facilitate pattern completion of concept features by retrieving attractor states representing multidimensional concept representations [47].
Multidimensional hippocampal representations may further support imagination of both episodes and concepts [48,49]. Hippocampus is thought to contribute to simulations of future experiences by flexibly recombining features from existing episodic memories; for example, retrieving a memory of visiting a college campus may help one to imagine meeting a new friend there (Figure 3) [1,48]. Consistent with this proposal, activation patterns measured in hippocampus during retrieval of episodic memories are reactivated during imagination of new episodes composed from the same elements [50]. Similarly, multidimensional hippocampal representations may support imagination in the conceptual domain. For example, if one is trying to imagine an ideal new family vehicle, mPFC may select relevant item dimensions such as cargo capacity and passenger capacity to be represented within the hippocampal-entorhinal system. Each of these dimensions may be related to multiple stimulus features (e.g., low passenger capacity is associated with smaller size, fewer doors, and fewer seats). Exploration of this feature space could then support imagination of novel concepts that involve novel feature combinations [11]; for example, one might imagine a vehicle that is intermediate between a sedan and an SUV, resulting in a new concept of a crossover vehicle (Figure 3). Consistent with this account, hippocampus represents conjunctions of individual concepts (e.g., tea, jelly) when they are combined to form a new concept (e.g., tea-jelly) [51]. At the same time, mPFC also demonstrates evidence of conjunctive representations and reflects the perceived value of the new concept, suggesting that it may have a role in evaluating hypothetical concepts [51].
Conclusions
A key challenge in successfully navigating our complex world is to identify important concepts and the features that define those concepts. Growing evidence suggests that hippocampus, entorhinal cortex, and mPFC support rapid concept learning by forming multidimensional cognitive maps of relevant features. Dimensionality reduction in mPFC may facilitate selection of behaviorally relevant features, guiding formation of attractor states in hippocampus that support concept generalization. Finally, hippocampal and entorhinal cognitive maps may guide recombination of concept features, supporting imagination of novel concepts.
Highlights.
Hippocampus and medial prefrontal cortex form multidimensional cognitive maps
Grid coding in entorhinal cortex supports navigation within cognitive maps
Cognitive maps support relational coding of both episodic memories and concepts
Hippocampal pattern completion supports generalization of concepts to new exemplars
Navigation of cognitive maps supports imagination of future events and new concepts
Acknowledgements
Thanks to Meghana Potturu for assistance with reviewing literature. This work was supported by the National Institutes of Health (R01 MH100121 to ARP; F32 MH114869 to NWM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behrens TEJ, Muller TH, Whittington JCR, Mark S, Baram AB, Stachenfeld KL, Kurth-Nelson Z: What Is a Cognitive Map? Organizing Knowledge for Flexible Behavior. Neuron 2018, 100:490–509. [DOI] [PubMed] [Google Scholar]
- 2.Constantinescu AO, O’Reilly JX, Behrens TEJ: Organizing conceptual knowledge in humans with a gridlike code. Science 2016, 352:1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack ML, Love BC, Preston AR: Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proc National Acad Sci 2016, 113:13203–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuck NW, Cai MB, Wilson RC, NIv Y: Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron 2016, 91:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichenbaum HB, Cohen NJ: Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 2014, 83:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottini R, Doeller CF: Knowledge Across Reference Frames: Cognitive Maps and Image Spaces. Trends Cogn Sci 2020, 24:606–619. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y: Orbitofrontal Cortex as a Cognitive Map of Task Space. Neuron 2014, 81:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Keefe J, Nadel L: The hippocampus as a cognitive map. Oxford University Press, USA; 1978. [Google Scholar]
- 9.Moser EI, Kropff E, Moser M-B: Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Neuroscience 2008, 31:69–89. [DOI] [PubMed] [Google Scholar]
- 10.Bellmund JLS, Cothi W de, Ruiter TA, Nau M, Barry C, Doeller CF: Deforming the metric of cognitive maps distorts memory. Nat Hum Behav 2020, 4:177–188. [DOI] [PubMed] [Google Scholar]
- 11.Bellmund JLS, Gärdenfors P, Moser EI, Doeller CF: Navigating cognition: Spatial codes for human thinking. Science 2018, 362:eaat6766. [DOI] [PubMed] [Google Scholar]; ** This theoretical review covers recent evidence that neural place and grid representations, which are thought to have a key role in spatial navigation, are also involved in representing concepts. The authors propose a theoretical framework for understanding concept representations in terms of attractor states within a conceptual feature space.
- 12.Theves S, Fernández G, Doeller CF: The hippocampus maps concept space, not feature space. J Neurosci 2020, 40:7318–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dusek JA, Eichenbaum HB: The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences 1997, 94:7109–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H: The Hippocampus, Memory, and Place Cells: Is It Spatial Memory or a Memory Space? Neuron 1999, 23:209–226. [DOI] [PubMed] [Google Scholar]
- 15.Theves S, Fernandez G, Doeller CF: The Hippocampus Encodes Distances in Multidimensional Feature Space. Curr Biol 2019, 29:1226–1231.e3. [DOI] [PubMed] [Google Scholar]
- 16.Mack ML, Love BC, Preston AR: Building concepts one episode at a time: The hippocampus and concept formation. Neurosci Lett 2018, 680:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Focusing on hippocampal contributions to concept formation, the authors review evidence that hippocampus integrates individual episodes to form generalizable concepts. They propose that hippocampus supports rapid concept learning through attention-weighted error-driven learning.
- 17.Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D: A Map for Social Navigation in the Human Brain. Neuron 2015, 87:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SA, Miller DS, Nili H, Ranganath C, Boorman ED: Map Making: Constructing, Combining, and Inferring on Abstract Cognitive Maps. Neuron 2020, 107:1226–1238.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This fMRI study examines how social hierarchies (based on popularity or competence of fictional people) are represented in the brain. The authors find that individual one-dimensional concept features become integrated into two-dimensional maps in hippocampus, entorhinal cortex, and mPFC.
- 19.Davis T, Love BC, Preston AR: Learning the Exception to the Rule: Model-Based fMRI Reveals Specialized Representations for Surprising Category Members. Cereb Cortex 2012, 22:260–273. [DOI] [PubMed] [Google Scholar]
- 20.Davis T, Xue G, Love BC, Preston AR, Poldrack RA: Global neural pattern similarity as a common basis for categorization and recognition memory. J Neurosci 2014, 34:7472–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falco ED, Ison MJ, Fried I, Quiroga RQ: Long-term coding of personal and universal associations underlying the memory web in the human brain. Nat Commun 2016, 7:13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey HG, Falco ED, Ison MJ, Valentin A, Alarcon G, Selway R, Richardson MP, Quiroga RQ: Encoding of long-term associations through neural unitization in the human medial temporal lobe. Nat Commun 2018, 9:4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeithamova D, Dominick AL, Preston AR: Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron 2012, 75:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlichting ML, Mumford JA, Preston AR: Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun 2015, 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowman CR, Zeithamova D: Abstract Memory Representations in the Ventromedial Prefrontal Cortex and Hippocampus Support Concept Generalization. J Neurosci 2018, 38:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collin SHP, Milivojevic B, Doeller CF: Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci 2015, 18:1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H: Hippocampal Representation of Related and Opposing Memories Develop within Distinct, Hierarchically Organized Neural Schemas. Neuron 2014, 83:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie S, Keene CS, Farovik A, Bladon J, Place R, Komorowski R, Eichenbaum H: Representation of memories in the cortical–hippocampal system: Results from the application of population similarity analyses. Neurobiol Learn Mem 2016, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton NW, Schlichting ML, Preston AR: Representations of common event structure in medial temporal lobe and frontoparietal cortex support efficient inference. Proceedings of the National Academy of Sciences 117:29338–29345. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In this fMRI study, the authors find evidence that memory representations in medial temporal lobe and mPFC form a hierarchical cognitive map that reflects common concepts that are shared across distinct events. The authors propose a model of how hierarchical cognitive maps guide reasoning about specific relationships between items.
- 30.Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B: Spatial Representation in the Entorhinal Cortex. Science 2004, 305:1258–1264. [DOI] [PubMed] [Google Scholar]
- 31.Moser EI, Moser M: A metric for space. Hippocampus 2008, 18:1142–1156. [DOI] [PubMed] [Google Scholar]
- 32.Klukas M, Lewis M, Fiete I: Efficient and flexible representation of higher-dimensional cognitive variables with grid cells. Plos Comput Biol 2020, 16:e1007796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittington JCR, Muller TH, Mark S, Chen G, Barry C, Burgess N, Behrens TEJ: The Tolman-Eichenbaum Machine: Unifying Space and Relational Memory through Generalization in the Hippocampal Formation. Cell 2020, 183:1249–1263.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This theoretical paper proposes a model of how hippocampus and entorhinal cortex represent a range of cognitive maps. The authors propose that general learning mechanisms support formation of both Euclidean cognitive maps that may be used in spatial navigation and more general graph structures that may encode relationships between concepts.
- 34.Stachenfeld KL, Botvinick MM, Gershman SJ: The hippocampus as a predictive map. Nat Neurosci 2017, 7:1951. [DOI] [PubMed] [Google Scholar]
- 35.Garvert MM, Dolan RJ, Behrens TEJ: A map of abstract relational knowledge in the human hippocampal-entorhinal cortex. Elife 2017, 6:e17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schapiro AC, Turk-Browne NB, Norman KA, Botvinick MM: Statistical learning of temporal community structure in the hippocampus. Hippocampus 2016, 26:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radulescu A, Niv Y, Ballard I: Holistic Reinforcement Learning: The Role of Structure and Attention. Trends Cogn Sci 2019, 23:278–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikenheiser AM, Schoenbaum G: Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci 2016, 17:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Place R, Farovik A, Brockmann M, Eichenbaum HB: Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci 2016, 19:992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G: Suppression of Ventral Hippocampal Output Impairs Integrated Orbitofrontal Encoding of Task Structure. Neuron 2017, 95:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komorowski RW, Manns JR, Eichenbaum HB: Robust Conjunctive Item-Place Coding by Hippocampal Neurons Parallels Learning What Happens Where. J Neurosci 2009, 29:9918–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varga NL, Morton NW, Preston AR: Schema, Inference, and Memory In: Oxford Handbook of Human Memory. Edited by Kahana MJ, Wagner AD. In press. [Google Scholar]
- 43.Mack ML, Preston AR, Love BC: Ventromedial prefrontal cortex compression during concept learning. Nat Commun 2020, 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Examining fMRI activity during concept learning, Mack, Preston, and Love find evidence of dimensionality reduction in mPFC that increases through learning. The decrease in dimensionality is dependent on the complexity of the concept learning problem, with patterns during low and medium complexity problems evincing greater dimensionality reduction. These results suggest that mPFC forms efficient representations of concept features.
- 44.Schapiro AC, Turk-Browne NB, Botvinick MM, Norman KA: Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philosophical Transactions Royal Soc B Biological Sci 2017, 372:20160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horner AJ, Bisby JA, Bush D, Lin W-J, Burgess N: Evidence for holistic episodic recollection via hippocampal pattern completion. Nat Commun 2015, 6:7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutler RA, Duff MC, Polyn SM: Searching for Semantic Knowledge: A Vector Space Semantic Analysis of the Feature Generation Task. Front Hum Neurosci 2019, 13:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon SH, Schapiro AC: Semantic Search as Pattern Completion across a Concept. Trends Cogn Sci 2020, 24:95–97. [DOI] [PubMed] [Google Scholar]
- 48.Schacter DL, Addis DR, Buckner RL: Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 2007, 8:657–661. [DOI] [PubMed] [Google Scholar]
- 49.Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D: Hippocampal amnesia disrupts creative thinking. Hippocampus 2013, 23:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakral PP, Madore KP, Addis DR, Schacter DL: Reinstatement of Event Details during Episodic Simulation in the Hippocampus. Cereb Cortex 2019, 30:2321–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barron HC, Dolan RJ, Behrens TEJ: Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci 2013, 16:1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]


