Supplemental digital content is available in the text.
KEY WORDS: Unmanned aerial vehicle, ground transportation, blood product
Abstract
BACKGROUND
Timely and safe distribution of quality blood products is a major challenge faced by blood banks around the world. Our primary objective was to determine if simulated blood product delivery to an urban trauma center would be more rapidly achieved by unmanned aerial vehicle (UAV) than by ground transportation. A secondary objective was to determine the feasibility of maintaining simulated blood product temperatures within a targeted range.
METHODS
In this prospective pilot study, we used two distinct methods to compare UAV flight duration and ground transport times. Simulated blood products included packed red blood cells, platelet concentrate, and fresh frozen plasma. For each blood product type, three UAV flights were conducted. Temperature was monitored during transport using a probe coupled to a data logger inside each simulated blood product unit.
RESULTS
All flights were conducted successfully without any adverse events or safety concerns reported. The heaviest payload transported was 6.4 kg, and the drone speed throughout all nine flights was 10 m/s. The mean UAV transportation time was significantly faster than ground delivery (17:06 ± 00:04 minutes vs. 28:54 ± 01:12 minutes, p < 0.0001). The mean ± SD initial temperature for packed red blood cells was 4.4°C ± 0.1°C with a maximum 5% mean temperature variability from departure to landing. For platelet concentrates, the mean ± SD initial temperature was 21.6°C ± 0.5°C, and the maximum variability observed was 0.3%. The mean ± SD initial fresh frozen plasma temperature was −19°C ± 2°C, and the greatest temperature variability was from −17°C ± 2°C to −16°C ± 2°C.
CONCLUSIONS
Unmanned aerial vehicle transportation of simulated blood products was significantly faster than ground delivery. Simulated blood product temperatures remained within their respective acceptable ranges throughout transport. Further studies assessing UAV transport of real blood products in populated areas are warranted.
LEVEL OF EVIDENCE
Therapeutic/care management, level IV.
Timely and safe distribution of quality blood products is a major challenge faced by blood banks around the world.1 This is particularly difficult in the context of mass casualty incidents when there is an increased demand for blood products because hemorrhaging patients are cared for in several different hospitals.
On October 18, 2018, a large-scale city-wide disaster simulation was conducted in Montreal, Québec, and involved one pediatric and two adult level 1 trauma centers.2 The crisis scenarios were designed to challenge hospital blood supply. Each center ran multiple simultaneous massive transfusion protocols for their sickest simulated patients. Héma-Québec, the non-for-profit organization responsible for management and distribution of blood products in the province of Québec, was called upon to deliver simulated blood products to participating hospitals as they rapidly ran out of O negative blood units. While the first courier delivery was rapid, subsequent deliveries took much longer because of delays in driving back and forth between the Héma-Québec distribution center and the hospitals. Indeed, ground transportation of blood products may often be limited by recurrent traffic jams and road closures. Furthermore, delivery of blood products by ambulance or police vehicle may not be possible in the context of mass casualty incidents because these vehicles are primarily dedicated to scene response and patient transport.
Recent studies have assessed the feasibility of delivering medical equipment by unmanned aerial vehicle (UAV) including flotation devices,3,4 automated external defibrillators,5,6 and chemistry,7 microbiology,8 and hematology samples.7,9 Mesar et al.10 successfully delivered a 4.5-kg payload containing medical equipment by UAV over 12 km.11 Amukele et al.9 assessed the quality of blood products after a UAV flight of 22 ± 4.5 minutes at 100-m altitude with continuous temperature logging. The flights were conducted away from populated areas, and their length was between 13- and 20-km air travel distance according to the UAV mean speed and flight duration. Each transport cooler contained 2 to 3 U, and the maximum payload weight was 1.9 kg. The blood products were tested after the flight, and no adverse impact was identified (i.e., no hemolysis, no clumping, target shipping temperature range was maintained).
There are no published reports of UAV transportation of blood products to urban trauma centers. Moreover, a comparison between ground and UAV transport times of medical products has never been performed in an urban environment.
PATIENTS AND METHODS
Study Objectives
The primary objective of this prospective proof of concept pilot study was to determine if simulated blood product delivery to an urban trauma center would be more rapidly achieved by UAV than by ground transportation. A secondary study objective was to explore the feasibility of using UAVs to deliver simulated blood products in an urban setting in terms of operations, technical challenges including transport container design and blood product temperature stability, population safety, and legal parameters and added value.
Ethical Considerations
The research protocol was submitted to the McGill University Health Centre Research Ethics Board. Relevant sections of the protocol were reviewed by the cochair, and it was determined that full board review was not required. A letter of exemption from the Research Ethics Board Review was sent to the principal investigator on August 16, 2019. While InDro Robotics (Salt Spring Island, BC, Canada) had no role in data collection, data analysis, and article writing, the company was involved in determination of maximum payload weight and the number of flights that could be done in the time available for data collection. InDro Robotics participated in the design of the payload container and conducted flight tests before data collection.
Payload for UAV Transportation of Blood Products
The thermoregulated shipping container was developed by Héma-Québec in collaboration with InDro Robotics to separately transport three types of simulated blood products: packed red blood cells (pRBCs), platelet concentrates (PCs), and fresh frozen plasma (FFP) at their specific target shipping temperatures (Table 1). Each of the corresponding blood product bags was filled with VpRBC = 313 mL, VPC = 260 mL, and VFFP = 297 mL of 0.9% saline solution to meet the current target final volumes at Héma-Québec. Since the purpose of transporting blood products by UAV in this study was to meet the needs in a crisis, the maximum transport time considered for the shipping container development was established at 90 minutes. The container consisted of a waterproof and light resistant bag (Enthusiast Gear) with a volume capacity of 15 L. A HOBO temperature probe coupled to a data logger (HOBO Pro v2; Onset, Bourne, MA) was inserted into each simulated blood product unit. Preliminary tests determined that the gear bag could not be used alone to maintain the blood product temperature within targeted ranges. Temperature stability within targeted ranges was achieved in the laboratory by placing blood products and their temperature probe into a plastic bag containing phase changing material (PCM). A second PCM was wrapped around the plastic bag and attached by an elastic. This whole unit was then inserted into a foam bag, which was placed into the gear bag (Fig. 1). Phase changing materials with a phase transition temperature of T = 5°C (PCM 5) were selected for pRBCs, while PCMs with a respective phase transition temperature of T = 22°C (PCM 22) and T = −22°C (PCM 22) were preferred for PCs and FFP.
TABLE 1.
Payload Weights for Components and Blood Products Transported by Drone
| Transported Products | Weight, kg | Total Weight, kg* |
|---|---|---|
| Components | ||
| Enthusiast Gear bag | 0.48 | n/a |
| PCM 5 and 22 | 0.70 | n/a |
| PCM 20 | 0.90 | n/a |
| pRBCs (313 mL) | ||
| 1× pRBC** | 0.51 | 2.4 |
| 3× pRBC† | 1.5 | 3.4 |
| Platelet concentrate (260 mL) | ||
| 1× PC** | 0.45 | 2.4 |
| 3× PC† | 1.4 | 3.3 |
| Fresh frozen plasma (297 mL) | ||
| 1× FFP** | 0.48 | 2.8 |
| 3× FFP† | 1.4 | 3.7 |
*Total weight includes Enthusiast Gear bag, 2 PCMs, and 1 or 3 blood products with HOBO.
**With 1 HOBO.
†With 3 HOBO.
n/a, not applicable.
Figure 1.

Drone carrying gear bag.
UAV Operation
For each blood product type, three UAV flights were conducted. Every flight duration was compared with ground transportation time. Blood product delivery by ground vehicle occurred from the Héma-Québec regional blood distribution center to the Montreal General Hospital (MGH), a level 1 trauma center located in downtown Montreal. Each UAV flight was 9.8-km long, corresponding to the aerial distance separating the Héma-Québec regional blood distribution center and MGH. Unfortunately, strict safety regulations prohibited drone take-off from the regional distribution center because of its location within 3 nautical miles (5.6 km) of the Pierre-Elliott-Trudeau International Airport. Therefore, the UAV took off from the MGH roof on the 20th floor (altitude, ~85 m), performed 10 loops (each 1-km long) above the Mount-Royal Park adjacent to MGH, and then landed on the same roof. This was done nine times in total: three flights transporting 3 U of simulated platelets at a target temperature of 20°C to 24°C; three flights transporting 3 U of simulated pRBCs at a target temperature of 1°C to 6°C; and three flights transporting 3 U of FFP at a target temperature below −20°C. These target temperatures were based on the Canadian Standards Association criteria for blood product transport over a 24-hour period.10 Every time the drone landed on the MGH roof to deliver the payload, the UAV pilot detached the payload bag, carried it to a research assistant, and made physical contact with that person. The research assistant then carried a different container located at a predetermined transition point on the roof and delivered it to the MGH blood bank on the seventh floor. This was done to ensure that the payload container would never be stuck inside a blocked hospital elevator, which would have prevented subsequent flights from being conducted.
Ground Transportation of Blood Products
Two research assistants drove a car from the Héma-Québec distribution center to the designated parking spot at MGH. Driver departure and UAV takeoff were simultaneous. Drivers followed directions provided by (Waze Mobile Ltd., Tel Aviv, Israel) as well as speed limits and traffic regulations. Once parked at MGH, drivers delivered the shipping box containing simulated blood products to the hospital blood bank. All drivers were required to complete two test drives before data collection. A research assistant was responsible for oversight of the ground transport team and management of communications with the drivers. Simulated blood products were transported using the thermoregulated shipping containers currently used at Héma-Québec for daily operations. This box was thoroughly tested by Héma-Québec and validated to meet the Canadian Standards Association criteria for blood product transport up to 24 hours.11 Each box transported by car contained one type of simulated blood product with a HOBO probe to monitor temperature. The composition of the ground transportation box and its internal content are described in Figure 2.
Figure 2.
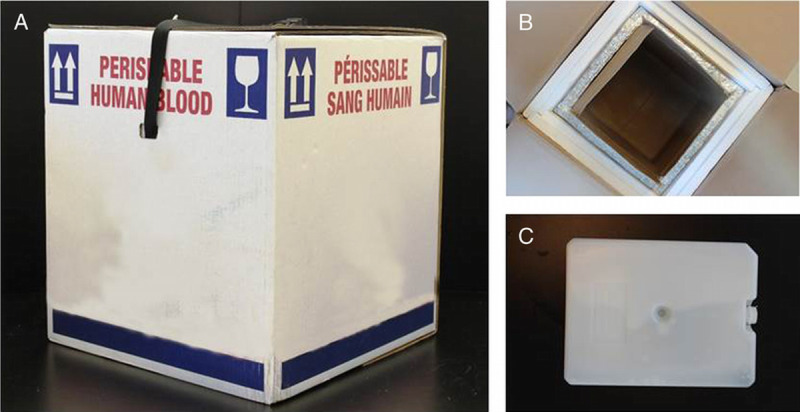
Ground transportation of blood products. (A) Standard Vacuum Insulated Panels (VIP) transport box for blood product. (B) Top view of the VIP container; the inner layer is made up of VIP panels protected by a foam middle layer, and the outer layer consist of cardboard. (C) An example of plastic phase change material used to maintain target temperature ranges with packed red blood cells (TTransition = 5°C), platelet concentrates (TTransition = 22°C), and fresh frozen plasma (TTransition = −22°C).
Time Monitoring
Transport times were monitored by two distinct methods: (1) time stamped messages on the (WhatsApp Inc., San Mateo, California) instant messaging app and (2) manual recording of time by a research assistant equipped with a chronometer and monitoring radio communications. Each transport was launched in the exact same way (Supplemental Digital Content, Supplementary Table 1, http://links.lww.com/TA/B810). The radio dispatcher launched each transport by saying “Activate Transport number X” while also sending a similar prewritten message on the WhatsApp data collection group. This dual communication system ensured redundancy and safety throughout data collection. The WhatsApp discussion was exported to a (TextEdit, Apple, California) format and saved once data collection was completed. Time stamped messages were used to determine car transport and drone flight times. The times required to deliver the simulated blood products from the MGH roof and from the parking lot to the hospital blood bank were also measured.
Statistical Analysis
The Wilcoxon test was used to compare the mean time of blood products delivery by UAV versus ground transportation. Descriptive statistics were used to analyze the secondary outcomes.
Safety Precautions
Redundant Communication Methods
Hospital Security, the local emergency medical service representative, and key research assistants were provided portable radios allowing communication on one common channel. Radio briefing was done, and radio communication tests were conducted before data collection. In addition, all research team members had access to their personal cell phone to make a phone call and/or use the research WhatsApp group called “Medi-Drones 2019” to communicate throughout the data collection process.
Security and Paramedic Support on Launching and Landing Sites
Local emergency medical service provided an ambulance staffed with primary care paramedics on standby at MGH during each data collection day; these paramedics were ready to respond in case of any emergency related to the research project.
UAV Operation Rehearsal
The entire research team and UAV pilots were present the day before data collection, which allowed for a thorough review of the research procedures and flight plan, as well as testing of UAV equipment.
Flight Cancellation for Weather Concerns
It was predetermined that UAV operation would be canceled in case of heavy precipitation, temperature above 40°C, or winds greater than 35 km/h. The drone operation supervisor was responsible for making the decision not to fly based on the most up-to-date weather forecast on the morning of each data collection day.
RESULTS
Drone Transportation
Data collection took place on September 23 and 24, 2019, in Montreal, Canada. The UAV operation was supervised by Mr. Philip Reece, CEO and founder of InDro Robotics, a company based in British Columbia, Canada, and specializing in commercial use of UAVs. The drone used for data collection was a modified M600pro with companion computer onboard and increased lift ability.
The total weight transported by the UAV for each blood product type including the water and light resistant container and PCMs was 5.4 kg for pRBCs and platelets, and 6.4 kg for FFP. The drone flew at a mean altitude of 69.82 m above take-off, and the distance flown for each flight was between 99,931.1 and 10,088.9 me depending on wind diversion. Drone speed throughout all nine flights was 10 m/s. All flights were conducted successfully without any adverse events or safety concerns reported. Two rain delays occurred. Data collection resumed as soon as rain ceased, and conditions were found to be safe by the drone operation supervisor.
Ground Transportation
The drivers followed directions provided by Waze and completed their transports without any incident. Ground transports were done at different times of the day but were always simultaneous with UAV transports.
Drone Versus Ground Transport Times
Unmanned aerial vehicle transportation times were significantly less than ground delivery times in all nine simulations (p < 0.0001). Data from the WhatsApp group were manually recorded by the research assistant monitoring radiocommunications (Table 2). The longest ground transport recorded (transport 3) was 00:38:10 minutes during rush hour (Table 3). Of note, there was greater variability in ground delivery times (00:25:45 to 00:38:10 minutes), whereas UAV flight durations were more constant throughout all nine flights (00:16:54 to 00:17:16 minutes). With both transport modalities, significant in-hospital transport delays (~4 minutes) were identified and largely related to the time spent waiting for an elevator to arrive.
TABLE 2.
Drone Versus Ground Transport Times for Nine Simulated Blood Product Deliveries
| Variable | Time, Mean ± SD | p |
|---|---|---|
| Radiocommunications data | ||
| Prehospital transport time | <0.0001 | |
| UAV | 00:17:06 ± 0:00:04 | |
| Car | 00:28:54 ± 0:01:12 | |
| In-hospital transit | 0.54 | |
| UAV | 00:04:04 ± 0:00:15 | |
| Car | 00:04:20 ± 0:00:19 | |
| Total transport time | <0.0001 | |
| UAV bag at blood bank | 00:21:11 ± 0:00:49 | |
| Car box at blood bank | 00:33:14 ± 0:03:56 | |
| WhatsApp data | ||
| Prehospital transport time | <0.0001 | |
| UAV | 00:17:05 ± 0:00:04 | |
| Car | 00:28:51 ± 0:01:12 | |
| In-hospital transit | 0.50 | |
| UAV | 00:04:04 ± 0:00:15 | |
| Car | 00:04:22 ± 0:00:21 | |
| Total transport time | <0.0001 | |
| UAV bag at blood bank | 00:21:00 ± 0:00:16 | |
| Car box at blood bank | 00:33:13 ± 0:01:19 |
TABLE 3.
Comparison of Drone and Ground Transport Times
| Variable | Transport Number | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Start time | |||||||||
| Date | Sep 23 | Sep 23 | Sep 23 | Sep 23 | Sep 24 | Sep 24 | Sep 24 | Sep 24 | Sep 24 |
| Time | 13:58:31 | 14:40:47 | 16:04:26 | 16:49:42 | 8:28:15 | 9:07:31 | 10:03:40 | 11:26:29 | 13:04:41 |
| Radiocommunications data | |||||||||
| Type of blood product | pRBCs | Platelets | FFP | Platelets | FFP | pRBCs | FFP | pRBCs | Platelets |
| UAV landed | 16:54.5 | 17:04.5 | 17:06.8 | 17:16.2 | 17:16.3 | 17:21.4 | 17:04.4 | 16:47.3 | 17:10.6 |
| UAV bag at blood bank | 22:02.2 | 21:37.5 | 20:59.2 | 21.51.1 | 20:40.9 | 22:09.9 | 21:11.1 | 19:39.3 | 20:33.1 |
| UAV hospital delay | 05:07.7 | 04:33.1 | 03:52.3 | 04:34.9 | 03:24.5 | 04:48.4 | 04:06.7 | 02:52.0 | 03:22.6 |
| Driver arrived | 22:07.7 | 30:34.4 | 34:21.5 | 33:09.0 | 29:13.1 | 27:21.1 | 28:21.7 | 28:23.9 | 26:33.9 |
| Car box at blood bank | 25:45.0 | 36:57.4 | 38:10.0 | 36:57.4 | 34:38.3 | 31:04.8 | 32:56.2 | 32:17.7 | 30:20.0 |
| Car hospital delay | 03:37.3 | 06:23.0 | 03:48.5 | 03:48.4 | 05:25.2 | 03:43.7 | 04:34.6 | 03:53.8 | 03:46.1 |
| Delta (car time − UAV time) | 03:42.8 | 15:19.9 | 17:10.9 | 15:06.3 | 13:57.4 | 08:54.9 | 11:45.2 | 12:38.4 | 09:46.9 |
| WhatsApp data | |||||||||
| Type of blood product | pRBCs | Platelets | FPP | Platelets | Plasma | pRBCs | Plasma | pRBCs | Platelets |
| UAV landed | 16:58.0 | 16:57.0 | 17:05.0 | 17:14.0 | 17:15.0 | 17:24.0 | 17:02.0 | 16:46.0 | 17:12.0 |
| UAV bag at blood bank | 22:05.0 | 21:29.0 | 21:04.0 | 21:51.0 | 20:38.0 | 22:08.0 | 21:08.0 | 19:37.0 | 20:33.0 |
| UAV hospital delay | 05:07.0 | 04:32.0 | 03:59.0 | 04:37.0 | 03:23.0 | 04:44.0 | 04:06.0 | 02:51.0 | 03:21.0 |
| Driver arrived | 22:11.0 | 30:31.0 | 34:18.0 | 33:09.0 | 29:10.0 | 27:18.0 | 28:14.0 | 28:18.0 | 26:32.0 |
| Car box at blood bank | 25:47.0 | 37:13.0 | 38:05.0 | 36:57.0 | 34:34.0 | 31:07.0 | 32:49.0 | 32:11.0 | 30:17.0 |
| Car hospital delay | 03:36.0 | 06:42.0 | 03:47.0 | 03:48.0 | 05:24.0 | 03:49.0 | 04:35.0 | 03:53.0 | 03:45.0 |
| Delta (car time − UAV time) | 03:36.0 | 15:44.0 | 17:01.0 | 15:06.0 | 13.56.0 | 08:59.0 | 11:41.0 | 12:34.0 | 09:44.0 |
Temperature Monitoring
Temperature was monitored successfully throughout each transport by UAV and by car. As expected, the internal temperature of all simulated blood products was maintained within their respective acceptable range for all ground transport experiments as the current thermoregulation containers at Héma-Québec were developed and thoroughly validated for up to 24 hours of transport (Table 4; Supplemental Digital Content, Supplementary Fig. 1, http://links.lww.com/TA/B809). More specifically, pRBCs were conditioned (1–6°C) before being packed. Their mean ± SD initial temperature was 3.7°C ± 0.2°C and did not change significantly during each drive (n = 3). A maximum of 0.7% variation from the initial pRBC temperature was observed. Platelet concentrates (n = 3) were also conditioned at their storage temperature (20–24°C) before each drive, resulting in a mean ± SD initial temperature of 21.0°C ± 0.8°C. The highest temperature variation from departure to arrival was <1°C. Fresh frozen plasma units (n = 3) were conditioned at T = −20°C before packaging, and their mean ± SD initial temperature was measured at −20.5°C ± 0.3°C. As observed with pRBCs and PCs, the internal temperature of FFP units did not significantly change during any ground transports, as the warmer FFP unit was measured at −20.2°C with a temperature variation close to the experimental error (<2%).
TABLE 4.
Comparison of Simulated Blood Product Temperatures With Transport by Drone Versus Ground
| Blood Product | Ground* | Air** | ||||
|---|---|---|---|---|---|---|
| TI, °C | TF, °C | Variation,† % | TI, °C | TF, °C | Variation,† % | |
| pRBC | ||||||
| Transport 1 | 3.4 | 3.4 | 0.0 | 4.4 ± 0.2 (4.3–4.6) | 4.7 ± 0.1 (4.5–4.8) | 4.8 |
| Transport 6 | 3.8 | 3.8 | 0.0 | 4.3 ± 0.1 (4.3–4.4) | 4.5 ± 0.1 (4.4–4.6) | 3.3 |
| Transport 8 | 3.8 | 3.9 | 0.7 | 4.4 ± 0.1 (4.2–4.5) | 4.6 ± 0.2 (4.5–4.8) | 5.0 |
| PC | ||||||
| Transport 2 | 20.5 | 20.6 | 0.1 | 21.0 ± 0.1 (21.0–21.2) | 21.0 ± 0.1 (21.0–21.2) | 0.0 |
| Transport 4 | 20.6 | 20.6 | 0.2 | 21.5 ± 0.1 (21.5–21.6) | 21.5 ± 0.1 (21.4–21.6) | 0.2 |
| Transport 9 | 22.0 | 22.0 | 0.3 | 22.1 ± 0.1 (22.1–22.2) | 22.2 ± 0.2 (22.0–22.4) | 0.3 |
| FFP | ||||||
| Transport 3 | −20.2 | −20.4 | 1.2 | −17 ± 2 (−18 to −16) | −16 ± 2 (−17 to −14) | 9.0 |
| Transport 5 | −20.7 | −20.6 | 0.5 | −20.6 ± 0.9 (−21.3 to −20.0) | −19.5 ± 0.7 (−19.9 to −19.0) | 6.0 |
| Transport 7 | −20.5 | −20.4 | 0.2 | −19.4 ± 0.4 (−19.7 to −19.2) | −18.5 ± 0.8 (−19.1 to −17.9) | 5.4 |
*n = 1.
**n = 3.
†Variation = (TI – TF)/TI × 100.
In the same fashion as for ground transport, each simulated product used for UAV transport was conditioned at its storage temperature before packaging. The mean ± SD initial temperature for pRBCs, calculated over all units (n = 9), was 4.4°C ± 0.1°C with a maximum 5% mean temperature variability from departure to landing. The internal temperature of all pRBC units remained within the acceptable range. For PCs, the mean ± SD initial temperature was 21.6°C ± 0.5°C for all units (n = 9). The internal temperature of all units did not change significantly during transport, and the maximum variability observed was 0.3%. At the time of packaging, the mean ± SD initial FFP temperature was calculated at −19°C ± 2°C for all units (n = 9), and the greatest internal temperature variability observed was from −17°C ± 2°C to a final −16°C ± 2°C, corresponding to the highest thermal variation observed (9%).
DISCUSSION
This is the first published study comparing ground versus UAV transport of simulated blood products to the blood bank of a hospital located in a densely populated urban area. Blood product temperatures were monitored successfully throughout each transport by UAV and by car. Unmanned aerial vehicle transportation time was significantly faster than ground delivery in all nine comparisons. Furthermore, the payload carried by UAV was heavier than has been reported in previous published studies (up to 6.4 kg).6,9
Data collection was completed prospectively without any safety issues. No technical problems were reported by either the drone pilots or the ground transportation team. There were no legal issues during data collection since all authorizations were obtained before conducting the study. Time was recorded accurately using two distinct methods. This study is limited by the use of simulated as opposed to real blood products, and flights were done within visual line of sight using the same launching and landing site.
The UAV flew in a predetermined loop, which overestimated the real transport time from the Héma-Québec blood product distribution center to MGH. Indeed, a linear flight would have allowed the UAV to fly at a higher speed, shortening the transport time by approximately 15%. In addition, the total distance flown by the drone on each flight was slightly longer than the actual aerial distance between the blood product distribution center and MGH (10 km vs. 9.8 km), which also contributed to overestimating the true flight time.
On the other hand, launching and landing from the same site are technically and logistically easier and possibly faster than doing it from two separate locations. Indeed, launching from a separate location than the landing site requires staff and batteries on two sites and possibly transporting equipment from the landing site back to the launching site between flights. Furthermore, data collection was conducted with pilots operating within visual line of sight. A linear flight from the Héma-Québec distribution center to MGH requires flying beyond visual line of sight (BVLOS), which is technically more difficult and potentially requires a delay to obtain clearance for takeoff from air traffic control. Since data were collected for this study in the context of a simulation with no real patients awaiting blood product delivery, it was felt that flying BVLOS over a densely populated area posed a safety concern. While the requirement that drones are operated within a visual line of sight limits the use of medical drones in many countries, regulators in Canada are increasingly issuing BVLOS permissions to first responders and industry users. This is a promising trend that is likely to spread to other countries, as UAV technology continues to advance and gain wider adoption.
At present, ground vehicles are able to transport much greater quantities of blood products compared with drones, and they can also carry multiple types of blood products at once. Multiple UAVs would be required to transport the equivalent blood supply carried by one ground vehicle. It is expected that technological advancements will eventually allow drones to transport much heavier payloads. Further developments in specialized UAV transport containers may eventually allow transport of different blood products on the same flight. Use of UAVs for interhospital transport of blood products is another topic that warrants further investigation.
Weather issues and particularly heavy precipitation can threaten the reliability of UAV transportation for daily operations. This may change with further technical developments in the drone industry. Unmanned aerial vehicles may potentially be most useful in case of mass casualty incidents when road closures or major traffic prevents efficient ground transportation of lifesaving blood products. Since operation of medical drones over populated areas can pose even greater risk in the event of a crash due to the nature of the payload, Transport Canada currently restricts such missions to real-life emergency situations (i.e., not simulated exercises or testing). Canadian Aviation Regulations restrict transport of payloads that include biohazardous material unless the operation is conducted in accordance with a special flight operations certificate.12 As the use of medical drones becomes more prevalent, these regulations may need to be modified to account for safe and secure packaging of potentially hazardous payloads and appropriate training for medical drone operators.
Transporting simulated FFP represented the biggest challenge precisely because of the greater temperature disparity with the environment. During winter, maintaining platelets within the targeted temperature range of 20°C to 24°C would be very challenging. Despite the higher thermal stress encountered by the shipping container, the use of PCM 20 on both sides of each FFP unit made it possible to keep all products frozen for the transport durations.
Simulated blood products were used in this study to follow a stepwise approach to evaluating the feasibility of UAV transport of blood products in an urban area. The transport container needed to be developed, validated, and tested. Furthermore, a variety of stakeholders who were not accustomed to working together had to learn to collaborate for research purposes. Amukele et al.9 has already demonstrated the stability of blood products after UAV flights away from populated areas. Further studies assessing UAV transport of real blood products in populated areas are warranted.
CONCLUSIONS
A drone delivering a payload weighing up to 6.4 kg launched from a level 1 trauma center and flew safely in an urban setting without any adverse events. Three different types of simulated blood products were successfully transported by UAV using dedicated shipping containers with thermoregulation capabilities. Unmanned aerial vehicle transportation of simulated blood products was significantly faster than ground transportation regardless of the time of the day, with a most striking time difference occurring during rush hour. This study also identified major delays in transporting blood products within the hospital itself, largely related to time spent waiting for elevators. The simulated blood product temperature was monitored during each UAV flight and remained within their respective acceptable ranges throughout transport. Considering weather and payload weight limitations, UAV drone transportation of blood products may currently be best used during mass casualty incidents, especially if ground transportation is limited.
Supplementary Material
AUTHORSHIP
V.H., R.F., D.B., F.d.C., and M.N. contributed to designing the study. V.H., R.F., D.B., M.-A.R., M.M., and F.G.-B. analyzed the data. All authors contributed to data collection and interpretation of the study results. All authors critically reviewed the article for important intellectual content and approved the final article.
ACKNOWLEDGMENT
We thank Timbercreek Asset Management/Timbercreek Communities and Ms. Nataliya Solomonyuk for their support in the realization of our project. We also thank Pierre-Marc Legris, Mohamed Merheb, Daoud Lairy, Jean-Sébastien Lavigne, Salah Hadjamara, and David Iannuzzi of the MUHC for their logistical assistance and Eddy Afram of the Corporation d’Urgences-santé for his contribution to safe data collection. Guylaine Desnoyers should also be thank for her expertise in blood banking. The authors also thank Percipient Research & Consulting for assistance with language editing.
DISCLOSURE
The authors declare no conflicts of interest. The Montreal General Hospital Foundation, Chaire de recherche et d’innovation en médecine d’urgence, Université Laval–Dessercom–CISSS Chaudière-Appalaches, Hema-Québec, Urgences-santé, EXO Tactik, and County of Renfrew Paramedic Service provided funding and in-kind contributions to this project.
Footnotes
Published online: October 2, 2020.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Valerie Homier, Email: valerie.homier@mcgill.ca.
Danny Brouard, Email: Danny.Brouard@hema-quebec.qc.ca.
Michael Nolan, Email: mnolan@countyofrenfrew.on.ca.
Marie-Andrée Roy, Email: maroyy@yahoo.ca.
Patricia Pelletier, Email: patricia.pelletier@mcgill.ca.
Melissa McDonald, Email: melissa.mcdonald@mail.mcgill.ca.
François de Champlain, Email: fdecha@videotron.ca.
Elene Khalil, Email: elene.khalil@mcgill.ca.
Frederic Grou-Boileau, Email: frederic.grou-boileau@mail.mcgill.ca.
REFERENCES
- 1.The Lancet Haematology Look! Up in the sky! It’s a bird. It’s a plane. It’s a medical drone! Lancet Haematol. 2017;4(2):e56. [DOI] [PubMed] [Google Scholar]
- 2.Services Québec Important exercice de simulation code orange en cours dans le réseau montréalais de la santé. Updated October 18, 2018. Available at: http://www.fil-information.gouv.qc.ca/Pages/Article.aspx?idArticle=2610181960. Accessed October 16, 2018.
- 3.Claesson A Svensson L Nordberg P, et al. Drones may be used to save lives in out of hospital cardiac arrest due to drowning. Resuscitation. 2017;114:152–156. [DOI] [PubMed] [Google Scholar]
- 4.Techspot Ripper lifeguard drone rescues stranded swimmers in Australia. Updated January 18, 2018. Available at: https://www.techspot.com/news/72822-little-ripper-lifeguard-drone-rescues-stranded-swimmers-australia.html. Accessed June 14, 2018.
- 5.Claesson A, Backman A, Ringh M, Svensson L, Nordberg P, Djarv T, Hollenberg J. Time to delivery of an automated external defibrillator using a drone for simulated out-of-hospital cardiac arrests vs emergency medical services. JAMA. 2017;317(22):2332–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheskes S, McLeod S, Nolan M, Snobelen P, Vaillancourt C, Brooks S, Dainty K, Chan T, Drennan I. Improving access to automated external defibrillators in rural and remote settings: a drone delivery feasibility study. J Am Heart Assoc. 2020;9(14):e016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amukele TK, Hernandez J, Snozek CLH, Wyatt RG, Douglas M, Amini R, Street J. Drone transport of chemistry and hematology samples over long distances. Am J Clin Pathol. 2017;148(5):427–435. [DOI] [PubMed] [Google Scholar]
- 8.Amukele TK, Street J, Carroll K, Miller H, Zhang SX. Drone transport of microbes in blood and sputum laboratory specimens. J Clin Microbiol. 2016;54(10):2622–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amukele T, Ness PM, Tobian AA, Boyd J, Street J. Drone transportation of blood products. Transfusion. 2017;57(3):582–588. [DOI] [PubMed] [Google Scholar]
- 10.Mesar T, Lessig A, King DR. Use of drone technology for delivery of medical supplies during prolonged field care. Journal of special operations medicine: a peer reviewed journal for SOF medical professionals. 2018;18:34–35. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Standards Association CAN/CSA-Z902-15 A National Standard of Canada. Blood and Blood Components: CSA 2015. Available at: https://www.scc.ca/en/standardsdb/standards/28352. Accessed June 13, 2020.
- 12.Government of Canada Canadian Aviation Regulations. Updated July 8, 2020. Available at: https://laws-lois.justice.gc.ca/eng/regulations/SOR-96-433/FullText.html#h-789. Accessed September 12, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


