Abstract
We sought to determine the associations among cerebral amyloid angiopathy (CAA), white matter rarefaction (WMR), circle of Willis atherosclerosis (CWA), and total microinfarct number with Braak neurofibrillary stage in postmortem individuals with and without Alzheimer disease (AD). Data from 355 cases of autopsied individuals with Braak stage I–VI who had antemortem consensus diagnoses of cognitively unimpaired (n = 183), amnestic mild cognitive impairment (n = 31), and AD dementia (n = 141) were used. The association between Braak stage and vascular lesions were individually assessed using multivariable linear regression that adjusted for age at death, APOE ε4 carrier status, sex, education, and neuritic plaque density. CAA (p = 0.007) and WMR (p < 0.001) were associated with Braak stage, independent of amyloid load; microinfarct number and CWA showed no association. Analyses of the interactions between APOE ε4 carrier status and vascular lesions found that greater WMR and positive ε4 carrier status were associated with higher Braak stages. These results suggest that CAA and WMR are statistically linked to the severity of AD-related NFT pathology. The statistical link between WMR and NFT load may be strengthened by the presence of APOE ε4 carrier status. An additional finding was that Lewy body pathology was most prevalent in higher Braak stages.
Keywords: Braak stage, Cerebral amyloid angiopathy, Mild cognitive impairment, Neurofibrillary tangles, White matter rarefaction
INTRODUCTION
Alzheimer disease (AD), the most common form of dementia, is the sixth leading cause of death in the United States, affecting over 5 million Americans and projected to cost the nation $305 billion in 2020 (1). It is characterized by the neuropathological hallmarks of amyloid plaques and neurofibrillary tangles (NFT) (2–4). The amyloid cascade hypothesis, which posits that the deposition of amyloid plaques leads to downstream NFT deposition and eventual neurodegeneration, has been one of the prevailing paradigms that has guided research and treatment efforts since the 1990s (5). However, ongoing research into pathways of AD pathogenesis has suggested alternatives that may be independent of amyloidosis.
In addition to amyloid plaques and NFTs, vascular lesions are found commonly in AD (6, 7). Cerebral amyloid angiopathy (CAA) has been shown to be a highly prevalent vascular lesion in AD (8–10) as has white matter rarefaction (WMR) (11–13). Clinical evidence has shown that white matter atrophy is associated with increased pulse pressure, which may be the result of age-related cerebrovascular stiffening (14). Recent evidence has also suggested that use of blood pressure-lowering medications was associated with a decreased risk of incident dementia or cognitive impairment (15). In addition, vascular pathologies, such as cortical microinfarcts and circle of Willis atherosclerosis (CWA), have been associated with cognitive decline and AD dementia (16, 17).
The current standard for quantifying NFT deposition in postmortem individuals is the Braak staging scheme (18, 19). Braak stages I and II are characterized by sparse NFT deposition in the entorhinal cortex, although some hippocampal tangles can also be found with Braak I and II. Braak stages III and IV make up the limbic stage with NFTs extending to the hippocampus, amygdala and medial temporal neocortex. According to successive National Institute on Aging-sponsored AD classification systems since 1997, individuals in this stage who also have threshold levels of neuritic plaques are termed as having “low” or “intermediate” likelihood for causing AD-related cognitive impairment or dementia (20–22). Braak stages V and VI represent the neocortical stage where there is diffuse NFT deposition throughout all neocortical lobes and, in the great majority of subjects, accompanied by frank dementia. The sequence of development has been conventionally thought to be that the deposition of amyloid plaques precede tangles (23) but this has been disputed, as Braak and others have argued that the near universal occurrence of entorhinal and limbic NFTs as part of normal aging should be regarded as the first stage of pathological AD (24, 25).
Aside from plaques and tangles, considerable evidence suggests that there is significant cerebrovascular contribution to AD pathogenesis. In particular, the notion that cerebrovascular damage may lead to NFT development has been proposed (26, 27) as a pathway to AD pathology that is parallel to the amyloid cascade hypothesis. Animal studies have suggested that cerebrovascular damage can result directly in tau hyperphosphorylation and NFT deposits in the cerebral cortex, perhaps as part of the immunological response to the vascular insult (28–30). Human studies have also suggested an association between vascular insult and tau pathology, with one study reporting vascular risk factors and amyloid plaques to be synergistic in their effects on pathological tau accumulation in the neocortex (27). Recent work by Malek-Ahmadi et al (2020) showed that the presence of CAA was associated with a >2-fold increased risk of being classified as Braak stage III compared with Braak I–II (26).
The role of APOE ε4 as a genetic risk factor for AD is well-established (31), as has its role as a risk factor for cerebrovascular and neurodegenerative disease (32–35). A recent study suggested that APOE ε4 carriers have a greater likelihood of blood-brain barrier integrity loss (35), making them more susceptible to cognitive decline and neurodegeneration (32).
Although previous studies have reported the association between CAA and NFT pathology (18, 22, 36), the extent to which other vascular lesions, such as WMR, CWA, and microinfarct number associate with NFT pathology has not been investigated. The intent of our study was to determine whether CAA, microinfarct number, WMR, or CWA were associated with tau pathology as defined by Braak stages I–VI in prospectively characterized autopsy subjects with varying antemortem clinical diagnoses intended to focus the investigation on a spectrum ranging from minimal to maximal Alzheimer’s pathology and not other pathologies. Additionally, interactions between APOE ε4 carrier status and vascular lesions on NFT load were investigated. We hypothesized that one or more of these vascular lesions would associate independently with Braak stage, and that APOE ε4 carriage would associate with a higher Braak stage than noncarriage for each of these lesions.
MATERIALS AND METHODS
Subject Selection
A database search was conducted of the Banner Sun Health Research Institute Arizona Study of Aging and Neurodegenerative Disorders (AZSAND)/Brain and Body Donation Program (BBDP) in Sun City, AZ (www.brainandbodydonationprogram.org). The operational details of this program are described elsewhere (37).
Selection of subjects was restricted to those with a premortem consensus diagnosis of cognitively unimpaired (CU), mild cognitive impairment (MCI), or AD at the last neurocognitive evaluation and a full postmortem neuropathological workup. Individuals with major neuropathological disorders including cases clinicopathologically defined as vascular dementia, hippocampal sclerosis, frontotemporal lobar degeneration with TDP-43 proteinopathy, Pick disease, progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, Huntington disease, large acute cerebral infarcts, motor neuron disease, and primary or metastatic malignant brain tumors were excluded.
All BBDP subjects gave written consent for study procedures, autopsy and sharing of de-identified data prior to enrollment. Approval for the study and its consenting procedures were approved by the Western Institutional Review Board of Puyallup, Washington.
Tissue Preparation and Neuropathological Diagnosis
The BBDP has rapid response teams that are on-call 24 hours a day, which allows for expedited transport to the morgue at Banner Sun Health Research Institute, and commencement of autopsy with a median postmortem interval of 3.0 hours. All subjects received identical neuropathological examinations, including summary semiquantitative brain density measures for neocortical total amyloid plaques and NFT as well as assignment of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque density and Braak neurofibrillary stage, as described previously (37). Lewy body pathology regional and summary density scoring were also assessed. CWA was graded by gross visual external inspection as previously published (38, 39). Cerebral WMR was graded in frontal, temporal, parietal and occipital lobes on large 40- or 80-μm sections stained with hematoxylin and eosin (40–45). The method is analogous to that used by neuroradiologists to grade leukoaraiosis or “small vessel disease” in the cerebral white matter with MRI. Rarefaction restricted to the immediate periventricular region, occupying 25% or less of the centrum semi-ovale, is termed “mild,” while “moderate” is used when this extends to between 25% and 50%, with “severe” being reserved for rarefaction involving >50%. A summary score was obtained by adding the scores from all lobes, with a maximum score of 12. CAA was graded on the same thick sections, primarily with the thioflavin S stain, and is a semiquantitative estimate of the density of involved blood vessels, again by analogy with the CERAD templates. Each region received a score of 0–3, with a maximum total score of 12. Brain infarcts were classified by estimated age (acute, subacute, old or chronic), location and size. Centrum ovale infarcts were defined as those that are restricted to the centrum ovale; if the infarct involved both cerebral cortex and centrum ovale, it was classified as a cerebral cortex infarct. Deep nuclei infarcts included those of the basal ganglia, thalamus, subthalamic regions and hypothalamus. Infratentorial infarcts were those involving the brainstem and/or cerebellum. A volume was estimated for each infarct, and summary figures were recorded for brain subdivisions as well as for the entire brain. Foci of cerebellar cortical sclerosis were recorded as infarcts and were generally microscopic in size class. Acute ischemic changes were not counted as infarcts but were separately noted. It is recognized that the actual numbers of microscopic infarcts in a brain are likely to be up to 1000 times higher than what is found with standard brain sampling (46). Infarcts are very common in the BBDP population, with 43% of all subjects having one or more autopsy-confirmed infarcts. For this study, seeking to understand the effects of vascular factors on NFT abundance and distribution, individuals with acute infarcts were excluded from the analysis.
Statistical Analysis
Demographic data were analyzed using one-way analysis of variance (ANOVA), Kruskal-Wallis test, and Chi-square analysis as appropriate. We tested independent associations of CAA, CWA, microinfarcts, and WMR with the Braak stage spectrum using multivariable linear regression models. All models adjusted for age at death, APOE ε4 carrier status, neuritic plaque load as defined by CERAD neuritic plaque density, years of education and sex.
Due to the relevance of APOE ε4 on both Alzheimer’s disease progression and cardiovascular disease (32–34), we then used separate statistical models to test for interaction of APOE ε4 carrier status with each of these lesions.
RESULTS
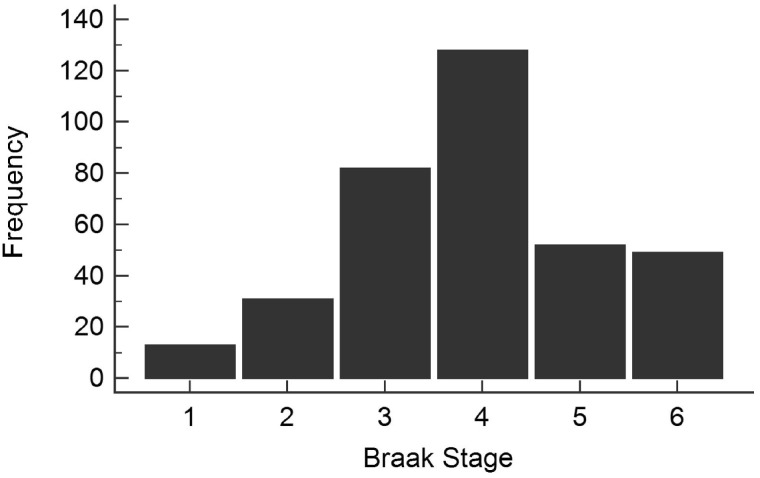
Data from 355 individuals were included in this study. Mean age at death was 87 ± 7.04 years, mean education was 14.8 ± 2.57 years, and 50% were female. The median postmortem interval was 3.00 (IQR = 2.50, 3.50) hours. Mean brain weight at autopsy was 1147.04 ± 125.37 g. We confirmed that Braak stage was normally distributed among the subjects (Fig. 1).
FIGURE 1.
Study sample Braak stage distribution. Braak stage frequency met the assumption of normality which allowed for linear regression models to be used.
Table 1 shows demographic and neuropathological characteristics stratified by Braak stage where the entorhinal stage encompasses Braak I–II, limbic stage includes Braak III–IV, and neocortical stage includes Braak V–VI. Age at death differed significantly among the 3 groups, with subjects in the limbic stage being the oldest and subjects in the entorhinal stage being the youngest. When age at death for limbic stage individuals was examined further, MCI cases had the oldest age at death (92.19 ± 4.82), followed by AD cases (90.67 ± 6.01) and CU cases (88.04 ± 6.37). Several studies have noted that the density of AD histopathological lesions drops in the older-old, perhaps due to poorer survival in those with more severe AD (47). Sex also differed significantly among the groups (p = 0.003), where, as previously reported (48), females were more likely to be in a higher Braak stage. Levels of education were not significantly different between groups. Consensus diagnosis associated with Braak stage (p < 0.001), with the majority (37/44) of individuals in the entorhinal stage being CU, and the majority (98/101) of individuals in the neocortical stage having a clinical diagnosis of AD dementia.
TABLE 1.
Demographic and Neuropathological Characteristics of Cases Evaluated by Braak Stage
| Braak I–II Entorhinal | Braak III–IV Limbic | Braak V–VI Neocortical | p Value | |
|---|---|---|---|---|
| n = 44 | n = 210 | n = 101 | ||
| Age at death (y) | 82.39 ± 5.84 | 89.04 ± 6.30 | 85.17 ± 7.50 | <0.001 |
| Sex Male/female (% male) | 31/13 (70%) | 92/118 (44%) | 55/46 (54%) | 0.003* |
| Education (y) | 15.36 ± 2.54 | 14.82 ± 2.53 | 14.50 ± 2.67 | 0.16 |
| Consensus diagnosis (CU/MCI/AD) | 37/3/4 | 145/26/39 | 1/2/98 | <0.001* |
| APOE ε4 Carrier/noncarrier (% carrier) | 8/35 (19%) | 41/167 (20%) | 56/44 (56%) | <0.001* |
| Postmortem interval (hours) | 2.94 (2.38, 3.50) | 2.91 (2.50, 3.50) | 3.00 (2.50, 3.50) | 0.92 |
| Brain weight at autopsy (g) | 1,236.61 ± 140.43 | 1,160.81 ± 108.77 | 1,079.40 ± 117.84 | <0.001 |
| Lewy body pathology (yes/no) | 10/34 | 59/151 | 58/43 | <0.001* |
| CERAD neuritic plaque density | <0.001* | |||
| None | 21 | 55 | 2 | |
| Sparse | 8 | 43 | 1 | |
| Moderate | 8 | 41 | 3 | |
| Frequent | 7 | 70 | 95 | |
| Circles of Willis atherosclerosis | 2.09 ± 1.64 | 1.96 ± 1.17 | 2.08 ± 1.15 | 0.56 |
| White matter rarefaction | 2.81 ± 2.80 | 3.66 ± 3.05 | 5.47 ± 3.40 | <0.001 |
| Microinfarct number | 0.02 ± 0.05 | 0.42 ± 1.95 | 0.54 ± 2.40 | 0.03 |
| Cerebral amyloid angiopathy | 1.57 ± 2.68 | 2.68 ± 3.29 | 5.69 ± 3.84 | <0.001 |
Mean ± SD; Median (interquartile range) for postmortem interval.
p value determined via Chi-square analysis. All others determined via Kruskal-Wallis analysis.
APOE ε4 carrier status also differed significantly among groups (p < 0.001), with 8/43 and 41/208 individuals in the entorhinal and limbic stages carrying the ε4 allele, respectively, while this ratio was 56/100 individuals in the neocortical stage. CERAD neuritic plaque density differed across the groups (p < 0.001) with 21/44 individuals having a CERAD density of 0 in the entorhinal stage, a heterogenous distribution of CERAD density in the limbic stage, and an overwhelming majority, 95/101, of individuals in the neocortical stage having a CERAD density of 3 (frequent). Lewy body pathology was absent in 64% of the subjects, but was most prevalent in the highest Braak stages (p < 0.001). Of the vascular lesions assessed, WMR (p < 0.001), CAA (p < 0.001), and microinfarct number (p = 0.031) differed significantly among the groups with the lowest amount of each lesion in the entorhinal stage and the highest amount of each lesion in the neocortical stage. CWA score did not differ significantly among Braak stage groups (p = 0.56).
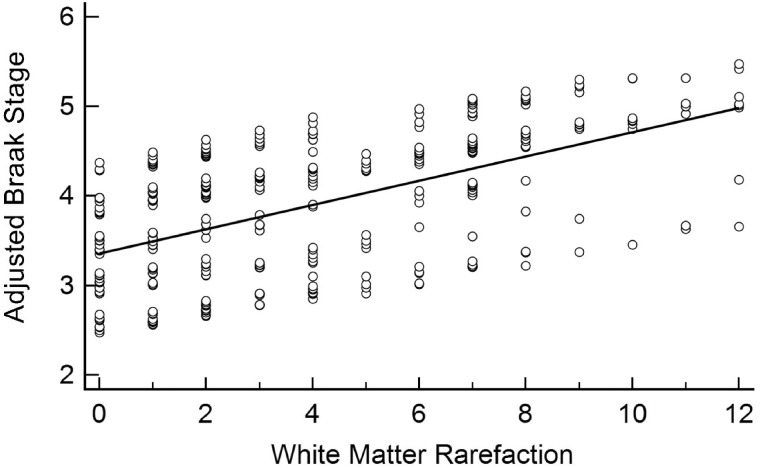
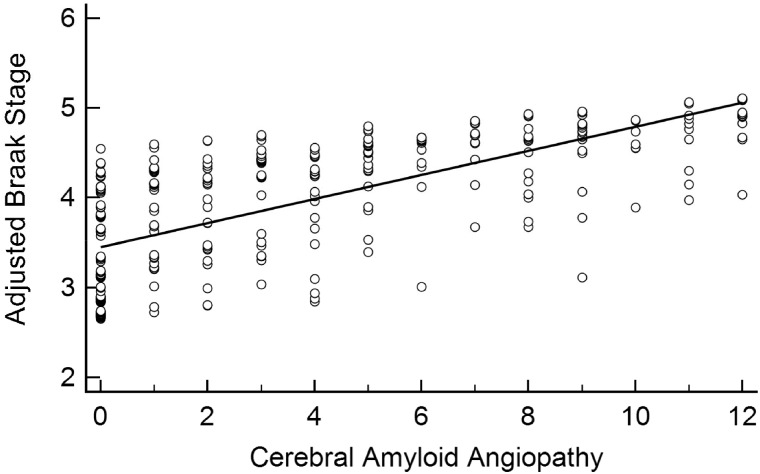
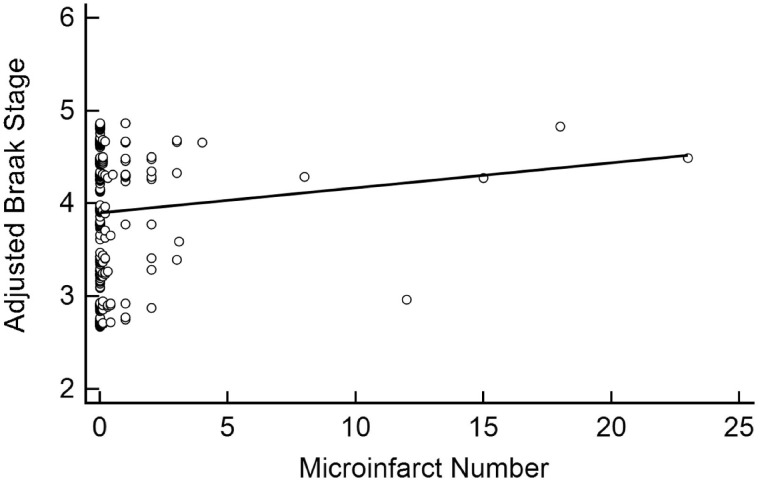
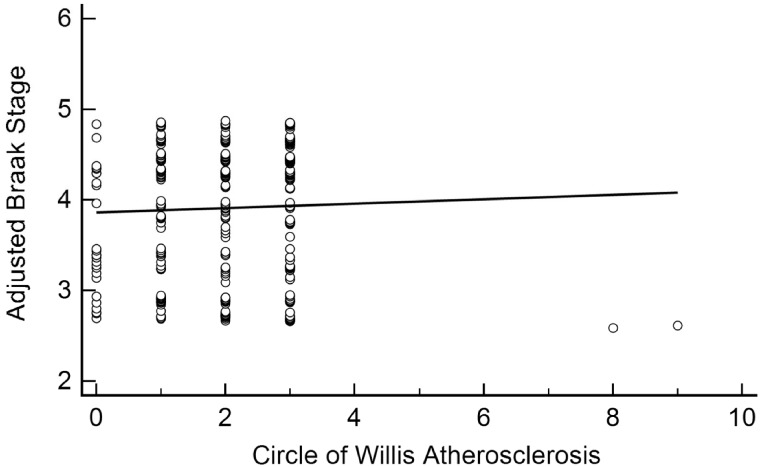
WMR was associated with Braak stage (β = 0.09, 95% CI: [0.06, 1.13], p < 0.001) even after adjusting for age at death, APOE ε4 carrier status, CERAD density, education, and sex (Table 2; Fig. 2). CAA also associated independently with Braak stage (β = 0.05, 95% CI: [0.01, 0.08], p = 0.01) after adjusting for these variables (Fig. 3). Neither microinfarct number (β = 0.02, 95% CI: [–0.03, 0.08], p = 0.44) nor CWA (β = –0.02, 95% CI: [–0.13, 0.09], p = 0.72) was associated with the Braak stage (Figs. 4 and 5).
TABLE 2.
Independent Effects and APOE ε4 Interactions of Vascular Lesions on Braak Stage
| Independent Effects |
Interactions With APOE ε4 |
|||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value | |
| CAA | 0.05 | (0.01, 0.08) | 0.01 | 0.05 | (–0.02, 0.12) | 0.15 |
| WMR | 0.09 | (0.06, 0.13) | <0.001 | 0.09 | (0.02, 0.17) | 0.01 |
| CWA | –0.02 | (–0.13, 0.09) | 0.72 | 0.02 | (–0.23, 0.26) | 0.90 |
| Microinfarct Number | –0.02 | (–0.03, 0.08) | 0.44 | –0.04 | (–0.17, 0.09) | 0.57 |
CAA, cerebral amyloid angiopathy; WMR, white matter rarefaction; CWA, circle of Willis atherosclerosis. All models adjusted for age at death, education, sex, APOE ε4 carrier status, and CERAD Neuritic Plaque Density.
FIGURE 2.
Linear association between white matter rarefaction and Braak stage. White matter rarefaction was significantly associated with Braak stage after adjusting for age at death, sex, education, APOE ε4 carrier status, and CERAD neuritic plaque density (β = 0.09, 95% CI: [0.06, 0.13], p < 0.001).
FIGURE 3.
Linear association between cerebral amyloid angiopathy and Braak stage. Cerebral amyloid angiopathy was significantly associated with Braak stage after adjusting for age at death, sex, education, APOE ε4 carrier status, and CERAD neuritic plaque density (β = 0.05, 95% CI: [0.01, 0.08], p = 0.01).
FIGURE 4.
Linear association between microinfarct number and Braak stage. Microinfarct number was not significantly associated with Braak stage after adjusting for age at death, sex, education, APOE ε4 carrier status, and CERAD neuritic plaque density (β = 0.02, 95% CI: [–0.03, 0.08], p = 0.44).
FIGURE 5.
Linear association between circle of Willis atherosclerosis and Braak stage. Circle of Willis Atherosclerosis was not significantly associated with Braak stage after adjusting for age at death, sex, education, APOE ε4 carrier status, and CERAD neuritic plaque density (β = –0.02, 95% CI: [–0.13, 0.09], p = 0.72).
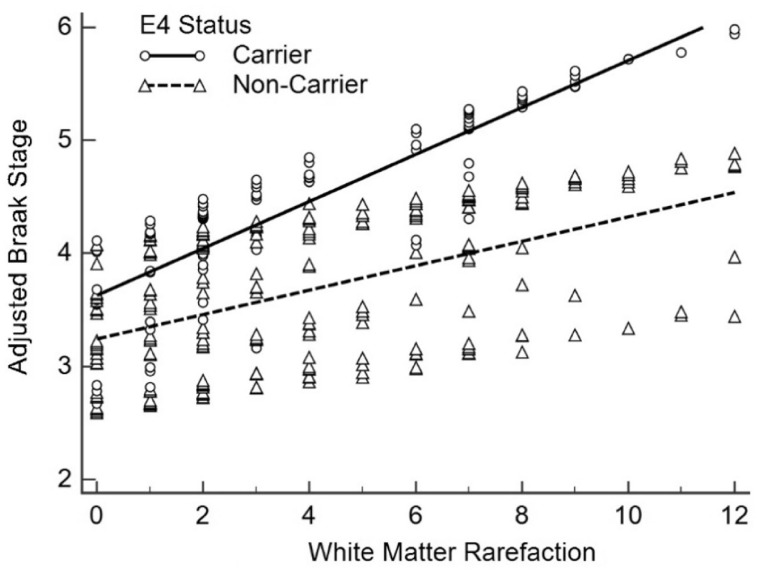
Table 2 shows the results of the APOE ε4 by vascular lesion interactions. WMR was found to significantly interact with APOE ε4 carrier status (β = 0.09, 95% CI: [0.02, 0.17], p = 0.01), showing a stronger association with Braak stage as an interactive term than WMR alone, though both were significant (Fig. 6). No significant interactions were found between APOE ε4 and CAA (β = 0.05, 95% CI: [–0.02, 0.12], p = 0.15), APOE ε4 and microinfarct number (β = –0.04, 95% CI: [–0.17, 0.09], p = 0.57), or APOE ε4 and CWA (β = 0.02, 95% CI: [–0.23, 0.26], p = 0.90). Region-wise analyses of WMR and CAA were carried out for the frontal, temporal, parietal, occipital lobes and are shown in Table 3. Frontal, temporal, and parietal CAA showed significant associations with Braak stage while occipital CAA did not. For WMR, all 4 regions were significantly associated with Braak stage.
FIGURE 6.
White matter rarefaction association with Braak stage stratified by APOE ε4 carrier status. White matter rarefaction (WMR) significantly interacted with APOE ε4 carrier status (β = 0.09, 95% CI: [0.02, 0.17], p = 0.01) after adjusting for age at death, sex, education, and CERAD neuritic plaque density, showing a stronger association to Braak stage as an interactive term than WMR alone.
TABLE 3.
Regional Analyses of White Matter Rarefaction and Cerebral Amyloid Angiopathy Associations With Braak Stage
| Region | Coefficient | 95% CI | p Value |
|---|---|---|---|
| CAA | |||
| Frontal | 0.16 | (0.04, 0.29) | 0.01 |
| Temporal | 0.22 | (0.08, 0.35) | 0.002 |
| Parietal | 0.18 | (0.05, 0.31) | 0.006 |
| Occipital | 0.05 | (–0.07, 0.17) | 0.39 |
| WMR | |||
| Frontal | 0.24 | (0.12, 0.36) | <0.001 |
| Temporal | 0.28 | (0.17, 0.40) | <0.001 |
| Parietal | 0.18 | (0.07, 0.28) | <0.001 |
| Occipital | 0.22 | (0.10, 0.33) | <0.001 |
DISCUSSION
We found that CAA and WMR were associated with NFT load among CU, MCI, AD autopsy subjects. The findings for CAA were consistent with previous studies. WMR showed both independent and APOE ε4-related associations with NFT load. The underlying mechanism of this association is still unclear; however, as it has been suggested that WMR in advanced AD may represent Wallerian degeneration of cortical neurons bearing NFT (49, 50). The finding that WMR had a stronger association with Braak stage in APOE ε4 carriers than in noncarriers suggests that one of the ways APOE ε4 might increase the risk of developing AD is through interactions with vascular and/or inflammatory pathways. For any given level of WMR, Braak stage was higher in APOE ε4 carriers than in noncarriers. However, WMR can be the result of several different vascular abnormalities and is akin to white matter hyperintensities observed in neuroimaging studies. Since the prevalence of white matter hyperintensities in older adults is high (51), the age-related effect of vascular lesions cannot be discounted despite controlling for age at death in the analyses. Thus, reported associations between vascular and AD pathologies may depend largely on a study’s context.
CWA was not associated with Braak stage, which differs from previous findings (39). Roher et al analyzed CWA differences based on neuropathological diagnosis whereas our analysis investigated the linear association between CWA severity and Braak stage (39). Also, it may be that differential survival of subjects with varying severity of CWA and NFTs could affect the relationship between CWA and Braak stage.
Our study confirms previous findings of greater Lewy body disease at higher Braak NFT stages (52).
The findings of this study add to and complement the existing literature on cerebrovascular associations with AD. The independent association of CAA and WMR with NFT burden suggests that AD-related tauopathies are not exclusively related to amyloid plaque burden. It is possible that amyloid plaque burden and cerebrovascular injury each represent parallel pathways toward NFT development. Although it is unclear whether there are specific cerebrovascular pathways contributing to NFT deposition in humans, their role is supported by experimental evidence in animal models (28–30). Neuroimaging evidence showing that cerebrovascular dysregulation precedes amyloid deposition (53) offers the possibility that amyloid and vascular pathways responsible for NFT development may work in parallel. If this is the case, then concomitant therapies for vascular and amyloid targets could prove to be effective in abrogating downstream NFT pathology. The significant interaction between WMR and APOE ε4 carrier status on Braak stage also suggests that APOE-directed treatments could significantly alter NFT load (54, 55).
Additional studies that examine the influence of genetic polymorphisms with known vascular functions and associations may help identify specific vascular pathways associated with AD pathology, particularly if these pathways are mediated via APOE. Vascular endothelial growth factor (VEGF) (56, 57), C-reactive protein (CRP) (58, 59), and methylenetetrahydrofolate.
(MTHFR) polymorphisms (60, 61) have shown to be associated with AD and are specific to vascular and inflammatory mechanisms that are thought to be common between cardiovascular disease and AD. Whether these genes, or others, interact with APOE to influence AD pathology remains to be seen, but it is reasonable to expect that future studies in this area need to consider the possible role of cardiovascular pathways in APOE-mediated AD pathology.
JBN was supported in part by the Midwestern University Kenneth A. Suarez Summer Research Fellowship. The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson's Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research.
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. 2020 Alzheimer's disease facts and figures. Alzheimer's Dement 2020;16:391–460. [DOI] [PubMed] [Google Scholar]
- 2. Laurent C, Buée L, Blum D.. Tau and neuroinflammation: what impact for Alzheimer's Disease and Tauopathies? Biomed J 2018;41:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller J, Budson A.. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Research 2018;7:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu D, Liu F.. Pathological changes of tau related to Alzheimer's disease. ACS Chem Neurosci 2019;10:931–44 [DOI] [PubMed] [Google Scholar]
- 5. Hardy JA, Higgins GA.. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992;256:184–5 [DOI] [PubMed] [Google Scholar]
- 6. Takeda S. Progression of Alzheimer's disease, tau propagation, and its modifiable risk factors. Neurosci Res 2019;141:36–42 [DOI] [PubMed] [Google Scholar]
- 7. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—the disregarded partner of Alzheimer's disease. Alzheimers Dement 2019;15:158–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar-Singh S. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes Brain Behav 2008;7:67–82 [DOI] [PubMed] [Google Scholar]
- 9. Brenowitz WD, Nelson PT, Besser LM, et al. Cerebral amyloid angiopathy and its co-occurrence with Alzheimer's disease and other cerebrovascular neuropathologic changes. Neurobiol Aging 2015;36:2702–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bos I, Verhey FR, Ramakers I, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther 2017;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Englund E, Brun A, Alling C.. White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain 1988;111:1425–39 [DOI] [PubMed] [Google Scholar]
- 12. Englund E, Brun A.. Frontal lobe degeneration of non-Alzheimer type. IV. White matter changes. Arch Gerontol Geriatr 1987;6:235–43 [DOI] [PubMed] [Google Scholar]
- 13. Roher AE, Tyas SL, Maarouf CL, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement 2011;7:436–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thorin-Trescases N, de Montgolfier O, Pinçon A, et al. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 2018;314:H1214–h1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA 2020;323:1934–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arvanitakis Z, Leurgans SE, Barnes LL, et al. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kövari E, Gold G, Herrmann FR, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology 2007;68:927–31 [DOI] [PubMed] [Google Scholar]
- 18. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 19. Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlson JO, Gatz M, Pedersen NL, et al. Antemortem prediction of Braak stage. J Neuropathol Exp Neurol 2015;74:1061–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 1997;18:S1–2. [PubMed] [Google Scholar]
- 22. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimers Dis 2012;2012:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duyckaerts C, Braak H, Brion JP, et al. PART is part of Alzheimer disease. Acta Neuropathol 2015;129:749–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braak H, Del Tredici K.. Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathol 2014;128:767–72 [DOI] [PubMed] [Google Scholar]
- 26. Malek-Ahmadi M, Perez SE, Chen K, et al. Braak stage, cerebral amyloid angiopathy, and cognitive decline in early Alzheimer's disease. J Alzheimers Dis 2020;74:189–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rabin JS, Yang HS, Schultz AP, et al. Vascular risk and beta-amyloid are synergistically associated with cortical tau. Ann Neurol 2019;85:272–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merlini M, Wanner D, Nitsch RM.. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer's disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol 2016;131:737–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basurto-Islas G, Gu JH, Tung YC, et al. Mechanism of tau hyperphosphorylation involving lysosomal enzyme asparagine endopeptidase in a mouse model of brain ischemia. J Alzheimers Dis 2018;63:821–33 [DOI] [PubMed] [Google Scholar]
- 30. Wen Y, Yang SH, Liu R, et al. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta 2007;1772:473–83 [DOI] [PubMed] [Google Scholar]
- 31. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat Commun 2020;11:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012;485:512–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med 2016;94:739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marais AD. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 2019;51:165–76 [DOI] [PubMed] [Google Scholar]
- 35. Main BS, Villapol S, Sloley SS, et al. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener 2018;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams S, Chalmers K, Wilcock GK, et al. Relationship of neurofibrillary pathology to cerebral amyloid angiopathy in Alzheimer's disease. Neuropathol Appl Neurobiol 2005;31:414–21 [DOI] [PubMed] [Google Scholar]
- 37. Beach TG, Adler CH, Sue LI, et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015;35:354–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 2006;113:13–21 [DOI] [PubMed] [Google Scholar]
- 39. Roher AE, Esh C, Kokjohn TA, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol 2003;23:2055–62 [DOI] [PubMed] [Google Scholar]
- 40. Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med 2003;9:112–22 [PMC free article] [PubMed] [Google Scholar]
- 41. Dugger BN, Clark CM, Serrano G, et al. Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates. J Neuropathol Exp Neurol 2014;73:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roher AE, Maarouf CL, Malek-Ahmadi M, et al. Subjects harboring presenilin familial Alzheimer's disease mutations exhibit diverse white matter biochemistry alterations. Am J Neurodegener Dis 2013;2:187–207. [PMC free article] [PubMed] [Google Scholar]
- 43. Castao EM, Maarouf CL, Wu T, et al. Alzheimer disease periventricular white matter lesions exhibit specific proteomic profile alterations. Neurochem Int 2013;62:145–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dugger BN, Adler CH, Shill HA, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord 2014;20:525–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roher AE, Weiss N, Kokjohn TA, et al. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry 2002;41:11080–90 [DOI] [PubMed] [Google Scholar]
- 46. Westover MB, Bianchi MT, Yang C, et al. Estimating cerebral microinfarct burden from autopsy samples. Neurology 2013;80:1365–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beach TG, Malek-Ahmadi M.. Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimer Dis 2020; doi: 10.3233/JAD-201213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Filon JR, Intorcia AJ, Sue LI, et al. Gender differences in Alzheimer disease: brain atrophy, histopathology burden, and cognition. J Neuropathol Exp Neurol 2016;75:748–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McAleese KE, Walker L, Graham S, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 2017;134:459–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leys D, Pruvo JP, Parent M, et al. Could Wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry 1991;54:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging 2004;15:365–7 [DOI] [PubMed] [Google Scholar]
- 52. Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018;14:330–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun 2016;7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mamun AA, Uddin MS, Bin Bashar MF, et al. Molecular insight into the therapeutic promise of targeting APOE4 for Alzheimer's disease. Oxid Med Cell Longev 2020;2020:5086250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamazaki Y, Zhao N, Caulfield TR, et al. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol 2019;15:501–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Del Bo R, Scarlato M, Ghezzi S, et al. Vascular endothelial growth factor gene variability is associated with increased risk for AD. Ann Neurol 2005;57:373–80 [DOI] [PubMed] [Google Scholar]
- 57. Smach MA, Charfeddine B, Othman LB, et al. 1154G/A and -2578C/A polymorphisms of the vascular endothelial growth factor gene in Tunisian Alzheimer patients in relation to beta-amyloid (1–42) and total tau protein. Neurosci Lett 2010;472:139–42 [DOI] [PubMed] [Google Scholar]
- 58. van Oijen M, de Maat MP, Kardys I, et al. Polymorphisms and haplotypes in the C-reactive protein gene and risk of dementia. Neurobiol Aging 2007;28:1361–6 [DOI] [PubMed] [Google Scholar]
- 59. Kok EH, Alanne-Kinnunen M, Isotalo K, et al. CRP gene variation affects early development of Alzheimer's disease-related plaques. J Neuroinflammation 2011;8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang B, Jin F, Kan R, et al. Association of MTHFR gene polymorphism C677T with susceptibility to late-onset Alzheimer's disease. J Mol Neurosci 2005;27:23–7 [DOI] [PubMed] [Google Scholar]
- 61. Mansoori N, Tripathi M, Luthra K, et al. MTHFR (677 and 1298) and IL-6-174 G/C genes in pathogenesis of Alzheimer's and vascular dementia and their epistatic interaction. Neurobiol Aging 2012;33:1003.e1001–e8. [DOI] [PubMed] [Google Scholar]