Abstract
BACKGROUND:
Blood transfusion is a lifesaving intervention for millions of recipients worldwide every year. Storing blood makes this possible but also promotes a series of alterations to the metabolism of the stored erythrocyte. It is unclear whether the metabolic storage lesion is correlated with clinically relevant outcomes and whether strategies aimed at improving the metabolic quality of stored units, such as hypoxic storage, ultimately improve performance in the transfused recipient.
STUDY DESIGN AND METHODS:
Twelve healthy donor volunteers were recruited in a two-arm cross-sectional study, in which each subject donated 2 units to be stored under standard (normoxic) or hypoxic conditions (Hemanext technology). End-of-storage measurements of hemolysis and autologous posttransfusion recovery (PTR) were correlated to metabolomics measurements at Days 0, 21, and 42.
RESULTS:
Hypoxic red blood cells (RBCs) showed superior PTR and comparable hemolysis to donor-paired standard units. Hypoxic storage improved energy and redox metabolism (glycolysis and 2,3-diphosphoglycerate), improved glutathione and methionine homeostasis, decreased purine oxidation and membrane lipid remodeling (free fatty acid levels, unsaturation and hydroxylation, acyl-carnitines). Intra- and extracellular metabolites in these pathways (including some dietary purines) showed significant correlations with PTR and hemolysis, though the degree of correlation was influenced by sulfur dioxide (SO2) levels.
CONCLUSION:
Hypoxic storage improves energy and redox metabolism of stored RBCs, which results in improved posttransfusion recoveries in healthy autologous recipients—a Food and Drug Administration gold standard of stored blood quality. In addition, we identified candidate metabolic predictors of PTR for RBCs stored under standard and hypoxic conditions.
More than 11 million red blood cell (RBC) units are transfused every year in the United States alone.1 This lifesaving intervention is made logistically possible by storage in the blood bank, which allows preservation of RBC units under refrigerated conditions for up to 42 days in most countries. However, storage is accompanied by the progressive accumulation of a series of biochemical and morphological modifications, collectively referred to as the “storage lesion.”2 During the first 2 weeks of storage, high-energy phosphate compounds—especially 2,3-diphosphoglycerate (2,3-DPG) are progressively consumed, owing to a combination of 1) slower rates of glycolytic enzyme activities at 1 to 6°C storage temperatures3; 2) progressive acidification of the intracellular pH in the closed system of a bag; 3) reversible and irreversible oxidation of the active site of rate-limiting glycolytic enzymes, like glyceraldehyde 3-phosphate dehydrogenase.4 As 2,3-DPG is consumed and hemoglobin affinity for oxygen increases, sulfur dioxide (SO2) levels progressively rise from approximately 60% to as high as greater than 95% by Storage Day 21.5 This phenomenon is paralleled by the accumulation of reactive oxygen species,6 which is aggravated by a progressive loss in the RBCs capacity to cope with oxidant stress.4 As a result, RBCs face a supraphysiological oxidant insult, ultimately resulting in the irreversible oxidation of critical structural and functional proteins such as hemoglobin, band 3, and peroxiredoxin 2.7-11 While RBCs are naturally equipped to cope with oxidant stress, most of these mechanisms either fail or are overwhelmed as a function of storage duration. Examples include 1) the progressive consumption and slower capacity to synthesize de novo reduced glutathione12; 2) the incapacity of the pentose phosphate pathway (PPP) to sustain the generation of nicotinamide adenine dinucleotide phosphate (NADPH) which is necessary to recycle oxidized glutathione4,13; 3) the failure/limited activity of purine oxidation salvage reactions in the mature erythrocyte; 4) the consumption of other antioxidant thiols (e.g., methionine), which is in part explained by the consumption of these metabolites by oxidant damage-repair enzymes9; and, 5) the failure of proteasomal-dependent degradation of irreversibly modified proteins.6
Ultimately, these processes cause the release of irreversibly oxidized proteins and metabolites (especially oxylipins) in the supernatants of stored units through a process of vesiculation.14 The increased vesiculation rate in stored RBCs negatively impacts the morphology of the erythrocyte, which loses the classic discocytic shape and acquires an echinocytic, spheroechinocytic, and, finally, spherocytic phenotype.6 As the morphological lesions accumulate in the stored RBC, its structural rigidity and susceptibility to lyse in the face of oxidant or mechanical insults increases.15 Consistently, some clinical trials have shown that transfusion of end-of-storage RBCs (older than 35 days) results in increased extravascular hemolysis.16
Despite a series of reassuring evidence from randomized clinical trials,17 it is still unclear whether the “age of blood” ultimately impacts clinical outcomes.2 As reassuring prospective evidence on the nonsuperiority of fresh blood versus standard of practice keeps accumulating (e.g., with respect to multiple organ failure in pediatric patients),18 meta-analyses of such evidence has shown that transfusion of packed RBCs with an age of 30 days or more was associated with an increased risk of in-hospital mortality compared to mean RBCs of less than 10 days.19 While some preliminary studies have shown that the metabolic impact of blood storage is detectable in the bloodstream of both healthy autologous recipients20 and patients with sickle cell disease receiving exchange transfusion,21 it is not known if the metabolic storage lesion correlates to any extent with clinically relevant variables. Elegant studies in rodent models have suggested that various mouse strains are differentially susceptible to storage-induced oxidant stress (especially to the lipid fraction),22,23 suggesting a significant role of donor biology on the progression and severity of the storage lesion, as well as the capacity of the stored RBC to circulate at 24 hours after transfusion, defined as chromium-51-labeled posttransfusion recovery (PTR). Despite the limitations of this assay (described extensively here24), PTR is one of the two gold standards of stored blood quality as per Food and Drug Administration and European Council guidelines, along with the propensity of stored RBCs to hemolyze as a function of storage duration. To the best of the authors’ knowledge, while studies in rodents have attempted to correlate metabolic measurements of the storage lesion to PTR and hemolysis, no similar studies have been performed in humans and only preliminary correlations of single metabolites have been reported.5
In the light of the above, it is clear that clinical trials on the age of blood may have been confounded not just by the age of the unit but also by factors related to the biology of the donor and the recipient. In other terms, units from some donors may store better than others for genetic, dietary, and/or environmental reasons, and the metabolic age of an RBC unit may differ from its chronological age.25 Findings from the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) demonstrate donor-dependent heterogeneity in the propensity of RBCs to hemolyze in vitro in response to storage duration, oxidative stress, and mechanical/osmotic insults.26 Heterogeneity in antioxidant pathways may thus impact the severity and progression of the storage lesion and the associated outcomes upon transfusion of those units into recipients. This heterogeneity can be in part explained by processing strategies (e.g., leukoreduction,27 storage additives28), dietary factors or other habits (e.g., exercise or smoking29), or genetic factors. For example, glucose 6-phosphate dehydrogenase deficiency—the most common enzymopathy in human that affects approximately 400 million people and approximately 13% of the African American donor population in some metropolitan areas30 —results in an impaired capacity to activate the PPP and thus an exacerbation of the redox storage lesion.31 In addition, interdonor heterogeneity in SO2 at the time of donation is a potentially compounding and unappreciated factor.5 Therefore, improving RBC storage conditions through an increased understanding of RBC metabolism could be a viable strategy to enhance RBC quality for all donated units, independent of donor-specific factors, and improve transfusion outcomes overall.
In the past 10 years, we have provided compelling evidence that hypoxic storage of RBCs ameliorates the storage lesion through a series of compounding mechanisms, including 1) the concomitant removal of carbon dioxide during the process of deoxygenation, which results in the intracellular alkalinization of the unit, and in turn favors the activation of glycolysis, the Rapoport-Luebering shunt and the activity of glucose 6-phosphate dehydrogenase; 2) improved preservation of DPG and redox metabolism, both by constraining reactive oxygen species–generating reactions and promoting adenosine triphosphate (ATP)-dependent glutathione synthesis; 3) preventing oxidation of functional and structural proteins, including hemoglobin, band 3, peroxiredoxin 2, and glyceraldehyde 3-phosphate dehydrogenase; and 4) improved end-of-storage morphology and decreased vesiculation.4,5,9,32-34 In the light of this evidence, we recently showed in a rodent model of trauma and hemorrhage that transfusion of end-of-storage hypoxic RBCs was superior to transfusion of conventional blood in correcting systemic hypoxia and preventing organ damage at a fraction of the transfused volume.35 Despite this encouraging evidence, until now only preliminary studies36 have been performed in humans to demonstrate the safety and efficacy of hypoxic blood transfusion. To this end, In the present study we hypothesized that hypoxic blood storage would outperform conventionally stored blood with respect to the energy and redox metabolic lesion, and that hypoxic storage of RBCs would not only meet the 75% PTR threshold required by the Food and Drug Administration (FDA) for licensing, but would also be superior to conventionally stored units donated by the same donor in a two-arm cross-sectional study. Finally, we describe the first comprehensive report of candidate metabolic correlates to the FDA gold standards of PTR and hemolysis in normoxic and hypoxic fresh and stored RBCs and supernatants.
MATERIALS AND METHODS
Clinical study
Twelve healthy donor volunteers were enrolled at the Hoxworth Blood Center under an FDA investigational new drug application and Institutional Review Board–approved protocol of the University of Cincinnati (number 2017-6190) and in conformity with the Declaration of Helsinki. Each subject donated 2 units with a minimum of 8 weeks between donations following FDA and AABB regulations. Both units were processed to obtain log4 leukofiltered RBC products (Haemonetics), which were randomized to be stored either under standard conditions in citrate phosphate double dextrose solution/additive solution-3, or under hypoxic storage with SO2 levels controlled at less than 20%. Deoxygenation of the units was performed through proprietary Hemanext Inc. technology and workflows, as extensively described.4,5,33 At the completion of the two-arm cross-sectional study, 24 units were stored under standard (n = 12) or hypoxic conditions (n = 12) for up to 42 days, with each donor acting as their own control. Each unit was sterilely docked and sampled at Storage Day 0 (defined as a starting timepoint within 24 hr of completion of processing), −21, and −42 for metabolomics and lipidomics analyses, SO2, partial pressure of oxygen, partial pressure of carbon dioxide, hemolysis, pH, ATP, 2,3-DPG, and lactate measurements, as previously described.32
Determination of PTR via 51Cr labeling
Autologous in vivo PTR studies were performed at Hoxworth Blood Center, as previously described.37 On Day 42, each unit was inspected for any signs of unusual hemolysis or discoloration indicative of bacterial growth. The unit was well mixed by hand (1 min), and approximately 15 mL of the RBCs removed and labeled with approximately 15 μCi of chromium-51 (51Cr) using standard techniques.37 Briefly, the labeling agent, 51Cr sodium chromate, was mixed aseptically with the RBCs at room temperature for 30 minutes. One double-volume saline wash was conducted. An aliquot of the final volume was reserved for assay as a standard, and the remaining labeled cells (approx. 10 mL) were injected into a free-flowing peripheral vein. Samples (5 mL each) were taken from a contralateral vein within the first 30 minutes after infusion, as well as at 24 hours. The samples were counted in a gamma counter to determine 51Cr activity. The activity of the samples from the first 30 minutes was back-extrapolated to determine a T0 activity. The amount of RBCs and the amount of radioactivity infused were determined based on the methods of Moroff and coworkers38 and the International Committee for Standardization in Hematology.39 All 51Cr specimens used for survival analysis were counted at the same time and corrected for decay. Areas under the curve (AUCs) were calculated by integrating the slope of 51Cr as a function of time over the first 24 hours (GraphPad Prism 8.0, GraphPad Software, Inc). Posttransfusion recovery at 24 hours and AUC were used for correlation to metabolomics and lipidomics measurements.
Sample processing and metabolite extraction
A volume of 50 μL of frozen RBC and supernatant aliquots was extracted 1:10 or 1:25, respectively in ice cold extraction solution (methanol:acetonitrile:water 5:3:2 v/v)9 or pure methanol.40 Samples were vortexed and insoluble material pelleted, as described,12,41 before further metabolomics and lipidomics analysis via ultra-high-pressure liquid chromatography coupled to mass spectrometry (Vanquish-Qexactive, Thermo Fisher), as extensively described in prior publications9 and detailed in Supplementary Methods, available as supporting information in the online version of this paper. Metabolite assignments were performed with a metabolomics data analyzer (MAVEN (Princeton University),42 as previously detailed.9,40 Graphs and statistical analyses (either one-tail paired t test or repeated measures analysis of variance [ANOVA]), as well as network analyses and circos plots of correlations were prepared with computer software (GraphPad Prism; GENE E, Broad Institute; the Metscape 2.0 plugin for Cytoscape43; MetaboAnalyst 4.044; and the OmicsNet plugin).45
RESULTS
Hypoxic storage of RBCs results in improved PTR
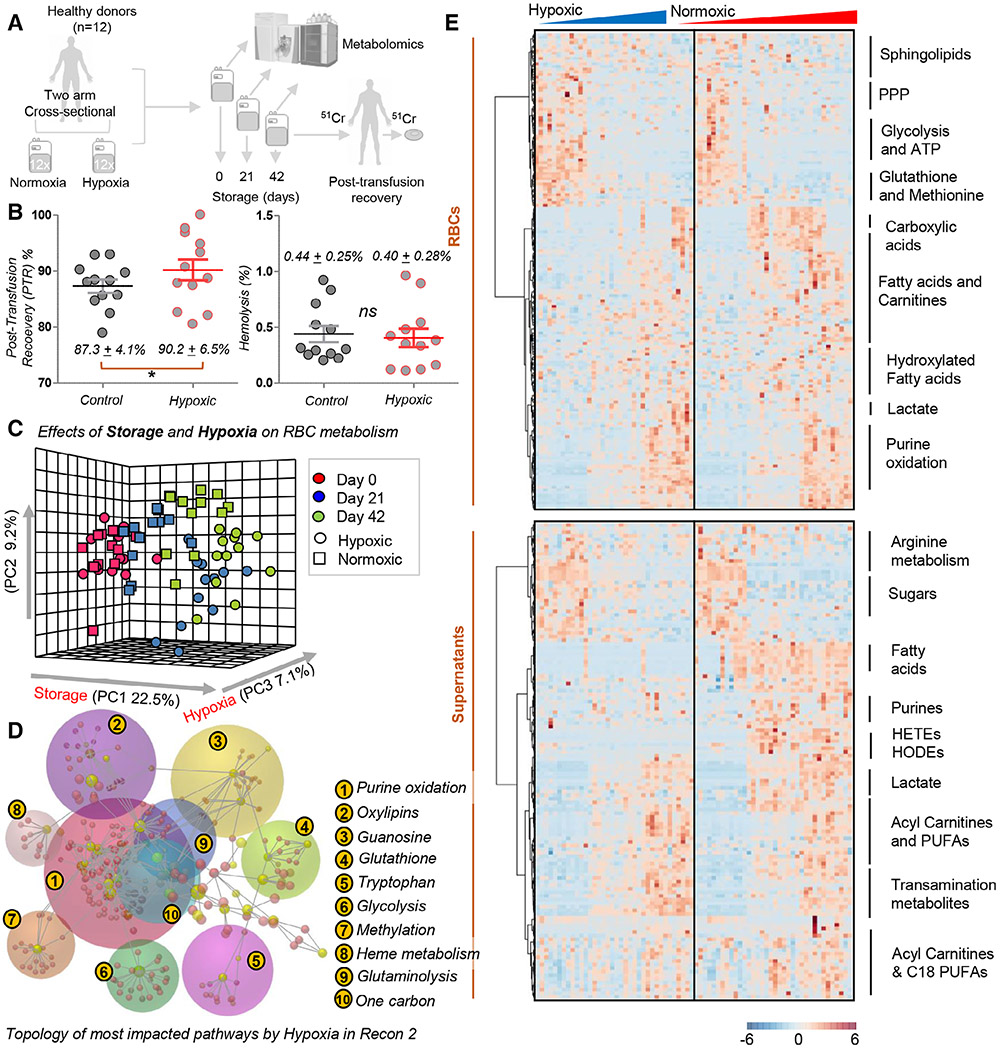
Twelve healthy donor volunteers were recruited to participate in a two-arm cross-sectional study. Each donor donated 2 units of blood over the time span of 3 months (Fig. 1A). The 2 units were randomized to be stored under standard (normoxic) and hypoxic conditions (n = 12 per group, each donor representing their own control). At the end of storage, we measured the two FDA gold standards of storage quality, hemolysis and PTR (i.e., the percentage of transfused RBCs that still circulate in the bloodstream of the recipient, 24 hours after autologous transfusion). All of the tested units met the FDA requirement of 75% or greater PTR. However, hypoxic RBCs were characterized by an increased PTR over standard RBCs (87.3 ± 4.1 in controls vs. 90.2 ± 6.5 in hypoxic tests; p < 0.05 one-tail paired t test; Fig. 1B). Notably, hypoxic storage improved PTR measurements in paired units donated by the same donor in 9 of 12 units (Fig. S1, available as supporting information in the online version of this paper), while hemolysis was improved in only one-half of the units, probably owing to the extra processing required for deoxygenation itself. As a result, no significant differences were observed between the two groups with respect to storage hemolysis, which was lower than the FDA-mandated 1% threshold in all the 24 units tested in this study (Fig. 1B).
Fig. 1.
Hypoxic storage results in superior PTR and different metabolic reprogramming in stored RBCs. Overview of the experimental design. Twelve healthy donor volunteers donated 2 units of blood (two-arm, cross-sectional study) that were stored either under standard (normoxic) or hypoxic conditions for up to 42 days (A). At the end of storage, Food and Drug Administration gold standards for storage quality were tested for all 24 units, showing superior 24-hr posttransfusion recovery (PTR) (p < 0.05, one-tail paired t test) and comparable hemolysis (<1% in all tested units) for hypoxic RBCs compared to standard units (B). Metabolic changes were determined in normoxic and hypoxic units as a function of storage duration and oxygen levels, as highlighted by principal component analysis (PCA) (C). Pathway analysis of significantly altered metabolites are reported in the form of a network in (D) or heat map with hierarchical clustering in (E).
The metabolic impact of blood storage under normoxic and hypoxic conditions
Metabolomic analyses were performed on RBCs and supernatants from the 12 normoxic and 12 hypoxic units, sterilely sampled at Storage Days 0, 21, and 42, consistent with prior studies.4,5,9,28 The results from this analysis are extensively reported in tabulated form in Table S1, available as supporting information in the online version of this paper.
Unsupervised analyses of metabolomics data were performed, including principal component (PC) analysis (Fig. 1C), network representation of metabolic pathways based on the significant metabolites by repeated-measures ANOVA (Fig. 1D) and hierarchical clustering analysis (Fig. 1E). Consistent with previous studies,46,47 storage duration had the strongest impact on the metabolic phenotypes of RBCs and supernatants (PC1, explaining 22.5% of the total variance). However, a significant impact of hypoxic storage was noted along PC2 and PC3 (Fig. 1C, approx. 10% of the total variance). Significant metabolites for RBC (top half of Fig. 1E) and supernatants (bottom half) were represented in the form of a heat map, with a summary of the main pathways annotated on the right-hand side of the panel (scalable versions of these maps, also including the metabolite names are provided in Figs. S2 and S3, available as supporting information in the online version of this paper). Omicsnet was used to generate a 3D network visualization of the main pathways affected by hypoxic storage, which included 1) purine oxidation, 2) oxylipins, 3) guanosine and purine metabolism, 4) glutathione homeostasis, 5) tryptophan and indole metabolism, 6) glycolysis, 7) methionine (and methylation) metabolism, 8) heme metabolism, 9) glutaminolysis, and 10) one-carbon metabolism (Fig. 1D).
Hypoxic RBCs display higher glycolysis, altered carboxylic acid, and redox metabolism
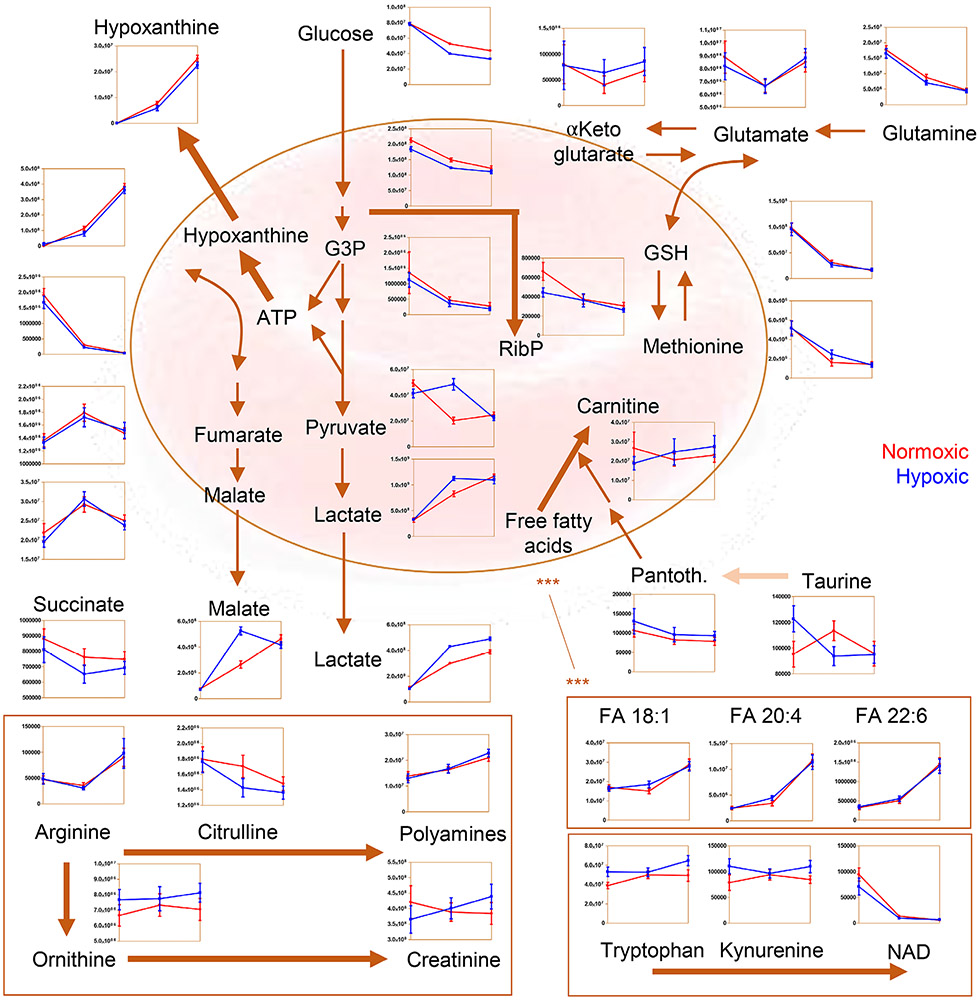
Previous studies on metabolic responses to hypoxic storage in the laboratory setting highlighted an increase in glycolysis and a decrease in oxidant stress markers in comparison to standard, normoxic storage strategies.4,5,9,32,48 While some of these changes recapitulated observations from hypoxic exposure in vivo—e.g., following exposure to high-altitude hypoxia49 —we wanted to test whether a commercially ready platform could phenocopy the benefits of hypoxic storage observed in our previous smaller scale, more controlled laboratory studies. We could thus confirm that hypoxic storage increased glucose consumption and lactate generation (Fig. 2), consistent with a decrease in pH compared to conventionally stored units. Higher glycolytic fluxes were accompanied by decreases in the steady-state levels of PPP metabolites at the early storage time points (Fig. 2), in keeping with previous observations.4 Consistent with the previously reported impact of hemoglobin oxygen saturation on the rate of carboxylic acid metabolism,50 hypoxic RBCs had lower levels of succinate and higher levels of malate compared to standard RBCs (Fig. 2). Decreased levels of glutathione oxidation and turnover, purine oxidation (e.g., hypoxanthine), tryptophan oxidation (e.g., kynurenine and its catabolites), and methionine oxidation were detected in hypoxic units (Fig. 2). Arginine metabolism was also differentially regulated, with higher levels of ornithine and lower levels of citrulline in hypoxic RBCs, and a similar trend in the accumulation of arginine as a function of storage duration (Fig. 2).
Fig. 2.
Overview of metabolites in central carbon and nitrogen metabolism affected by normoxic (red) or hypoxic (blue) storage at Storage Days 0, 21, and 42. Pantoth. = Pantothenic acid.
RBC metabolic correlates to PTR in normoxic and hypoxic units
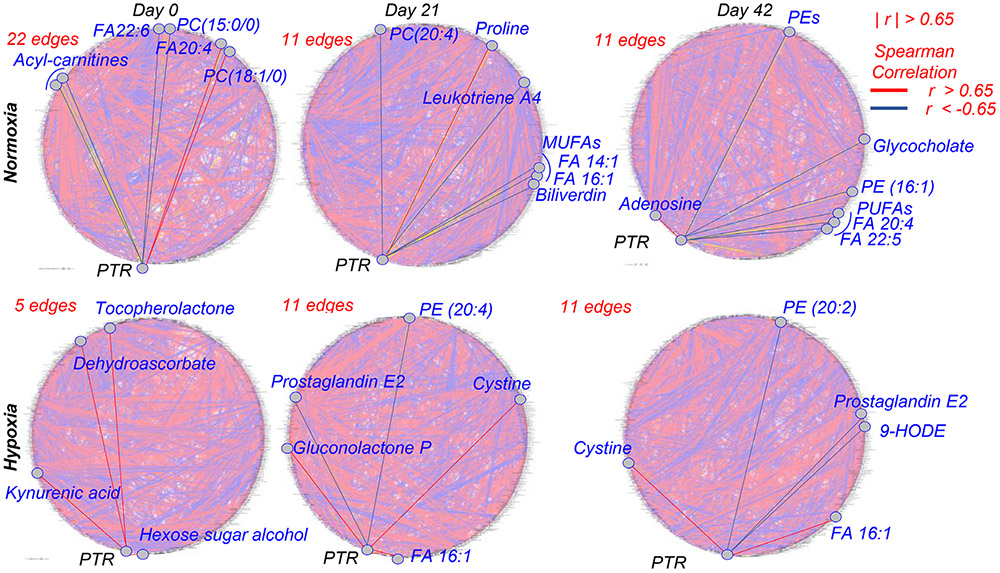
While previous studies had extensively characterized the metabolic lesion in stored RBCs and supernatants, to the best of the authors’ knowledge no previous study has compared metabolic readouts to the FDA gold standard of PTR. While such studies have been extensively performed in rodent models,22,23 similar studies in humans have been limited to single-candidate metabolic markers like hypoxanthine.5 As such, in the present study we correlated metabolic measurements in RBCs and supernatants from units stored under standard normoxic conditions and hypoxia. Results are extensively reported in tabulated form in Table S1, available as supporting information in the online version of this paper. Circos plots were generated based on correlation data through the Metscape 2.0 plugin for Cytoscape for intracellular metabolites. Briefly, RBC metabolites were graphed around a circumference and connected to other metabolites with an edge if the module of the Spearman correlation between their relative abundances was higher than 65% (∣r∣ >0.65), and color coded in red or blue to visualize a positive or negative correlation, respectively. This representation offers the opportunity to visually appreciate the impact of storage duration, exposure to hypoxia and storage under hypoxic conditions on RBC metabolism (Fig. 3). This phenomenon, referred to as the metabolic linkage,51 is based on the concept that fluxes through different metabolic pathways are constrained by shared substrate/product availability. Therefore, small molecule metabolites involved in pathways that cross regulate will be characterized by a high degree of correlation. Alterations of the degree of cross regulation between metabolites in these pathways can occur as a function of environmental changes (e.g., normoxia vs. hypoxia and storage). Perturbation of a pathway as a function of (hypoxic) storage will thus be reflected in cross-regulated pathways. This representation also affords the opportunity to highlight RBC metabolites with significant correlations to PTR at Storage Days 0, 21, and 42 (left to right) under normoxic (top row) or hypoxic conditions (Fig. 3, bottom row). Notably, acyl-carnitines, free fatty acids (FAs; especially mono and polyunsaturated: 14:1, 16:1, 18:1; 20:4; 22:5; 22:6) were among the top negative correlates in normoxic cells at any tested storage day. Some of these correlates were preserved under hypoxic conditions (e.g., FA 16:1; 9-hydroxyoctadecadienoic acid [9-HODE] and prostaglandin E2 isobars). Purines and their deaminated counterparts (adenosine, hypoxanthine, xanthine, hydroxyisourate, NADPH, ATP, adenosine monophosphate [AMP], inosine monophosphate) were positively and negatively correlated to PTR, respectively, in both normoxic and hypoxic units. However, hypoxic RBCs, especially at Days 0 and 42 showed additional correlations to PTR that were not observed or were not as strong in normoxic units. This list includes metabolites in glycolysis (hexose phosphate isobars, fructose bisphosphate), several carnitines, tryptophan metabolites (anthranilate and kynurenic acid) spermidine and dehydroascorbate (Fig. 3, bottom row).
Fig. 3.
Circos plot of correlation networks among metabolites and PTR. Circos plots were generated for normoxic (top row) and hypoxic (bottom row) RBCs at Days 0, 21, and 42 (from left to right). Metabolites were connected by an edge if the module of the Spearman correlation between their relative levels or with end-of-storage PTR was higher than 65%. Edges in red highlight metabolites with significant correlation with end-of-storage PTR at each time point in each condition. For those instances in which >10 edges were identified, selected metabolites were graphed among the most significant ones for representative pathways.
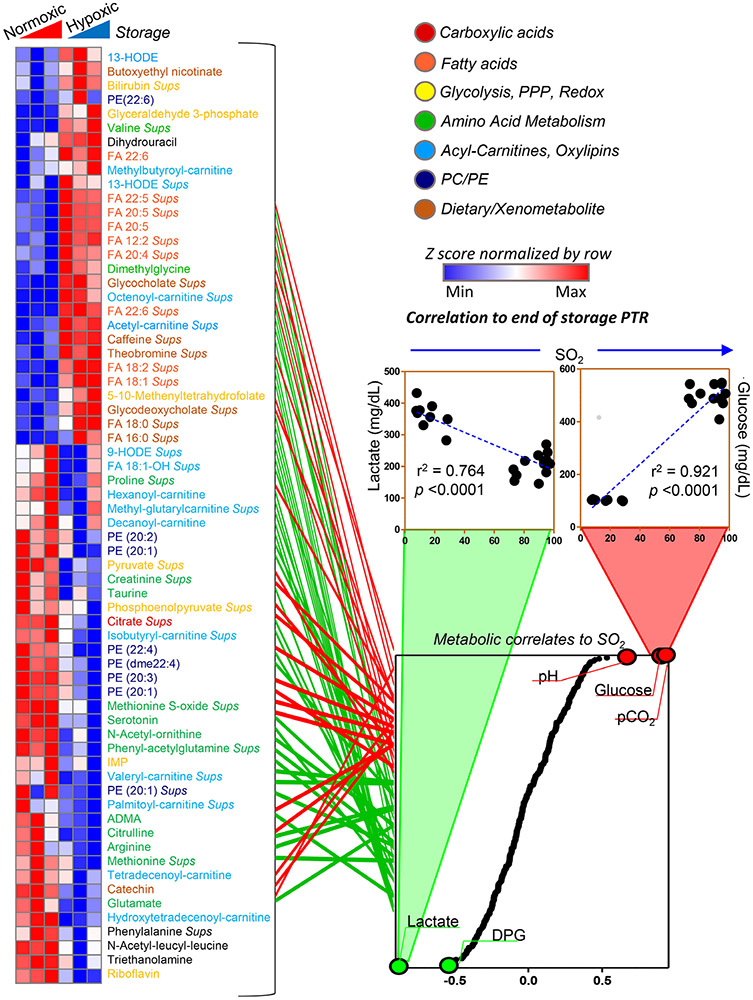
Further elaboration of these data was performed via hierarchical clustering of correlation values at Days 0, 21, and 42 in normoxic and hypoxic RBCs (Fig. 4). This analysis highlighted that the levels of some metabolites are candidate markers of PTR at any given storage day in normoxic RBCs but not in hypoxic RBCs, and vice versa (Fig. 4). These metabolites are color coded by pathway in Fig. 4, and include amino acids, acyl-carnitines and oxylipins, phosphatidylcholines and ethanolamines, and xeno/dietary metabolites (caffeine, theobromine, catechin). Of note, metabolites in this heat were among the top ones influenced by oxygen levels, as gleaned by their correlation to SO2 levels (Fig. 4, bottom right panel). As expected, glucose consumption (r2 = 0.921; p < 0.0001) and lactate generation (r2 = 0.764; p < 0.0001) accompanied by pH acidification were by far the top-ranking metabolites in this analysis.
Fig. 4.
Significant metabolic correlates to PTR differ in a storage-independent hypoxia-dependent fashion, as noted in the heat map on the left-hand side. Metabolites are color coded by metabolic pathway, according to the legend in the top right corner. Some metabolites showed significant (module of Spearman) correlation to PTR in normoxia, but not in hypoxia at any given storage day and vice versa (red in heat map). Some of the metabolites with high correlation with PTR in normoxia were only significant in hypoxic RBCs at the end of storage. All these metabolites were part of pathways significantly affected by SO2 levels of the unit. Indeed, the levels of these metabolites showed significant positive (bottom right panel in red) or negative (green) correlations to SO2 levels. Correlation curves are shown for glucose and lactate, two representative metabolites of glycolysis and the most differentially regulated pathway between normoxic and hypoxic RBCs.
Finally, a subset of metabolites was identified by unsupervised clustering as potential good predictors of PTR in normoxic RBCs, and hypoxic RBCs only at Storage Day 42 (top of the heat map in Fig. 4). This subgroup included several oxylipins (9-HODE; FA 18:1-OH), acyl-carnitines (hexanoyl, methylglutaryl, decanoyl), and amino acids (proline).
Metabolic correlates to hemolysis, PTR, and other physiological measurements in RBCs and supernatants
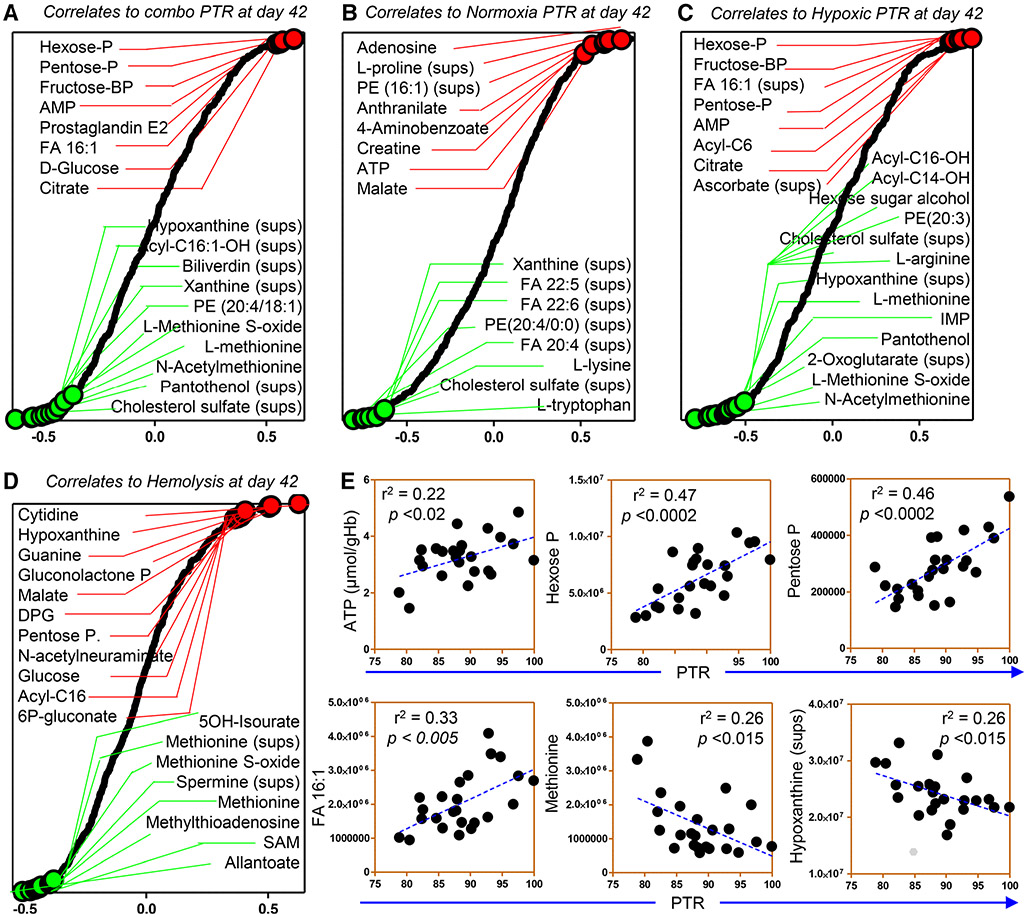
Upon finalization of the unsupervised network analyses described above on RBC metabolites and PTR, we performed additional elaborations to visualize a digested version of the data. The results of these analyses are extensively reported in Table S1, available as supporting information in the online version of this paper. In Fig. 5, we highlighted the top correlates to PTR in all units (A), normoxic units (B), or hypoxic units (C), and hemolysis independently of the condition (D). Hemolysis was treated in a combined fashion, owing to the lack of significant differences between the two conditions. Consistent with older reports,52 the high-energy phosphate compound ATP had moderate but significant correlation to PTR (Fig. 5A). Other metabolites like hexose and pentose sugar phosphate compounds (e.g., glucose 6-phosphate, ribose 5-phosphate, or other isobar, which could not be resolved in the current analyses), glycolytic metabolites (glucose, fructose bisphosphate), and other purines (AMP) and deamination products, methionine and its metabolites (methionine sulfoxide), free FAs, and oxylipins/hydroxylated FAs (e.g., consistent with previous reports in rodents22) showed stronger and more significant correlations with PTR (Fig. 5A). Notably, supernatant levels of several metabolites were stronger predictors than their intracellular counterparts, including several oxylipins (Fig. 5A and B). Elevated intracellular carnitines and free FAs (especially monounsaturated and relative hydroxylated forms), and the coenzyme A precursor pantetheine were instead negative predictors of PTR, in both normoxic and hypoxic units (Fig. 5B, C), suggestive of poor PTR in the face of extensive membrane lipid remodeling. Finally, high-energy phosphate compounds (ATP, adenosine diphosphate, and AMP), oxidized purines (hypoxanthine, as previously reported5) and other oxidized purines (xanthine), sulfur-containing compounds (methionine, methionine sulfoxide), and tryptophan metabolites (anthranilate, kynurenic acid, and methyldioxyindole, a putative metabolite of microbiome origin) displayed strong correlation with PTR and AUC (Fig. 5A, Fig. S1, available as supporting information in the online version of this paper). Of note, N-acetylmethionine—a marker of proteolysis as this acetylated amino acid is released from proteolysis of the N-term initiator methionine—was among the top negative correlates with PTR in hypoxic RBCs (Fig. 5C). Increased markers of NADPH metabolism (hexose phosphate, markers of PPP activation, Fig. 5A-C; and riboflavin, biliverdin, Figs. 3 and 4, markers of NADPH-dependent biliverdin reductase B activity53), were positively and negatively associated with PTR, respectively (Fig. 5C). Increased levels of carboxylic acids (malate and citrate, but not 2-oxoglutarate) were also positive correlates to PTR in normoxic and hypoxic RBCs (Fig. 5B, C).
Fig. 5.
Ranked Day 42 metabolic correlates to PTR measurements. PTR—in the tested units: all (A), normoxic (B) or hypoxic (C), and hemolysis (D). Highlights of representative correlation curves (E).
Correlation of Day 42 metabolites to hemolysis highlighted a significant negative correlation between the levels of methionine, purine oxidation metabolites, and cross-talk between the two pathways via salvage reactions, including methionine, methionine sulfoxide, S-adenosylmethionine, methylthioadenosine, polyamines (e.g., cadaverine, spermine), hypoxanthine and 5OH-isourate, guanine, and guanosine diphosphate (connected to this pathway via the activity of the enzyme hypoxanthine guanosine phosphoribosyltransferase; Fig. 5D). Markers of PPP activation (gluconolactone phosphate, 6-phosphogluconate, and pentose phosphate isobars) were also positively correlated to hemolysis (Fig. 5D).
DISCUSSION
During the past decade, omics investigations of human RBC units stored using all currently licensed storage addifives37,54,55 helped to identify the impact of processing strategies (including leukoreduction27 and storage solutions28) and donor variability28,31 on the molecular heterogeneity of stored units. In the face of a growing body of evidence documenting the storage lesion, little is known about whether any of these metabolic changes that accumulate during storage in the blood bank ultimately impact RBC performance upon transfusion in vivo.56 Efforts to overcome such limitations have been brought about by Zimring and colleagues, who showed that some metabolic lesions correlate with PTR in rodent models of blood transfusion. However, only a subset of these metabolites may be mechanistically, not just correlatively, associated with PTR, as they propagate across generations of mice that have been genetically manipulated in a fine way to isolate single biologic variables (e.g., the ferroreductase STEAP3).22,23 While such studies are not feasible in humans owing to obvious limitations, other programmatic efforts like the REDS-III study have set out to associate donor genetic factors (e.g., polymorphisms in genes relevant to RBC structural integrity and function) to in vitro outcomes such as hemolysis and hemoglobin increments in the recipients of those units.29 Metabolomics measurements in a subset of the units from the REDS-III study have already been reported,28 though correlations to clinically relevant outcomes are still pending.
In the light of these considerations, the rationale behind the present study was to 1) modify blood processing strategies with the goal to mitigate the storage metabolic lesion, an outcome that we had shown to be achievable through hypoxic storage of RBCs4,5,9,32-34; and 2) to determine whether improvements in the metabolic state of the stored erythrocyte would result in improved measurements with respect to the two FDA standards of RBC storage quality, i.e., end-of-storage hemolysis and PTR. As a result, we report that hypoxic storage results in superior PTR compared to paired units stored under standard normoxic conditions. We confirm the metabolic benefits of hypoxic storage, ultimately resulting in improved energy and redox metabolism. Finally, we show metabolites in pathways that respond to alteration of SO2 levels ranked among the top correlates to PTR and hemolysis. This first-of-its-kind report confirms and expands on previous evidence in humans and rodents of a role of purine5 and lipid oxidation22,23 (e.g., hypoxanthine and 9-HODE) as candidate markers of hemolysis and PTR. The identification of such markers paves the way for interventional studies that leverage mechanistic interventions to prevent the accumulation of purine and lipid oxidation products and thus enhance stored blood quality.
By highlighting correlations between acyl-carnitines, CoA precursors (panthothenol), free FA levels, and membrane lipid unsaturation and hydroxylation, we conclude that membrane lipid remodeling is a potentially targetable pathway that can be leveraged to improve RBC PTR. Future studies will be necessary to test whether dietary interventions aimed at modifying fatty acyl composition through food intake or supplementation of lipid-targeting antioxidants (vitamin E, carnitines57) ultimately impact this phenotype. Interestingly, dietary purines like caffeine metabolites had already been preliminarily reported as potential markers of PTR in independent cohorts.58 Polymorphisms in cytochrome P450 1A2 have been suggested to underlie the heterogeneity in caffeine metabolism and the potential impact/correlation this may have with the capacity of transfused RBCs to circulate upon transfusion. Others reported here, like catechin and theobromine (dietary) or methyldioxindole and glycine-conjugated bile acids (bacterial origin/metabolism), have not been reported in previous studies. For other metabolites like purines (including AMP and ATP) and purine deamination (including hypoxanthine, xanthine, and urate), their cross-talk to sulfur metabolism via methionine and recycling pathways (that generate polyamines) had been previously documented as hallmarks of the progression of the storage lesion,5,9 although these were in the absence of a direct measurement of a potentially clinically relevant outcomes such as PTR.
Several limitations of the present study must be acknowledged. First of all, correlative analyses were performed on metabolic measurements on a total of 24 units (paired normoxic and hypoxic units from the same 12 donors). Owing to the elevated numbers of observations, despite post hoc correction for multiple observations performed here (false discovery rate), some of the reported findings may just result from spurious correlation and will need to be confirmed prospectively in independent cohorts. The small sample size of the cohort recruited for this study may also be insufficient to appreciate the impact of the heterogeneity of donor biology—including donor sex, ethnicity, and age—with respect to hemolysis26 and PTR59 parameters. Undoubtedly, RBCs have to survive storage without hemolyzing and circulate long enough upon transfusion to ensure the delivery an efficacious “dose” of transfused blood. However, such conditions are necessary but not sufficient to ensure that transfused RBCs are actually functional. Future studies will be necessary to determine whether the metabolic markers of PTR reported here would also be predictive of direct measurements of the RBC “physiome,” that is, measurements of the RBC physiological profile that are affected by RBC metabolism (e.g., oxygen affinities, deformability, control of cell volume, control of vasoactive effectors, redox “buffering” capacity). In prior work on rodent models of shock, limited improvements in PTR (approx. 3%) did not mirror a remarkably superior performance of hypoxic RBCs in resuscitating hemorrhaged rats.35 Units donated by the same donors (as is the case in the present study) tend not to show consistent reproducible levels of spontaneous hemolysis across multiple donations.60 In addition, spontaneous hemolysis does not recapitulate the RBC propensity to hemolyze following additional, more physiologically relevant insults, such as oxidant, osmotic, or mechanical stress.60 In addition, PTR in healthy subjects does not suffice to provide information on the actual RBC recovery in nonhealthy, heterologous patients. Several studies have highlighted that PTR of allogenically transfused RBC in patients with acute inflammatory disorders is significantly reduced,61,62 while in patients with immature innate immune systems, the PTR is increased or is insensitive to age-mediated storage.63
Despite the limitations noted above, in the present study we report that hypoxic storage ameliorates the metabolic phenotypes of stored RBCs (lower markers of oxidant stress to lipids and purines and higher levels of high-energy phosphate compounds) and improves PTR in a two-arm cross-sectional clinical study powered to appreciate significant effects on this parameter. We thus report the first comprehensive correlative analysis of metabolic parameters to hemolysis and PTR, suggesting potential candidate markers in fresh and stored units to predict end of storage blood quality. We highlight that storage under normoxic and hypoxic conditions significantly impacts differential pathways related to energy and redox homeostasis and, as such, future studies aimed at validating the candidate markers of stored blood quality will have to take into account processing strategies in the study design. Larger studies will be necessary to validate and expand on the present observations by taking into account the role of donor biology on transfusion outcomes and markers thereof. Finally, identification of energy and redox metabolic pathways that correlate with potentially clinically relevant variables may inform new strategies to intentionally manipulate these very pathways, for example, with the use of novel storage additives aimed at maximizing the quality of RBCs stored under hypoxic conditions.
Supplementary Material
Appendix S1. Supporting information
Table S1. Supporting information.
ACKNOWLEDGMENTS
TY, ADu, and JAC designed the study; SN, SS, FM, NR, and JAC supervised and performed the clinical trial; ADA, TN, and DS performed metabolomics analyses; ADA prepared figures and tables and drafted the first version of the manuscript; ADA and JAC reviewed critically the manuscript text. All the authors contributed critically to the finalization of the paper.
AD was supported by funds from the Boettcher Webb-Waring Investigator Early Career Award 2017, RM1GM131968 by the National Institute of General and Medical Sciences, R01HL146442 and R01H148151 by the National Heart, Lung and Blood Institutes. TY, AD and JAC are supported by NHLBI R44 HL132172.
ABBREVIATIONS:
- 2,3-DPG
2,3-diphosphoglycerate
- 9-HODE
9-hydroxyoctadecadienoic acid
- AMP
adenosine monophosphate
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- AUCs
areas under the curve
- 51Cr
chromium-51
- FA
Fatty acid
- FDA
Food and Drug Administration
- NADPH
nicotinamide adenine dinucleotide phosphate
- PPP
pentose phosphate pathway
- PTR
posttransfusion recovery
- SO2
sulfur dioxide
Footnotes
CONFLICT OF INTEREST
SN, SS, DS, FM, and NR have disclosed no conflicts of interest. ADA and TN are founders of Omix Technologies Inc and Altis Biosciences LLC. ADA and JAC are consultants for Hemanext Inc, with which ADu and TY are affiliated.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus 2019;17:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurkovich JT, Zielinski DC, Yang L, et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J Biol Chem 2017;292:19556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016;128:e32–42. [DOI] [PubMed] [Google Scholar]
- 5.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018;103:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alessandro A, D’Amici GM, Vaglio S, et al. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica 2012;97:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion 2016;56:421–6. [DOI] [PubMed] [Google Scholar]
- 8.Bayer SB, Hampton MB, Winterbourn CC. Accumulation of oxidized peroxiredoxin 2 in red blood cells and its prevention. Transfusion 2015;55:1909–18. [DOI] [PubMed] [Google Scholar]
- 9.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018;58:2978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinalducci S, Ferru E, Blasi B, et al. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus 2012;10(Suppl 2):s55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Apolipoprotein J/clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence. PLoS One 2011;6:e26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017;57:325–36. [DOI] [PubMed] [Google Scholar]
- 13.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion 2000;40:353–60. [DOI] [PubMed] [Google Scholar]
- 14.Delobel J, Prudent M, Tissot J-D, et al. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteomics Clin Appl 2016;10:257–66. [DOI] [PubMed] [Google Scholar]
- 15.Blasi B, D’Alessandro A, Ramundo N, et al. Red blood cell storage and cell morphology. Transfus Med 2012;22:90–6. [DOI] [PubMed] [Google Scholar]
- 16.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest 2017;127:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus 2017;15:112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinella PC, Tucci M, Fergusson DA, et al. Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically ill pediatric patients: a randomized clinical trial. JAMA 2019;322:2179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng MSY, David M, Middelburg RA, et al. Transfusion of packed red blood cells at the end of shelf life is associated with increased risk of mortality - a pooled patient data analysis of 16 observational trials. Haematologica 2018;103:1542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alessandro A, Reisz JA, Zhang Y, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv 2019;3:884–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culp-Hill R, Srinivasan AJ, Gehrke S, et al. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion 2018;58:2797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howie HL, Hay AM, de Wolski K, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv 2019;3:2272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016;101:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis RO, Mahajan S, Rapido F, et al. Reexamination of the chromium-51-labeled posttransfusion red blood cell recovery method. Transfusion 2019;59:2264–75. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Zimring JC, Busch M. Chronological storage age and metabolic age of stored red blood cells: are they the same? Transfusion 2019;59:1620–3. [DOI] [PubMed] [Google Scholar]
- 26.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertinhez TA, Casali E, Baroni F, Berni P, Baricchi R, Spisni A. A comparative study of the effect of leukoreduction and pre-storage leukodepletion on red blood cells during storage. Front Mol Biosci [Internet]. 2016. April 21 [cited 2019 May 7];3:13 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4839302/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS III – Omics. Transfusion 2019;59:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roubinian NH, Plimier C, Woo JP, et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood 2019;134:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis RO, Jhang J, Hendrickson JE, et al. Frequency of glucose-6-phosphate dehydrogenase-deficient red blood cell units in a metropolitan transfusion service. Transfusion 2013;53:606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion 2015;55:2659–71. [DOI] [PubMed] [Google Scholar]
- 32.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, et al. CO2 -dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion 2016;56:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang 2007;92:22–31. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus 2010;8:220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams AT, Jani VP, Nemkov T, et al. Transfusion of anaerobically or conventionally stored blood after hemorrhagic shock. Shock 2020;53(3):352–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida T, AuBuchon JP, Dumont LJ, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion 2008;48:2096–105. [DOI] [PubMed] [Google Scholar]
- 37.Cancelas JA, Dumont LJ, Maes LA, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion 2015;55:491–8. [DOI] [PubMed] [Google Scholar]
- 38.Moroff G, Sohmer PR, Button LN. Proposed standardization of methods for determining the 24-hour survival of stored red cells. Transfusion 1984;24:109–14. [DOI] [PubMed] [Google Scholar]
- 39.Recommended method for radioisotope red-cell survival studies. International Committee for Standardization in Haematology. Br J Haematol 1980;45:659–66. [DOI] [PubMed] [Google Scholar]
- 40.Reisz JA, Zheng C, D’Alessandro A, et al. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. Methods Mol Biol 1978;2019:121–35. [DOI] [PubMed] [Google Scholar]
- 41.Nemkov T, Hansen KC, Dumont LJ, et al. Metabolomics in transfusion medicine. Transfusion 2016;56:980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melamud E, Vastag L, Rabinowitz JD. Metabolomic analysis and visualization engine for LC–MS data. Anal Chem 2010;82:9818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karnovsky A, Weymouth T, Hull T, et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinforma 2012;28:373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018;46(W1):W486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou G, Xia J. Using OmicsNet for network integration and 3D visualization. Curr Protoc Bioinformatics 2019;65:e69. [DOI] [PubMed] [Google Scholar]
- 46.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016;128:e43–50. [DOI] [PubMed] [Google Scholar]
- 47.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion 2015; 55:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst 2013;9:1196–209. [DOI] [PubMed] [Google Scholar]
- 49.D’Alessandro A, Nemkov T, Sun K, et al. AltitudeOmics: red blood cell metabolic adaptation to high altitude hypoxia. J Proteome Res 2016;15:3883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med 2017;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’alessandro A, Nemkov T, Reisz J, et al. Omics markers of the red cell storage lesion and metabolic linkage. Blood Transfus 2017;15:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roussel C, Buffet PA, Amireault P. Measuring post-transfusion recovery and survival of red blood cells: strengths and weaknesses of chromium-51 labeling and alternative methods. Front Med (Lausanne) [Internet]. 2018. May 15 [cited 2019 Sep 30];5:130 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5962717/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paukovich N, Xue M, Elder JR, et al. Biliverdin reductase B dynamics are coupled to coenzyme binding. J Mol Biol 2018;430(18 Pt B):3234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion 2018;58:1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolfsson Ó, Sigurjonsson ÓE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang 2017;112:326–35. [DOI] [PubMed] [Google Scholar]
- 56.Zimring JC. Widening our gaze of red blood storage haze: a role for metabolomics. Transfusion 2015;55:1139–42. [DOI] [PubMed] [Google Scholar]
- 57.Arduini A, Holme S, Sweeney JD, et al. Addition of L-carnitine to additive solution-suspended red cells stored at 4 degrees C reduces in vitro hemolysis and improves in vivo viability. Transfusion 1997;37:166–74. [DOI] [PubMed] [Google Scholar]
- 58.Yerrabothala S, Tsongalis GJ, Fu X, et al. Correlation between red blood cell survival and cytochrome P450 1A2 enzyme activity. Blood 2013;122:3658–8. [Google Scholar]
- 59.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 2008;48:1053–60. [DOI] [PubMed] [Google Scholar]
- 60.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion 2018;59:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luten M, Roerdinkholder-Stoelwinder B, Schaap NPM, et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion 2008;48:1478–85. [DOI] [PubMed] [Google Scholar]
- 62.Zeiler T, Müller JT, Kretschmer V. Flow-cytometric determination of survival time and 24-hour recovery of transfused red blood cells. Transfus Med Hemother 2003;30:14–9. [Google Scholar]
- 63.Nalbant D, Cancelas JA, Mock DM, et al. In premature infants there is no decrease in 24-h post-transfusion allogeneic red cell recovery after 42 days of storage. Transfusion 2018;58:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Table S1. Supporting information.