To the Editor:
Drugs targeting the immune system are a promising new approach for treatment of schizophrenia. In search of methods for patient stratification, great effort has been invested into positron emission tomography studies using radioligands that bind to the 18-kDa translocator protein (TSPO), a glial cell marker, yielding seemingly inconclusive results (1–12). To address this issue, we previously conducted an individual participant data meta-analysis, pooling data from all 5 published studies at the time that employed second-generation TSPO radioligands that show superior signal-to-noise ratio compared with the first TSPO radioligand, (R)-[11C]PK11195 (13). Contrary to the originally expected hypothesis of an upregulation of TSPO, we observed strong evidence of lower total distribution volume (VT)—an index of radioligand binding to TSPO—in psychosis patients compared with healthy control subjects (14).
Concurrently, an additional study was published using the radioligand [18F]PBR111 in patients with schizophrenia, showing an interaction effect between patient status and age (11). This study was subsequently included in a new meta-analysis by Marques et al., which showed no difference in VT between patients and control subjects in studies using second-generation TSPO radioligands (15). However, this meta-analysis only made use of summary statistics, such that the results are not directly comparable to Plavén-Sigray et al. (14). Since then, data collection has been completed for a further study examining first-episode psychosis patients and control subjects using [11C]PBR28 (16). Here, we aim to reconcile the differences in conclusions from TSPO positron emission tomography studies in psychosis, at the levels of both individual studies and meta-analyses. To this end, we conducted a new individual participant data meta-analysis, making use of all of the now-available data obtained using second-generation TSPO radioligands, using a preregistered analysis plan agreed upon by representatives of all included studies (see https://github.com/pontusps/TSPO_psychosis). The primary objective was to re-evaluate the hypotheses of 1) higher, 2) lower, or 3) no difference in radioligand binding between patients and healthy control subjects. Secondary objectives were to assess the effects of antipsychotic medication on TSPO levels, as well as relationships between TSPO and disease duration, symptom measures, and age-group interaction effects.
The pooled individual participant data amounted to 99 patients and 109 healthy control subjects. The analysis approach was based on our previous study, whose main outcomes were VT values from the frontal cortex, temporal cortex, and hippocampus. Only data without partial volume correction were used, to allow for more homogeneous pooling. A linear mixed-effects model was used to examine patient–control subject differences in VT, while accounting for the nested structure of data (varying intercepts and slopes for all included studies), with genotype as covariate. Age, sex, medication, duration of illness, and symptom severity were all examined as covariates. We also specified an additional model including a group-by-age interaction effect to test the hypothesis of Ottoy et al. (11). Alongside p values, Bayes factors (BFs) were applied to examine the relative support for the hypotheses of higher, lower, or no change of TSPO levels in patients compared with healthy control subjects [see (14) for specification of priors].
In all three regions, BFs showed moderate to strong support (all BFs > 5) for lower VT in patients as compared with no difference (all p < .005) and strong support (all BFs > 10) for lower VT compared with higher VT in patients (Figure 1). There was no effect of antipsychotic medication on TSPO (all p > .6), and no association was observed between VT and disease duration or symptom levels (all p > .5). We found no age-by-group interaction effect on VT in any of the examined regions (all p > .6) (for full results, see https://github.com/pontusps/TSPO_psychosis).
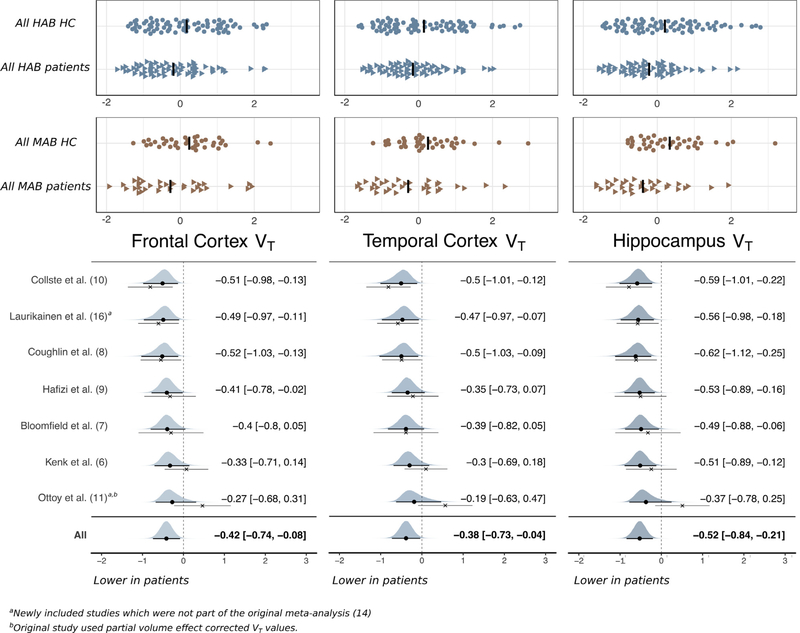
Figure 1.
Standardized differences in VT between patients with psychosis and HC subjects. The top panel shows the TSPO VT values of all individual patients and HC subjects, subdivided into HAB and MAB groups, respectively. The data have been standardized within genotype and study with a mean of 0 and an SD of 1, in order to allow for visualization of compiled data obtained using different radioligands. The lower panel shows the estimated standardized difference in VT using a Bayesian linear effects model, with study as random effect. The black circle denotes the posterior mean, and the thick line denotes the 95% credible interval of the estimated random slopes (study specific effects); these are also presented in text next to the plots. The cross denotes the patient–control subject mean difference in raw data (together with its 95% confidence interval) without performing linear mixed-effects modeling. Hence, the difference between the dot and the cross displays the model shrinkage toward the mean. HAB, high-affinity binder; HC, healthy control; MAB, mixed-affinity binder; TSPO, 18-kDa translocator protein; VT, total distribution volume.
While the magnitude of the observed differences is reduced compared with those observed in Plavén-Sigray et al. (14), the conclusions remain unchanged. The result of no elevation of TSPO in second-generation radioligand studies is in agreement with the meta-analysis by Marques et al. (15). Instead, the current meta-analysis found patients to have lower VT compared to control subjects. This could be explained by an increased sensitivity in our analysis, since the individual participant data approach allows for control of potential confounding effects, such as age, sex, or medication, and estimation of the effect sizes using the data directly, rather than relying on the specific summary statistics reported in the original publications (17). We chose not to include patient–control subject comparisons using (R)-[11C]PK11195 because the outcome measures used in these studies show low reliability and consistency (18–20), likely owing to low specific binding of this radioligand (13,21). Hence, based on the best available evidence so far, we conclude that TSPO is lower in patients with psychotic disorders as compared with healthy control subjects.
The presence of lower TSPO in patients does not necessarily abrogate the hypothesis of proinflammatory activation in psychosis. While TSPO has been shown to be sensitive in systemic inflammation paradigms (22,23), TSPO is not specific to microglia (24,25), and in vitro data have shown a lack of correspondence between TSPO and proinflammatory activation (26,27), although conflicting data exist (28,29). For schizophrenia specifically, the lack of a relationship between TSPO and cell- and activation-specific markers was recently confirmed in a postmortem study (30). In addition, body mass index was recently reported to influence TSPO values in a large sample of healthy control subjects (31). This could be a potential confounder for the present analysis, but it could also offer a future line of investigation into disease mechanisms and risk factors underlying psychotic disorders.
Although we observed no associations between symptom severity or duration of illness and VT, it is noteworthy that the three studies displaying the strongest effects (8,10,16) included patients in an early phase of the disorder with a majority being unmedicated or drug naïve. On the opposite side of the meta-analytical estimate is the study by Ottoy et al. (11), which included a sample of patients with longer duration of illness and expressing more severe symptoms (32). To further examine this pattern, longitudinal studies monitoring the in vivo expression of immune markers in patients from the onset throughout different stages of the disorder are warranted.
Acknowledgments and Disclosures
This work was supported by Swedish Research Council Grant No. 523-2014-3467 (to SC); the Swedish Society for Medical Research (to PP-S); the Lundbeck Foundation (to PP-S); Medical Research Council-UK Grant No. MC-A656-5QD30 (to ODH), Wellcome Trust Grant No. 094849/Z/10/Z (to ODH); the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust (to ODH); King’s College London (ODH); National Institute of Mental Health Grant Nos. EB024495 (to JMC), NS100847 (to JMC), and R01MH100043 (to RM); the One Mind - Gifford Foundation (to JMC); and Academy of Finland Grant Nos. 267982 (to JH) and 323036 (to HL).
SC has received grant support from AstraZeneca as a coinvestigator and has served as a speaker for Otsuka. RM received travel support and speaking fees from Janssen for invited talks. LDP, MM, and JO received support from Janssen R&D to fund their study. RM has received travel support and speaker fees from Janssen and consulting fees from Otsuka-Lundbeck Canada. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Angelini, AstraZeneca, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Janssen, Lundbeck, Leyden-Delta, Mylan, Neurocrine, Otsuka, Sunovion, Rand, Recordati, and Roche. All other authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Pontus Plavén-Sigray, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Region Stockholm, Stockholm, Sweden; Neurobiology Research Unit, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Granville J. Matheson, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Region Stockholm, Stockholm, Sweden
Jennifer M. Coughlin, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medical Institutions, Baltimore, Maryland Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Sina Hafizi, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Heikki Laurikainen, Department of Psychiatry, University of Turku and Neuropsychiatric Imaging Group, Turku PET Centre, Turku University Hospital, Turku, Finland.
Julie Ottoy, Molecular Imaging Center Antwerp, University of Antwerp, Antwerp, Belgium.
Livia De Picker, Collaborative Antwerp Psychiatric Research Institute (CAPRI), University of Antwerp, Antwerp, Belgium.
Pablo Rusjan, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Jarmo Hietala, Department of Psychiatry, University of Turku and Neuropsychiatric Imaging Group, Turku PET Centre, Turku University Hospital, Turku, Finland.
Oliver D. Howes, Institute of Psychiatry, Psychology and Neuroscience, King’s College London MRC London Institute of Medical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom; Hammersmith Hospital; and Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom.
Romina Mizrahi, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Manuel Morrens, Collaborative Antwerp Psychiatric Research Institute (CAPRI), University of Antwerp, Antwerp, Belgium.
Martin G. Pomper, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medical Institutions, Baltimore, Maryland Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Simon Cervenka, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Region Stockholm, Stockholm, Sweden.
References
- 1.Van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. (2008): Microglia activation in recent-onset schizophrenia: A quantitative (R)-[11 C] PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. [DOI] [PubMed] [Google Scholar]
- 2.Doorduin J, De Vries EFJ, Willemsen ATM, De Groot JC, Dierckx RA, Klein HC (2009): Neuroinflammation in schizophrenia-related psychosis: A PET study. J Nucl Med 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 3.Banati R, Hickie IB (2009): Therapeutic signposts: Using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med J Aust 190:S26. [DOI] [PubMed] [Google Scholar]
- 4.Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, et al. (2016): In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: A [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry 21:1672–1679. [DOI] [PubMed] [Google Scholar]
- 5.Di Biase MA, Zalesky A, O’keefe G, Laskaris L, Baune BT, Weickert CS, et al. (2017): PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry 7:e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, et al. (2015): Imaging neuroinflammation in gray and white matter in schizophrenia: An in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. (2015): Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [11C] PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, et al. (2016): In vivo markers of inflammatory response in recent-onset schizophrenia: A combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, et al. (2017): Imaging microglial activation in untreated first-episode psychosis: A PET study with [18F]FEPPA. Am J Psychiatry 174:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, et al. (2017): Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 22:850–856. [DOI] [PubMed] [Google Scholar]
- 11.Ottoy J, De Picker L, Verhaeghe J, Deleye S, Kosten L, Sabbe B, et al. (2018): [18F] PBR111 PET imaging in healthy controls and schizophrenia: Test-retest reproducibility and quantification of neuroinflammation. J Nucl Med 59:1267–1274. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Doef TF, De Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, et al. (2016): In vivo (R)-[11C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr 2:16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita M, Kobayashi M, Ikawa M, Gunn RN, Rabiner EA, Owen DR, et al. (2017): Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176—based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res 7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plavén-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, et al. (2018): Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: A meta-analysis using individual participant data. Biol Psychiatry 84:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, et al. (2018): Neuroinflammation in schizophrenia: Meta-analysis of in vivo microglial imaging studies. Psychol Med 49:2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurikainen H, Vuorela A, Toivonen A, Reinert-Hartwall L, Trontti K, Lindgren M, et al. (2020): Elevated serum chemokine CCL22 levels in first-episode psychosis: Associations with symptoms, peripheral immune state and in vivo brain glial cell function. Transl Psychiatry 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TudurSmith C, Marcucci M, Nolan SJ, Iorio A, Sudell M, Riley R, et al. (2016): Individual participant data meta-analyses compared with meta-analyses based on aggregate data. Cochrane Database Syst Rev 9:MR000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plavén-Sigray P, Cervenka S (2019): Meta-analytic studies of the glial cell marker TSPO in psychosis-a question of apples and pears? A commentary on ‘Neuroinflammation in schizophrenia: Metaanalysis of in-vivo microglial imaging’ by Marques et al et al. Psychol Med 49:1583–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matheson GJ, Plavén-Sigray P, Forsberg A, Varrone A, Farde L, Cervenka S (2017): Assessment of simplified ratio-based approaches for quantification of PET [11C]PBR28 data. EJNMMI Res 7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plavén-Sigray P, Matheson GJ, Cselényi Z, Jučaite A, Farde L, Cervenka S (2018): Test-retest reliability and convergent validity of (R)-[11C] PK11195 outcome measures without arterial input function. EJNMMI Res 8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Jiang T, Telu S, Zoghbi SS, Gunn RN, Rabiner EA, et al. (2017): 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. J Cereb Blood Flow Metab 38:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, et al. (2015): Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A 112:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillmer AT, Holden D, Fowles K, Nabulsi N, West BL, Carson RE, Cosgrove KP (2017): Microglial depletion and activation: A [11 C] PBR28 PET study in nonhuman primates. EJNMMI Res 7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notter T, Coughlin JM, Sawa A, Meyer U (2018): Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol Psychiatry 23:36–47. [DOI] [PubMed] [Google Scholar]
- 25.Veronese M, Marques T, Bloomfield PS, Rizzo G, Singh N, Jones D, et al. (2017): Kinetic modelling of [11C]PBR28 for 18kDa translocator protein PET data: A validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cereb Blood Flow Metab 38:1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, et al. (2017): Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab 37:2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayan N, Mandhair H, Smyth E, Dakin SG, Kiriakidis S, Wells L, et al. (2017): The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLoS One 12:e0185767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR, et al. (2020): Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 68:280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannestad J, Gallezot J-D, Schafbauer T, Lim K, Kloczynski T, Morris ED, et al. (2012): Endotoxin-induced systemic inflammation activates microglia:[11C] PBR28 positron emission tomography in nonhuman primates. Neuroimage 63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneeboer MAM, van der Doef T, Litjens M, Psy NBB, Melief J, Hol EM, et al. (2019): Microglial activation in schizophrenia: Is translocator 18 kDa protein (TSPO) the right marker? Schizophr Res 215:167–172. [DOI] [PubMed] [Google Scholar]
- 31.Tuisku J, Plavén-Sigray P, Gaiser EC, Airas L, Al-Abdulrasul H, Brück A, et al. (2019): Effects of age, BMI and sex on the glial cell marker TSPO—A multicentre [11 C] PBR28 HRRT PET study. Eur J Nucl Med Mol Imaging 46:2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Picker L, Ottoy J, Verhaeghe J, Deleye S, Fransen E, Kosten L, et al. (2019): State-associated changes in longitudinal [18F]-PBR111 TSPO PET imaging of psychosis patients: Evidence for the accelerated ageing hypothesis? Brain Behav Immun 77:46–54. [DOI] [PubMed] [Google Scholar]