Abstract
Objective
Preliminary findings suggest a relationship between lower serum 25-hydroxyvitamin D [25(OH)D] levels and incidence and severity of COVID-19. The aim of this study was to evaluate the relationship between vitamin D status at admission and different markers of inflammation, coagulation, and sepsis in hospitalized patients with COVID-19.
Method
We conducted a retrospective study on 137 consecutive patients with SARS-CoV-2 infection and available data on serum 25(OH)D levels, who were admitted to our Institution between March 1 and April 30, 2020. Patients were divided into two groups: survivors (n = 78; 57%) and non-survivors (n = 59; 43%).
Results
At admission, all patients showed hypovitaminosis D. Median total serum 25(OH)D levels at admission were significantly higher in survivors than non-survivors (12 ng/mL vs 8 ng/mL; p < 0.01). Non-survivors exhibited significantly higher median levels of white blood cell (WBC) count, neutrophil-to-lymphocyte count ratio (NLR), high-sensitivity C-reactive protein (hsCRP), ferritin, interleukin 6 (IL-6), D-dimer, fibrinogen, and procalcitonin (PCT) compared to survivors at three different time points during hospitalization. In a multivariate analysis performed by a logistic regression model, serum 25(OH)D levels were significantly inversely associated with risk of COVID-19-related in-hospital mortality (odds ratio, 0.91; 95% confidence interval, 0.85–0.98; p = 0.01). According to receiver operating characteristic curve analysis, hsCRP, NLR, ferritin, and D-dimer were the best predictive biomarkers for poor prognosis of COVID-19, whereas IL-6, PCT, fibrinogen, 25(OH)D, WBC count, and tumor necrosis factor alpha (TNF-α) may serve as supportive biomarkers for worse clinical course of the disease.
Conclusions
We found a markedly high prevalence (100%) of hypovitaminosis D in patients admitted to hospital with COVID-19, suggesting a possible role of low vitamin D status in increasing the risk of SARS-CoV-2 infection and subsequent hospitalization. The inverse association between serum 25(OH)D levels and risk of in-hospital mortality observed in our cohort suggests that a lower vitamin D status upon admission may represent a modifiable and independent risk factor for poor prognosis in COVID-19.
Keywords: Vitamin D status, inflammatory markers, mortality, SARS-CoV-2, cytokine storm, COVID-19
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in the city of Wuhan (China) in December 2019 and was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020. Over recent months, COVID-19 has placed unprecedented strain on healthcare systems worldwide, posing serious threats to global health. Italy was the first Western country to be hit by the COVID-19 outbreak. Older age and underlying comorbid conditions (such as cardiovascular disease, hypertension, obesity, and diabetes mellitus) have emerged as major risk factors for mortality related to COVID-19 (1–7). A dysregulated immune response resulting in the so-called “cytokine release syndrome” (also known as “cytokine storm”) has been shown to play a critical role in the pathophysiology of the most severe cases of COVID-19 (8,9). Patients with severe manifestations of COVID-19 exhibit significantly increased circulating levels of C-reactive protein and several pro-inflammatory cytokines and chemokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin 6 (IL-6), and interferon-gamma (IFN-γ) (10–12). In turn, increased circulating levels of such cytokines result in a systemic hyperinflammatory state characterized by increased activity of CD8+ cytotoxic T cells, augmented differentiation of T helper (Th) 17 cells, and reduced activity of regulatory T cells (13). These abnormal immune responses can lead to acute respiratory distress syndrome and multiorgan failure (13–15). An increase in neutrophil counts and a marked reduction of peripheral lymphocyte counts (primarily CD4+ and CD8+ T cells) have also been reported in severe cases of COVID-19, with the degree of lymphopenia correlating with disease severity (11,16). Severe cases of COVID-19 also exhibit abnormal coagulation results, which consist of significantly elevated concentrations of fibrinogen, D-dimer, and other fibrin degradation products, along with significantly longer prothrombin time and activated partial thromboplastin time (11,16,17). These findings suggest the development of overt disseminated intravascular coagulation (17).
Over the last decade, several mechanistic studies have shown that vitamin D exerts anti-inflammatory and immunomodulatory properties, beyond its well-established role in the regulation of calcium and bone homeostasis (18). Vitamin D has been shown to play a pivotal role in the regulation of both innate and adaptive immune responses, promoting antiviral effector mechanisms, reducing the expression of several pro-inflammatory cytokines, and favoring tolerogenic responses (18–22). Preclinical evidence supports that calcitriol (the active metabolite of vitamin D, also referred to as 1,25-dihydroxyvitamin D3) exerts various effects on both innate and adaptive immune systems, resulting in induction of anti-inflammatory pathways and immune tolerance. With regard to innate immunity, calcitriol is able to induce the transcription of antimicrobial peptides (e.g., cathelicidin and defensin β2) in several human cell lines (keratinocytes, myeloid cells, monocytes/macrophages, and neutrophils) (23–26). Also, calcitriol (a) promotes the differentiation of monocytes/macrophages and enhances their chemotactic and phagocytic capacity (27,28); (b) inhibits the synthesis of pro-inflammatory cytokines (including IL-6 and TNF-α) by monocytes and macrophages (29); (c) reduces macrophage surface expression of major histocompatibility complex-class II molecules, thus decreasing the macrophage antigen presentation and T cell stimulatory ability (30,31); (d) promotes the shift of macrophage polarization from M1 phenotype (pro-inflammatory or “classically activated” macrophages) toward M2 phenotype (anti-inflammatory or “alternatively activated” macrophages) (32); and (e) modulates the differentiation and function of dendritic cells, rendering them more tolerogenic and reducing their antigen-presenting capacity (33–37). With regard to adaptive immunity, calcitriol up-regulates regulatory T cells (38) and promotes the shift of T cells from an “effector” toward a “regulatory” and anti-inflammatory phenotype by reducing Th1 and Th17 cell differentiation and favoring Th2 cell differentiation (39–41). Additionally, immune cells are both vitamin D targets and local producers of vitamin D (42). Indeed, functional vitamin D receptor has been identified in almost all immune cells, including neutrophils, T cells, and antigen-presenting cells (macrophages and dendritic cells) (43–46) as well as in human airway epithelial cells (47). In addition, several immune cells (e.g., macrophages, dendritic cells, and T- and B-lymphocytes) have been found to express the vitamin D–activating enzymes 25- and 1α-hydroxylase (30,48–51). In vitro studies also suggest that vitamin D plays an important role in local “respiratory homeostasis” either by inducing the expression of antimicrobial peptides or by directly affecting the replication of respiratory viruses (47).
Vitamin D deficiency represents a global pandemic afflicting more than 1 billion individuals across all age groups worldwide (52). Moreover, there is an overlap between risk factors for vitamin D deficiency and severe COVID-19 (such as Black or Asian ethnic origin, older age, and obesity) (53). Hence, over the last few months, several researchers have suggested vitamin D deficiency as an independent risk factor for COVID-19 infection and adverse outcomes in the context of established disease (53–56). Similarly, there has been a growing interest in a potential role for vitamin D as an adjuvant immunomodulatory agent able to prevent SARS-CoV-2 infection or counteract the development of the cytokine release syndrome and improve outcomes in the setting of COVID-19 (53,54,57). Therefore, we conducted a retrospective cohort study among patients with COVID-19 admitted to our Institution during the Italian COVID-19 outbreak, comparing the levels of inflammatory markers at admission between survivors and non-survivors with confirmed SARS-CoV-2 infection. Our study primarily aimed to measure serum 25-hydroxyvitamin D [25(OH)D] levels upon inpatient admission, in order to evaluate the relationship between vitamin D status and different markers of inflammation, coagulation, and sepsis in this population.
Methods
Study design and participants
We conducted a retrospective study including patients with confirmed SARS-CoV-2 infection who were consecutively admitted to our Institution (Tor Vergata University Hospital-PTV, Rome, Italy) between March 1 and April 30, 2020. Serum 25(OH)D levels were measured at admission in all patients, as per our institutional protocol. The study was reviewed and approved by the Ethics Committee of University of Rome Tor Vergata (Registration Number: 141/20, July 23, 2020). At admission, all patients provided written informed consent to anonymous data collection and analysis for research purposes. Patients were divided into two groups according to the outcomes of survival and death, namely survivors and non-survivors. For each group, we considered the length of hospital stay. In addition, the length of stay in the intensive care unit (ICU) was considered as a clinical marker of disease severity (need for invasive mechanical ventilation).
Data collection and informed consent
The medical records of patients were independently reviewed by two members of our research team. Epidemiological, clinical, radiological, and laboratory data were collected through electronic medical records (Modulab®) and recorded in an anonymous inpatient COVID-19 database.
Laboratory examination
Initial diagnosis of COVID-19 was made by an infectious disease specialist based on clinical symptoms (cough, fever, dyspnea, and/or anosmia) and imaging tests (chest X-ray and/or computed tomography) indicative of acute respiratory tract infection and COVID-19 pneumonia. Laboratory confirmation of SARS-CoV-2 infection was made from nasopharyngeal swab samples obtained upon hospital admission and analyzed through real-time reverse transcription polymerase chain reaction (RT-PCR) for 2019-nCoV RNA extraction according to the manufacturer’s instructions (RT-PCR kit Seegene AllplexTM 2019-nCoV Assay, Seegene, Seoul, South Korea). Hematology and biochemical parameters were measured on blood, serum, and plasma samples collected upon admission to the emergency department, infectious disease unit, or ICU. White blood cell (WBC) count was determined by using automated hematological analyzer (Dasit-Sysmex, Milan, Italy). We also determined the neutrophil-to-lymphocyte ratio (NLR) as a marker of systemic inflammation (58) (normal values range between 0.78 and 3.53) (59).
Serum levels of high-sensitivity C-reactive protein (hsCRP; reference range 0–5 mg/L) were measured by using an immunoturbidimetric method (Abbott Diagnostics, Milan, Italy). Serum levels of IL-6 (reference range: 0–50 pg/mL) were measured using chemiluminescence method (IMMULITE 2000 instrument, Siemens, Milan, Italy). Serum levels of TNF-α (reference range: 0–12.4 pg/mL) were measured using the enzyme-linked immunosorbent assay (ELISA) technique (DRG, International Instruments GmbH, Marburg, Germany). Serum levels of ferritin (reference range: 21.81–274.66 ng/mL), procalcitonin (PCT; reference range: 0.01–0.50 ng/mL) were measured using the chemiluminescence method (Architect Instrument, Abbott, Milan, Italy). Total serum 25(OH)D was measured by electrochemiluminescence (Abbott Architect Instrument, Milan, Italy), with the limit of quantitative value at 2.2 ng/mL at 20% coefficient variation. Plasma fibrinogen concentrations (reference range: 200–400 mg/dL) were measured using the Clauss method (ACL-TOP instrumentation, Werfen, Milan, Italy). Plasma D-dimer levels (reference range: 0–500 ng/mL) were measured by ACL-TOP instrumentation (Werfen, Milan, Italy).
Hematological and biochemical parameters were measured at three different time points: (1) time of hospital admission (T1), (2) midpoint of hospitalization (T2), and (3) 1 day before discharge or death (T3) for survivors and non-survivors, respectively. Total serum 25(OH)D levels were only measured at admission (T1).
Statistical analysis
Descriptive statistics such as frequency, percentage, mean and standard deviation (SD), median, and percentiles were calculated. Both the histogram and the Kolmogorov-Smirnov test of normality (p value < 0.05) were used to check whether the data were normally distributed.
In the presence of a normal distribution of data, parametric tests were used such as analysis of variance with Bonferroni post hoc test in the case of more than two variables, or t test in the case of two variables. Non-parametric tests, such as Kruskal-Wallis test (variables with more than two categories) and Mann-Whitney U test (variables with two categories), were used to test differences between different groups. A p value of less than 0.05 was considered statistically significant in all statistical analyses. The comparison between the percentages was performed using the χ2 test. Multivariate analysis performed by a logistic regression model was used to determine the independent association of serum 25(OH)D levels (expressed as continuous variable) and risk of COVID-19-related in-hospital mortality. We initially included in the logistic regression model the following covariates: (1) continuous variables: 25(OH)D, age, body mass index (BMI), WBC count, NLR, hsCRP, fibrinogen, D-dimer, IL-6, TNF-α, ferritin, and PCT and (2) categorical variables: sex, hypertension and cardiovascular disease (CVD), diabetes mellitus, obesity (expressed as a BMI value of ≥30 kg/m2), and malignancy (active malignancy or history of previous malignancy). After backward elimination process, WBC count, hsCRP, IL-6, TNF-α, ferritin, PCT, BMI (continuous variable), hypertension and CVD, diabetes mellitus, and malignancy were excluded from the model because of the p value > 0.1. p values < 0.05 were considered statistically significant. All statistical analyses were performed using MedCalc Version 18.2.18 (MedCalc Software Ltd, Ostend, Belgium). Receiver operating characteristic (ROC) curve analysis was performed to assess sensitivity and specificity of different potential predictive biomarkers for worse clinical course and adverse outcomes of COVID-19. The area under the curve (AUC) was used to assess the prognostic accuracy. AUC values ranging from 0.5 to 1.0 indicate the prognostic marker discriminatory ability for COVID-19 morbidity and mortality.
Results
Patient demographics, admission characteristics, and length of stay
A total of 137 consecutive patients admitted to our Institution (between March 1 and April 30, 2020) were enrolled into this retrospective single-center study. All patients had laboratory-confirmed cases of COVID-19, were Caucasian, and resided in the Lazio region. Participant demographics, admission characteristics, and length of stay are shown in Table 1. Table 2 lists the prevalence of major comorbidities in our study population. Participants included 89 males (65%) and 48 females (35%). Patients were divided into two groups according to the outcomes of survival and death, namely survivors (n = 78; 57%) and non-survivors (n = 59; 43%). Mean age in the survivor group was significantly lower than that in the non-survivor group (65 ± 13 vs 70 ± 14 years, respectively; p = 0.01). The survivor group included 43 males (55%) and 35 females (45%), while the non-survivor group included 46 males (78%) and 13 females (22%). The percentage of males was significantly higher in non-survivors compared to survivors (78% vs 55%, p < 0.005), whereas the percentage of females was significantly lower in non-survivors compared to survivors (22% vs 45%, p < 0.005). Median values of BMI between survivors and non-survivors were comparable (Table 1). However, the percentage of obese patients (defined as a BMI value of ≥30 kg/m2) was significantly higher in non-survivors compared to survivors (29% vs 15%; n = 17 vs 12; p = 0.007) (Table 2). Among survivors, there were 27 patients (35%) with hypertension and CVD, 8 patients (10%) with diabetes mellitus, and 7 patients (9%) with active or previous malignancy. Among non-survivors, there were 25 patients (42%) with hypertension and CVD, 6 patients (10%) with diabetes mellitus, and 9 patients (15%) with active or previous malignancy. There was no statistically significant difference in the percentage of hypertension and CVD, diabetes mellitus, and active or previous malignancy between survivors and non-survivors (Table 2).
Table 1.
Participant Demographics, Admission Characteristics, and Length of Stay in Hospital and ICU
| No. of patients | 137 | ||||
|---|---|---|---|---|---|
| Male, n (%) | 89 (65%) | ||||
| Female, n (%) | 48 (35%) | ||||
|
SURVIVORS n = 78 (57%) |
Age (years) |
BMI (kg/m2) |
Length of hospital stay (days) | Length of stay in ICU (days) | |
| Minimum | 34 | 22 | 3 | 0 | |
| Maximum | 89 | 38.5 | 75 | 33 | |
| Mean | 65 | 28 | 30 | 3 | |
| Median | 64 | 27.5 | 25 | 0 | |
| SD | 13 | 3.3 | 18 | 7 | |
| 25th–75th percentile | 55 − 76 | 26 − 29 | 17 − 41 | 0 − 0 | |
| p value compared to non-survivors | p = 0.01 | p = 0.5 | p < 0.001 | p < 0.001 | |
|
NON-SURVIVORS n = 59 (43%) |
Age (years) |
BMI (Kg/m2) |
Length of in-hospital stay (days) | Length of stay in ICU (days) | |
| Minimum | 34 | 23 | 4 | 0 | |
| Maximum | 95 | 37 | 65 | 34 | |
| Mean | 70 | 29 | 15 | 8 | |
| Median | 73 | 28.5 | 14 | 7 | |
| SD | 14 | 3 | 10 | 8 | |
| 25th–75th percentile | 61 − 80 | 27 − 31 | 8 − 20 | 0 − 13 | |
|
p value compared to survivors |
p = 0.01 | p = 0.5 | p < 0.001 | p < 0.001 | |
BMI = body mass index, ICU = intensive care unit, SD = standard deviation.
Table 2.
Prevalence of Major Comorbidities in the Study Population
| Comorbidity |
SURVIVORS n = 78 (57%) |
NON-SURVIVORS n = 59 (43%) |
p value |
|---|---|---|---|
| Obesity (BMI ≥30 kg/m2), n (%) | 12 (15%) | 17 (29%) | 0.007 |
| Hypertension and CVD, n (%) | 27 (35%) | 25 (42%) | 0.4 |
| Diabetes mellitus, n (%) | 8 (10%) | 6 (10%) | 0.1 |
| Active or previous malignancy, n (%) | 7 (9%) | 9 (15%) | 0.27 |
BMI = body mass index; CVD = cardiovascular disease. Percentages refer to the total number of patients within each group (survivor group and non-survivor group).
All patients received the same standard care for the treatment of COVID-19 (as per our institutional protocol) consisting of a combination therapy with dexamethasone plus hydroxychloroquine and lopinavir/ritonavir administered shortly after the admission. None of the patients reported vitamin D supplementation prior to hospital admission. The mean length of hospital stay was 30 ± 18 days and 15 ± 10 days for survivors and non-survivors, respectively (p < 0.001). With regard to length of stay in the ICU, survivors and non-survivors spent a mean time in the ICU of 3 ± 7 days and 8 ± 8 days, respectively (p < 0.001) (Table 1).
Comparison of hematological and biochemical parameters and pro-inflammatory cytokines in survivors and non-survivors
Hematological and biochemical parameters and pro-inflammatory cytokines were measured at three different time points: (1) time of hospital admission (T1), (2) midpoint of hospitalization (T2), and (3) 1 day before discharge or death (T3) for survivors and non-survivors, respectively. Total serum 25(OH)D levels were only measured at admission (T1). Baseline values at the time of hospital admission (T1) and changes in hematological and biochemical parameters and pro-inflammatory cytokines at different time points during hospitalization (T2 and T3) are shown in Figure 1 (expressed as median values) and Figure 2 (expressed as median values, with the addition of SD and interquartile ranges) and listed in Supplementary Table S1. Values of hematological and biochemical parameters in survivors (SU) and non-survivors (NSU) were compared at each time point, namely: T1-SU vs T1-NSU; T2-SU vs T2-NSU; and T3-SU vs T3-NSU. Kruskal-Wallis non-parametric test was used to test differences between groups at different time points.
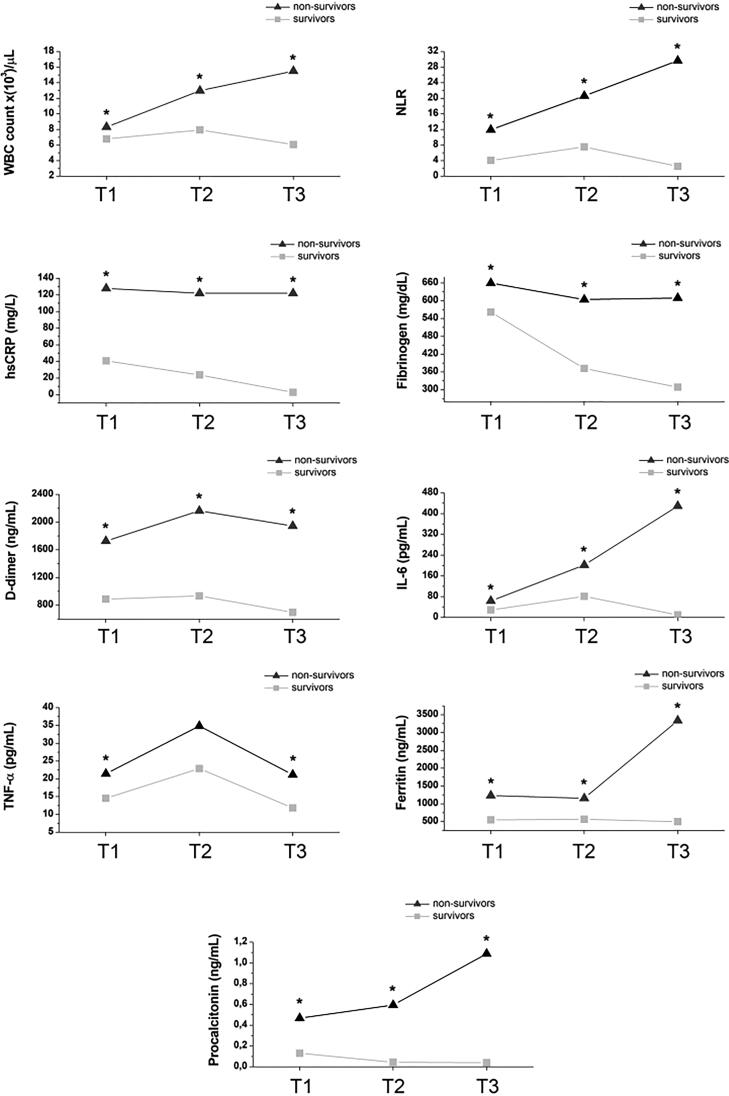
Figure 1.
Baseline values at the time of hospital admission (T1) and changes in hematological and biochemical parameters and pro-inflammatory cytokines at different time points during hospitalization (T2 and T3). All parameters are expressed as median values at each time point. Values of hematological and biochemical parameters in survivors (SU) and non-survivors (NSU) were compared at each time point, namely T1-SU vs T1-NSU; T2-SU vs T2-NSU; and T3-SU vs T3-NSU. At each time point, asterisks (*) indicate statistical significance.
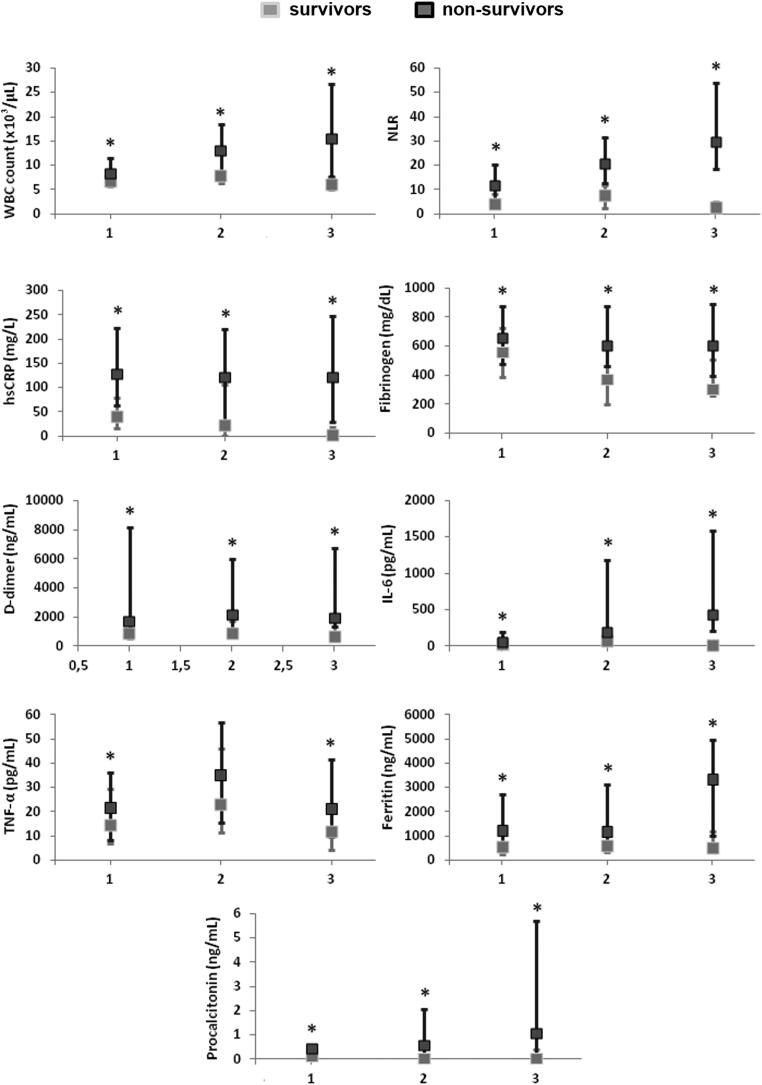
Figure 2.
Baseline values at the time of hospital admission (T1) and changes in hematological and biochemical parameters and pro-inflammatory cytokines at different time points during hospitalization (T2 and T3). All parameters are expressed as median values at each time point, with the addition of standard deviation and interquartile ranges. Values of hematological and biochemical parameters in survivors (SU) and non-survivors (NSU) were compared at each time point, namely T1-SU vs T1-NSU; T2-SU vs T2-NSU; and T3-SU vs T3-NSU. At each time point, asterisks (*) indicate statistical significance.
WBC count and NLR
In survivors, median WBC count tended to increase from T1 (7 × 103/µL) to T2 (8 × 103/µL) and normalized at T3 (6 × 103/µL). In non-survivors, median WBC count was higher than survivors at T1 (8 × 103/µL), increased at T2 (13 × 103/µL), and remained above the normal range at T3 (15.5 × 103/µL).
In survivors, median NLR was 4.1 at T1, 7.5 at T2, and 2.5 at T3. In non-survivors, median NLR was markedly high at T1 (NLR: 12) and sharply increased at T2 (NLR: 21) and T3 (NLR: 30). The difference in median WBC count and NLR between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
hsCRP
Median hsCRP values were above the normal range at all time points in survivors: T1 (41 mg/L), T2 (24 mg/L), and T3 (3 mg/L). However, median hsCRP values were markedly higher in non-survivors at all time points, from T1 (128 mg/L) to T2 (122 mg/L) and T3 (122 mg/L). The difference in hsCRP values between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
Fibrinogen
In survivors, median fibrinogen levels were above the normal range at T1 (560 mg/dL), whereas they decreased and normalized at T2 (370 mg/dL) and T3 (310 mg/dL). In non-survivors, median fibrinogen levels were increased at all time points: T1 (660 mg/dL), T2 (600 mg/dL), and T3 (610 mg/dL). The difference in fibrinogen levels between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
D-dimer
In survivors, median D-dimer levels were above the normal range at all time points, although they tended to decrease at T3: T1 (890 ng/mL), T2 (936 ng/mL), and T3 (699 ng/mL). In non-survivors, median D-dimer levels were abnormally high at all time points: T1 (1728.5 ng/mL), T2 (2164 ng/mL), and T3 (1945.5 ng/mL). The difference in D-dimer levels between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
Pro-inflammatory cytokines and markers of sepsis and inflammation
IL-6
In survivors, median IL-6 levels were normal at T1 (29 pg/mL), whereas they increased at T2 (81.5 pg/mL) and normalized at T3 (10 pg/mL). On the contrary, in non-survivors, median IL-6 levels were slightly increased at T1 (64 pg/mL), whereas they dramatically increased at T2 (202 pg/mL) and T3 (430 pg/mL). The difference in IL-6 levels between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
TNF-α
In both survivors and non-survivors, TNF-α levels showed a similar Gaussian distribution. At T1, median TNF-α levels were slightly increased in both survivors and non-survivors: T1-SU (14.5 pg/mL) and T1-NSU (21 pg/mL). At T2, median TNF-α levels further increased in both survivors and non-survivors: T2-SU (23 pg/mL) and T2-NSU (35 pg/mL). At T3, median TNF-α levels decreased and normalized in survivors (T3-SU: 12 pg/mL), whereas they decreased without normalizing in non-survivors (T3-NSU: 21 pg/mL). Although non-survivors exhibited higher TNF-α levels at all time points compared to survivors, statistical significance between survivors and non-survivors was only observed at T1 (T1-SU vs T1-NSU; p < 0.05) and T3 (T3-SU vs T3-NSU; p < 0.05).
Ferritin
In survivors, median ferritin levels were above the normal range at all time points in both survivors and non-survivors. In survivors, median ferritin levels were as follows: T1 (550 ng/mL), T2 (568.5 ng/mL), and T3 (501 ng/mL). In non-survivors, median ferritin levels were markedly elevated at T1 (1234 ng/mL) and T2 (1158 ng/mL), while they sharply increased at T3 (3342.5 ng/mL). The difference in ferritin levels between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
PCT
In survivors, median PCT levels remained within the normal range at all time points: T1 (0.13 ng/mL), T2 (0.04 ng/mL), and T3 (0.04 ng/mL). In non-survivors, median PCT values were normal at T1 (0.47 ng/mL), whereas they were mildly elevated at T2 (0.6 ng/mL) and T3 (1.1 ng/mL). The difference in PCT levels between survivors and non-survivors was statistically significant at all time points (T1-SU vs T1-NSU, T2-SU vs T2-NSU, T3-SU vs T3-NSU; p < 0.05).
25-hydroxyvitamin D [25(OH)D]
Total serum 25(OH)D levels represent the most reliable biomarker of vitamin D status (60). At admission, all 137 patients showed hypovitaminosis D, defined as serum 25(OH)D levels <30 ng/mL according to the Endocrine Society guidelines on evaluation, treatment and prevention of vitamin D deficiency (61).
Relationship between serum 25(OH)D levels and mortality
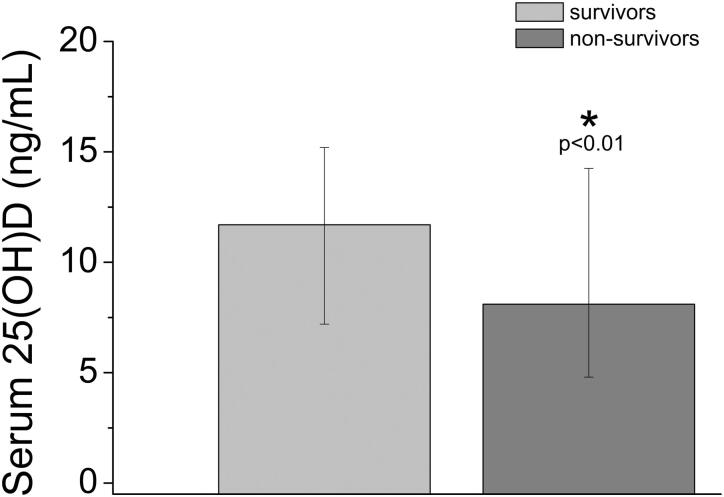
In our cohort, median total serum 25(OH)D levels at admission were significantly higher in survivors than non-survivors (12 ng/mL [25th–75th percentile: 7–15] vs 8 ng/mL [25th–75th percentile: 5–14]; p < 0.01) (Figure 3). We also evaluated 25(OH)D level as continuous variable through a logistic regression analysis to determine the independent association of 25(OH)D and in-hospital mortality. In our final logistic regression model after adjusting for major confounders (age, sex, obesity, NLR, fibrinogen, D-dimer), there was a significant inverse association between serum 25(OH)D levels and risk of COVID-19-related in-hospital mortality (odds ratio [OR], 0.91; 95% confidence interval [CI] 0.85–0.98; p = 0.01; Table S2).
Figure 3.
Median and interquartile ranges of total serum 25-hydroxyvitamin D [25(OH)D] levels at admission in survivors and non-survivors. At admission, survivors showed significantly higher median total serum 25(OH)D levels compared to non-survivors (12 ng/mL vs 8 ng/mL).
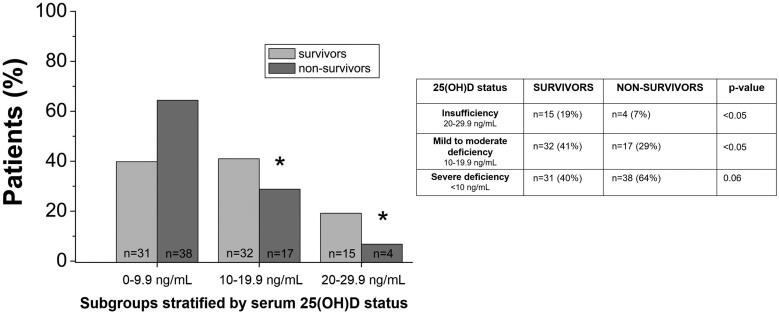
Subgroups stratified by 25(OH)D status at admission
Both survivors (n = 78) and non-survivors (n = 59) were further stratified into different subgroups according to their serum 25(OH)D status at admission (T1), as follows:
Group 1: survivors with severe vitamin D deficiency, defined as serum 25(OH)D levels <10 ng/mL (n = 31; 40% of all survivors).
Group 2: survivors with mild to moderate vitamin D deficiency, defined as serum 25(OH)D levels between 10 and 19.9 ng/mL (n = 32; 41% of all survivors).
Group 3: survivors with vitamin D insufficiency, defined as serum 25(OH)D levels between 20 and 29.9 ng/mL (n = 15; 19% of all survivors).
Group 4: non-survivors with severe vitamin D deficiency, defined as serum 25(OH)D levels <10 ng/mL (n = 38; 64% of all non-survivors).
Group 5: non-survivors with mild to moderate vitamin D deficiency, defined as serum 25(OH)D levels between 10 and 19.9 ng/mL (n = 17; 29% of all non-survivors).
Group 6: non-survivors with vitamin D insufficiency, defined as serum 25(OH)D levels between 20 and 29.9 ng/mL (n = 4; 7% of all non-survivors) (Supplementary Table S3).
Mortality in subgroups stratified by 25(OH)D status at admission
Differences in median serum 25(OH)D levels and percentages of patients between survivor and non-survivor subgroups stratified by 25(OH)D status (group 1 vs group 4; group 2 vs group 5; group 3 vs group 6) were tested using the Kruskal-Wallis non-parametric test.
In survivor subgroups, the median value of serum 25(OH)D was 6 ng/mL in group 1 [25(OH)D < 10 ng/mL], 16 ng/mL in group 2 [25(OH)D between 10 and 19.9 ng/mL], and 25 ng/mL in group 3 [25(OH)D between 20 and 29.9 ng/mL]. In non-survivor subgroups, the median value of serum 25(OH)D was 5 ng/mL in group 4 [25(OH)D < 10 ng/mL], 13 ng/mL in group 5 [25(OH)D between 10 and 19.9 ng/mL], and 24 ng/mL in group 6 [25(OH)D between 20 and 29.9 ng/mL].
In non-survivors, the percentage of patients was higher (64%) in group 4 [25(OH)D < 10 ng/mL], but it tended to decrease in group 5 (29%) [25(OH)D between 10 and 19.9 ng/mL] and group 6 (7%) [25(OH)D between 20 and 29.9 ng/mL] (Supplementary Table S3, Figure 4). Moreover, the percentage of patients was significantly higher in the survivor subgroup with mild to moderate vitamin D deficiency compared to the non-survivor subgroup with mild to moderate vitamin D deficiency (group 2 vs group 5: 41% vs 29%, respectively; p < 0.05), as well as in the survivor subgroup with vitamin D insufficiency compared to the non-survivor subgroup with vitamin D insufficiency (group 3 vs group 6: 19% vs 7%, respectively; p < 0.05). Conversely, there was a trend toward a lower percentage of patients in the survivor subgroup with severe vitamin D deficiency compared to the non-survivor subgroup with severe vitamin D deficiency (group 1 vs group 4: 40% vs 64%, respectively; p = 0.06) (Supplementary Table S3, Figure 4).
Figure 4.
Percentage of patients within survivor and non-survivor subgroups stratified by serum 25-hydroxyvitamin D [25(OH)D] status.
Length of hospital stay in subgroups stratified by 25(OH)D status at admission
Among survivors, mean length of hospital stay was 33 ± 17 days in group 1 [25(OH)D < 10 ng/mL], 29 ± 20 days in group 2 [25(OH)D between 10 and 19.9 ng/mL], and 25 ± 13 days in group 3 [25(OH)D between 20 and 29.9 ng/mL] (Supplementary Table S4). Among non-survivors, mean length of hospital stay was 15 ± 10 days in group 4 [25(OH)D < 10 ng/mL], 15 ± 9 days in group 5 [25(OH)D between 10 and 19.9 ng/mL], and 19.5 ± 8 days in group 6 [25(OH)D between 20 and 29.9 ng/mL] (Supplementary Table S4). Among survivors, length of hospital stay decreased within groups that showed higher 25(OH)D levels, although statistical significance between different survivor subgroups was not reached. Overall, length of hospital stay was greater in all survivor groups compared to non-survivor groups stratified by 25(OH)D status, although statistical significance was observed only between survivor and non-survivor subgroups with mild to moderate and severe vitamin D deficiency (group 1 vs group 4, p < 0.05; group 2 vs group 5, p < 0.05) (Supplementary Table S4).
Length of stay in ICU in subgroups stratified by 25(OH)D status at admission
Among survivors, mean length of stay in the ICU was 4 ± 9 days in group 1 [25(OH)D < 10 ng/mL], 3 ± 7 days in group 2 [25(OH)D between 10 and 19.9 ng/mL], and 0.6 ± 2 days in group 3 [25(OH)D between 20 and 29.9 ng/mL] (Supplementary Table S4).
Among non-survivors, mean length of stay in the ICU was 8 ± 7 days in group 4 [25(OH)D < 10 ng/mL], 8 ± 10 days in group 5 [25(OH)D between 10 and 19.9 ng/mL], and 8.5 ± 6 days in group 6 [25(OH)D between 20 and 29.9 ng/mL] (Supplementary Table S4). The difference in mean length of stay in the ICU was statistically significant between all survivor and non-survivor groups stratified by 25(OH)D status (group 1 vs group 4, group 2 vs group 5, group 3 vs group 6; p < 0.05) (Supplementary Table S4).
Hematological and biochemical parameters and pro-inflammatory cytokines in subgroups stratified by 25(OH)D status at admission
Values of hematological parameters, biochemical parameters, and pro-inflammatory cytokines measured at the time of hospital admission (T1) in survivor and non-survivor subgroups stratified by serum 25(OH)D status are presented in Supplementary Appendix 1 and Supplementary Table S5. With regard to the analysis examining the relationship between 25(OH)D status and hematological and biochemical parameters and pro-inflammatory cytokines, we only took into account measurements performed at T1 (time of hospital admission) because this was the same time point at which data on serum 25(OH)D levels were available.
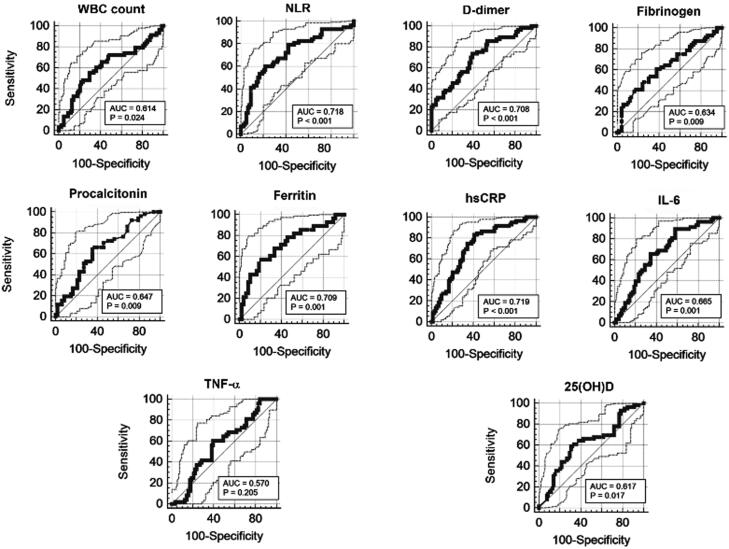
Predictive biomarkers for COVID-19 morbidity and mortality
ROC curve analysis and AUC were performed to identify reliable predictive biomarkers for worse clinical course and adverse outcomes (expressed as mortality) of COVID-19. The AUC provides an overall measure of accuracy of different analytes. Based on AUC values of different analytes at admission, we observed that hsCRP (AUC = 0.719), NLR (AUC = 0.718), ferritin (AUC = 0.709), and D-dimer (AUC= 0.708) at admission were the best predictive biomarkers for adverse outcome of COVID-19 (death). On the other hand, IL-6 (AUC = 0.665), PCT (AUC = 0.647), fibrinogen (AUC = 0.634), 25(OH)D (AUC = 0.617), WBC count (AUC = 0.614), and TNF-α (AUC = 0.570) could be considered as supportive biomarkers for disease severity and mortality (Figure 5, Table 3).
Figure 5.
Receiver operating characteristic curve analysis performed for different hematological and biochemical parameters.
Table 3.
Area Under the ROC Curve (AUC), Sensitivity, and Specificity of Different Markers of Inflammation, Coagulation, and Sepsis Measured Upon Hospital Admission (T1)
| WBC | NLR | hsCRP | Fibrinogen | D-dimer | IL-6 | TNF-α | Ferritin | PCT | 25(OH)D | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 55 | 61 | 83 | 41 | 74 | 90 | 60 | 57 | 65 | 59 |
| Specificity (%) | 71 | 78 | 59 | 82 | 61 | 42 | 60 | 79 | 65 | 70 |
| Cutoff | >8.2 | >8.3 | >53.7 | >741 | >1015 | >14.6 | >18.6 | >1103 | >0.2 | <8.7 |
| AUC; 95% CI | 0.614; 0.527 to 0.696 | 0.718; 0.634 to 0.791 |
0.719; 0.635 to 0.794 |
0.634; 0.542 to 0.719 |
0.708; 0.621 to 0.786 |
0.665; 0.575 to 0.746 |
0.570; 0.472 to 0.663 |
0.709; 0.594 to 0.808 |
0.647; 0.544 to 0.740 |
0.617; 0.53 to 0.699 |
ROC = receiver operating characteristic, CI = confidence interval, WBC = white blood cell, NLR = neutrophil-to-lymphocyte count ratio, hsCRP = high-sensitivity C-reactive protein, IL-6 = interleukin 6, TNF-α = tumor necrosis factor alpha, PCT = procalcitonin, 25(OH)D = 25-hydroxyvitamin D.
Discussion
Our study suggests an involvement of low vitamin D status as a potential risk factor for SARS-CoV-2 infection and COVID-19-related hospitalization. First, we found a markedly high prevalence of hypovitaminosis D (100%), which was present in all 137 patients upon admission, in accordance with findings observed in another study (62). These findings are also in line with those observed in a recent large, real-word, population-based study, which identified an independent and significant association between a low 25(OH)D status (<30 ng/mL) and the increased likelihood of COVID-19 infection (63). In a univariate analysis from the aforementioned study, low 25(OH)D levels were also significantly associated with an increased likelihood of hospitalization due to COVID-19 (63). A single-center retrospective cohort study further confirmed that likely deficient vitamin D status was associated with increased risk for COVID-19 (defined by a positive PCR test result) (64). In addition, a smaller retrospective cohort study found significantly lower serum 25(OH)D levels in SARS-CoV-2 PCR–positive patients compared to negative patients (65). Accordingly, a retrospective observational analysis conducted in the US among 191,779 patients with SARS-CoV-2 results performed from mid-March through mid-June 2020 showed that the SARS-CoV-2 positivity rate was higher in patients with deficient 25(OH)D values (<20 ng/mL) compared to patients with adequate values (30–34 ng/mL) and those with values ≥55 ng/mL (66).
In our cohort, median total serum 25(OH)D levels at admission were significantly higher in survivors than non-survivors (12 vs 8 ng/mL). In non-survivors, median total serum 25(OH)D levels at admission were therefore indicative of severe vitamin D deficiency (<10 ng/mL). In the multivariate analysis performed by a logistic regression model, serum 25(OH)D levels were significantly inversely associated with risk of COVID-19-related in-hospital mortality (OR, 0.91; 95% CI, 0.85–0.98; p = 0.01; Table S2), independent of age, sex, BMI, markers of inflammation, coagulation and sepsis, and presence of major comorbidities (hypertension and CVD, diabetes mellitus, obesity, and active malignancy or history of previous malignancy).
Length of stay in the ICU was considered as a clinical marker of disease severity due to the need for invasive mechanical ventilation. Non-survivors showed a significantly shorter length of in-hospital stay than survivors (mean: 15 vs 30 days), as well as a significantly longer length of stay in the ICU compared to survivors (mean: 8 vs 3 days) (Table 1). With regard to subgroups stratified by 25(OH)D status, we observed that in survivors the length of hospital stay tended to be shorter in patients who had higher levels of 25(OH)D (Supplementary Table S4), suggesting that a higher vitamin D level may favor a faster recovery. Accordingly, a recent prospective cohort study conducted in hospitalized patients with and without COVID-19 aged ≥65 years found a significantly higher incidence of noninvasive ventilation support and high dependency unit admission among patients with vitamin D deficiency in the COVID-19-positive group. Importantly, study participants were considered vitamin D-replete in presence of 25(OH)D levels >30 nmol/L (corresponding to >12 ng/mL) (67).
In keeping with our findings, another Italian retrospective observational study conducted among 42 hospitalized patients with acute respiratory failure due to COVID-19 found a high prevalence of hypovitaminosis D (81%), and a survival analysis showed that patients with severe vitamin D deficiency [defined as 25(OH)D levels <10 ng/mL] exhibited a significantly higher mortality risk after 10 days of hospitalization compared to those with 25(OH)D levels ≥10 ng/mL (50% vs 5%, p = 0.019) (62). Another retrospective study conducted among 185 hospitalized patients with COVID-19 found that vitamin D deficiency [defined as serum total 25(OH)D levels <12 ng/mL] at the time of admission was associated with higher risk of invasive mechanical ventilation and death (68). De Smet et al. (69) recently documented that male patients with COVID-19 exhibited progressively lower 25(OH)D levels with advancing disease radiologic stage (assessed by chest computed tomography), and vitamin D deficiency (<20 ng/mL) at admission was significantly and independently associated with COVID-19 mortality.
Thus, a lower vitamin D status may partly account for adverse clinical outcomes and mortality in hospitalized patients with COVID-19. In particular, our results—together with the findings from the aforementioned studies (62,67,68)—may suggest the existence of a baseline 25(OH)D threshold falling within a given range (probably between 8 and 12 ng/mL), which might predict poor clinical outcomes in hospitalized patients with COVID-19. However, large prospective studies are needed to confirm this hypothesis.
In our cohort, non-survivors also exhibited significantly higher levels of markers of inflammation, coagulation, and sepsis (WBC count, NLR, hsCRP, ferritin, IL-6, D-dimer, fibrinogen, and PCT) compared to survivors at all time points (T1, T2, T3) during hospitalization. Non-survivors also showed significantly higher levels of the pro-inflammatory cytokine TNF-α compared to survivors at all time points (T1, T2, T3), although statistical significance was reached only at T1 and T3. Similar findings have recently been confirmed in other studies comparing asymptomatic patients or those with mild to moderate COVID-19 and patients with severe COVID-19 (70,71), as well as in studies comparing hospitalized COVID-19-negative and COVID-19-positive patients (67). A lower vitamin D status may have contributed, at least in part, to exacerbating systemic inflammation and cytokine release syndrome among non-survivors in our cohort. This explanation may be reasonable in light of the anti-inflammatory and immunomodulatory properties of vitamin D (18,19,21).
A recent Spanish retrospective case-control study (72) found that mean serum 25(OH)D levels were significantly lower in 216 hospitalized patients with COVID-19 compared to 197 sex-matched population-based controls (14 ± 7 vs 20.9 ± 7.4 ng/mL, respectively). Patients with COVID-19 also showed a higher prevalence of vitamin D deficiency—defined as serum 25(OH)D < 20 ng/mL—compared to controls (82% vs 47%). Furthermore, 25(OH)D levels significantly and inversely correlated with serum ferritin and D-dimer levels (72). Similarly, a Chinese case-control study (73) found that median serum 25(OH)D levels were significantly lower in hospitalized patients with COVID-19 compared to healthy controls (55.6 vs 72 nmol/L; 22.2 vs 28.8 ng/mL, respectively). In addition, authors found significantly higher rates of vitamin D deficiency—defined as serum 25(OH)D < 20 ng/mL—in COVID-19 cases (41.9%) compared to healthy controls (11.1%). Among COVID-19 cases, median serum 25(OH)D levels were significantly lower in severe/critical cases (38.2 nmol/L; 15.2 ng/mL) than in mild/moderate cases (56.6 nmol/L; 22.6 ng/mL). Interestingly, ROC curve analysis identified a serum 25(OH)D level of 41.19 nmol/L (16.47 ng/mL) as a potential threshold to predict risk of SARS-CoV-2 infection and disease severity (73).
In our cohort, the percentage of obese patients was significantly higher among non-survivors compared to survivors, further supporting a critical role of obesity as a risk factor for poor prognosis of COVID-19, as it has recently been demonstrated by other groups (6,7). However, obese patients frequently exhibit hypovitaminosis D due to a number of possible reasons, including volumetric dilution of vitamin D in the large fat mass of such individuals (74), blunted catecholamine-induced release of vitamin D3 and 25(OH)D3 from adipose tissue (75), as well as altered activity and expression of vitamin D-metabolizing enzymes in the liver and extrahepatic tissues (75–77). Thus, hypovitaminosis D may be one of the drivers of poor prognosis in obese patients with COVID-19.
Overall, findings from our study further suggest that lower vitamin D levels may partly contribute to worsening the clinical course and prognosis in hospitalized patients with COVID-19. Importantly, preliminary findings from intervention studies (including randomized placebo-controlled trials) conducted among hospitalized patients with COVID-19 suggest that vitamin D supplementation (in different doses and formulations) may promote viral clearance and reduce the severity of the disease (78–80). Cross-sectional clinical studies have shown that lower vitamin D levels are significantly associated with acute respiratory tract infections (81–83). A British cohort study showed that each 10-nmol/L (4-ng/mL) increase in serum 25(OH)D levels was associated with a 7% lower risk of respiratory infection, after adjustment for adiposity, lifestyle, and socioeconomic factors (84). A meta-analysis by Martineau et al. (85) demonstrated that vitamin D supplementation protected against acute respiratory tract infections, and protective properties of vitamin D were stronger in participants with baseline serum 25(OH)D levels of <25 nmol/L (corresponding to <10 ng/mL, indicative of severe vitamin D deficiency) compared to those with baseline levels of ≥25 nmol/L (≥10 ng/mL). Brenner et al. (86) recently assessed the association between serum 25(OH)D levels and mortality from respiratory diseases over a 15-year follow-up period in a cohort of 9548 adults aged 50 to 75 years. Authors found that participants with vitamin D insufficiency and deficiency exhibited strongly increased respiratory mortality compared to those with sufficient vitamin D status (86). Of note, a serum 25(OH)D level of approximately ≥40 ng/mL may provide protection against acute viral respiratory infections, as it has been showed in a prospective cohort study conducted in 198 healthy adults (87). Therefore, attainment and maintenance of target serum 25(OH)D levels of 40 to 60 ng/mL may be critical in order to achieve the immunomodulatory properties of vitamin D in vivo and to effectively prevent acute respiratory tract infections, including SARS-CoV-2 infection (19,54,57). According to our ROC curve analysis, hsCRP (AUC = 0.719), NLR (AUC = 0.718), ferritin (AUC =0.709), and D-dimer (AUC= 0.708) at admission were the best predictive biomarkers for adverse outcomes of COVID-19. NLR is a well-known marker of systemic inflammation (58), which has previously been suggested as a useful prognostic marker for hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease (88). Importantly, NLR has recently been shown to effectively predict mortality in hospitalized patients with COVID-19 (89). According to our ROC curve analysis, IL-6 (AUC = 0.665), PCT (AUC = 0.647), fibrinogen (AUC = 0.634), 25(OH)D (AUC= 0.617), WBC count (AUC =0.614), and TNF-α (AUC = 0.570) may be considered as supportive biomarkers for COVID-19 mortality. The measurement of these analytes may also serve as a panel of supportive biomarkers for worse clinical course of COVID-19. Similarly, other studies showed that TNF-α (90) and PCT (91) upon admission are reliable predictors of disease severity and death in hospitalized patients with COVID-19.
Finally, in our cohort, mean age of the survivor group was significantly lower than that of the non-survivor group (65 ± 13 vs 70 ± 14 years, respectively). Older age has emerged as one of the main risk factors for severe COVID-19 since the start of the pandemic (92). Intriguingly, older adults also represent individuals at high risk for vitamin D deficiency, as skin storage depots of 7-dehydrocholesterol (the precursor of vitamin D) and the human skin capacity to synthesize cholecalciferol upon sunlight exposure both decrease with age (93). In particular, age older than 70 years is an important risk factor for impaired vitamin D status (94). Hence, aging-associated vitamin D deficiency may represent one of the drivers of COVID-19-related mortality in older adults by potentially triggering systemic inflammatory responses and endothelial dysfunction (95).
We acknowledge that our findings must be interpreted with caution due to a series of major limitations of the present study, including the retrospective database design, the small sample size, the lack of information on chronic lung disease, as well as the lack of a healthy control group. Moreover, as a single-center study, we cannot generalize our results to other settings. Conversely, the main strength of the study is the assessment of vitamin D status of participants upon admission. Our results could also be analyzed in future meta-analyses of observational studies assessing the vitamin D status in patients with COVID-19. More detailed data regarding the pharmacological treatments employed for the management of COVID-19 in our patients will be further assessed.
Conclusion
Our study found a high prevalence of hypovitaminosis D in patients admitted to hospital with COVID-19, suggesting a possible role of low vitamin D status in increasing the risk of SARS-CoV-2 infection and subsequent hospitalization. We also found that non-survivors exhibited significantly lower vitamin D levels at admission compared to survivors, as well as higher levels of markers of inflammation, coagulation, and sepsis. Serum 25(OH)D levels were significantly inversely associated with the risk of COVID-19-related in-hospital mortality, independent of age, sex, markers of inflammation, coagulation and sepsis, and major comorbidities. We therefore suggest that a lower vitamin D status upon admission may be a modifiable risk factor and early predictive marker for adverse outcomes and mortality in hospitalized patients with COVID-19. However, larger prospective studies should be conducted to further confirm the existence of a causal relationship between hypovitaminosis D and SARS-CoV-2 infection and COVID-19 severity. Moreover, large multicenter, randomized, double-blind controlled trials are needed to address whether vitamin D supplementation can effectively reduce the risk of SARS-CoV-2 infection, as well as COVID-19-related hospitalization, morbidity, and mortality.
Supplementary Material
Acknowledgments
We acknowledge all medical staff involved in the diagnosis and treatment of patients with COVID-19 at Tor Vergata University Hospital-PTV.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- CI
confidence interval
- AUC
area under the curve
- BMI
body mass index
- COVID-19
coronavirus disease 2019
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- hsCRP
high-sensitivity C-reactive protein
- ICU
intensive care unit
- IFN-γ
interferon-gamma
- IL-1β
interleukin-1 beta
- IL-6
interleukin 6
- NLR
neutrophil-to-lymphocyte count ratio
- OR
odds ratio
- PCT
procalcitonin
- ROC curve
receiver operating characteristic curve
- RT-PCR
reverse transcription polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation Th = T helper
- TNF-α
tumor necrosis factor alpha
- WBC
white blood cell
- WHO
World Health Organization
Authors’ contributions
MI and MM designed the research project, wrote the paper, supervised the project, and equally contributed to the manuscript. AB collected and retrieved clinical data, analyzed results, and contributed to the research project. MP performed statistical analysis. SL and MN performed, collected, and retrieved biochemical data. SB, AF, MIa, MA, and VC supervised the research project and reviewed the manuscript. All authors edited the manuscript. No honorarium, grant, or other forms of payment were received by authors to write this manuscript.
Disclosure statement
No author has potential conflicts of interest to disclose.
References
- 1.Leiva Sisnieguez CE, Espeche WG, Salazar MR.. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55(6):2001148. doi: 10.1183/13993003.01148-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, The Northwell COVID-19 Research Consortium, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattar N, McInnes IB, McMurray JJV.. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rottoli M, Bernante P, Belvedere A, Balsamo F, Garelli S, Giannella M, Cascavilla A, Tedeschi S, Ianniruberto S, Rosselli Del Turco E, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020;183(4):389–97. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamara A, Tahapary DL.. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14(4):655–9. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B.. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–20. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangalmurti N, Hunter CA.. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infante M, Ricordi C, Alejandro R, Caprio M, Fabbri A.. Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Rev Anti Infect Ther. 2020;19(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore JB, June CH.. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 14.Ye Q, Wang B, Mao J.. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaim S, Chong JH, Sankaranarayanan V, Harky A.. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caprio M, Infante M, Calanchini M, Mammi C, Fabbri A.. Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord. 2017;22(1):27–41. doi: 10.1007/s40519-016-0312-6. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri A, Infante M, Ricordi C.. Editorial - Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev Med Pharmacol Sci. 2020;24(7):4048–52. doi: 10.26355/eurrev_202004_20876. [DOI] [PubMed] [Google Scholar]
- 20.Infante M, Ricordi C, Padilla N, Alvarez A, Linetsky E, Lanzoni G, Mattina A, Bertuzzi F, Fabbri A, Baidal D, et al. The role of vitamin D and omega-3 PUFAs in islet transplantation. Nutrients. 2019;11(12):2937. doi: 10.3390/nu11122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charoenngam N, Holick MF.. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, Chavez C, Alvarez A, Baidal D, Alejandro R, et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11(9):2185. doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 24.Gombart AF, Borregaard N, Koeffler HP.. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 25.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–65. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 27.Griffin MD, Xing N, Kumar R.. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Soruri A, Gieseler RK, Peters JH.. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38(6):535–40. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Leung DYM, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E.. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J, Rutgeerts O, Saint-Arnaud R, Bouillon R, Mathieu C, et al. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin Exp Immunol. 2000;120(1):139–46. doi: 10.1046/j.1365-2249.2000.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C.. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217(12):1292–300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Zhou M, Guo Y, Song Z, Liu B.. 1,25-Dihydroxyvitamin D3 promotes high glucose-induced M1 macrophage switching to M2 via the VDR-PPARγ signaling pathway. Biomed Res Int. 2015;2015:157834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V.. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164(9):4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 34.Penna G, Adorini L.. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 35.Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, Maghazachi AA, Belardelli F, Adorini L, Gessani S, et al. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174(1):270–6. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira GB, van Etten E, Verstuyf A, Waer M, Overbergh L, Gysemans C, Mathieu C.. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab Res Rev. 2011;27(8):933–41. doi: 10.1002/dmrr.1275. [DOI] [PubMed] [Google Scholar]
- 37.Saul L, Mair I, Ivens A, Brown P, Samuel K, Campbell JDM, Soong DY, Kamenjarin N, Mellanby RJ.. 1,25-Dihydroxyvitamin D3 restrains CD4+ T cell priming ability of CD11c + dendritic cells by upregulating expression of CD31. Front Immunol. 2019;10:600. doi: 10.3389/fimmu.2019.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LSK, Lammas DA, Raza K, Sansom DM, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C.. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543). Diabetes. 2000;49(8):1301–7. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 40.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A.. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 41.Bouillon R, Lieben L, Mathieu C, Verstuyf A, Carmeliet G.. Vitamin D action: lessons from VDR and Cyp27b1 null mice. Pediatr Endocrinol Rev. 2013;10(Suppl 2):354–66. [PubMed] [Google Scholar]
- 42.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C.. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Prietl B, Treiber G, Pieber TR, Amrein K.. Vitamin D and immune function. Nutrients. 2013;5(7):2502–21. Jul doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC.. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Nakayama Y, Horiuchi H, Ohta T, Komoriya K, Ohmori H, Kamimura T.. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol Immunotoxicol. 2002;24(3):335–47. doi: 10.1081/iph-120014721. [DOI] [PubMed] [Google Scholar]
- 46.White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13(1):21–9. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 47.Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA.. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1):rmv.1909. [DOI] [PubMed] [Google Scholar]
- 48.Monkawa T, Yoshida T, Hayashi M, Saruta T.. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000;58(2):559–68. doi: 10.1046/j.1523-1755.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 49.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC.. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8(3):285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 50.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C.. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2005;21(1):37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE.. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–65. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 53.Martineau AR, Forouhi NG.. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8(9):735–6. doi: 10.1016/S2213-8587(20)30268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP.. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glinsky GV. Tripartite combination of candidate pandemic mitigation agents: Vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8(5):129. doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercola J, Grant WB, Wagner CL.. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12(11):3361. doi: 10.3390/nu12113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebadi M, Montano-Loza AJ.. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;74(6):856–9. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S.. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016:8191254. doi: 10.1155/2016/8191254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M.. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J.. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017;8(6):947–57. doi: 10.3945/an.117.015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 62.Carpagnano GE, Di Lecce V, Quaranta Vn Zito, Buonamico E, Capozza E.. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2020. Aug 9:1–7.doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, Frenkel‐Morgenstern M.. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. Febs J. 2020;287(17):3693–702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J.. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M.. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):13591359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF.. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van Den Abbeele K, Mandal AKJ, Missouris CG.. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2020. Aug 27. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U.. Vitamin d deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA.. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2020. Nov 25. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S.. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, Hadadi A, Montazeri M, Nasiri M, Shirvani A, et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15(9):e0239799. doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020. Oct 27. doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye K, Tang F, Liao X, Shaw BA, Deng M, Huang G, et al. Does serum vitamin D level affect COVID-19 infection and its severity?-A case-control study. J Am Coll Nutr. 2020. Oct 13:1–8.doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- 74.Drincic AT, Armas LA, Van Diest EE, Heaney RP.. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20(7):1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 75.Di Nisio A, De Toni L, Sabovic I, Rocca MS, De Filippis V, Opocher G, Azzena B, Vettor R, Plebani M, Foresta C, et al. Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. J Clin Endocrinol Metab. 2017;102(7):2564–74. doi: 10.1210/jc.2016-3591. [DOI] [PubMed] [Google Scholar]
- 76.Elkhwanky M-S, Kummu O, Piltonen TT, Laru J, Morin-Papunen L, Mutikainen M, Tavi P, Hakkola J.. Obesity represses CYP2R1, the vitamin D 25-hydroxylase, in the liver and extrahepatic tissues. JBMR Plus. 2020;4(11):e10397. doi: 10.1002/jbm4.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roizen JD, Long C, Casella A, O’Lear L, Caplan I, Lai M, Sasson I, Singh R, Makowski AJ, Simmons R, et al. Obesity decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin D. J Bone Miner Res. 2019;34(6):1068–73. doi: 10.1002/jbmr.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM.. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. 2020. Nov 12. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 80.Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, Annweiler C.. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. 2doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–7. [DOI] [PubMed] [Google Scholar]
- 82.Ginde AA, Mansbach JM, Camargo CA.. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E.. On the epidemiology of influenza. Virol J. 2008;5:29. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berry DJ, Hesketh K, Power C, Hyppönen E.. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106(9):1433–40. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 85.Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brenner H, Holleczek B, Schöttker B.. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12(8):2488. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML.. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao C, Liu X, Tang Z.. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. COPD. 2017;12:2285–90. doi: 10.2147/COPD.S141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gan J, Li J, Li S, Yang C.. Leucocyte subsets effectively predict the clinical outcome of patients with COVID-19 pneumonia: a retrospective case-control study. Front Public Health. 2020;8:299. doi: 10.3389/fpubh.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu R, Han C, Pei S, Yin M, Chen X.. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2):106051. doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ.. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(2):505–14. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacLaughlin J, Holick MF.. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R.. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 95.Caprio M, Mammi C, Rosano GM. Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci. 2012;8(1):4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.