Abstract
Food allergies are the result of immune responses that cause adverse reactions to foods. Immune responses to foods may produce a spectrum of symptoms and disorders, including acute allergic reactions and anaphylaxis, food protein-induced allergic proctocolitis, food protein-induced enterocolitis syndrome, food-dependent exercise-induced anaphylaxis, and oral allergy syndrome (pollen-food allergy syndrome). Food allergic responses also contribute to chronic inflammatory disorders such as eosinophilic esophagitis and atopic dermatitis. Although food allergy affects people from infancy through adulthood, there are allergic features that differ according to age (i.e., presentation, triggers, and natural course) that have important implications for diagnosis, prognosis, and management. New food allergies can develop at any age and we propose similarities in the etiology of de novo food allergy whether in infancy or adulthood. The approach to managing food allergy changes dramatically over the life course, and physicians and patients must respond accordingly to optimize care. Food allergy therapies are emerging and the efficacy and safety of these interventions could differ by age group of those treated. In this review, we highlight interesting observations on the etiology and characteristics of food allergy presenting at different ages, and discuss clinical management as it relates to life stage.
Keywords: natural history, peanut allergy, IgE, skin prick test
INTRODUCTION
Food allergy is defined as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”1 The immune pathology distinguishes food allergy from other adverse reactions that can occur from foods—e.g., intolerance, poisoning, and pharmacologic affects.1 One may surmise that characteristics of food allergy differ substantially from infancy through adulthood. Reviews and guidelines have emphasized that food allergies are more common in children than adults, that specific foods such as milk or egg are more common triggers for children and typically resolve, and that specific food allergic disorders are typical in specific age groups (e.g., protein-induced enterocolitis syndrome in infants and young children).1–4 Emerging observations that we review here have questioned some of these tenets, and provide interesting insights into the evolving epidemiology and pathophysiology of food allergy with implications for diagnosis, prevention, and treatment. Additionally, it is clear that managing food allergies differs substantially for individuals at different ages and life stages, for which responsibilities and daily risks evolve through infancy, childhood, adolescence, young adulthood, for pregnant mothers, adults facing occupational exposure, and other situations that affect adults through their lifetime. While data are sparse for many aspects of these topics, we review practical implications with guidance toward managing food allergy through the life course.
EPIDEMIOLOGY: AGE-RELATED INSIGHTS
There are many challenges in estimating food allergy prevalence.1, 4, 5 Self-reported food allergy typically overestimates prevalence compared with estimates based upon a diagnosis determined by allergy testing, particularly when oral food challenges (OFC) are used, which remains the gold standard for food allergy diagnosis. The types of food allergic reactions included in estimates of food allergy prevalence can also affect the estimates; for example, estimates differ whether mild allergic reactions from pollen-food allergy syndrome (PFAS) are included. In a 2015–2016 U.S. household survey with 38,408 responses for children, Gupta et al. reported symptoms clearly consistent with acute, IgE-mediated reactions—excluding probable PFAS—which was used to define convincing cases of food allergy.6 This study also evaluated whether each reported allergy was physician-diagnosed, as well as the specific testing (e.g. confirmatory skin prick tests (SPT), serum food-specific IgE (sIgE), or OFC). The authors reported that the overall prevalence of convincing IgE-mediated food allergy was 7.6% (95% confidence interval [CI]:7.1–8.1%) after excluding the 4% whose parent-reported food allergy did not meet inclusion criteria. Two-thirds of the children with convincing food allergies had a physician diagnosis. The most common food allergens were peanut (2.2%), milk (1.9%), shellfish (1.3%), and tree nuts (1.2%). The rate of food allergy was 2.8% in infants under age 1 year, which peaked to 10% at age 2 years, and was 7.1% in adolescents 14–17 years. Cow’s milk was the most common food allergen in early life, present among approximately 50% of convincingly food-allergic <1 year-olds, 40% of food-allergic 1–2 year-olds and 30% of food allergic 3–5 year-olds. Among children ages 6–10 years, peanut surpassed cow’s milk allergy in prevalence, present among 1 in 3 food-allergic children, compared to 1 in 4 who were convincingly milk allergic. By early adolescence, tree nut and shellfish allergies also exceeded cow’s milk allergy in prevalence, each present in approximately 1 in 5 food-allergic children.
Using a similar approach, Gupta et al. concomitantly surveyed 40,443 adults (age 18 years and older) and found convincing food allergies in 10.8% (95% CI, 10.4–11.1%), with an additional 8.2% reporting reaction symptomatology deemed inconsistent with an IgE-mediated reaction.7 Among all adults, the most common allergies were shellfish (2.9%), milk (1.9%), peanut (1.8%), tree nuts (1.2%) and fin fish (0.9%). Table 1 provides rates of convincing food allergies for selected foods and selected ages from these two studies,6, 7 and also indicates the rate of new-onset food allergy among the adults with the specific allergy. Remarkably, approximately half of US food-allergic adults report developing at least one of their food allergies during adulthood, with shellfish allergy responsible for the largest number of such cases. Although the national survey did not report allergic reactions to alcoholic beverages, these occur and are, by default, observed in teenagers and adults.8
Table 1.
| Food | All Children | All Adults | <1 yr | 2 yr | 6–10 yr | 14–17 yr | 18–29 yr | 40–49 yr | ≥60 yr | Percent of adults with adult-onset allergy |
|---|---|---|---|---|---|---|---|---|---|---|
| Any | 7.6 | 10.8 | 2.8 | 10.0 | 8.0 | 7.1 | 11.3 | 10.0 | 8.8 | 48 |
| Peanut | 2.2 | 1.8 | 0.6 | 2.4 | 2.6 | 2.1 | 2.5 | 1.8 | 0.8 | 17.5 |
| Tree nut | 1.2 | 1.2 | 0.2 | 1.1 | 1.4 | 0.9 | 1.6 | 1.1 | 0.6 | 34.6 |
| Milk | 1.9 | 1.9 | 1.5 | 4.3 | 1.9 | 1.1 | 2.4 | 2.0 | 1.9 | 22.7 |
| Shellfish | 1.3 | 2.9 | 0.2 | 1.1 | 1.5 | 1.5 | 2.8 | 2.5 | 2.6 | 48.2 |
| Fin fish | 0.6 | 0.9 | 0.1 | 0.6 | 0.6 | 0.6 | 1.1 | 0.8 | 0.6 | 39.9 |
| Egg | 0.9 | 0.8 | 0.4 | 1.4 | 0.9 | 0.5 | 1.1 | 0.7 | 0.5 | 29.0 |
| Wheat | 0.5 | 0.8 | 0.4 | 1.0 | 0.5 | 0.4 | 1.0 | 0.8 | 0.6 | 52.6 |
| Soy | 0.5 | 0.6 | 0.4 | 0.9 | 0.5 | 0.2 | 0.7 | 0.6 | 0.4 | 45.4 |
| Sesame | 0.2 | 0.2 | 0.1 | 0.2 | 0.3 | 0.1 | 0.3 | 0.2 | 0.1 | 25.7 |
It is generally thought that some childhood food allergies, such as those to milk and egg, are more likely to resolve than others, such as peanut, tree nuts, fish, and shellfish allergies, which frequently persist into adulthood.9 The 2015–2016 U.S. surveys6, 7 (Table 1) also provide interesting data suggesting that higher-than-anticipated numbers of adults have food allergies to “childhood” allergens, with, in some cases, surprisingly high rates of new-onset allergy to typical “childhood” allergens (e.g., milk 22.7%, egg 29%, wheat 52.6%, and soy 45.4%). It is also notable that in childhood, males predominate and in adulthood females predominate (Figure 1).6, 7 In a chart review of 171 Chicago-area patients with clinically-confirmed food allergies, 15% were adult-onset.10 These studies excluded PFAS, which may be the most common type of adult food allergy.11 Rates are quite different across the globe. In a study of 8 European countries using symptoms and evidence of IgE sensitization to define probable food allergy in general population surveys of adults, the lowest rate of allergy was in Athens (0.3%) and the highest in Zurich (5.6%).12 The European surveys included adults with PFAS. A study of Israeli young adults (ages 17–18) that included OFCs confirmed a prevalence of 0.67%, with tree nuts (0.28%), milk (0.16%), peanut (0.14%) as most common.13
Figure 1.
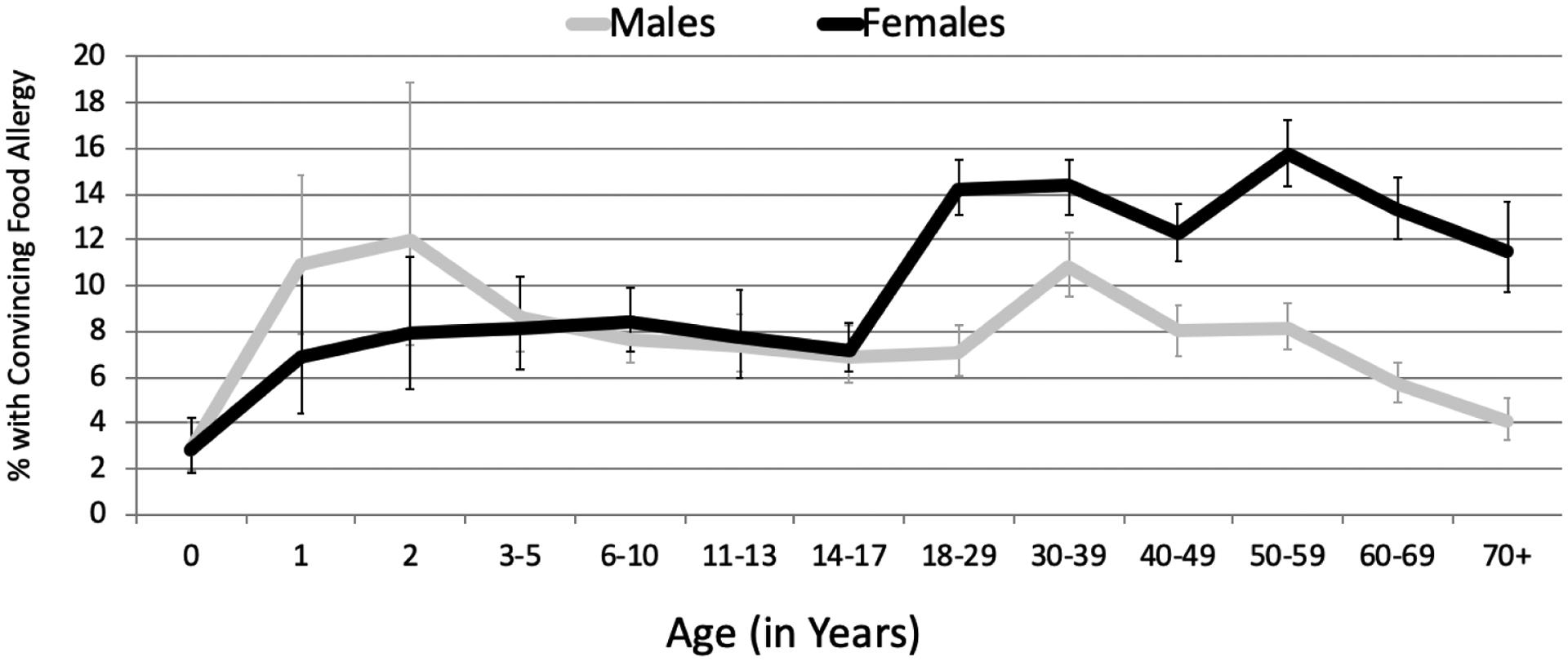
Age- and sex-specific prevalence estimates of convincing IgE-mediated food allergy
These epidemiological findings, especially the high rates in westernized countries, if confirmed by replication/validation studies, reflect an even greater urgency for clinicians to identify primary and secondary prevention approaches, in addition to treatment approaches, and address this new, concerning trend toward greater pediatric allergy persistence, and higher rates of adult-onset (age 18 years and older) allergies.
DOES ETIOLOGY DIFFER BY AGE?
Food allergy is the result of numerous genetic and environmental factors, resulting in a lack or loss of tolerance to certain foods.14–18 Immune alteration and/or digestion/absorption of the foods may influence allergy occurrence, which matches the notion that infants and children are at greater risk than adults for developing food allergies. Mounting evidence for the “dual allergen exposure hypothesis” of Gideon Lack19–22 suggests that non-ingestion exposures via the skin, especially on inflamed skin, with a lack of oral exposure, can result in allergic sensitization. This has led to the encouragement of early ingestion of peanut for infants as a prevention strategy, particularly for infants with atopic dermatitis (AD).23, 24 In a study of twins, Kivisto et al25 found a significantly higher concordance rate for peanut allergy among monozygotic (MZ) twins than among dizygotic (DZ) twins (0.64 vs 0.07), strengthening the evidence of heritability of peanut allergy, and found that AD was a significant risk factor for food allergy, independent of genetic factors, highlighting the potential importance of AD control and prevention among children to reduce the risk of food allergy.
It has also been reported that children with AD and positive IgE antibodies to specific foods have a fairly significant risk of developing acute food-allergic reactions when foods are removed from their diet to treat the AD.26, 27 For example, 1 in 5 patients with food-triggered atopic dermatitis and no previous history of IgE mediated food hypersensitivity reactions developed new immediate reactions to a variety of newly avoided foods (e.g., cow’s milk, peanut, fish, wheat and others), with nearly one-third of such patients experiencing anaphylaxis.27 This circumstance, as well as other examples of food allergies in children following removal of an ostensibly tolerated food,28–30 suggests that atopic individuals may be in a state of natural desensitization that can be lost with allergen avoidance. Clearly, the airway is also a powerful sensitizing route of exposure because PFAS occurs despite ingestion of fruits with the proteins that are homologous to the pollen.31
Do these notions of alteration in gut permeability, skin and lung exposure as a sensitizing route, and loss of desensitization apply to adult-onset food allergy? The evidence suggests the answer is “yes.” The reports that acid suppressors may be a risk factor for adult food allergy,32, 33 and also reports of food-dependent, exercise-induced anaphylaxis (FDEIA) occurring to otherwise tolerated foods (another example of alteration in gut permeability)34 suggest that adults may be prone to gut-level disturbances that may affect food-allergy outcomes. Many of the foods accounting for adult-onset food allergies, such as shellfish or tree nuts, are not eaten regularly and loss of a desensitized state may be an explanation, given periods of no oral exposure. There are also examples of new-onset acute allergic reactions to milk in atopic adults who avoided milk.35 Occupational/airborne and skin exposure may sensitize adults--not just young children--with AD. For example, cases of adult-onset milk,36 and lupin37 allergy have been attributed to occupational skin and respiratory exposure, and adult soy allergy may be triggered by pollen exposure.38 The skin as a sensitizing route for adult food allergy is also demonstrated by alpha-gal syndrome from tick bites39, 40 as well as cases of milk/cheese,41, 42 wheat43, 44, and soy45 allergies in adults who use cosmetics and skin-care products containing these ingredients. Although more studies are needed, it is possible that the etiology of risks for developing food allergies do not change significantly over the life course, although the absolute risks may be different and likely decrease with age.
MANIFESTATIONS AND DISORDERS: FOCUS ON AGE
In the following sections, we discuss the age-relevant features of the following food allergic disorders and in Table 2 summarize the key age-related features and natural course of the disorders.
Table 2.
Characteristics of food allergic disorders emphasizing life course
| Disorder | Age-related Features | Natural Course |
|---|---|---|
| IgE-mediated food allergies/anaphylaxis |
|
|
| Food Protein-induced allergic proctocolitis (FPIAP) |
|
|
| Food protein-induced enterocolitis (FPIES) |
|
|
| Eosinophilic esophagitis (EoE) |
|
|
| Atopic dermatitis (AD) |
|
|
| Pollen food allergy syndrome |
|
|
Acute allergic reactions and anaphylaxis
IgE-mediated acute allergic reactions can vary from mild to severe. Pollen-related food allergy, discussed later, involves sensitization to primarily fruits and vegetables and tends to be mild, while anaphylaxis is more often triggered by foods such as peanut, tree nuts, fish, and shellfish and tends to be severe—sometimes fatal. Fatalities from allergic reactions are rare overall, but appear to be slightly more common among children,46, 47 possibly reflecting certain risk factors in adolescents and young adults such as delaying epinephrine injections.48 Infants less than one year of age seem to have milder symptoms compared to older children with the main symptoms being hives, rash or vomiting and less commonly respiratory or cardiovascular.49 The recent report of two child deaths from OFC underscore the importance of maintaining up-to-date national and international registries on food-associated fatalities.50 Gupta et al estimated the proportion of food-allergic adults7 and children6 in the US who have experienced at least one severe reaction—characterized by reports of specific symptoms across multiple organ systems (e.g., hives, swelling, difficulty swallowing, throat tightening, vomiting, wheezing, dizziness, etc.). A history of at least one “severe” reaction over the lifetime was estimated to be present in 42.3% (95% CI, 39.1–45.4%) of US food-allergic children and 51.1% (95% CI, 49.3–52.9) of US food-allergic adults. Among children and adults, those foods with the highest rates of severe reactions were identical (child rate / adult rate): peanut (59.2%/67.8%), tree nut (56.1%/61.3%), shrimp (51.1%/56.6%) and fish (49.0%/56.5%). All of the major food allergens (milk, egg, wheat, soy, peanut, tree nuts, fish, shellfish, sesame) had severe reaction rates over 27%. In these studies, interestingly, the rate of severe reactions to milk were 25.3% in children versus 39.3% in adults, and for egg severe reactions were 28.1% in children versus 39.4% in adults. In these adults, allergies represented mostly childhood-onset allergies, suggesting persistence of the more severe phenotypes. Also, when asked about healthcare utilization, one in five children reported a visit to the ED for a food allergic reaction in the past year compared to one in ten adults. Overall, there appear to be some differences in severity and anaphylaxis rates across the age spectrum, but the triggers of severe reactions are substantially similar.
Food protein-induced allergic proctocolitis (FPIAP)
FPIAP presents in infants who are generally healthy but have visible specks or streaks of blood mixed with mucous in the stool.1 Food-specific IgE is typically undetectable in infants with FPIAP. The diagnosis is clinical and includes noting improvement in symptoms with dietary exclusion and a lack of systemic symptoms, vomiting, diarrhea, and poor growth. Biopsies of the rectal or colonic mucosa are not typically undertaken for diagnosis but when obtained, there is eosinophilic infiltration.51 FPIAP is considered a disease of infancy that resolves in the first year of life.1, 51, 52 However, eosinophilic colitis (as a specific diagnosis) and colonic eosinophilia (from a variety of triggers or part of systemic illness) are well-described in adults.53 Eosinophilic colitis is grouped among eosinophilic gastrointestinal disorders, with varied symptoms and etiologies; however, some patients present with blood in the stool and association with atopy and food allergy. A Canadian report described 7 adults with FPIAP, including a 70-year-old and a 57-year-old having bloody diarrhea and food “sensitivities” to cow’s milk and other foods. The symptoms were self-limiting. Whether there is any specific relationship between FPIAP of infancy to the food-related subtypes of eosinophilic colitis in adults remains unexplored and there are no long-term outcome studies in infants with FPIAP.
FPIES-Food protein-induced enterocolitis (FPIES)
FPIES is a non-IgE-mediated food allergy that typically presents in infancy, with repetitive protracted vomiting that begins approximately 1 to 4 hours following ingestion of the trigger food (“acute” FPIES reaction).54 Vomiting may be accompanied by lethargy; pallor and diarrhea may follow. Severe reactions can progress to hypothermia, methemoglobinemia, acidemia, and hypotension, all mimicking sepsis. A “chronic” form of FPIES may occur when the offending food is ingested regularly. The triggers are classically milk, soy, oat, and rice, but these triggers vary internationally, with fish being a more common trigger in Italy and Spain.54, 55 This typically infant-onset disorder usually resolves, however the number of affected children appears to be growing. For example, a recent US population-based cross-sectional prevalence survey estimated that 0.51% (95%CI: 0.42–0.62) of the pediatric population aged <18 years had physician-diagnosed FPIES at some point in their lifetime. While it is important to note these “physician-diagnosed” cases were parent-reported and not clinically confirmed by the study epidemiologists, these rates are consistent with cumulative FPIES incidence rates during infancy of 0.34–0.70 reported by single-center birth cohorts in Israel and Spain.56 These pediatric estimates can be contrasted with the 0.22% (95%CI:0.17–0.28) of adults in the aforementioned US population-based survey reporting that they themselves had received a physician-diagnosis of FPIES at some point in their lifetime.57
Reports of FPIES in adults are increasing, and some nuances are apparent compared with the disorder in infants/children. What may be the first case report of adult FPIES was published in 2012 and described a 53-year-old male whose trigger, verified by OFC, was scallops.58 Subsequent chart reviews of 31 adults from Australia59 and 20 adults from Canada60 showed a range of FPIES triggers (mostly shellfish, fish, milk, egg, wheat), adult-onset, with the trigger being previously tolerated, and symptoms similar in timing and pattern to infant FPIES. These adult-case series revealed some distinctions compared with infants and children because those affected have different trigger foods and were predominantly females (infant FPIES predominantly affects males). Interestingly, these sex differences mirror findings in IgE-mediated food allergy, which disproportionately impacts male infants, and female adults.6, 7, 57 Whether the pathophysiology of the adult onset form is distinct from the infantile/early child form remains to be determined and the natural course of FPIES in adults is also unexplored.
Eosinophilic esophagitis (EoE)
EoE is a chronic inflammatory disorder characterized by eosinophilic inflammation of the esophagus resulting in esophageal dysfunction.61 An esophageal biopsy is undertaken when there is a suggestive history of such esophageal dysfunction (dysphagia, food impaction, food refusal, failure to progress with food introduction, heartburn, regurgitation, vomiting, chest pain, odynophagia, abdominal pain, and malnutrition). The diagnosis requires the presence of esophageal eosinophilia (≥15 eosinophils per high-powered microscope field). Food is an important EoE trigger and management can include dietary elimination of allergens, often undertaken empirically. Medical management involves off-label use of corticosteroids, such as swallowing puffs from an asthma inhaler, and the use of proton-pump inhibitors.
Children and adults may have different EoE presenting characteristics.62, 63 Infants and young children may experience reflux symptoms, vomiting, pain, and poor growth; older children, adolescents, and adults describe not only heartburn but also dysphagia with solid/chunky foods, chest pain, and experience food impaction.62, 64–66 Endoscopy and biopsy findings may differ with age, based on increasing fibrosis and stenosis with time.66, 67 In a retrospective review of 426 patients with biopsies fulfilling EoE criteria at the University of Michigan, fewer adults than children were diagnosed with EoE, referred to an allergist, started on swallowed corticosteroids, or had repeated evaluations. Treatments described previously can be effective across age groups, including dietary elimination for adults.68 However, adults are more likely to experience stenosis and require esophageal dilatation. EoE appears to be persistent.62, 67 Ridolo et al. compared risk factors associated with EoE that was refractory to treatment in adults and children.69 In children, risks for refractory disease were female gender and high visual-analog scale scores at follow-up; for adults, risks were longer periods of follow-up, diagnostic delay, use of antibiotics during infancy, and food allergies. Estimates from a 2016 population-based survey70 found that 0.16% (95%CI: 0.12–0.22) of the US pediatric population and 0.18% (95%CI: 0.14–0.23) of US adults reported lifetime physician-diagnosed EoE— corresponding to roughly 550,000 patients. Notably, this cross-sectional survey also found that the highest rates of lifetime physician-diagnosed EoE were reported by male respondents aged 30–39 [0.35% (95%CI: 0.20–0.61)]. Overall, EoE appears to have different presenting features and findings over the life course but much of this may be related to the length of time patients experience chronic inflammation.
Atopic dermatitis (AD)
Experts conclude that about one-third of children with moderate-to-severe AD also have food allergy.71 However, there is more controversy about the degree to which food allergy contributes to chronic AD and whether or to what degree eliminating foods from the diet improves AD in infants vs children vs adults.1, 71, 72 Studies of diets that eliminate specific targeted foods or common food allergens in children suggest that at least a subset of them may improve AD, and this approach can be supported for diets excluding specific foods in select patients, depending on their age.73 If using this approach, clinicians must carefully evaluate patients to prove a relationship between the specific food and AD to avoid unnecessary avoidance diets.72 Although AD in infants and young children can resolve, there is a well-recognized increased risk of sequential progression from AD to other atopic diseases, including food allergy, allergic rhinitis, allergic asthma, and allergic rhinoconjunctivitis, a process referred to as the atopic march.74 In addition, there are concerns that elimination diets to treat AD in infants and children may lead to nutritional deficits, reduced quality of life, and possible anaphylactic reactions to previously tolerated foods.27, 72 Although recent reviews describe clinical evidence from over 24 studies pointing to a causal role of food allergy in at least some infants and children with AD,72, 73 there is little information about adults. A randomized, three-week, double-blind trial of 33 hospitalized adult patients with severe AD used an antigen-free formula or placebo diet and found no significant difference between diet groups of 25 evaluable patients.75 However, in another trial, 37 adults with AD and birch pollen sensitization were fed a diet eliminating birch-related foods followed by double-blind, placebo-controlled OFC, and 17 adults (46%) reacted with increased AD.76 The possible role of food allergy in adult AD is clearly understudied. There are also no studies evaluating the role of food allergies triggering AD over the life course and overall, especially in adults, this remains an under-investigated area.
Pollen-food allergy syndrome (PFAS)/oral allergy syndrome (OAS)
PFAS/OAS occurs in individuals with pollen allergy or those sensitized to pollens.1 Affected individuals typically report oral or throat pruritus when ingesting raw fruits or vegetables that have proteins homologous to the pollen proteins. The trigger food proteins are easily denatured by heat or digestion, hence the typically mild and localized symptoms. Systemic reactions may occur either because allergy to the food is due to stable proteins, a primary food allergy not related to cross-reacting labile proteins, or augmentation factors (large amounts of food, exercise, etc.) that increase reaction severity. The etiology is related to pollen sensitization and therefore the syndrome is not expected to present in infancy or early childhood before pollen exposure. Although PFAS prevalence varies by region according to pollen exposure, prevalence rates overlap between children and adults.77 A review of the literature as of 2018 reported PFAS prevalence from 4.7% to over 20% among children and 13% to 58% among adults.31 Clearly, this is a common allergy that affects a broad age range. In a study of 1360 children in Italy with pollen-related allergic rhinitis, 23.9% reported PFAS/OAS, and a longer duration of allergic rhinitis symptoms was related to developing PFAS/OAS.78 This finding may be a sign that individuals living in areas with more pollen seasons have a higher rate of PFAS, and reflects the higher range of prevalence in adults. Although the descriptions of PFAS/OAS are similar across the life course and the disorder may be persistent, studies have not reported long-term outcomes over the lifespan.
NATURAL COURSE
Various studies suggest that IgE-mediated allergies to milk, egg, wheat, and soy typically resolve in childhood, while allergies to peanut, tree nuts, fish, and shellfish are generally persistent.9, 79–81 There are limited data on the natural course of allergies during adulthood. In a study of infants followed into childhood with persistent (n=28) vs transient (n=30) egg allergy, a decline in specific IgE (rather than increase in egg IgG4 alone) was associated with natural tolerance.82 In another study, a small group of adults (n=13) with wheat allergy was followed over 5 years, and 9 (69.2%) became wheat tolerant, suggesting resolution is possible, but the study was limited by size and possibly patient selection.83 As shown in Table 1, adults allergic to most of the common food allergens had carried that allergy since childhood, indicating the majority of adult food allergies begin in childhood and are persistent. However, recent US survey data suggest that adult-onset food allergy may be more common than previously acknowledged—affecting up to half of food-allergic adults.7 Non-IgE mediated allergies of infancy and childhood—FPIAP and FPIES—usually resolve.1, 54 EoE84 and PFAS appear to be persistent, but their natural history across the lifespan is not well-studied via prospective, longitudinal approaches.
MANAGEMENT ACROSS THE LIFE COURSE
Managing food allergies involves avoiding the allergen and preparing to recognize and treat an allergic reaction or anaphylaxis. The responsibility for managing food allergy changes dramatically over the life course. Table 3 summarizes the challenges to managing food allergies from infancy through adulthood. From birth until adolescence, supervising adults play a critical role in ensuring safety. Infants are entirely dependent on their parents or guardians, or those in day care, but supervising infants may be easier than supervising toddlers who are able to independently grab food and require additional observation, possibly from paid caregivers and school personnel to whom responsibilities are delegated.85–87 Grade-school children require sharing of responsibilities between the child and adults. Depending upon developmental abilities, children can gradually be given responsibilities that are often undertaken with continued supervision from adults, such as informing adults of their allergies and any allergic symptoms, not sharing foods, reading ingredient labels, informing restaurants of their allergy, and carrying their medications.
Table 3.
Challenges to food allergy management change across the lifespan.
| Age group | Responsibility (primary stakeholders) | Primary challenges |
|---|---|---|
| Infant | Parent/guardian, day care |
|
| Toddler | Parent/guardian, day care |
|
| Grade school children | Patient, parent/guardian, school |
|
| High school adolescents | Patient, parent/guardian, school |
|
| College/young adults | Patient |
|
| Adults | Patient |
|
Transition of responsibilities from adults to the allergic child is a process. In separate studies, Simons et al. asked pediatric allergists88 and parents89 about the timing of transferring responsibilities. Allergists indicated that transferring responsibilities to recognize anaphylaxis and appropriately use epinephrine should not occur earlier than ages 9 to 11 years but by 12 to 14 years, adolescents should be able to recognize anaphylaxis symptoms, carry an autoinjector, and self-inject.88 Interestingly, parents expected that this shift toward child self-management would occur at earlier ages: by 6 years for describing anaphylaxis and by 6 to 11 years for using an epinephrine autoinjector.89 It is important to note that these surveys stated that a supervising adult would ultimately be responsible for recognizing and treating anaphylaxis.
The greatest transitions for transferring responsibilities for self-management occur in adolescents and young adults during high school and college. In a study of 190 children and 59 adolescents and their parents, children and parents generally reported congruent expectations for who was primarily responsible for identifying allergic reactions, following dietary restrictions, and explaining their allergy to others.90 However, parental and adolescent perceptions of who is responsible for self-management differed dramatically, with adolescents much more likely to report that they are fully responsible for their allergy management compared to their parents—who were more likely to view responsibilities as shared or fully parental. Increasing independence means potentially taking increasing risks, with a Canadian study finding that peanut-allergic teenagers, and youth living with a single parent had elevated risk of accidental allergen exposure.91 These longitudinal findings are consistent with cross-sectional surveys in Australian and US adolescents with food allergy reporting high rates of not carrying their epinephrine, not informing others of their allergy, and ingesting foods that are potentially risky.92, 93 In another survey of adolescents and young adults,94 less risky behavior was associated with individuals having peanut allergy, those with supportive female friends, overprotective mothers, teachers aware of the food allergy, and individuals with a history of being bullied and having an educational 504 (disability) plan. A different study95 comparing determinants of epinephrine carriage behaviors among US children and adolescents reported that the perceived frequency of epinephrine carriage by family and friends was a significant predictor of actual epinephrine carriage among adolescents aged 13–17 years, but not younger children. Similarly, food allergy-related quality of life was much more influenced by perceived family and social support among food-allergic adolescents compared to their younger counterparts, suggesting that efforts to improve peer support for anaphylaxis management might be particularly beneficial for food-allergic adolescents. Finally, a European study of children ages 8–12 years found that, even when food-allergic children and their parents agree on the perceived severity of the child’s allergy, parents may systematically under-estimate the adverse psychosocial impact of the child’s food allergy, relative to the child’s own perceived impairment.96 Therefore, providers should be attentive to potential differences in perceived disease burden within parent-child dyads and consider how they might mediate and address such differential concerns to maximize food allergy-related quality of life. Food allergy management of college-aged individuals appears particularly poor.97 Dyer et al.98 suggested that colleges can help by increasing awareness of food allergy signs and symptoms, establishing roles and responsibilities for adults and stakeholders, and notifying stakeholders on campus about those students with allergies.
Managing food allergy into adulthood has not been extensively studied, but clearly requires addressing food allergies for employees in the workplace, during pregnancy, and during travel, and often, responsibilities fall to a person who may be responsible for many others. Data from recent US population-based surveys6, 7 indicate that patient-reporting of having a current epinephrine prescription declines with age (Figure 2). While approximately 2 in 3 children/adolescents with physician-confirmed FA reported a current epinephrine auto-injector prescription, this dropped to 1 in 3 among patients aged 50+. By age 60, fewer than 1 in 3 patients with physician-confirmed FA and a history of FA-related ED visits reported a current epinephrine auto-injector prescription. While these data are limited by the fact that epinephrine carriage practices were not assessed, they nonetheless suggest that greater investigation of FA management practices among older adults are warranted, given the relative dearth of data In these populations. The allergist who counsels patients with food allergies over the life span should consider how disease management challenges may vary at different developmental phases and discuss approaches as appropriate. Practical management includes counseling on avoidance (label reading, cross contact, restaurant, and food service, etc), approaches to grade school and college, bullying and emotional aspects of living with food allergy, travel, and recognizing allergic reactions, and emergency management.1 These topics are reviewed in greater detail elsewhere.1, 85, 86, 94, 99–101
Figure 2.
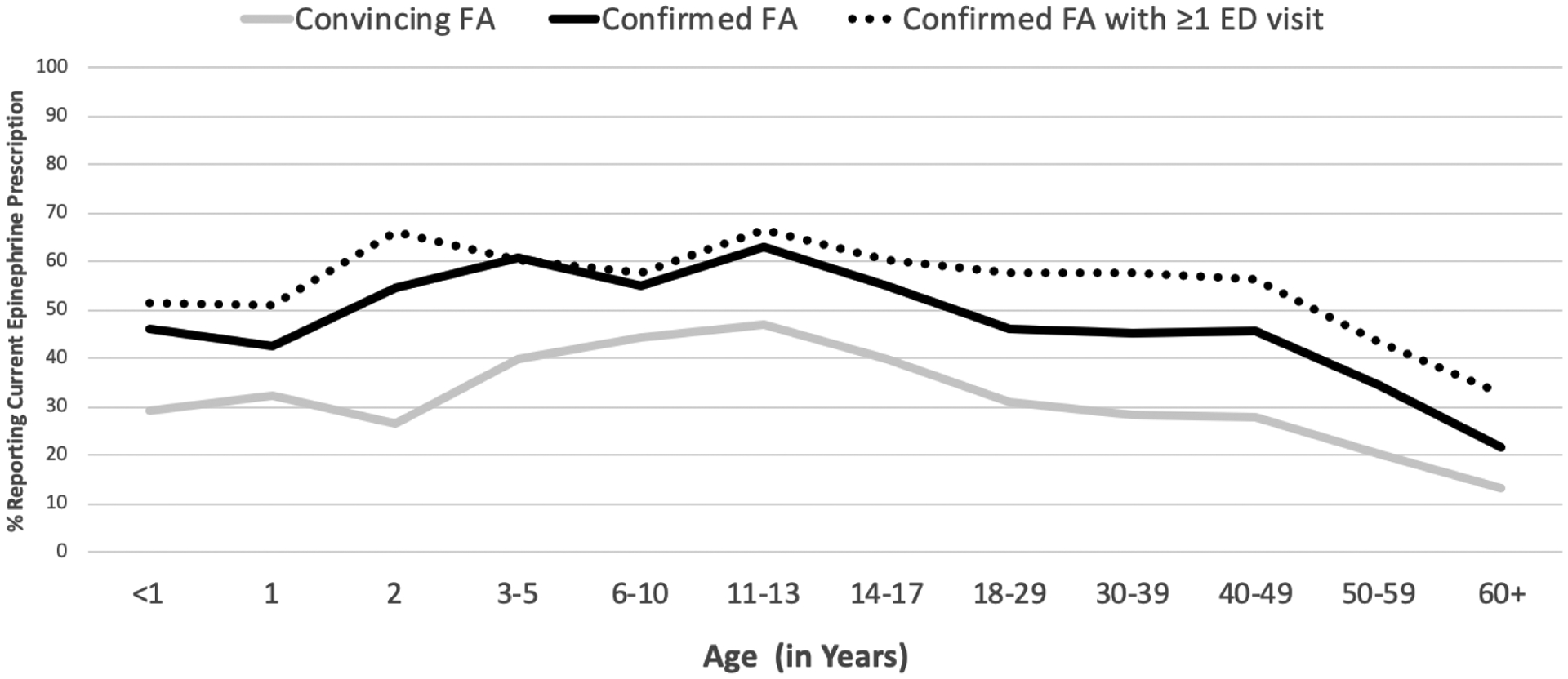
Age-specific report of current epinephrine auto-injector prescription
TREATMENT
There are many emerging therapies for food allergy, and their safety and efficacy are a consideration when considering the age group to which they are applied. Studies already suggest that epicutaneous immunotherapy with a commercial product may be ineffective in older children.102, 103 Oral immunotherapy (OIT) or sublingual immunotherapy (SLIT) may also be more effective, or have longer lasting effects, or induce prolonged remission in very young children compared with patients in other age groups.104–106 In children and young adults (mean age 8.7 years) enrolled in a double-blinded trial, low- and high-dose vital wheat gluten OIT induced desensitization in approximately half of the patients after 1 year of treatment. Two years of low-dose wheat gluten OIT desensitized 30%, and 13% had sustained unresponsiveness.107 In another long-term randomized, blinded trial of 120 adults and children (ages 7–55 years) with peanut allergy over 156 weeks, Chinthrajah et al108 found that peanut OIT could desensitize individuals with peanut allergy to 4000 mg peanut protein but discontinuing or even reducing to 300 mg daily, could increase the likelihood of regaining clinical reactivity to peanut. No differences in clinical responses in adult vs children were seen. Changing how providers inform patients about non-life-threatening symptoms is one promising avenue for improving treatment. Howe et al109 surveyed 50 families with allergy-challenged adolescents and found that compared with those families informed that symptoms are side effects, those informed that symptoms can signal desensitization were less anxious, less likely to contact staff about symptoms, experienced fewer non-life-threatening symptoms as doses increased, and less likely to skip/reduce doses.
Further, in a randomized trial with omalizumab to test continued versus discontinued dosing in multi-food allergic children and young adults (5–22 years), Andorf et al110 found sustained sensitization after omalizumab-facilitated multi-OIT was best achieved by maintaining OIT dosing of either 300 mg or 1 g of each food allergen instead of discontinuing multi-OIT: similar efficacy and safety was shown in all ages. OIT and SLIT have both shown promise in treating peanut and milk allergy, across different ages. In all ages with common food allergies, a combination of SLIT and OIT may induce a significant increase in challenge thresholds with fewer adverse events.111
Current immunotherapy studies in infants, children, and adults are encouraging and the lengthy treatment period and relatively high rates of adverse reactions are being addressed through the use of adjunctive therapy, such as anti-IgE antibodies, Chinese herbal therapy, and probiotics.112–115 With our increased understanding of the molecular mechanisms involved in food allergies and other atopic and immune diseases, we have made much progress in identifying and developing other biologics. Besides anti-IL4Rα and anti-IL-33, which is currently being evaluated for food allergies,116 other biologics that alter immune response have been developed and are in varying stages of preclinical and clinical development or have been approved for specific diseases. In both children and adults, there are common mechanisms underlying atopic diseases and asthma and an understanding of the mechanisms underlying one disease can assist with our understanding and treatments of other immune diseases. Biomarkers for diagnosis and prognosis may soon assist us with identifying those patients at different ages best positioned to benefit from immunotherapy.112 Our analysis of a large standard food challenge dataset in adults found that readily obtainable biomarker values and patient demographics may help predict OFC outcomes.117 Recent advances in high-throughput technologies such as mass cytometry (CyTOF) and next-gen sequencing (NGS) along with concomitant advances in data analytics have enabled us to monitor single cells, increasing the research focus on upstream cellular factors involved in the efficacy of immunotherapy for food allergies, particularly the role of T cells. As our appreciation of different T cell subsets (memory and naïve which differ in infants, children, and adults) and their plasticity increases, the initial simplistic view that restoring Th1/Th2 balance by decreasing Th2 or increasing Th1 responses can ameliorate food allergy is being enhanced by a more complex model involving other T cell subsets, particularly Treg.118
Future studies must aim to further unravel the mechanisms related to the initiation or resolution of food allergy over the life course and apply this knowledge to identify better methods of prevention and treatment, which likely need to be adjusted from infancy through adulthood.
SUMMARY
There are many manifestations of food allergy, and for some disorders, the presentation and course are unique to the patient’s age. It is remarkable that some aspects of food allergy such as common triggers of severe reactions (peanut, tree nuts, shellfish) or mild reactions (fruits and vegetables related to pollen sensitization) are similar over the lifespan, and the etiology of new-onset food allergy may also be similar over the lifespan. Clearly, management strategies must change with age to address different potential obstacles. As new therapeutics emerge, it will be important to consider their potential impact at different ages.
Supplementary Material
Abbreviations:
- AD
atopic dermatitis
- CI
confidence interval
- EoE
eosinophilic esophagitis
- FDEIA
food-dependent, exercise-induced anaphylaxis
- FPIAP
Food protein-induced allergic proctocolitis
- FPIES
Food protein-induced enterocolitis
- OAS
oral allergy syndrome
- OFC
oral food challenge
- PFAS
pollen-food allergy syndrome
- SPT
skin prick test
Footnotes
Conflicts/Declarations
S. H. Sicherer reports royalty payments from UpToDate and from Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, from Food Allergy Research and Education, and from HAL Allergy; and personal fees from the American Academy of Allergy, Asthma and Immunology as Deputy Editor of the Journal of Allergy and Clinical Immunology: In Practice, outside of the submitted work.
C. M. Warren reports grant funding from the National Institute of Environmental Health Sciences, the National Institute of Allergy and Infectious Diseases, and the Sunshine Charitable Foundation.
Christopher Dant has no conflicts of interest and nothing to declare
R. S. Gupta reports research support from the National Institutes of Health (NIH), Stanford Sean N. Parker Center for Food Allergy & Asthma Research, UnitedHealth Group, National Confectioners Association (NCA), ThermoFisher Scientific, Genentech, and Food Allergy Research and Education (FARE); and serves as a consultant/advisor for FARE, Aimmune Therapeutics, BEFOREBrands, Allergenis, Kaleo Inc., and DBV Technologies
K. C. Nadeau reports royalty payments from patents from Stanford University; grants from the National Institute of Allergy and Infectious Diseases, from Food Allergy Research and Education; co-founder of Alladapt, IgGenix, Latitude, and BeforeBrands, and she is on the Board of Scientific Counselors of the National Institutes of Health.
References
- 1.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol 2010; 126:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–19. [DOI] [PubMed] [Google Scholar]
- 3.Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014; 134:1016–25. [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences E, and Medicine. Finding a path to safety in food allergy: Assessment of global burden, causes, prevention, management, and public policy. Washington, DC, 2016. [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018; 141:41–58. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018; 142 (6). pii: e20181235. doi: 10.1542/peds.2018-1235. Epub 2018 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open 2019; 2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pita JS, Sousa N, Bartolome B, Loureiro C, Bom AT. Beer: an uncommon cause of anaphylaxis. BMJ Case Rep 2019; 12(1). pii: e227723. doi: 10.1136/bcr-2018-227723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage J, Sicherer S, Wood R. The Natural History of Food Allergy. J Allergy Clin Immunol Pract 2016; 4:196–203. [DOI] [PubMed] [Google Scholar]
- 10.Kamdar TA, Peterson S, Lau CH, Saltoun CA, Gupta RS, Bryce PJ. Prevalence and characteristics of adult-onset food allergy. J Allergy Clin Immunol Pract 2015; 3:114–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SC, Kim SR, Park KH, Lee JH, Park JW. Clinical Features and Culprit Food Allergens of Korean Adult Food Allergy Patients: A Cross-Sectional Single-Institute Study. Allergy Asthma Immunol Res 2019; 11:723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons SA, Burney PGJ, Ballmer-Weber BK, Fernandez-Rivas M, Barreales L, Clausen M, et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. The Journal of Allergy and Clinical Immunology: In Practice 2019; 7:1920–8. [DOI] [PubMed] [Google Scholar]
- 13.Nachshon L, Schwartz N, Elizur A, Schon Y, Cheryomukhin M, Katz Y, et al. The Prevalence of Food Allergy in Young Israeli Adults. J Allergy Clin Immunol Pract 2019; 7:2782–9. [DOI] [PubMed] [Google Scholar]
- 14.Berin MC. Mechanisms that define transient versus persistent food allergy. J Allergy Clin Immunol 2019; 143:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson HA, O’Mahony L, Burks AW, Plaut M, Lack G, Akdis CA. Mechanisms of food allergy. J Allergy Clin Immunol 2018; 141:11–9. [DOI] [PubMed] [Google Scholar]
- 16.Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018; 73:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol 2016; 137:984–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhondalay GK, Rael E, Acharya S, Zhang W, Sampath V, Galli SJ, et al. Food allergy and omics. J Allergy Clin Immunol 2018; 141:20–9. [DOI] [PubMed] [Google Scholar]
- 19.Lack G Epidemiological risks for food allergy. The Journal of Allergy and Clinical Immunology 2008; 121(6):1331–6. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Fox AT, Sasieni P, Du TG, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009; 123:417–23. [DOI] [PubMed] [Google Scholar]
- 21.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015; 135:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher HR, Keet CA, Lack G, du Toit G. Preventing Peanut Allergy: Where Are We Now? J Allergy Clin Immunol Pract 2019; 7:367–73. [DOI] [PubMed] [Google Scholar]
- 23.Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR Jr., Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 2017; 139:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greer FR, Sicherer SH, Burks AW, Committee On N, Section On A, Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019; 143; 143(4). pii: e20190281. doi: 10.1542/peds.2019-0281. [DOI] [PubMed] [Google Scholar]
- 25.Kivisto JE, Clarke A, Dery A, De Schryver S, Shand G, Huhtala H, et al. Genetic and environmental susceptibility to food allergy in a registry of twins. J Allergy Clin Immunol Pract 2019; 7:2916–8. [DOI] [PubMed] [Google Scholar]
- 26.Flinterman AE, Knulst AC, Meijer Y, Bruijnzeel-Koomen CA, Pasmans SG. Acute allergic reactions in children with AEDS after prolonged cow’s milk elimination diets. Allergy 2006; 61:370–4. [DOI] [PubMed] [Google Scholar]
- 27.Chang A, Robison R, Cai M, Singh AM. Natural History of Food-Triggered Atopic Dermatitis and Development of Immediate Reactions in Children. J Allergy Clin Immunol Pract 2016; 4:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larramendi CH, Martin EM, Pascual MC, Fiandor A, Diaz Pena JM. Possible consequences of elimination diets in asymptomatic immediate hypersensitivity to fish. Allergy 1992; 47:490–4. [DOI] [PubMed] [Google Scholar]
- 29.Barbi E, Gerarduzzi T, Longo G, Ventura A. Fatal allergy as a possible consequence of long-term elimination diet. Allergy 2004; 59:668–9. [DOI] [PubMed] [Google Scholar]
- 30.Ho H-E, Chehade M. Development of IgE-mediated immediate hypersensitivity to a previously tolerated food following its avoidance for eosinophilic gastrointestinal diseases. J Allergy Clin Immunol Pract 2018; 6:649–50. [DOI] [PubMed] [Google Scholar]
- 31.Carlson G, Coop C. Review Article: Pollen food allergy syndrome (PFAS): A review of current available literature. Ann Allergy Asthma Immunol 2019; 123(4):359–365. doi: 10.1016/j.anai.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J.Allergy Clin.Immunol 2007; 119:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. The Journal of Allergy and Clinical Immunology 2008; 121:1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldweg AM. Food-Dependent, Exercise-Induced Anaphylaxis: Diagnosis and Management in the Outpatient Setting. J Allergy Clin Immunol Pract 2017; 5:283–8. [DOI] [PubMed] [Google Scholar]
- 35.Nachshon L, Goldberg MR, Elizur A, Appel MY, Levy MB, Katz Y. Food allergy to previously tolerated foods: Course and patient characteristics. Ann Allergy Asthma Immunol 2018; 121:77–81. [DOI] [PubMed] [Google Scholar]
- 36.Quirantes Sierra B, Lara Jimenez A, Skodova M. Sensitization to cow’s milk protein in a dairy worker. Occup Med (Lond) 2017; 67:579–80. [DOI] [PubMed] [Google Scholar]
- 37.Crespo JF, Rodriguez J, Vives R, James JM, Reano M, Daroca P, et al. Occupational IgE-mediated allergy after exposure to lupine seed flour. J Allergy Clin Immunol 2001; 108:295–7. [DOI] [PubMed] [Google Scholar]
- 38.Minami T, Fukutomi Y, Saito A, Sekiya K, Tsuburai T, Taniguchi M, et al. Frequent episodes of adult soybean allergy during and following the pollen season. J Allergy Clin Immunol Pract 2015; 3:441–2. [DOI] [PubMed] [Google Scholar]
- 39.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol 2015; 135:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, et al. Investigation into the alpha-Gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract 2019:2348–2358.e4. doi: 10.1016/j.jaip.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bito T, Kanda E, Tanaka M, Fukunaga A, Horikawa T, Nishigori C. Cows milk-dependent exercise-induced anaphylaxis under the condition of a premenstrual or ovulatory phase following skin sensitization. Allergol Int 2008; 57:437–9. [DOI] [PubMed] [Google Scholar]
- 42.Voskamp AL, Zubrinich CM, Abramovitch JB, Rolland JM, O’Hehir RE. Goat’s cheese anaphylaxis after cutaneous sensitization by moisturizer that contained goat’s milk. J Allergy Clin Immunol Pract 2014; 2:629–30. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi T, Ito T, Kawakami H, Fuzishiro K, Hirano H, Okubo Y, et al. Eighteen cases of wheat allergy and wheat-dependent exercise-induced urticaria/anaphylaxis sensitized by hydrolyzed wheat protein in soap. Int J Dermatol 2015; 54:e302–5. [DOI] [PubMed] [Google Scholar]
- 44.Fukutomi Y, Taniguchi M, Nakamura H, Akiyama K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy 2014; 69:1405–11. [DOI] [PubMed] [Google Scholar]
- 45.Yagami A, Suzuki K, Nakamura M, Sano A, Iwata Y, Kobayashi T, et al. Case of anaphylactic reaction to soy following percutaneous sensitization by soy-based ingredients in cosmetic products. J Dermatol 2015; 42:917–8. [DOI] [PubMed] [Google Scholar]
- 46.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy 2013; 43:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J Allergy Clin Immunol Pract 2017; 5:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006 1. J Allergy Clin Immunol 2007; 119:1016–8. [DOI] [PubMed] [Google Scholar]
- 49.Samady W, Trainor J, Smith B, Gupta R. Food-induced anaphylaxis in infants and children. Ann Allergy Asthma Immunol 2018; 121:360–5. [DOI] [PubMed] [Google Scholar]
- 50.Upton J, Alvaro M, Nadeau K. A perspective on the pediatric death from oral food challenge reported from the Allergy Vigilance Network. Allergy 2019; 74:1035–6. [DOI] [PubMed] [Google Scholar]
- 51.Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr.Gastroenterol.Nutr 2005; 41:16–22. [DOI] [PubMed] [Google Scholar]
- 52.Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 2006; 117:e760–e8. [DOI] [PubMed] [Google Scholar]
- 53.Impellizzeri G, Marasco G, Eusebi LH, Salfi N, Bazzoli F, Zagari RM. Eosinophilic colitis: A clinical review. Dig Liver Dis 2019; 51:769–73. [DOI] [PubMed] [Google Scholar]
- 54.Nowak-Wegrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2017; 139:1111–26 e4. [DOI] [PubMed] [Google Scholar]
- 55.Miceli Sopo S, Monaco S, Badina L, Barni S, Longo G, Novembre E, et al. Food protein-induced enterocolitis syndrome caused by fish and/or shellfish in Italy. Pediatr Allergy Immunol 2015; 26:731–6. [DOI] [PubMed] [Google Scholar]
- 56.Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: a large-scale, prospective population-based study. The Journal of Allergy and Clinical Immunology 2011; 127:647–53. [DOI] [PubMed] [Google Scholar]
- 57.Nowak-Wegrzyn A, Warren CM, Brown-Whitehorn T, Cianferoni A, Schultz-Matney F, Gupta RS. Food protein-induced enterocolitis syndrome in the US population-based study. J Allergy Clin Immunol 2019; 144:1128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes BN, Boyle RJ, Gore C, Simpson A, Custovic A. Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol 2012; 130:1199–200. [DOI] [PubMed] [Google Scholar]
- 59.Tan JA, Smith WB. Non-IgE-mediated gastrointestinal food hypersensitivity syndrome in adults. J Allergy Clin Immunol Pract 2014; 2:355–7. [DOI] [PubMed] [Google Scholar]
- 60.Du YJ, Nowak-Wegrzyn A, Vadas P. FPIES in adults. Ann Allergy Asthma Immunol 2018; 121:736–8. [DOI] [PubMed] [Google Scholar]
- 61.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018; 155:1022–33 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markowitz JE, Clayton SB. Eosinophilic Esophagitis in Children and Adults. Gastrointest Endosc Clin N Am 2018; 28:59–75. [DOI] [PubMed] [Google Scholar]
- 63.Rassbach W, Rubenstein JH, Elkins M, DeMatos V, Greenson JK, Greenhawt M. Age-based differences in the diagnosis and management of esophageal eosinophilia. J Allergy Clin Immunol Pract 2015; 3:81–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N.Engl.J Med 2004; 351:940–1. [DOI] [PubMed] [Google Scholar]
- 65.DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa’ad AH, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. The Journal of Allergy and Clinical Immunology 2010; 126:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79:577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, Smout A, Bredenoord AJ. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol 2018; 113:836–44. [DOI] [PubMed] [Google Scholar]
- 68.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12:1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ridolo E, Martignago I, Pellicelli I, Incorvaia C. Assessing the Risk Factors for Refractory Eosinophilic Esophagitis in Children and Adults. Gastroenterol Res Pract 2019; 2019:1654543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cianferoni A, Warren CM, Brown-Whitehorn T, Schultz-Matney F, Nowak-Wegrzyn A, Gupta RS. Eosinophilic esophagitis and allergic comorbidities in a US population-based study. Allergy Allergy. 2019. December 17. doi: 10.1111/all.14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergmann MM, Caubet JC, Boguniewicz M, Eigenmann PA. Evaluation of Food Allergy in Patients with Atopic Dermatitis. Journal of Allergy and Clinical Immunology: In Practice 2013; 1:22–8. [DOI] [PubMed] [Google Scholar]
- 72.Eigenmann PA, Beyer K, Lack G, Muraro A, Ong PY, Sicherer SH, et al. Are avoidance diets still warranted in children with atopic dermatitis? Pediatr Allergy Immunol. 2020. January;31(1):19–26. doi: 10.1111/pai.13104. [DOI] [PubMed] [Google Scholar]
- 73.Lim NR, Lohman ME, Lio PA. The Role of Elimination Diets in Atopic Dermatitis-A Comprehensive Review. Pediatr Dermatol 2017; 34:516–27. [DOI] [PubMed] [Google Scholar]
- 74.Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J Allergy Clin Immunol 2019; 143:894–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munkvad M, Danielsen L, Hoj L, Povlsen CO, Secher L, Svejgaard E, et al. Antigen-free diet in adult patients with atopic dermatitis. Acta Dermatologica Venereologica 1984; 64:524–8. [PubMed] [Google Scholar]
- 76.Reekers R, Busche M, Wittmann M, Kapp A, Werfel T. Birch pollen-related foods trigger atopic dermatitis in patients with specific cutaneous T-cell responses to birch pollen antigens. J Allergy Clin Immunol 1999; 104:466–72. [DOI] [PubMed] [Google Scholar]
- 77.Mastrorilli C, Cardinale F, Giannetti A, Caffarelli C. Pollen-Food Allergy Syndrome: A not so Rare Disease in Childhood. Medicina (Kaunas) 2019; 55(10). pii: E641. doi: 10.3390/medicina55100641.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dondi A, Tripodi S, Panetta V, Asero R, Businco AD, Bianchi A, et al. Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol 2013; 24:742–51. [DOI] [PubMed] [Google Scholar]
- 79.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol 2014; 133:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol 2014; 133:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sicherer SH, Wood RA, Perry TT, Jones SM, Leung DYM, Henning AK, et al. Clinical factors associated with peanut allergy in a high-risk infant cohort. Allergy 2019; 74:2199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andorf S, Bunning B, Tupa D, Cao S, Long AJ, Borres MP, et al. Trends in egg specific immunoglobulin levels during natural tolerance and oral immunotherapy. Allergy 2019. doi: 10.1111/all.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scibilia J, Rossi Carlo M, Losappio Laura M, Mirone C, Farioli L, Pravettoni V, et al. Favorable Prognosis of Wheat Allergy in Adults. J Investig Allergol Clin Immunol 2019; 29:118–23. [DOI] [PubMed] [Google Scholar]
- 84.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J.Allergy Clin.Immunol 2007; 119:731–8. [DOI] [PubMed] [Google Scholar]
- 85.Venter C, Sicherer SH, Greenhawt M. Management of Peanut Allergy. J Allergy Clin Immunol Pract 2019; 7:345–55. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Bingemann T, Russell AF, Young MC, Sicherer SH. The Allergist’s Role in Anaphylaxis and Food Allergy Management in the School and Childcare Setting. J Allergy Clin Immunol Pract 2018; 6:427–35. [DOI] [PubMed] [Google Scholar]
- 87.Greiwe JC, Pazheri F, Schroer B. Nannies’ knowledge, attitude, and management of food allergies of children: an online survey. J Allergy Clin Immunol Pract 2015; 3:63–7. [DOI] [PubMed] [Google Scholar]
- 88.Simons E, Sicherer SH, Simons FE. Timing the transfer of responsibilities for anaphylaxis recognition and use of an epinephrine auto-injector from adults to children and teenagers: pediatric allergists’ perspective. Ann.Allergy Asthma Immunol 2012; 108:321–5. [DOI] [PubMed] [Google Scholar]
- 89.Simons E, Sicherer SH, Weiss C, Simons FE. Caregivers’ perspectives on timing the transfer of responsibilities for anaphylaxis recognition and treatment from adults to children and teenagers. J Allergy Clin Immunol Pract 2013; 1:309–11. [DOI] [PubMed] [Google Scholar]
- 90.Annunziato RA, Rubes M, Ambrose M, Caso N, Dillon M, Sicherer SH, et al. Allocation of food allergy responsibilities and its correlates for children and adolescents. J Health Psychol 2015; 20:693–701. [DOI] [PubMed] [Google Scholar]
- 91.Cherkaoui S, Ben-Shoshan M, Alizadehfar R, Asai Y, Chan E, Cheuk S, et al. Accidental exposures to peanut in a large cohort of Canadian children with peanut allergy. Clin Transl Allergy 2015; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sampson MA, Munoz-Furlong A, Sicherer SH. Risk-taking and coping strategies of adolescents and young adults with food allergy. The Journal of Allergy and Clinical Immunology 2006; 117:1440–5. [DOI] [PubMed] [Google Scholar]
- 93.Robinson M, Koplin JJ, Field MJ, Sasaki M, Peters RL, McWilliam V, et al. Patterns of Carriage of Prescribed Adrenaline Autoinjectors in 10-to 14-Year-Old Food-Allergic Students: A Population-Based Study. The Journal of Allergy and Clinical Immunology: In Practice 2019; 7:437–43. [DOI] [PubMed] [Google Scholar]
- 94.Warren CM, Dyer AA, Otto AK, Smith BM, Kauke K, Dinakar C, et al. Food Allergy-Related Risk-Taking and Management Behaviors Among Adolescents and Young Adults. J Allergy Clin Immunol Pract 2017; 5:381–90 e13. [DOI] [PubMed] [Google Scholar]
- 95.Warren CM, Zaslavsky JM, Kan K, Spergel JM, Gupta RS. Epinephrine auto-injector carriage and use practices among US children, adolescents, and adults. Ann Allergy Asthma Immunol 2018; 121:479–91. [DOI] [PubMed] [Google Scholar]
- 96.van der Velde JL, Flokstra-de Blok BM, Dunngalvin A, Hourihane JO, Duiverman EJ, Dubois AE. Parents report better health-related quality of life for their food-allergic children than children themselves. Clin Exp Allergy 2011; 41:1431–9. [DOI] [PubMed] [Google Scholar]
- 97.Greenhawt MJ, Singer AM, Baptist AP. Food allergy and food allergy attitudes among college students. The Journal of Allergy and Clinical Immunology 2009; 124:323–7. [DOI] [PubMed] [Google Scholar]
- 98.Dyer AA, O’Keefe A, Kanaley MK, Kao LM, Gupta RS. Leaving the nest: Improving food allergy management on college campuses. Ann Allergy Asthma Immunol 2018; 121:82–9 e5. [DOI] [PubMed] [Google Scholar]
- 99.Herbert L, Shemesh E, Bender B. Clinical Management of Psychosocial Concerns Related to Food Allergy. J Allergy Clin Immunol Pract 2016; 4:205–13. [DOI] [PubMed] [Google Scholar]
- 100.Marchisotto MJ, Harada L, Kamdar O, Smith BM, Waserman S, Sicherer S, et al. Food Allergen Labeling and Purchasing Habits in the United States and Canada. J Allergy Clin Immunol Pract 2017; 5:345–51. [DOI] [PubMed] [Google Scholar]
- 101.Sicherer SH, Allen K, Lack G, Taylor SL, Donovan SM, Oria M. Critical Issues in Food Allergy: A National Academies Consensus Report. Pediatrics 2017; 140(2). pii: e20170194. doi: 10.1542/peds.2017-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 2017; 139:1242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, et al. Effect of Varying Doses of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Exposure Among Patients With Peanut Sensitivity: A Randomized Clinical Trial. JAMA 2017; 318:1798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol 2017; 139:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Investigators PGoC, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 2018; 379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 106.Kim EH, Yang L, Ye P, Guo R, Li Q, Kulis MD, et al. Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2019; 144(5):1320–1326.e1. doi: 10.1016/j.jaci.2019.07.030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nowak-Wegrzyn A, Wood RA, Nadeau KC, Pongracic JA, Henning AK, Lindblad RW, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol 2019; 143:651–61. [DOI] [PubMed] [Google Scholar]
- 108.Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019; 394:1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Howe LC, Leibowitz KA, Perry MA, Bitler JM, Block W, Kaptchuk TJ, et al. Changing Patient Mindsets about Non-Life-Threatening Symptoms During Oral Immunotherapy: A Randomized Clinical Trial. J Allergy Clin Immunol Pract 2019; 7:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andorf S, Purington N, Kumar D, Long A, O’Laughlin KL, Sicherer S, et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine 2019; 7:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W, Sindher SB, Sampath V, Nadeau K. Comparison of sublingual immunotherapy and oral immunotherapy in peanut allergy. Allergo J Int 2018; 27:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sampath V, Sindher SB, Zhang W, Nadeau KC. New treatment directions in food allergy. Ann Allergy Asthma Immunol 2018; 120:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burks AW, Sampson HA, Plaut M, Lack G, Akdis CA. Treatment for food allergy. J Allergy Clin Immunol 2018; 141:1–9. [DOI] [PubMed] [Google Scholar]
- 114.Virkud YV, Wang J, Shreffler WG. Enhancing the Safety and Efficacy of Food Allergy Immunotherapy: a Review of Adjunctive Therapies. Clin Rev Allergy Immunol 2018; 55:172–89. [DOI] [PubMed] [Google Scholar]
- 115.Vickery BP, Ebisawa M, Shreffler WG, Wood RA. Current and Future Treatment of Peanut Allergy. J Allergy Clin Immunol Pract 2019; 7:357–65. [DOI] [PubMed] [Google Scholar]
- 116.Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019; 4(22). pii: 131347. doi: 10.1172/jci.insight.131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sindher S, Long AJ, Purington N, Chollet M, Slatkin S, Andorf S, et al. Analysis of a Large Standardized Food Challenge Data Set to Determine Predictors of Positive Outcome Across Multiple Allergens. Front Immunol 2018; 9:2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sampath V, Nadeau KC. Newly identified T cell subsets in mechanistic studies of food immunotherapy. J Clin Invest 2019; 129:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


