Abstract
Background: Uptake of genetic testing for heritable conditions is increasingly common. In families with known autosomal dominant genetic cancer predisposition syndromes (CPS), testing youth may reduce uncertainty and provide guidance for future lifestyle, medical, and family building considerations. The goals of this systematic review were to examine: (1) how parents and their children, adolescents, and young adults (CAYAs) communicate and make decisions regarding testing for CPS and (2) how they communicate and make decisions about reproductive health/family building in the context of risk for CPS.
Methods: Searches of MEDLINE/Pubmed, CINAHL, Web of Science, and PsycINFO yielded 4161 articles since January 1, 2000, which contained terms related to youth, pediatrics, decision-making, genetic cancer predispositions, communication, and family building.
Results: Articles retained (N = 15) included five qualitative, six quantitative, and four mixed-method designs. Parents generally agreed testing results should be disclosed to CAYAs at risk or affected by genetic conditions in a developmentally appropriate manner. Older child age and child desire for information were associated with disclosure. Greater knowledge about risk prompted adolescents and young adults to consider the potential impact on future relationships and family building.
Conclusions: Most parents believed it was their responsibility to inform their CAYAs about genetic testing results, particularly to optimize engagement in recommended preventative screening/lifestyle behaviors. Disclosing test results may be challenging due to concerns such as young age, developmental appropriateness, and emotional burden. Additional research is needed on how CPS risk affects CAYAs' decisions about reproductive health and family building over time.
Keywords: communication, family building, cancer predisposition syndrome, genetics, oncology
Introduction
Uptake of genetic testing for heritable conditions has increased due to lower cost, greater insurance coverage, and accessibility. Inherited genetic mutations play a prominent role in approximately 5%–10% of cancers.1 Common heritable cancer predisposition syndromes (CPS) include Hereditary Breast and Ovarian Cancer (HBOC) and Lynch syndrome. Although rarer, Li-Fraumeni Syndrome (LFS), DICER-1 syndrome, and Von Hippel Lindau are increasingly diagnosed due to the availability of gene panel testing.1 Individuals with a personal or family history suggestive of one or more CPS are generally referred to a genetic counselor for testing, education, and counseling about current and future implications.1
In families with known autosomal dominant (HBOC, LFS) genetic CPS, testing youth may reduce uncertainty and provide guidance for future lifestyle, medical, and reproductive considerations, but there is also potential for distress and psychosocial harm.2 A report by the American Society of Human Genetics (ASHG) encouraged parents to defer pre-symptomatic testing for adult-onset conditions until later adolescence or adulthood to ensure the child is mature enough to participate in the decision.3 However, family communication and decision-making regarding genetic testing for CPS are understudied in families of at-risk children, adolescents, and young adults (CAYAs). Adolescence and young adulthood are marked by increasing autonomy, but reliance on parents for medical decision-making is common.4,5 Among some CPS, parents report a desire for developmentally appropriate communication of test results to CAYAs to allow for necessary medical interventions.6 In addition to providing opportunities to engage in recommended screening/preventative behaviors, early knowledge about CPS allows CAYAs to consider reproductive implications (including contraception and future family building).7 Misconceptions about fertility and the heritability of cancer are common in childhood cancer survivorship and may lead to anxiety surrounding fertility and unplanned pregnancies.8,9 Many parents are also unaware of survivor's parenthood goals.4 These data suggest early conversations about reproductive health/family building are warranted in the setting of CPS.
A recent meta-analysis in families affected by inherited genetic conditions showed early disclosure of genetic test results facilitated care planning and reproductive decision making, while later disclosure led to family tensions.7 Clinicians have an important role in guiding parents through the medical/psychosocial effects of genetic testing on their children. Guidance often includes developmentally appropriate discussion of results, engagement in recommended screening and preventative behaviors, and reproductive considerations.
Previous systematic reviews have focused mainly on psychosocial effects of genetic testing among adults10 and attitudes toward testing children,11 and/or have been limited to HBOC.12 More recently, reviews have focused on awareness, knowledge, and attitudes toward genetic testing, but have not specifically examined CPS and family communication surrounding future family building.13,14 The goals of this systematic review were to examine (1) how families communicate and make decisions regarding testing for CPS and (2) how they communicate and make decisions about reproductive health/family building in the context of risk for CPS.
Methods
Procedure
Literature search
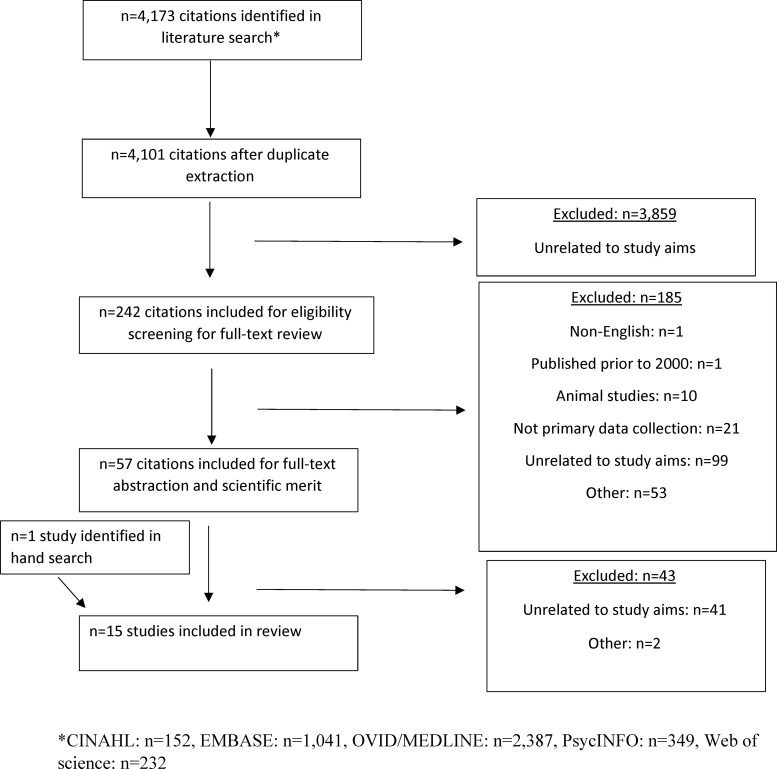
A comprehensive search of several databases (Ovid, Medline In-Process & Other Non-Indexed Citations, CINAHL, PsycINFO, Web of Science, and Embase) was conducted by a medical librarian using preferred reporting items for systematic reviews and meta-analysis guidelines (Fig. 1). The search terms were chosen in collaboration with the team of authors and suggested synonyms by the above programs to make the search as comprehensive as possible. The search was conducted using a variety of expert searching techniques, including Boolean operators, and included MeSH terms, when available, “Genetic Counseling,” “Hereditary Cancer,” “Reproductive Health,” “Family Planning Services,” and “Genetic Predisposition to Disease.” The search also used natural language and free text terms such as “provider communication,” “shared decision making,” “psychosocial factors,” “family characteristics,” and “decision making aids.” Search results were exported to a reference manager, and both digitally and manually identified duplicates were discarded. Resulting abstracts were transferred into Covidence© and reviewed for relevance, and the full-text articles were reviewed for inclusion and data extraction.
FIG. 1.
PRISMA flow chart. PRISMA, preferred reporting items for systematic reviews and meta-analysis.
Inclusion and exclusion criteria
Ten authors independently screened study titles and abstracts in five pairs. If an article was identified by either author for potential inclusion, it was reviewed by a doctoral-level faculty member. If included, the article underwent full-text review. Included articles were (1) human studies; (2) published in the last 20 years (January 2000 through March 2020); (3) written in English; (4) empirical full-length articles in peer-reviewed journals (no reviews, commentaries, guidelines, or case reports); and (5) focused on how parents and CAYAs (no age range specified) made decisions about CPS testing and/or reproductive health/family building. Consensus between at least two authors was required for inclusion/exclusion.
Scientific merit
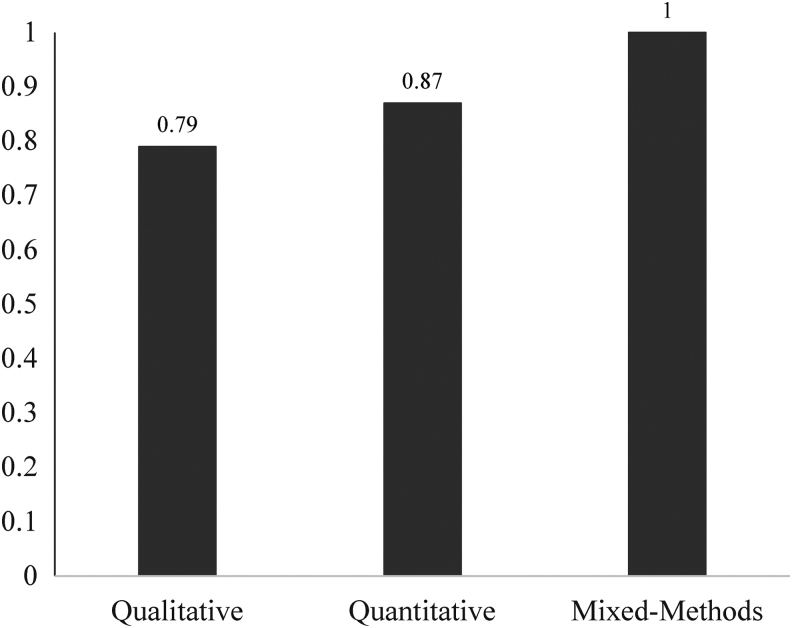
Studies were rated on 11 criteria derived from quantitative and qualitative publishing guidelines and a previous systematic review.15 Mixed-methods studies were rated on the 18 unique criteria that spanned qualitative and quantitative methods. For each criterion, articles were scored from 1 (little/no evidence of meeting the criterion) to 3 (good evidence). Ratings were averaged to calculate a total scientific merit score (range: 1–3). The 15 articles were double coded for scientific merit, which ranged from 1.90 to 2.55 (mean = 2.23, standard deviation [SD] = 0.24) for qualitative studies, 1.73–2.55 (mean = 2.18, SD = 0.34) for mixed-methods studies, and 2.18–2.82 (mean = 2.55, SD = 0.24) for quantitative studies. All articles were rated by two authors to ensure inter-rater reliability. Intraclass correlations demonstrated high inter-rater reliability (Fig. 2). Low-merit studies included poorly controlled analyses, unvalidated measures, poor sampling, and/or low statistical power.
FIG. 2.
Scientific merit score.
Data extraction
Authors used a detailed spreadsheet to systematically extract data about study, sample, methodology, and outcomes.
Results
Available literature
The search returned 152 results from CINAHL, 1041 from EMBASE, 2399 from OVID/MEDLINE (12 removed as duplicates leaving 2387), 349 in PsycINFO, and 232 from Web of Science for a total of 4161 articles. The articles were entered into a management software (i.e., Covidence), where more duplicates were removed for a total of 4101 articles. Of these, 4087 did not meet inclusion criteria, resulting in 15 articles (Fig. 1): 5 qualitative (33%), 6 quantitative (40%), and 4 mixed-method designs (27%). Most studies were cross-sectional and included surveys and semistructured interviews (see Tables 1–3).
Table 1.
Qualitative Studies
| AuthorsRef. | Location | Design | Purpose | Sample | Primary findings | Scientific merit |
|---|---|---|---|---|---|---|
| Rowland et al.16 | United Kingdom | Interview with thematic analysis | Examine experiences of parents, children, and young people when discussing genetic risk in families affected by BRCA gene mutations, and how information shared impacts children and young people's views about future risk | Parents, children, or teens testing positive or at risk of carrying the BRCA 1/2 gene; N = 27 from 11 families; 13 children or young people (ages 10–21) and 14 parents | Most parents (primarily mothers) disclosed information. At 12–14 years, discussions primarily focused on how risk affected parent's health. At 15–17 years, focus was on risk to child's health. Discrepancy between parent perception of risk and amount of risk they convey to children sometimes resulted in knowledge gaps among children. Most young people wanted testing if risk info was communicated to them. Greater knowledge of BRCA mutation caused young people to consider the impact that breast cancer risk may have on future relationships and offspring; parents reported children expressing desire for genetic testing to better understand these risks. Males were less likely to perceive risk due to communication from the parents. All families believed it is parents' responsibility to communicate risk info. Themes: Family communication of risk information; selective communication of genetic risks information; children and young people's understandings of genetic risk info; and implications for future decision making. |
1.90 |
| Schultz et al.17 | United States | Qualitative/semistructured interview | Examine the influence of aspects of adolescence on the perspectives of parents regarding Li-Fraumeni testing and discussions of genetic cancer risk | Diagnosis: LFS; N = 46 parents from 39 families (32 mothers and 14 fathers); 92 children (not interviewed); 30 parents had children older than 13, ages of parents not reported | Parents felt the period of adolescence is an important time to discuss genetic testing and LFS. Aspects facilitating discussion: cognitive and developmental appropriateness; increased cancer risk and need for medical screening; genetic knowledge impacting behaviors and habits; preparation for transition to adult care; and reproductive risks. Aspects complicating discussion: negative emotional impact; misunderstandings; added burden; and negative impact on self-image and future planning. Parents felt informing adolescents was crucial to manage medical responsibility in the future. Parents discussed complexities of reproductive choices in the context of genetic risk. Themes: aspects of adolescence supporting or complicating LFS testing and discussions; importance of knowing tumor status in regard to health care decisions; relationship of LFS status and adolescent sexual behaviors; and relationship of LFS status and adolescent risk-taking behaviors. |
2.36 |
| Clarke et al.21 | Canada | Qualitative/semistructured interview | Examine experiences of BRCA1/2 women carriers in communicating genetic risk information to their children | Mothers testing positive for BRCA1/2; N = 24; mean age = 45.4 (SD not reported) | Three phases of disclosure: (1) pre-disclosure, where parents consider the impact the result has on them (fear of getting cancer, loss of future goals, anticipating child reactions, concern for daughters' future, and discrimination), thinking about the context of disclosure (e.g., age of child and emotions, and pressure to disclose), and consequences of not telling (unintentional disclosure and dishonesty); (2) disclosure, including a range of ways they disclosed, from a plan to disclosing unintentionally, emotionality of disclosure; (3) impact of disclosure, including fear of positive result for child, guilt about inheritance, and opinions about when to test; need to provide children with increased emotional support; and limited control over testing timeline for child after disclosure. | 2.18 |
| Bradbury et al.18 | United States | Qualitative/semistructured interview | Examine the content and method of genetic testing result disclosure, understanding and perceptions of hereditary risk, and psychosocial and health-related impact of this communication | Children of BRCA mutation carriers; N = 35; 22 kids (10 male and 12 female), median age = 26 (18–33); 13 parents (11 moms and 2 dads), median age = 48 (43–66) | Parents frequently disclosed to their children; parent with mutation shared result. Most had a good understanding initially, which improved over time; some sought additional information; some were frightened/scared about results; and about half reported their initial reaction after disclosure was different than parents perceived. Being female and older, and already having children were reasons for undergoing testing. |
2.18 |
| Alderfer et al.40 | United States | Qualitative/semistructured interview | Examine expectations of genetic testing | Families getting genetic testing for cancer; N = 12 (8 female and 4 male); mean = 17.8, SD = 4.82 (12–25) | Themes: perspectives on offering genetic testing to children, perceived advantages (allow for disease prevention) and disadvantages (negative emotions) of testing involving children in the decision to test, and psychosocial/behavioral impact of testing. | 2.55 |
LFS, Li-Fraumeni Syndrome; SD, standard deviation.
Table 2.
Quantitative Studies
| AuthorsRef. | Location | Design | Purpose | Sample | Measures | Primary findings | Scientific merit |
|---|---|---|---|---|---|---|---|
| Douma et al.25 | Netherlands | Cross-sectional | Examine attitudes and experiences regarding childhood DNA testing, PND, and PGD within families at high risk for FAP | Familial adenomatous polyposis patients, carriers, noncarriers, partners; N = 525 FAP family members, 131 partners; FAP mean age: 43.6 (14.1, 16–84); partners mean: 46.0 (11.5, 21–79) | Demographic questionnaire; attitudes toward DNA testing in childhood questionnaire; experiences with DNA testing of children questionnaire; influence of FAP on desire to have children questions; attitudes toward PND and PGD questionnaire; experiences with PND and PGD questionnaire; psychosocial variable questionnaire | Seventy-two percent had a clinical geneticist explain test results to children (with or without parents). Twenty percent informed children of results themselves without a physician present, Four percent had not yet informed children due to feeling children were too young or because they were noncarriers. Thirty-four believe <12 years is appropriate age for testing; 38% preferred 12–16 years due to wanting the child to be able to understand the testing process. Fifty-two percent believed children should be tested individually at a fixed age; 37% wanted all children in a family tested at one time; 4% thought testing should not be done on children at all. |
2.82 |
| Hamilton et al.22 | United States | Longitudinal, prospective | Examine the effects of open parent-child and parent-co-parent communication and perceived quality of parenting relationship on communication of cancer risk within the family | Mothers undergoing BRCA testing; N = 204, 102 parenting dyads; mothers mean age: 46.0 (6.0); partners mean age: 48.1 (6.8) | Demographic interview; clinical record abstraction; Parenting Alliance Measure; cognitive appraisals of parent-child communication questions (interview); Parent-Adolescent Communication | Open communication between mother and child, and partner and child, was associated with open communication 6 months after getting genetic test results. Both the mother-child and partner-child relationship qualities have important implications regarding communication about cancer risk. |
2.18 |
| Aktan-Collan et al.19 | Finland | Cross-sectional | Examine whether Lynch Syndrome carriers in Finland inform their offspring of mutation, possibility of predictive genetic testing, and outcomes of this information and describe challenges in disclosure, wish for professional support, and impact of sex on communication | Lynch syndrome mutation carriers over 40 years old with offspring; N = 248, 121 men and 127 women; mean age = 56.4 (9.0); men mean age: 56.9 (8.8); female mean age: 55.8 (9.2) | Sharing of result questionnaire; outcome of communication questionnaire; offspring attitudes toward testing questionnaire; challenges to disclosure questionnaire; opinions on a family genetic appointment; opinions on who should disclose and how | Eighty-three percent reported having disclosed results to at least one adult child. Thirteen percent had chosen not to disclose due to age, maturity, difficulty with making contact, and too painful. Sixty-six percent with underage children had not disclosed because of age. Younger parents more likely to be nondisclosing, but other demographic factors were unrelated. Sixty-nine percent of first-born adult children got tested after learning about genetic risk. Sixty-seven percent parents disclosed alone (82% of women), 28% with someone else (men more likely to have a support person than women—45% vs. 12%), 5% had someone else disclose; and 44% disclosed to all children at once. Children's risk of cancer most difficult to discuss. Thirty-seven percent felt parents should inform children; 1/3 felt that health care professionals should also be involved. |
2.64 |
| Patenaude et al.23 | Na | Intervention | Examine factors predicting patterns of disclosure of BRCA1/2 test results to first-degree relatives among women tested in a clinical protocol | Personal or family history of breast, ovarian, or other cancer consistent with BRCA1/2 heredity with posterior probability of carrying an altered gene of ≥10% based on published probabilities or Bayesian calculations and documentation of participants or family member cancer diagnosis; N = 273 women; age only reported by category: n = 87 women ≤40, 97 women between 41 and 50, and 89 women older than 50 | Family communication measure | Female children were told of mother's genetic testing result more often than males. Children 6–13 were told their mother's results less often than children ≥30 years. Adolescents (14–17 years old) and young adults (18–30 years old) were informed as often as those ≥30 years |
2.55 |
| Tercyak et al.41 | United States | Qualitative/Group education session with optional results disclosure; follow-up telephone interview | Examine parents' communication behaviors about their BRCA test results to their children and examine relationship between parent disclosure to children and parent psychological functioning after testing | Parents with BRCA/hereditary breast cancer; N = 133 (109 mothers, 24 fathers); mean age = 39.8, SD = 8.6 | Mothers were most likely to disclose mutation status to their children. Those utilizing more active coping and with higher general baseline stress or higher general post-test distress were more likely to share results with kids; parents either shared with all of the children or none (not examined by age). |
2.36 | |
| Conley et al.26 | United States | Cross-sectional | Assesses (1) to whom black women disclose genetic test results and (2) if patterns of disclosure vary based on test result (e.g., BRCA1/2 positive, negative, and VUS) | Black women (N = 149) with invasive; breast cancer diagnosed age ≤50 years; mean age = 44.9; SD = 6.2 | Reasons for disclosing genetic test results questionnaire; Familial relationships | Disclosure to female relatives was greater than disclosure to males. Compared to those who tested negative or had a VUS, BRCA1/2-positive women were significantly less likely to disclose results to their daughters. Informing patients/family about cancer risk is generally not being recognized as a benefit of genetic testing. |
2.73 |
FAP, familial adenomatous polyposis; PGD, preimplantation genetic diagnosis; PND, prenatal diagnosis; VUS, variant of uncertain significance.
Table 3.
Mixed-Methods Studies
| Authors | Location | Design | Purpose | Sample | Measures | Primary Findings | Scientific Merit |
|---|---|---|---|---|---|---|---|
| Grosfeld et al.27 | Netherlands | Mixed methods/cross-sectional and semistructured interview | Examine psychological reactions of 22 parental couples and 3 single parents after disclosure of genetic test results of their children | Parents of children receiving genetic testing for MEN2; MEN2A; FMTC; N = 47, 22 parental couples and 3 single parents; mean age = 35.9 (28–47) | Impact of event scale; Spielberger State Anxiety Inventory; Symptom Checklist 90 (General severity index); Interview | Parents who were informed that their child was a gene carrier reacted with resignation, showed moderate to high levels of test-related and general anxiety, but few psychological complaints; distress was greater in low SES families. Daily activities disturbed in 43% of parents informed their child was a carrier. Little impact on parent's future perspective of the child's future. |
2.18 |
| Clarke et al.24 | United Kingdom | Mixed methods/survey and semistructured interview | Examine mothers' experiences of communicating with survivors of retinoblastoma | Mothers of retinoblastoma survivors. N = 39 mothers; mean age = 38.4 (29–45) | Demographics; Summary measure of mothers' beliefs about impact of comm. on child coping; Interview | Mothers had spoken with their children about diagnosis and treatment. but less about genetic risk. Child age and info seeking about results were associated with disclosure. Mothers may need more guidance in communicating about the disease. |
2.27 |
| Patenaude et al.28 | Mixed methods | Examine (1) what daughters, ages 18–24 years, of BRCA1/2 mutation carriers understand about their 50% chance of carrying a BRCA1/2 mutation and about risk reduction or management options for mutation carriers, (2) the extent and nature of daughters' cancer-related distress, and (3) the effects of knowing mother's mutation status on daughters' future plans | Daughters of mothers who tested positive for BRCA1/2; N = 40 daughters; median age = 21 (18–24) | Qualitative telephone interview; Demographics; Brief Symptom Inventory 18; Impact of Event Scale; Breast Cancer Genetic Counseling Knowledge Questionnaire | Daughters lacked knowledge about genetics; over 1/3 of daughters reported cancer-related distress; genetic knowledge raised future concerns especially concerning having kids. | 2.55 | |
| Segal et al.20 | Canada | Mixed methods/cross-sectional and semistructured interview | Examine the content and process of disclosure from BRCA1/2 carriers to their offspring | Mothers testing positive for BRCA1/2; N = 31 mothers; mean age = 47.7 (34–59) | Age of child was most significant factor in decision to disclose. Similar results were found between what was actually discussed in families (e.g., preventative measures) and the anticipated disclosure topics for parents waiting to have discussions (carrier's reason for testing and preventative measures). Disclosure tended to happen alone with child and within a week of learning the results. Women who disclosed reported being closer to their child. |
1.73 |
SES, socioeconomic status.
Communication between parents and CAYAs regarding CPS results (n = 13)
Four studies showed parents agreed test results should be disclosed to the CAYA at risk or affected by genetic conditions in a developmentally appropriate manner.16–19 Disclosure happened quickly—one study reported conversations within a week of parents receiving results.20 Parents felt it was important to inform CAYAs of results to facilitate future medical management.17
One study defined three phases of HBOC test result disclosure: (1) predisclosure: parents considered the impact of the result on the CAYA (e.g., fear of getting cancer and concern for daughters' future), (2) disclosure: planned disclosure and disclosing unintentionally, and (3) impact of disclosure: fear of positive result for CAYA, guilt about inheritance, and opinions about when to test.21 Communication from parents was influenced by their perceptions of how the at-risk CAYA would handle the information and how it would affect their behaviors (e.g., coping and communication).22
Three studies (among HBOC kindreds) showed sons were less likely to receive information from their parents regarding test results and were more likely to perceive lower personal risk than daughters.16,18,23 One study found the affected parent was most likely to disclose mutation status,21 while another study found mothers were more likely to disclose information regardless of being the affected parent.16 Mothers noted feeling closer to their CAYA after disclosure occurred, but also needed additional guidance regarding communication of cancer risk.20,24 They communicated with CAYAs more about diagnosis and treatment, and less about genetic risk.24 One study found families preferred to have a clinical geneticist explain test results to their CAYA, and another found one-third of parents believed a medical provider should be involved in the conversation.19,25 Finally, black women with a positive breast cancer mutation were less likely to disclose results to daughters than those who tested negative.26
Developmental considerations (n = 7)
Two studies found CAYAs desire testing for hereditary CPS.16,24 Older child age and desire for information were associated with disclosure.24 Reasons not to disclose genetic test results to CAYAs included parental concerns about maturity/developmental readiness and/or emotional difficulty of disclosure.19,25 Younger age was the most significant reason parents chose not to disclose risk to CAYA.20,24 In one study, one-third of parents believed 12 years was the youngest age appropriate for testing, and almost half believed 12–16 was the ideal age for understanding the testing process.25 Another study found when the child was between 12 and 14, discussions focused on how genetic risk impacted parent health.16 When the CAYA was older (15–17 years), discussions emphasized risk to the child's health.16 Families felt discussing risk of the CAYA developing cancer was particularly challenging.19 Knowledge gaps were found in CAYAs' understanding of their genetic risk, due to parents trying to protect them and not accurately conveying risk.16
Reproductive considerations (n = 4)
Two studies included parents' perspectives of their at-risk CAYA's reproductive health/future family building.17,27 While communication about test results had little impact on parents' perspectives of their children's futures,27 parents acknowledged the challenges of future family building decisions within the context of genetic risk.17 In addition, parents were more likely to have discussions of risk with CAYAs if there were reproductive implications, and parents discussed reproductive complexities and sexual health within the context of risk.17 However, challenges arose from these discussions, such as negative self-image from test results and disrupted future planning.17
Two studies reported on perspectives of future planning among CAYAs in the context of CPS.16,28 Daughters who were informed of risk reported cancer-related distress, particularly regarding family building compared to sons.28 Greater knowledge of their breast cancer mutation influenced CAYAs to consider the impact risk would have on future relationships, children, and decisions about family building.16 Due to these potential effects, young people had a desire to undergo testing to better understand these risks.16
Discussion
This review examined decision making and communication between providers, parents, and CAYAs regarding testing, disclosure of results, and implications for family building in HBOC and other CPS. Although most parents believed it was their responsibility to inform CAYAs about genetic testing results (for optimizing preventative/lifestyle behaviors), some families preferred to have a medical provider participate in the conversation. Similar to other research showing parents have difficulty disclosing sensitive information to children,29 parents found disclosing genetic test results to offspring was challenging due to young age, developmental concerns, and emotional burden.
Examining perspectives about reproductive health/family building was an aim of this review, yet we found limited research on the topic. Studies examining parents' perspectives were conflicting, with some showing parents of at-risk CAYAs were not worried about their child's future family building, and others acknowledging their child would have challenges in reproductive decision making. However, at-risk female AYAs (particularly HBOC) often reported distress about their reproductive future/family building due to positive test results. Those at risk were more inclined to get testing to better understand these potential impacts on their future. Notably, parents of CAYAs with cancer tend to underestimate their reproductive concerns,4,30 and uncertainty about the ability to have biological children may cause distress and negatively affect quality of life.8,31–34 While CPS are different in that youth from affected families may not necessarily develop cancer, it is important to have early and ongoing family building conversations to minimize future distress and regret. The scope of reproductive counseling in CPS should be broad, including future family building goals, impact on future children, prevention of unplanned pregnancies, and preimplantation genetic testing.
This systematic review highlights the need for more research to inform best practices on testing and counseling youth at different ages/developmental stages about medical and reproductive implications of CPS. Mothers were the primary communicator of results to children, but clinicians can provide guidance for parents who struggle to articulate the implications of test results on future cancer risk and family building. Notably, sons were less likely to receive information from parents about HBOC test results and perceived themselves at lower risk compared to daughters. Clinicians should be aware of this discrepancy and inform men of their increased risk for both prostate and breast cancer.35
Early testing would facilitate timely counseling, yet raises concerns about psychosocial impact on the child. Many parents worry testing for CPS in childhood may compromise the child's autonomy and result in genetic discrimination.36 The ASHG report states unless immediate clinical intervention is warranted, parents should defer predictive/pre-symptomatic testing for adult-onset conditions until adulthood, or until the child can make medical decisions in a mature manner.3 Informed consent for genetic testing is important, and testing should be done only with the patient's “best interest” in mind.3 However, there are also benefits to testing CAYAs to inform future medical care and family planning. Individuals at risk may also benefit psychologically from learning they did not inherit the CPS.36 Despite guidelines generally advising against genetic testing of minor children for adult-onset conditions, current guidelines recommend taking the child's best interest into account by acknowledging circumstances in which it may be beneficial to test for these conditions (e.g., resolution of high anxiety and mature adolescent interest).37 Varied decision making is evident in practice: individual practitioners and parents vary in their willingness to test children, and may be more likely to recommend or ask for testing for children who are mature older adolescents and/or if there are perceived benefits to the child (e.g., encouraging healthy behaviors).38,39 More research is needed to understand best practices for giving CAYAs' results of genetic testing, given the benefits, yet protecting the CAYAs' autonomy.
This review had several limitations. Only a small portion of articles identified were deemed eligible for inclusion. Our decision to include only CPS was intended to inform future research within oncology populations, but we acknowledge our narrow criteria. Our review included only articles in English, and most studies were conducted in the United States, limiting generalizability. However, there are several strengths, including examination of communication about family building, an understudied area within the context of genetic CPS, in a broader range of CAYA conditions.
Finally, medical and psychosocial providers should partner with families affected by CPS to make decisions about the optimal timing and manner in which risk information is communicated to their children. As genetic testing becomes more common, families will need guidance about implications for medical planning and future family building. Research is needed to understand what interventions may best assist families with communicating results to CAYAs. Longitudinal studies should examine satisfaction with decisions about genetic risk communication and reproductive health, focusing on differing needs based on age and developmental stage. While genetic testing presents unique ethical and clinical considerations, it is important to understand the experiences of patients and families with inherited CPS and to minimize distress and psychosocial harm when disclosing risk.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
NIH-NCI K08CA237338-01 (Dr. Nahata) and T32HS026120 (Dr. Sutter).
References
- 1. Lee-May Chen SM Hereditary cancer syndromes and risk assessments. The American College of Obestricians and Gynecologists. Obstet Gynecol. 2019;134(6):e143–9 [Google Scholar]
- 2. Duncan RE, Gillam L, Savulescu J, et al. “You're one of us now”: young people describe their experiences of predictive genetic testing for Huntington disease (HD) and familial adenomatous polyposis (FAP). Am J Med Genet C Semin Med Genet. 2008;148C(1):47–55 [DOI] [PubMed] [Google Scholar]
- 3. Botkin JR, Belmont JW, Berg JS, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97(1):6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nahata L, Morgan TL, Ferrante AC, et al. Congruence of reproductive goals and fertility-related attitudes of adolescent and young adult males and their parents after cancer treatment. J Adolesc Young Adult Oncol. 2019;8(3):335–41 [DOI] [PubMed] [Google Scholar]
- 5. Arnett JJ Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469. [PubMed] [Google Scholar]
- 6. Rahm AK, Cragun D, Hunter JE, et al. Implementing universal Lynch syndrome screening (IMPULSS): protocol for a multi-site study to identify strategies to implement, adapt, and sustain genomic medicine programs in different organizational contexts. BMC Health Serv Res. 2018;18(1):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowland E, Metcalfe A. Communicating inherited genetic risk between parent and child: a meta-thematic synthesis. Int J Nurs Stud. 2013;50(6):870–80 [DOI] [PubMed] [Google Scholar]
- 8. Ellis SJ, Wakefield CE, McLoone JK, et al. Fertility concerns among child and adolescent cancer survivors and their parents: a qualitative analysis. J Psychosoc Oncol. 2016;34(5):347–62 [DOI] [PubMed] [Google Scholar]
- 9. Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13(10):689–99 [DOI] [PubMed] [Google Scholar]
- 10. Broadstock M, Michie S, Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8(10):731–8 [DOI] [PubMed] [Google Scholar]
- 11. McGill B, Wakefield C, Vetsch J, et al. Children and young people's understanding of inherited conditions and their attitudes towards genetic testing: a systematic review. Clin Genet. 2019;95(1):10–22 [DOI] [PubMed] [Google Scholar]
- 12. Borry P, Stultiëns L, Nys H, Dierickx K. Attitudes towards predictive genetic testing in minors for familial breast cancer: a systematic review. Crit Rev Oncol Hematol. 2007;64(3):173–181 [DOI] [PubMed] [Google Scholar]
- 13. Hann KE, Freeman M, Fraser L, et al. Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health. 2017;17(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warby M, Wakefield CE, Vetsch J, Tucker KM. Families' and health care professionals' attitudes towards Li-Fraumeni syndrome testing in children: a systematic review. Clin Genet. 2019;95(1):140–50 [DOI] [PubMed] [Google Scholar]
- 15. Long KA, Lehmann V, Gerhardt CA, et al. Psychosocial functioning and risk factors among siblings of children with cancer: an updated systematic review. Psychooncology. 2018;27(6):1467–79 [DOI] [PubMed] [Google Scholar]
- 16. Rowland E, Plumridge G, Considine A-M, Metcalfe A. Preparing young people for future decision-making about cancer risk in families affected or at risk from hereditary breast cancer: a qualitative interview study. Eur J Oncol Nurs. 2016;25:9–15 [DOI] [PubMed] [Google Scholar]
- 17. Schultz CL, Alderfer MA, Lindell RB, et al. The influence of adolescence on parents' perspectives of testing and discussing inherited cancer predisposition. J Genet Couns. 2018;27(6):1395–404 [DOI] [PubMed] [Google Scholar]
- 18. Bradbury AR, Patrick-Miller L, Pawlowski K, et al. Learning of your parent's BRCA mutation during adolescence or early adulthood: a study of offspring experiences. Psychooncology. 2009;18(2):200–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aktan-Collan KI, Kääriäinen HA, Kolttola EM, et al. Sharing genetic risk with next generation: mutation-positive parents' communication with their offspring in Lynch syndrome. Fam Cancer. 2011;10(1):43–50 [DOI] [PubMed] [Google Scholar]
- 20. Segal J, Esplen MJ, Toner B, et al. An investigation of the disclosure process and support needs of BRCA1 and BRCA2 carriers. Am J Med Genet A. 2004;125(3):267–72 [DOI] [PubMed] [Google Scholar]
- 21. Clarke S, Butler K, Esplen MJ. The phases of disclosing BRCA1/2 genetic information to offspring. Psychooncology. 2008;17(8):797–803 [DOI] [PubMed] [Google Scholar]
- 22. Hamilton JG, Mays D, DeMarco T, Tercyak KP. Modeling the dyadic effects of parenting, stress, and coping on parent–child communication in families tested for hereditary breast-ovarian cancer risk. Fam Cancer. 2016;15(4):513–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patenaude AF, Dorval M, DiGianni LS, et al. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–6 [DOI] [PubMed] [Google Scholar]
- 24. Clarke S-A, Sheppard L, Eiser C. Mothers' explanations of communicating past health and future risks to survivors of childhood cancer. Clin Child Psychol Psychiatry. 2008;13(1):157–70 [DOI] [PubMed] [Google Scholar]
- 25. Douma KF, Aaronson NK, Vasen HF, et al. Attitudes toward genetic testing in childhood and reproductive decision-making for familial adenomatous polyposis. Eur J Hum Genet. 2010;18(2):186–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conley CC, Ketcher D, Reblin M, et al. The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns. 2020;29(3):410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grosfeld FJ, Beemer FA, Lips CJ, et al. Parents' responses to disclosure of genetic test results of their children. Am J Med Genet. 2000;94(4):316–23 [DOI] [PubMed] [Google Scholar]
- 28. Patenaude AF, Tung N, Ryan PD, et al. Young adult daughters of BRCA1/2 positive mothers: what do they know about hereditary cancer and how much do they worry? Psychooncology. 2013;22(9):2024–31 [DOI] [PubMed] [Google Scholar]
- 29. Nahata L, Quinn GP, Tishelman AC. Counseling in pediatric populations at risk for infertility and/or sexual function concerns. Pediatrics. 2018;142(2):e20181435. [DOI] [PubMed] [Google Scholar]
- 30. Quinn GP, Knapp C, Murphy D, et al. Congruence of reproductive concerns among adolescents with cancer and parents: pilot testing an adapted instrument. Pediatrics. 2012;129(4):e930–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nilsson J, Jervaeus A, Lampic C, et al. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod. 2014;29(12):2704–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stinson JN, Jibb LA, Greenberg M, et al. A qualitative study of the impact of cancer on romantic relationships, sexual relationships, and fertility: perspectives of Canadian adolescents and parents during and after treatment. J Adolesc Young Adult Oncol. 2015;4(2):84–90 [DOI] [PubMed] [Google Scholar]
- 33. Stein DM, Victorson DE, Choy JT, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3(2):75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer. 2014;22(10):2805–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(23):1811–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Committee on Bioethics. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001;107(6):1451–5 [DOI] [PubMed] [Google Scholar]
- 37. Kesserwan C, Friedman Ross L, Bradbury AR, Nichols KE. The advantages and challenges of testing children for heritable predisposition to cancer. Am Soc Clin Oncol Educ Book. 2016;35:251–69 [DOI] [PubMed] [Google Scholar]
- 38. Borry P, Goffin T, Nys H, Dierickx K. Attitudes regarding predictive genetic testing in minors: a survey of European clinical geneticists. Am J Med Genet C Semin Med Genet. 2008;148C(1):78–83 [DOI] [PubMed] [Google Scholar]
- 39. Bradbury AR, Patrick-Miller L, Pawlowski K, et al. Should genetic testing for BRCA1/2 be permitted for minors? Opinions of BRCA mutation carriers and their adult offspring. Am J Med Genet C Semin Med Genet. 2008;148C(1):70–7 [DOI] [PubMed] [Google Scholar]
- 40. Alderfer MA, Lindell RB, Viadro CI, et al. Should genetic testing be offered for children? The perspectives of adolescents and emerging adults in families with Li-Fraumeni syndrome. J Genet Couns. 2017;26(5):1106–1115 [DOI] [PubMed] [Google Scholar]
- 41. Tercyak KP, Hughes C, Main D, et al. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42(3):213–224 [DOI] [PubMed] [Google Scholar]