Abstract
Background:
Mentor MemoryGel Breast Implants were approved by the U.S. Food and Drug Administration in November of 2006. Patients in the Core clinical study supporting this approval were followed for 10 years.
Methods:
This prospective, multicenter, clinical study included primary augmentation, revision augmentation, primary reconstruction, and revision reconstruction patients implanted with smooth or Siltex Texture MemoryGel Implants. Incidence, severity, and method of resolution for all postoperative complications were assessed on per-patient and per-implant bases. The primary effectiveness endpoints were overall mean change in chest circumference and bra cup size following the implantation procedure.
Results:
Primary augmentation (n = 552), revision augmentation (n = 145), primary reconstruction (n = 251), and revision reconstruction (n = 60) patients were enrolled in the study. Kaplan-Meier estimated 10-year cumulative incidence rates for key complications at the subject level for Baker grade III/IV capsular contracture were as follows: primary augmentation, 12.1 percent; revision augmentation, 24.4 percent; primary reconstruction, 20.5 percent; and revision reconstruction, 36.9 percent. For infection, rates were as follows: primary augmentation, 1.6 percent; revision augmentation, 1.4 percent; primary reconstruction, 6.2 percent; and revision reconstruction, 0 percent. For explantation with or without replacement, rates were as follows: primary augmentation, 11.6 percent; revision augmentation, 24.1 percent; primary reconstruction, 33.4 percent; and revision reconstruction; 37.8 percent. For rupture, rates were as follows: primary augmentation, 24.2 percent; revision augmentation, 23.7 percent; primary reconstruction, 32.7 percent; and revision reconstruction, 38.7 percent. For any reoperation, rates were as follows: primary augmentation, 25.5 percent; revision augmentation, 43.6 percent; primary reconstruction, 49.0 percent; and revision reconstruction, 50.7 percent.
Conclusion:
The results of this study demonstrate that MemoryGel Implants are safe and effective for use in women undergoing breast augmentation or reconstruction.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, IV.
MemoryGel Breast Implants (Mentor Worldwide LLC, Irvine, Calif.), approved by the U.S. Food and Drug Administration in 2006 for breast augmentation in women aged 22 years and older and for breast reconstruction in women of any age, consist of a single-lumen, round silicone elastomer shell, with a patch on the posterior side, and filled with a cohesive silicone gel. The 10-year prospective clinical study, initiated in September of 2000, was designed to assess the safety and effectiveness of MemoryGel Implants in women undergoing primary breast augmentation, primary breast reconstruction, or revision surgery. These findings extend the previously reported 3- and 6-year results,1,2 which demonstrated that MemoryGel Implants were safe and effective. In this article, we present final safety and effectiveness data from the MemoryGel Core Study with 10 years of follow-up.
PATIENTS AND METHODS
Study Design
The study was designed and conducted in accordance with the U.S. Food and Drug Administration’s then-current draft of “Guidance for Saline, Silicone Gel, and Alternative Breast Implants: Final Guidance for Industry and Food and Drug Administration Staff” (final version dated February 11, 2003), and safety analyses were conducted in accordance with the November 17, 2006 “Saline, Silicone Gel, and Alternative Breast Implants: Guidance for Industry and FDA Staff” that superseded the 2003 document (https://www.fda.gov/media/71081/download). The study evaluation schedule summary, measures of patient satisfaction, assessments of quality of life, protocol for magnetic resonance imaging substudy, and statistical analyses have been described elsewhere.1 Briefly, at each scheduled follow-up visit through 10 years, the following procedures and evaluations were performed: nipple and breast sensitivity assessment, breast measurements, capsular contracture assessment, concomitant medications, quality-of-life questionnaires (at 1-, 2-, 3-, 4-, 6-, 8-, and 10-year visits), adverse event evaluation, magnetic resonance imaging scan on subset of patients (1-, 2-, 4-, 6-, 8-, and 10-year visits), and Rheumatic Disease Diagnosis Questionnaire (if investigator believed, in his or her medical opinion, that the patient’s symptoms warranted a rheumatologic examination, rheumatologic confirmation was to be performed).
Patients
Eligible patients were women who were candidates for primary breast augmentation, primary breast reconstruction, or revision surgery. Although the study presented here enrolled augmentation patients who were aged 18 years or older, primary augmentation with MemoryGel Implants is currently indicated for women aged 22 years or older in the United States. Each patient provided written informed consent, and the study was approved by the institutional review board at each site. Women who were pregnant, had nursed a child within 3 months of study enrollment, were previously implanted with any silicone implant other than breast implants, had a confirmed diagnosis of rheumatic disease, currently had a condition that could compromise or complicate wound healing (except reconstruction patients), had a diagnosis of active cancer (only augmentation patients), had an infection or abscess, demonstrated tissue characteristics incompatible with implant placement (e.g., tissue damage resulting from radiation therapy, inadequate tissue, or compromised vascularity), had a premalignant breast disease without a subcutaneous mastectomy, had an untreated or inappropriately treated breast malignancy, without mastectomy, or who had any condition that would make magnetic resonance imaging prohibitive were excluded from this study.
Safety Analyses
All patients undergoing implantation with a study device were included in the safety analysis. If a study device was explanted, data up to and including the date of the explantation were included in all analyses. As a condition of the 2006 U.S. Food and Drug Administration approval, patients who underwent explantation continued to be followed for safety through 10 years, even if a study device was not reimplanted.
Incidence of postoperative complications were analyzed using the Kaplan-Meier method. All event rates presented here are at the subject level, unless otherwise specified. The severity, resolution, treatment required, and causality of the complications were assessed in addition to reoperations and explantations.
Magnetic Resonance Imaging Substudy
A subset of randomly selected patients underwent magnetic resonance imaging at the 1-, 2-, 4-, 6-, 8-, and 10-year visits after implantation in an attempt to estimate the overall rupture rate (magnetic resonance imaging cohort A). However, beginning in November of 2006, as a condition of U.S. Food and Drug Administration approval, all patients enrolled in the study from this point onward were required to undergo magnetic resonance imaging evaluation at the same times as magnetic resonance imaging cohort A (i.e., 6, 8, and 10 years after surgery; magnetic resonance imaging cohort B). An implant was considered to be ruptured if the investigator reported rupture as an adverse event, the most recent magnetic resonance imaging evaluation indicated that on implant evaluation there was rupture or indeterminate rupture, or on soft-tissue evaluation there was a judgment of definite extracapsular silicone or indeterminate extracapsular silicone. If the implant was explanted and returned to and physically examined by Mentor and determined not to be ruptured, it was not counted as a rupture. Kaplan-Meier survival analyses of time to rupture were performed to estimate the cumulative incidence of rupture.
Effectiveness Analyses
The primary effectiveness endpoints were the overall mean change in chest circumference and the overall mean increase in bra cup size, to be assessed principally in the primary augmentation cohort. The overall mean changes and standard deviation from the preoperative assessment were calculated for circumferential chest size and cup size increase. The Wilcoxon signed rank test was performed to test for statistical significance.
Secondary effectiveness was based on changes in quality of life. Each quality-of-life endpoint was summarized using descriptive statistics (mean, median, standard deviation, and minimum and maximum). Global patient satisfaction, assessed by asking the patient whether she would make the same decision to undergo breast implant surgery, was an additional effectiveness endpoint. Frequency counts, percentages, and 95 percent confidence intervals for the proportion of patients who would make the same decision to undergo surgery were tabulated for each follow-up visit. Immediate postmastectomy patients were excluded from these analyses for the primary reconstruction cohort and the overall patient population.
RESULTS
Patient Demographics
A total of 1008 patients (receiving 1898 implants) across 48 sites in the United States were included in four cohorts: primary augmentation (n = 552 patients), revision augmentation (n = 145), primary reconstruction (n = 251), and revision reconstruction (n = 60). The overall 10-year follow-up rate across all cohorts was 62 percent, equivalent to approximately 95.3 percent patient retention from each year prior (primary augmentation, 57 percent; revision augmentation, 64 percent; primary reconstruction, 73 percent; and revision reconstruction, 67 percent). Demographic characteristics are summarized in Table 1.
Table 1.
Demographic Characteristics*
| Characteristic | Augmentation | Reconstruction | ||
|---|---|---|---|---|
| Primary (n = 552) | Revision (n = 145) | Primary (n = 251) | Revision (n = 60) | |
| Median age, yr | 34 | 43 | 46 | 51 |
| Age range, yr | 18–65 | 20–63 | 18–79 | 29–72 |
| Race | ||||
| Caucasian | 483 (87.5) | 134 (92.4) | 231 (92.0) | 56 (93.3) |
| Asian | 17 (3.1) | 2 (1.4) | 3 (1.2) | 1 (1.7) |
| African American | 11 (2.0) | 2 (1.4) | 7 (2.8) | 2 (3.3) |
| Other | 41 (7.4) | 7 (4.8) | 10 (4.0) | 1 (1.7) |
| Marital status | ||||
| Married | 313 (56.7) | 86 (59.3) | 173 (68.9) | 40 (66.7) |
| Never married | 135 (24.5) | 25 (17.2) | 35 (13.9) | 5 (8.3) |
| Divorced | 81 (14.7) | 26 (17.9) | 30 (12.0) | 13 (21.7) |
| Separated | 17 (3.1) | 3 (2.1) | 5 (2.0) | 1 (1.7) |
| Widowed | 6 (1.1) | 5 (3.4) | 8 (3.2) | 1 (1.7) |
| Education | ||||
| <12 yr | 7 (1.3) | 0 (0) | 3 (1.2) | 2 (3.3) |
| High school graduate | 74 (13.4) | 26 (17.9) | 42 (16.7) | 9 (15.0) |
| Some college | 215 (38.9) | 56 (38.6) | 66 (26.3) | 20 (33.3) |
| College graduate | 189 (34.2) | 45 (31.0) | 85 (33.9) | 16 (26.7) |
| Postgraduate | 60 (10.9) | 17 (11.7) | 47 (18.7) | 9 (15.0) |
| Missing | 7 (1.3) | 1 (0.7) | 8 (3.2) | 4 (6.7) |
| Previous breast surgery (excluding mastectomy) | ||||
| No | 535 (96.9) | 89 (61.4) | 180 (71.7) | 22 (36.7) |
| Yes | 17 (3.1) | 55 (37.9) | 71 (28.3) | 38 (63.3) |
| Missing | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) |
| Smoking history | ||||
| Never smoked | 328 (59.4) | 79 (54.5) | 151 (60.2) | 29 (48.3) |
| Currently smoker | 107 (19.4) | 25 (17.2) | 21 (8.4) | 8 (13.3) |
| Former smoker | 117 (21.2) | 41 (28.3) | 79 (31.5) | 23 (38.3) |
*Values are No. (%) unless otherwise stated.
Effectiveness
For patients in the primary augmentation cohort and in the other three cohorts, the overall mean changes over the course of the study in circumferential chest size were positive and highly statistically significant (overall mean change of all cohorts was 6.1 cm; p < 0.001). For the primary augmentation cohort, there was a statistically significant mean increase of 1.8 bra cup sizes from baseline (p < 0.0001).
At the 10-year follow-up visit, 97.6 percent of patients who answered the question (523 of 536) indicated that they would make the same decision to undergo breast implant surgery (primary augmentation, 97.1 percent; revision augmentation, 98.8 percent; primary reconstruction, 99.1 percent; and revision reconstruction, 94.4 percent). Similarly, at 10-year follow-up, among patients who had any type of reoperation, 98.2 percent indicated that they would make the same decision to undergo breast implant surgery.
Postoperative Complications and Resolution
The 10-year Kaplan-Meier estimated cumulative incidence rates for key postoperative complications are shown in Table 2 and Figure 1. In the overall patient population, 48.3 percent of reported complications (excluding rupture/indeterminate rupture) did not receive any treatment, 36.9 percent were treated by a secondary procedure, and 14.3 percent were treated with medication. Only 0.8 percent of the complications resulted in hospitalization.
Table 2.
Ten-Year Kaplan-Meier Estimated Cumulative Incidence Rates of Occurrence of Key Complications
| Complications | Augmentation | Reconstruction | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary (n = 552) | Revision (n = 145) | Primary (n = 251) | Revision (n = 60) | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Key complications | ||||||||
| Any reoperation | 25.5 | 21.9–29.5 | 43.7 | 35.8–52.4 | 49.0 | 42.6–55.7 | 50.7 | 38.7–64.0 |
| Implant-related reoperation* | 11.94 | 9.39–15.13 | 21.03 | 15.09–28.89 | 23.00 | 17.60–29.73 | 28.41 | 18.07–42.91 |
| Capsular contracture Baker grade III/IV | 12.1 | 9.6–15.2 | 24.4 | 18.1–32.5 | 20.5 | 15.5–26.7 | 36.9 | 25.0–52.2 |
| Explantation with or without replacement | 11.6 | 9.1–14.8 | 24.1 | 17.7–32.3 | 33.4 | 27.6–40.1 | 37.8 | 26.7–51.7 |
| Explantation with replacement with study device | 7.4 | 5.4–10.2 | 13.6 | 8.6–21.1 | 19.8 | 14.9–25.9 | 24.8 | 15.0–39.2 |
| Rupture rates for MRI cohort A patients (suspected or confirmed) | 24.2 | 17.0–33.9 | 23.7 | 12.3–42.8 | 32.7 | 23.2–44.8 | 38.8 | 19.1–67.9 |
| Rupture rates for MRI cohort A implants (suspected or confirmed) | 14.9 | 10.7–20.6 | 16.5 | 9.3–28.3 | 24.3 | 17.4–33.3 | 25.8 | 12.1–49.8 |
| Rupture rates for MRI cohort B patients (suspected or confirmed) | 21.4 | 15.3–29.5 | 7.5 | 2.5–21.6 | 36.1 | 24.3–51.4 | 43.9 | 22.3–73.5 |
| Rupture rates for MRI cohort B implants (suspected or confirmed) | 12.5 | 9.1–17.2 | 6.3 | 2.7–14.6 | 28.1 | 19.2–40.0 | 44.4 | 24.8–70.1 |
| Infection | 1.6 | 0.9–3.1 | 1.4 | 0.4–5.5 | 6.2 | 3.8–10.1 | 0 | |
| Other complications ≥5% in at least one cohort | ||||||||
| Nipple sensation changes† | 12.8 | 10.2–16.0 | 13.6 | 8.9–20.4 | 2.1 | 0.9–5.0 | 4.0 | 1.0–15.2 |
| Breast mass | 5.6 | 3.9–7.9 | 6.0 | 3.0–11.6 | 8.6 | 5.5–13.4 | 5.2 | 1.7–15.1 |
| Breast pain† | 2.9 | 1.8–4.8 | 3.2 | 1.2–8.2 | 5.2 | 2.9–9.2 | 5.2 | 1.7–15.2 |
| Patient dissatisfaction | 0.4 | 0.1–1.5 | 3.6 | 1.5–8.5 | 4.8 | 2.5–9.2 | 9.0 | 3.4–23.0 |
| Granuloma | 0.2 | 0.0–1.3 | 2.3 | 0.8–7.1 | 0 | 5.0 | 1.6–14.7 | |
| Implant malposition/displacement† | 1.0 | 0.4–2.5 | 2.3 | 0.7–7.0 | 2.3 | 1.0–5.5 | 6.7 | 2.6–16.9 |
| Metastatic disease | 0 | 0 | 6.9 | 4.2–11.2 | 3.8 | 1.0–14.6 | ||
| Lack of projection | 0 | 0 | 1.0 | 0.2–3.8 | 5.5 | 1.8–16.3 | ||
| Symmastia | 0.2 | 0.0–1.7 | 0 | 0 | 5.0 | 1.6–14.7 | ||
MRI, magnetic resonance imaging.
*Implant-related complications included capsular contracture, rippling, infection, hematoma/seroma, and rupture.
†Mild occurrences excluded.
Fig. 1.
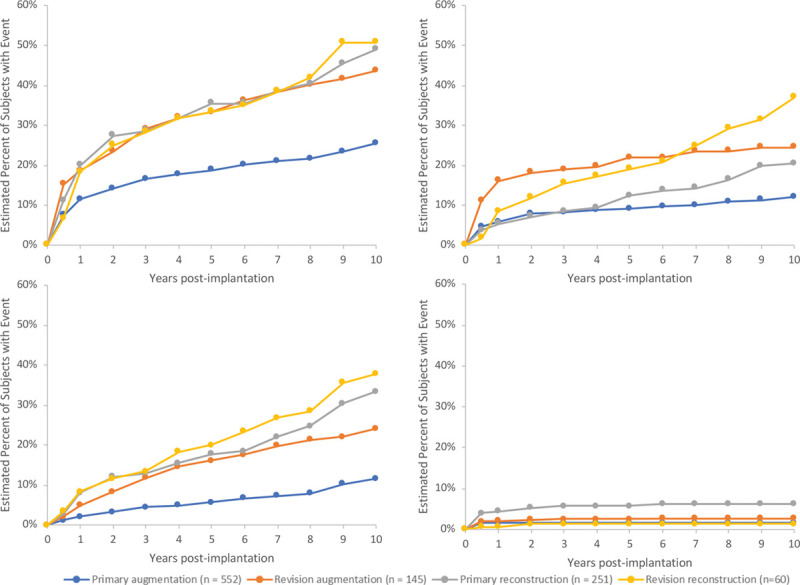
Kaplan-Meier estimated cumulative incidence rates of (above, left) reoperation, (above, right) capsular contracture Baker grade III and IV, (below, left) explantation with or without replacement, and (below, right) infection.
Reoperations
The 10-year estimated cumulative incidence rates for any reoperation and implant-related complications (including only reoperations because of capsular contracture, rippling, infection, hematoma/seroma, and rupture) are listed in Table 2. The primary reasons for reoperation that occurred at a rate of greater than or equal to 10 percent in at least one patient cohort were capsular contracture (Baker grade II, III, or IV), breast mass, rupture, and asymmetry (Fig. 2). The number of reoperations and additional operations are listed in Table 3. Types of additional surgical procedures through 10 years that occurred at a rate of greater than or equal to 10 percent in at least one patient cohort are listed in Table 4.
Fig. 2.
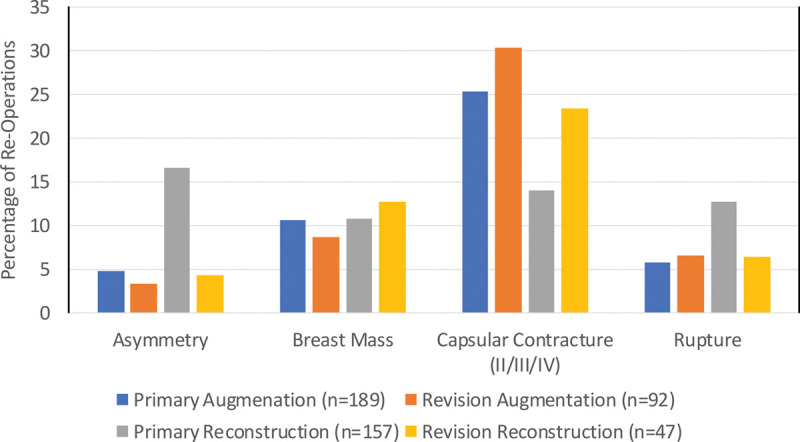
Primary reason for reoperation through 10 years (frequency ≥10 percent in at least one cohort).
Table 3.
Numbers of Reoperations and Additional Surgical Procedures through 10 Years
| Augmentation | Reconstruction | |||
|---|---|---|---|---|
| Primary | Revision | Primary | Revision | |
| No. | 552 | 145 | 251 | 60 |
| No. of patients who had reoperations | 133 | 61 | 115 | 30 |
| No. of reoperations | 189 | 92 | 157 | 47 |
| Additional surgical procedures | 329 | 172 | 320 | 94 |
Table 4.
Types of Additional Surgical Procedures through 10 Years*
| Primary Augmentation | Revision Augmentation | Primary Reconstruction | Revision Reconstruction | |
|---|---|---|---|---|
| No. | 329 | 172 | 320 | 94 |
| Explantation | 105 | 61 | 116 | 31 |
| Explantation with replacement with study device | 61 | 31 | 62 | 19 |
| Explantation without replacement with study device | 44 | 30 | 54 | 12 |
| Capsulectomy | 55 | 29 | 25 | 10 |
| Capsulotomy | 31 | 23 | 39 | 5 |
| Biopsy | 29 | 13 | 23 | 13 |
*Greater than or equal to 10% in at least one cohort.
Explantation
The most commonly reported reasons for explantation that occurred at a rate of greater than or equal to 10 percent in at least one patient cohort through 10 years were size change, capsular contracture (Baker grade II, III, or IV), rupture, and asymmetry (Fig. 3). Of the 189 patients whose devices were explanted, 108 (57.1 percent) were reimplanted with a study device.
Fig. 3.
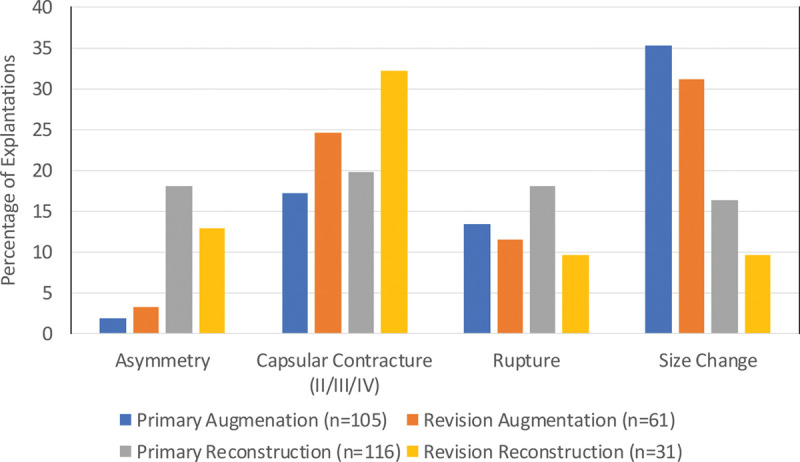
Primary reason for explantation through 10 years (frequency ≥10 percent in at least one cohort).
Rupture
Patient accounting for both the original magnetic resonance imaging substudy cohort (magnetic resonance imaging cohort A) and the non–magnetic resonance imaging cohort who underwent magnetic resonance imaging evaluation starting in 2006 as a condition of approval (magnetic resonance imaging cohort B) is presented in Figure 4. For the 420 patients enrolled in magnetic resonance imaging cohort A (primary augmentation, n = 202; revision augmentation, n = 56; primary reconstruction, n = 134; and revision reconstruction, n = 28), the overall 10-year follow-up rate was 53 percent (primary augmentation, 46 percent; revision augmentation, 48 percent; primary reconstruction, 68 percent; and revision reconstruction, 58 percent). The overall Kaplan-Meier estimated cumulative rupture rates (suspected or confirmed) for patients and implants are presented in Table 2 and Figure 5. There were 77 suspected or confirmed ruptured implants (primary augmentation, n = 31; revision augmentation, n = 11; primary reconstruction, n = 29; and revision reconstruction, n = 6) among 64 patients (primary augmentation, n = 25; revision augmentation, n = 8; primary reconstruction, n = 25; and revision reconstruction, n = 6) in magnetic resonance imaging cohort A. Of these 77 implants in 64 patients, at 6-year follow-up, rupture was detected in 20 implants in 17 patients, and at 8-year follow-up, rupture was detected in 40 implants in 33 patients. Mean time to rupture for the 77 implants was 7.9 years. Seventy-five of the 77 implants were considered silent ruptures. The overall Kaplan-Meier estimated cumulative silent rupture rates at 10 years were 27.3 percent and 18.1 percent for patients and implants, respectively. The overall Kaplan-Meier estimated cumulative symptomatic rupture rates based on the magnetic resonance imaging cohort at 10 years were 0.6 percent and 0.3 percent for patients and implants, respectively. The cumulative incidence rate of confirmed rupture in magnetic resonance imaging cohort A on a patient and implant level is reported in Table 5.
Fig. 4.
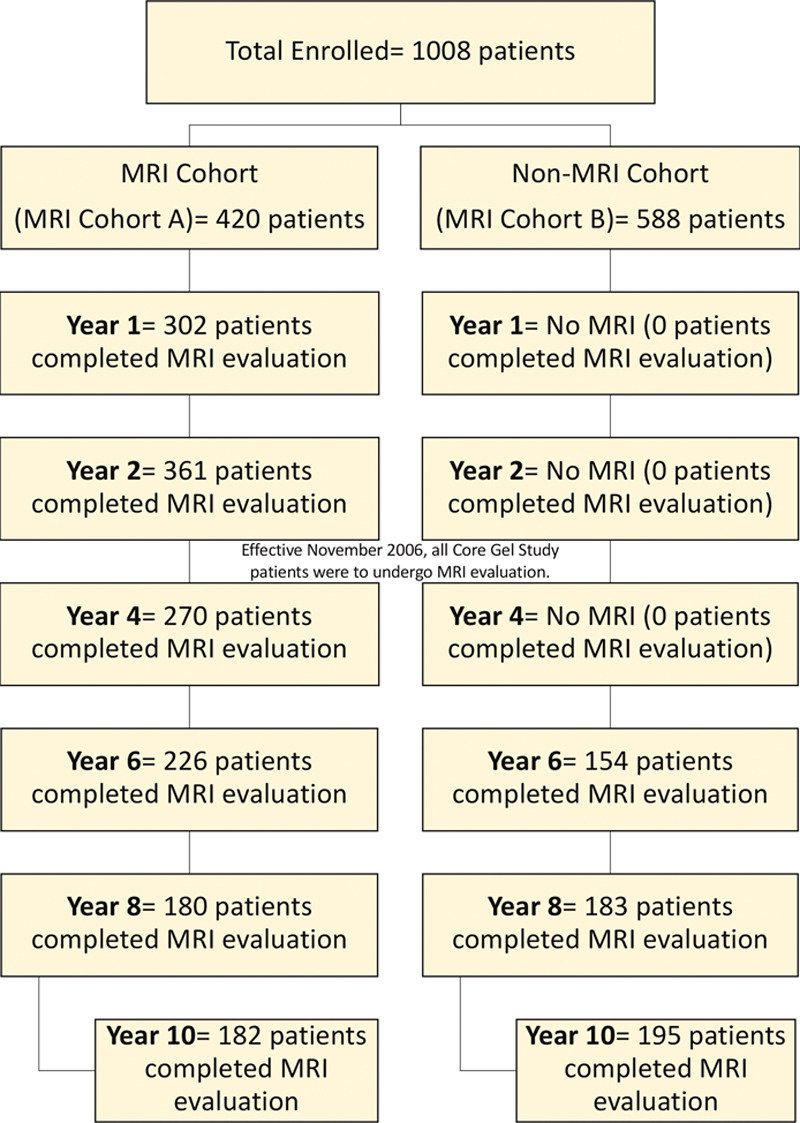
Patients in magnetic resonance imaging (MRI) cohorts A and B who completed magnetic resonance imaging evaluation by year.
Fig. 5.
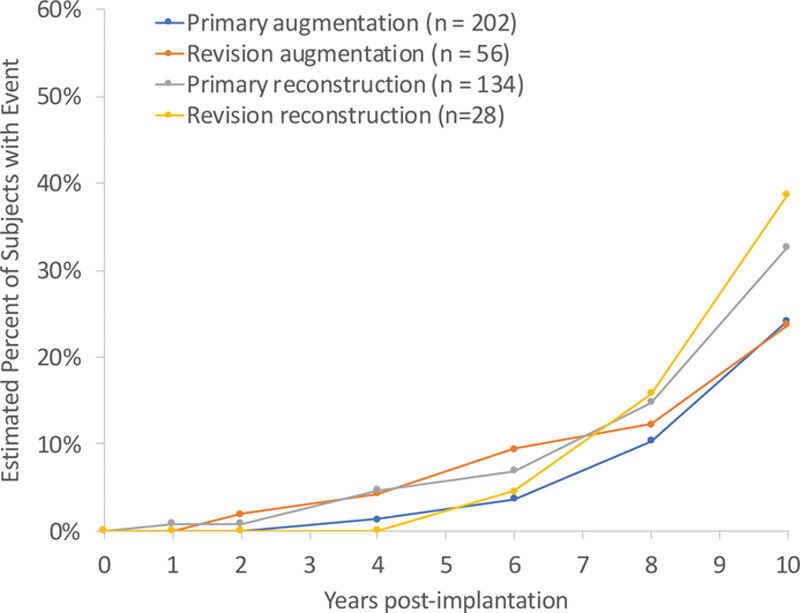
Kaplan-Meier estimated cumulative incidence of rupture by patient.
Table 5.
Cumulative Incidence Rate of Confirmed Rupture in Magnetic Resonance Imaging Cohorts A and B
| Augmentation | Reconstruction | |||||||
|---|---|---|---|---|---|---|---|---|
| Primary | Revision | Primary | Revision | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| MRI cohort A | ||||||||
| Implant level | 7.36 | 4.53–11.84 | 9.85 | 4.71–20.01 | 18.98 | 12.91–27.41 | 12.26 | 3.87–35.14 |
| Patient level | 9.81 | 5.49–17.21 | 13.85 | 5.83–30.92 | 23.04 | 15.02–34.39 | 17.65 | 5.83–30.92 |
| MRI cohort B | ||||||||
| Implant level | 4.63 | 2.64–8.06 | 7.90 | 5.68–10.93 | 21.29 | 13.50–32.65 | 25.56 | 10.35–54.95 |
| Patient level | 7.61 | 4.14–13.76 | 2.63 | 0.37–17.25 | 27.70 | 17.29–42.54 | 23.64 | 8.20–57.27 |
MRI, magnetic resonance imaging.
For the remainder of patients enrolled in magnetic resonance imaging cohort B, the overall 10-year follow-up rate was 41 percent (primary augmentation, 37 percent; revision augmentation, 45 percent; primary reconstruction, 55 percent; and revision reconstruction, 44 percent). The overall Kaplan-Meier estimated cumulative rupture rates at 10 years are listed in Table 2. There were 68 suspected or confirmed ruptured implants (primary augmentation, n = 34; revision augmentation, n = 5; primary reconstruction, n = 21; and revision reconstruction, n = 8) among 56 patients (primary augmentation, n = 29; revision augmentation, n = 3; primary reconstruction, n = 18; and revision reconstruction, n = 6). Of these 68 implants in 56 rupture patients, at 6-year-follow up, rupture was detected in 15 implants in 13 patients, and at 8-year follow-up, rupture was detected in 35 implants in 30 patients. Mean time to rupture for the 68 implants was 8.3 years. Sixty-five of the 68 implants were considered silent ruptures. The overall Kaplan-Meier estimated cumulative silent and symptomatic rupture rates at 10 years were 22.8 percent and 0.6 percent for patients, and 14.7 percent and 0.5 percent for implants, respectively. The cumulative incidence rate of confirmed rupture in magnetic resonance imaging cohort B on a patient and implant level is reported in Table 5.
In total (including magnetic resonance imaging cohorts A and B), 81 confirmed ruptured implants were removed, seven suspected ruptured implants (including one not ruptured at explantation) were removed, and 41 suspected ruptured implants were not removed (unknown, two confirmed ruptures and 14 suspected ruptures). Rupture was reported as the reason for implant removal in 45 implants (primary augmentation, n = 14; revision augmentation, n = 7; primary reconstruction, n = 21; and revision reconstruction, n = 2) in 38 patients. Four additional implants (primary augmentation, n = 2; revision augmentation, n = 1; primary reconstruction, n = 0; and revision reconstruction, n = 0) in four patients were removed because of suspected rupture. Two implants (revision augmentation, n = 1; and primary reconstruction, n = 1) in two patients were removed because of suspected rupture, but the implants were intact on explantation. Fifty-four suspected ruptures were not removed.
Deaths
Overall, 28 deaths occurred through 10-year follow-up: two in the primary augmentation cohort (lung cancer, n = 1; and acute alcohol intoxication, n = 1), two in the revision augmentation cohort (suicide, n = 1; and primary brain carcinoma, n = 1), 23 in the primary reconstruction cohort (cancer, n = 21; hypertrophic cardiomyopathy, n = 1; and unknown but according to the site “probably cancer,” n = 1), and one in the revision reconstruction cohort (metastatic breast cancer).
Stillbirth
Among study participants, there was one incidence of stillbirth of 291 pregnancies.
Breast Cancer
During the 10-year follow-up, 10 new diagnoses of breast cancer in eight patients were reported (primary augmentation, n = 3; revision augmentation, n = 2; primary reconstruction, n = 1; and revision reconstruction, n = 2).
Connective Tissue, Autoimmune, and Rheumatic Disease
Twenty-three patients had 29 newly confirmed diagnoses of connective tissue, autoimmune, or rheumatic disease during the 10-year follow-up period. These included fibromyalgia (n = 6), rheumatoid arthritis (n = 4), Sjögren syndrome (n = 3), systemic lupus erythematosus (n = 3), other inflammatory arthritis (n = 2), Raynaud syndrome (n = 2), carpal tunnel syndrome (n = 1), chronic fatigue syndrome (n = 1), Hashimoto thyroiditis (n = 1), other connective disorder (n = 1), pyoderma gangrenosum (n = 1), sarcoidosis (n = 1), scleroderma (n = 1), spondyloarthropathies (n = 1), and an unknown type of arthritis (n = 1).
DISCUSSION
For the primary augmentation cohort, the 10-year cumulative estimated risk rates for key complications were as follows: any reoperation, 25.5 percent; suspected or confirmed rupture, 24.2 percent (magnetic resonance imaging cohort A); capsular contracture (Baker grade III and IV), 12.1 percent; explantation, 11.6 percent; and infection, 1.6 percent. As to be expected, and consistent with the literature,3–5 the incidence rates of key complications, including reoperations, were higher in the revision than in the primary cohorts for augmentation and reconstruction procedures, and higher in the reconstruction than in the augmentation cohorts for primary and revision procedures. Notably, there was a relatively low rate of malposition observed across cohorts, with only a 1.0 percent cumulative incidence rate in primary augmentation patients.
Often, aesthetic concerns, not medical complications, are the driving force for reoperation. These elective revisions that are cosmetic in nature can elevate the reoperation rate without distinguishing between medically necessary and elective reoperation.6 As suggested by Tebbetts7 and Spear,6 we have also presented an implant-specific reoperation rate that included reoperations attributable to capsular contracture, rippling, infection, hematoma/seroma, and rupture, as it has been suggested that the implant-specific reoperation can be more informative when interpreting breast implant safety and efficacy outcomes.8
The overall Kaplan-Meier estimated rupture rates (suspected or confirmed) for magnetic resonance imaging cohorts A and B are presented in Table 2. It should be noted that these rates may be overestimates because of the strict definition of rupture used in this study: indeterminate ruptures were considered to be ruptures; and disagreement between a local and a central reviewer, who reviewed all magnetic resonance imaging scans, was considered as a rupture. The rates presented in Table 2 include both suspected (based on magnetic resonance imaging evaluation alone) and confirmed (based on surgical removal of the implant) ruptures. This study also used the most rigorous calculation method to determine the rupture rate using data for patients up until the time of their last magnetic resonance imaging examination (or removal of the device). Follow-up for subjects without a rupture was censored at the date of their last magnetic resonance imaging scan. Ruptures are most often silent; therefore, using an office visit rather than magnetic resonance imaging as a screening method may result in missing silent ruptures and a falsely low estimated rupture incidence.9 If the calculation method took into account the last office visit, the estimated rupture rate decreased to 19.3 percent for primary augmentation patients. The precision of the 24.2 percent (95 percent CI, 17.0 to 33.9 percent) Kaplan-Meier estimated cumulative rupture rate (suspected or confirmed) for primary augmentation patients at 10 years is also influenced by the relatively lower follow-up rate, which decreases the precision of the calculation, leading to relatively wide confidence intervals. Understanding the limitations of imaging in determining rupture, we also present the Kaplan-Meier estimated cumulative rupture rates of only those ruptures that were confirmed on explantation of 9.81 percent on a patient level and 7.36 percent on an implant level for the primary augmentation cohort (Table 5). The crude rupture rates (obtained by dividing the number of ruptures by the total number of patients enrolled in the study) in those patients who had primary breast augmentation (magnetic resonance imaging cohorts A and B) were 9.8 percent (54 of 552) by patient and 5.8 percent (65 of 1130) by implant. These rates may be potentially underestimated because of the relatively lower follow-up of 46 percent. Conversely, the Kaplan-Meier formula looks at longitudinal occurrence of discrete events using censored observations (e.g., incomplete data such as individuals lost to follow-up, discontinuation of the study), leading to a presumably more accurate, less biased estimated risk of confirmed and unconfirmed rupture, most of which were silent ruptures. The prevalence of silent ruptures compared to symptomatic ruptures likely contributes to the low U.S. rupture complaint rate between November of 2006 and December of 2019 of 0.7 percent for more than 2 million MemoryGel Breast Implants. It is important to note that silent ruptures do not manifest clinically significant symptoms and, therefore, although these patients are given the choice of surgery or observation, approximately one-third in our study did not undergo device removal. Other studies have shown that most patients do not undergo additional reoperations.9 One study focused on addressing the health implications resulting from an untreated silicone breast implant rupture demonstrated that, of the women with intracapsular rupture (n = 77), 90 percent (n = 69) showed no changes over a 2-year period between the first and second magnetic resonance imaging evaluations.10 This suggests that, often, explantation of the implant is not required, as no specific significant risk was associated with intracapsular ruptured implants. Along these lines, rupture accounted for a low number of reoperations across cohorts. When comparing across studies, it is critical to note the timing of when the magnetic resonance imaging scans were obtained and which methods were used to collect and calculate the rupture rates, as these can significantly impact the reported outcome.9 Furthermore, rupture is a time-related complication. and rupture rates tend to increase notably around 6 to 10 years after implantation.9 For example, the 6-year cumulative incidence by Kaplan-Meier of rupture (suspected or confirmed) of MemoryGel breast implants was 1.1 percent for primary augmentation, 11.6 percent for revision augmentation, 3.8 percent for primary reconstruction, and 5.9 percent for revision reconstruction,2 similar to the 1 percent rupture rate reported in a retrospective analysis comparing postoperative outcomes between patients implanted with Allergan (Allergan, Inc., Dublin, Ireland) versus Mentor implants after 6.8 years’ follow-up.11 In the present study, approximately half of the observed implant ruptures were identified at the 10-year follow-up.
Separate analyses examining the differences in complication and reoperation rates for smooth and textured devices were included in the original statistical analysis plan and have been reported elsewhere and highlight the risk-reduction benefits of textured implants.12 Briefly, the incidence of capsular contracture leading to reoperation in subglandular primary augmentation patients was significantly lower in patients implanted with textured (4.21 percent; 95 percent CI, 1.60 to 10.85 percent) versus smooth devices (19.84 percent; 95 percent CI, 12.52 to 30.63 percent; p = 0.0016). In primary reconstruction patients, the incidence of asymmetry with reoperation was significantly lower in those patients implanted with textured (3.88 percent; 95 percent CI, 1.63 to 9.13 percent) versus smooth implants (11.10 percent; 95 percent CI, 6.29 to 19.19 percent; p = 0.0169).
Importantly, no patients in this Core study were diagnosed with breast implant-associated anaplastic large cell lymphoma (n = 701 textured-surface implants included in the study). Although this study was not designed to statistically evaluate potential cause-and-effect associations, which would require well-designed, controlled, epidemiologic studies, there was no evidence of an association between the study device and incidence (or recurrence) of breast cancer or connective tissue/autoimmune/rheumatic disease. Twenty-nine confirmed new diagnoses of connective tissue, autoimmune, or rheumatic disease were reported in 23 patients during the 10-year follow-up period. With a total of 8469 person-years of follow-up across all four cohorts, this represents an annual incidence rate of 3.4 new diagnoses per 1000 person-years. Four confirmed new diagnoses of rheumatoid arthritis were reported during the 10-year follow-up period. This represents an annual incidence rate of 0.5 per 1000 person-years. By comparison, among the plastic surgery control patients in the study by Brinton et al., there were 49 cases of rheumatoid arthritis observed in 23,724 person-years of follow-up, corresponding to an annual incidence rate of 2.1 per 1000.13 Ten new diagnoses of breast cancer in eight patients were reported during the 10-year follow-up period, representing an annual incidence rate of 1.2 per 1000 person-years. In a separate study by Brinton et al., there were 136 cases of breast cancer observed in 96,675 person-years of follow-up, corresponding to an annual incidence rate of 1.4 per 1000.14 The study event rate of stillbirths was one of 291 pregnancies (0.34 percent), as compared to 6.05 per 1000 deliveries in 2012 in a study using data from the U.S. National Statistics System.15 Thus, in this study, there is no evidence of an association between MemoryGel Implants and incidence of connective tissue/autoimmune/rheumatic disease or breast cancer. This is consistent with other epidemiologic studies, which found no association between silicone breast implants and breast cancer or rheumatoid arthritis.16–19
CONCLUSION
The 10-year follow-up results from this study demonstrate that MemoryGel Implants are safe and effective for use in adult patients undergoing breast augmentation or breast reconstruction.
ACKNOWLEDGMENTS
This trial was sponsored by Mentor Worldwide LLC. The authors are grateful to Gene Poggio, Ph.D., Rong Lin, M.D., M.P.H., and John Leopold for biostatistical support.
Footnotes
This trial is registered under the name “Core Gel Study of the Safety and Effectiveness of Mentor Round Low Bleed Silicone Gel-filled Mammary Prostheses,” ClinicalTrials.gov identification no. NCT00753922 (https://www.clinicaltrials.gov/ct2/show/NCT00753922).
Disclosure: Dr. Caplin is a consultant and clinical investigator for Mentor Worldwide, LLC, and Establishment Labs. Dr. Calobrace has no financial disclosures to report. Dr. Wixtrom is a consultant for Mentor Worldwide, LLC. Drs. Estes and Canady are employees of Mentor Worldwide, LLC. This study was sponsored by Mentor Worldwide, LLC.
By reading this article, you are entitled to claim one (1) hour of Category 2 Patient Safety Credit. ASPS members can claim this credit by logging in to PlasticSurgery.org Dashboard, clicking “Submit CME,” and completing the form.
REFERENCES
- 1.Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg. 2007;120:19S–29S; discussion 30S–32S. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham B, McCue J. Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33:440–444. [DOI] [PubMed] [Google Scholar]
- 3.Hammond DC, Migliori MM, Caplin DA, Garcia ME, Phillips CA. Mentor Contour Profile Gel implants: Clinical outcomes at 6 years. Plast Reconstr Surg. 2012;129:1381–1391. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: Core study results at 6 years. Aesthet Surg J. 2012;32:709–717. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Murphy DK, Slicton A, Walker PSInamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(Suppl 1):8S–16S; discussion 17S–18S. [DOI] [PubMed] [Google Scholar]
- 6.Spear SL. Redefining reoperations. Plast Reconstr Surg. 2008;122:1279–1280. [DOI] [PubMed] [Google Scholar]
- 7.Tebbetts JB. Reoperations as a benchmark: The rhetoric, the logic, and the patient. Plast Reconstr Surg. 2008;122:662–665. [DOI] [PubMed] [Google Scholar]
- 8.Codner MA, Mejia JD, Locke MB, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127:1300–1310. [DOI] [PubMed] [Google Scholar]
- 9.Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: A review. Gland Surg. 2017;6:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmich LR, Vejborg IM, Conrad C, et al. Untreated silicone breast implant rupture. Plast Reconstr Surg. 2004;114:204–214; discussion 215–216. [DOI] [PubMed] [Google Scholar]
- 11.Martin SV, Ho W, Khan K.An extended 7-year review of textured breast implants for primary breast augmentation: Allergan versus Mentor. Ann Breast Surg. 2019;3:14. [Google Scholar]
- 12.Wixtrom RN, Garadi V, Leopold J, Canady JW. Device-specific findings of imprinted-texture breast implants: Characteristics, risks, and benefits. Aesthet Surg J. 2020;40:167–173. [DOI] [PubMed] [Google Scholar]
- 13.Brinton LA, Buckley LM, Dvorkina O, et al. Risk of connective tissue disorders among breast implant patients. Am J Epidemiol. 2004;160:619–627. [DOI] [PubMed] [Google Scholar]
- 14.Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 2000;11:819–827. [DOI] [PubMed] [Google Scholar]
- 15.MacDorman MF, Reddy UM, Silver RM. Trends in stillbirth by gestational age in the United States, 2006-2012. Obstet Gynecol. 2015;126:1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiting VB, Hölmich LR, Brandt B, et al. Long-term health status of Danish women with silicone breast implants. Plast Reconstr Surg. 2004;114:217–226; discussion 227–228. [DOI] [PubMed] [Google Scholar]
- 17.Brinton LA. The relationship of silicone breast implants and cancer at other sites. Plast Reconstr Surg. 2007;120(Suppl 1):94S–102S. [DOI] [PubMed] [Google Scholar]
- 18.Deapen D. Breast implants and breast cancer: A review of incidence, detection, mortality, and survival. Plast Reconstr Surg. 2007;120(Suppl 1):70S–80S. [DOI] [PubMed] [Google Scholar]
- 19.Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342:781–790. [DOI] [PubMed] [Google Scholar]


