Abstract
There is an urgent need to understand the underlying mechanisms contributing to thrombotic and inflammatory complications during COVID‐19. Data from independent groups have identified that platelets are hyperreactive during COVID‐19. Platelet hyperreactivity is accompanied by changes in platelet gene expression, and enhanced interactions between platelets and leukocytes. In some patients, SARS‐CoV‐2 mRNA has been detected in platelets. Together, this suggests that SARS‐CoV‐2 may interact with platelets. However, controversy remains on which receptors mediate SARS‐CoV‐2 platelet interactions. Most, but not all, transcriptomic and proteomic analyses fail to observe the putative SARS‐CoV‐2 receptor, angiotensin converting enzyme‐2, or the cellular serine protease necessary for viral entry, TMPRSS2, on platelets and megakaryocytes. Interestingly, platelets express other known SARS‐CoV‐2 receptors, which induce similar patterns of activation to those observed when platelets are incubated with SARS‐CoV‐2. This article explores these findings and discusses ongoing areas of controversy and uncertainty with regard to SARS‐CoV‐2 platelet interactions.
Keywords: ACE2, COVID‐19, platelets, SARS‐CoV‐2, thrombosis
1. INTRODUCTION
SARS‐CoV‐2 coronavirus emerged in Wuhan, China, in December 2019, and has infected more than 35 million people worldwide.1 The associated disease, COVID‐19, has claimed 1 million lives as of October 2020.1 While notable manifestations in COVID‐19 include acute respiratory distress syndrome, it is now well recognized that thrombosis and cardiovascular manifestations also contribute greatly to morbidity and mortality from COVID‐19.2., 3. Thrombotic complications have been observed in hospitalized patients with COVID‐19, which are manifested by elevated levels of D‐dimer and fibrin degradation products as well as macrothrombi and small vessel thrombosis in multiple organs.2., 3., 4., 5. However, the pathophysiologic cellular drivers of these thrombotic complications are poorly understood.
Platelets are small, cellular fragments derived from their parent cells, megakaryocytes. As platelets are anucleate, megakaryocytes are generally thought to package the molecular factors necessary for platelets to function before they are released into the circulation. In addition to their traditional roles in hemostasis, platelets play critical roles during infectious diseases, which are often associated with a heightened risk of thrombosis. Autopsies from COVID‐19 patients have revealed platelet‐rich thrombi in microcapillaries in the lungs, heart, kidneys, and skin and abnormally elevated numbers of megakaryocytes in heart and lungs further pointing to dysregulated hemostasis in patients infected with SARS‐CoV‐2.6., 7., 8.
2. PLATELET ACTIVATION AND HYPERACTIVTY IN COVID‐19
Recently, several independent studies have demonstrated that platelets are activated in COVID‐19 patients.9., 10., 11., 12., 13., 14. Platelets from COVID‐19 patients are hyperreactive in comparison to healthy donors when activated with traditional platelet agonists such as adenosine diphospate, collagen, and thrombin. This increase in platelet reactivity appears to be, in part, dependent on increased protein kinase C delta, extracellular signal‐regulated kinases, and p38 signaling, resulting in increased degranulation and thromboxane B2 generation.9., 13. Furthermore, platelets from COVID‐19 patients release extracellular vesicles as well as dense and alpha granule cargo into the blood, including serotonin, soluble P‐selectin, soluble CD40L, platelet‐derived growth factor, and platelet factor 4.10., 11., 12., 13. In addition, COVID‐19 induces significant changes to the platelet transcriptome, which are distinct in many ways from transcriptional changes observed in influenza and sepsis.9
Numerous factors can lead to platelet activation and hyperreactivity in COVID‐19 patients. Platelets may activate as a primary result of alterations in gene expression or secondarily to the generation of increased soluble pro‐coagulant molecules, such as fibrinogen.2., 15. During the overwhelming inflammation that prevails in COVID‐19, the accumulation of cytokines and other factors may also activate platelets—among a number of other potential mechanisms. In addition to these indirect mechanisms, viruses can directly activate platelets or megakaryocytes, including dengue virus, influenza virus, human immunodeficiency virus‐1 (HIV‐1), and encephalomyocarditis virus, offering another potential mechanism for increased platelet activation and hyperreactivity during SARS‐CoV‐2 infection.16., 17., 18., 19., 20., 21. Viruses can also activate platelets through indirect interactions with FcγRIIA, a mechanism that may take place uniquely if antibodies directed against SARS‐CoV‐2, or cross‐reacting antibodies against more common coronaviruses that generate minor cold symptoms in humans (229E, NL63, OC43, and HKU1), are present.22 A mechanism in which SARS‐CoV‐2 directly activates megakaryocytes and platelets appears possible as two independent studies have reported the presence of SARS‐CoV‐2 mRNA in platelets of some COVID‐19 patients.9., 12. However, how SARS‐CoV‐2 interacts with megakaryocytes and platelets has not fully been elucidated and remains controversial.
3. POTENTIAL RECEPTORS FOR SARS‐COV‐2 BINDING TO MEGAKARYOCYTES AND PLATELETS
A putative receptor for the binding and entry of SARS‐CoV‐2 virus to cells is angiotensin converting enzyme‐2 (ACE2).23 The receptor is highly expressed by nasopharyngeal airway epithelial cells as well as alveolar epithelial cells, vascular endothelial cells, and lung macrophages,24 which likely explains the notable acute respiratory distress syndrome in COVID‐19 patients. In addition to ACE2, the cellular serine protease TMPRSS2 is necessary for cleaving the S protein on SARS‐CoV‐2, which allows for fusion of viral and cellular membrane and subsequent viral entry to the cell.25 The expression of ACE2 and TMPRSS2 in platelets and megakaryocytes has not been specifically examined until now. Published work by Manne et al did not detect ACE2 or TMPRSS2 in CD45‐depleted platelets from either healthy donors or COVID‐19 patients using a number of complementary transcriptomic and proteomic assays, including RNA‐seq analysis, real‐time polymerase chain reaction, and western blot analysis.9 These findings were confirmed and extended by Zaid et al using similar techniques, and also demonstrated that ACE2 protein was not present in human platelets using immunocytochemistry.13 Consistent with this prospective work, retrospective analyses of previously published deep sequencing and microarray datasets from our groups and others,12., 16., 26., 27., 28., 29., 30., 31. have not identified any ACE2 or TMPRSS2 expression in CD34+‐derived, cultured human megakaryocytes or platelets from healthy donors. Furthermore, ACE2 and TMPRSS2 are not expressed in isolated human platelets during acute infectious diseases, including influenza, dengue, and sepsis.16., 29. Proteomic approaches based on mass spectrometry on isolated human platelets have also failed to identify ACE2 or TMPRSS2 protein in platelets.32 RNA‐seq analyses using platelets and megakaryocytes from mice also reveal a lack of ACE2 and TMPRSS2 expression.31., 33. In contrast, a recent study by Zhang et al observed robust ACE2 and TMPRSS2 mRNA and protein expression on platelets from healthy humans and mice.12 These investigators also used in vitro assays and in vivo ACE2 transgenic mice to report that both complete SARS‐CoV‐2 virus as well as the SARS‐CoV‐2 spike protein are able to induce platelet activation.
How might we reconcile these apparently discordant findings on whether platelets and megakaryocytes express ACE2 and TMPRSS2? Differences in ethnicities may explain the discrepant observations of ACE2 and TMPRSS2 RNA expression as Manne et al9 and Zaid et al13 included individuals from North America and North Africa while Zhang et al12 studied individuals from Asia. Interestingly, ACE2 and TMPRSS2 RNA were below the limit of detection in a publicly available microarray‐based platelet transcriptomics dataset which includes a relatively large proportion of healthy Black individuals.30 Alternatively, reported differences may be due to differences in methods to isolate platelets for RNA0based assays. Both Manne et al9 and Zaid et al13 used CD45+ beads to bind and thereby deplete any residual leukocytes from washed platelet preparations. In contrast, Zhang et al12 used gel‐purified platelets. While a monocyte‐specific marker (eg, CD14) was used to confirm the absence of white blood cells, perhaps lymphocytes, natural killer cells, and other white blood cells lacking CD14 expression were inadvertently present in platelet preparations used for RNA studies. For protein‐based assays, differences in antibody binding epitopes and/or non‐specific binding may have played a role. The work by Zhang et al12 also provided intriguing mechanistic evidence of platelet hyperactivation following the injection of SARS‐CoV‐2 spike protein in K18 human ACE2 transgenic mice. As human ACE2 expression is driven by the epithelial cell cytokeratin‐18 promoter,34 it remains uncertain as to whether human ACE2 was expressed by megakaryocytes and platelets in these transgenic mice and how these observations can be reconciled with those made in humans. Future studies are needed to confirm whether the transgenic expression of the receptor in mice reflects the expression profile in humans.
While the definitive answer of whether platelets express ACE2 and TMPRSS2 remains to be solved, it is very important to not lose sight of consistencies between these reports, and how concordant findings advance our understanding and are foundational for further studies. Not only did all studies demonstrate platelet activation in SARS‐CoV‐2 infection, they also confirmed that the SARS‐CoV‐2 virus can be found within platelets. This suggests that there may be ACE2‐independent mechanisms whereby SARS‐CoV‐2 directly interacts with, and possibly enters, platelets. What could this receptor be? In contrast to ACE2 and TMPRSS2, CD147 (basigin) is a highly glycosylated transmembrane protein robustly expressed in blood cells.35., 36. CD147 is believed to also be an alternative receptor for SARS‐CoV37 and SARS‐CoV‐238 as well as for HIV‐1 and measles.39., 40. The expression of CD147 in blood cellular lineages is suggested to be more elevated in male than female individuals, and is upregulated in conditions of asthma, chronic obstructive pulmonary disease, and obesity,35 which is consistent with the reported risks factors underlying complications in COVID‐19. However, the role of CD147 in SARS‐CoV‐2 infection remains controversial as additional studies have been unable to observe binding of the SARS‐CoV‐2 spike protein to CD147.41 While the role of CD147 remains unclear in SARS‐CoV‐2 infection, RNA‐seq16., 26., 29., 30., 31. and proteomic analysis indicate robust expression of CD147 on platelets with an estimated 2000 copies per platelet.32 Interestingly, previous studies from our group and others suggest engagement of CD147 through homotypic or heterotypic interactions induces P‐selectin expression, CD40L release, and platelet adherence similar to findings observed when SARS‐CoV‐2 interacts directly with platelets.36., 42. The studies by Zhang et al12 would also support a direct CD147 interaction with SARS‐Cov‐2 and the spike protein as they used competitive inhibition through the use of recombinant ACE2 and antibodies against the spike protein on SARS‐CoV‐2 to probe regulators of SARS‐CoV‐2–dependent platelet activation rather than only blocking platelet ACE2 specifically. Thus, there remains the possibility other receptors such as CD147 are responsible for mediating SARS‐CoV‐2 platelet interactions. Of note, red blood cells, which are more numerous than platelets in circulating blood, also express CD14743 and may compete against platelet CD147 for SARS‐CoV‐2 virus binding.
In addition to CD147, emerging evidence suggests CD26 may play a role in SARS‐CoV‐2 infection. Previous studies have demonstrated Middle East respiratory syndrome (MERS)‐CoV utilizes CD26 to infect cells and recent structural studies suggest CD26 might also bind SARS‐CoV‐2.44 However, a query of prior platelet RNA‐seq and proteomic datasets suggest that platelets and megakaryocytes do not express CD26, either under healthy baseline conditions or during acute infectious disease settings, including COVID‐19.9., 32. While these findings certainly deserve confirmation in future studies, currently available evidence would indicate that interactions between SARS‐CoV‐2 and platelets are unlikely to occur only through CD26. In addition to direct virus interaction with megakaryocytes and platelets, it is possible SARS‐CoV‐2 virus released into extracellular vesicles from infected white blood cells or endothelial cells can be internalized by platelets, resulting in SARS‐CoV‐2 virus positive platelets through indirect uptake of viral particles contained inside extracellular vesicles.45., 46., 47., 48.
4. CONCLUSIONS AND FUTURE DIRECTIONS
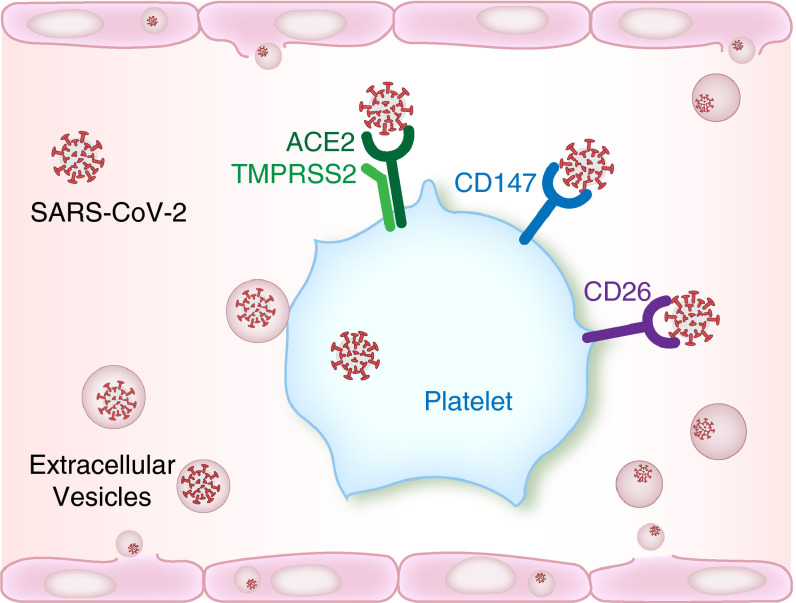
Independent studies from across the globe have shed light on platelet responses during SARS‐CoV‐2 infections. These studies have consistently identified that platelets are hyperreactive in COVID‐19, and this hyperreactivity may contribute to injurious host thrombo‐inflammatory responses. There also remains the possibility that platelets may serve as a cellular reservoir for SARS‐CoV‐2 infection, replication, and spread. Further studies elucidating these mechanisms are considered to be a high research priority. Additionally, understanding whether or not there are population‐based differences in the expression of putative SARS‐CoV‐2 entry receptors on platelets is critical. This is particularly important in light of emerging clinical data demonstrating a disproportionate burden of disease among some populations. In addition—or perhaps as an alternative mechanism to—ACE2 and TMPRSS2, other platelet receptors and mechanisms may regulate SARS‐CoV‐2 engagement of platelets (Figure 1 ).
FIGURE 1.
Possible mechanism(s) for SARS‐CoV‐2 interactions with platelets. A subset of platelets from COVID‐19 patients contain SARS‐CoV‐2 mRNA. Angiotensin converting enzyme‐2 (ACE2) and TMPRSS2 are the primary mechanism for entry of SARS‐CoV‐2 into cells, but the expression of these proteins on platelets remains controversial. Manne et al9and Zaid et al13recently observed the absence of both proteins in platelets based on RNA‐seq, real‐time polymerase chain reaction analysis, and proteomic approaches.9., 13.In contrast, Zhang et al12demonstrated expression of both ACE and TMPRSS2 on platelets.12It remains possible that SARS‐CoV‐2 may use ACE2‐independent binding partners to interact with platelets, including CD147 and CD26. Extracellular vesicles containing SARS‐CoV‐2 released from infected endothelial cells may also interact with platelets, therefore allowing interactions and viral cellular entry independent of direct virus binding
CONFLICT OF INTEREST
MTR is a member of the Scientific Advisory Board for Acticor Biotech SAS and holds a relevant patent.
AUTHOR CONTRIBUTIONS
Drafting of the manuscript: RAC, MTR and EB; concept and design: all authors; critical revision and editing of the manuscript: all authors.
Acknowledgments
National Heart, Lung, and Blood Institute R01HL130541 R01HL142804
Health Services Research and Development I01 CX001696
Fonds de Recherche du Québec en Santé
National Institute on Aging K01AG059892 R01AG048022 R56AG059877
Footnotes
Manuscript handled by: Katsue Suzuki‐Inoue
Final decision: Katsue Suzuki‐Inoue, 26 October 2020
Funding informationThis work was supported by grants from the NIH (K01AG059892 to RAC, and R01HL142804, R01AG048022, R56AG059877, and R01HL130541 to MTR). This work was also supported in part by Merit Review Award Number I01 CX001696 to MTR from the United States (US) Department of Veterans Affairs Clinical Sciences R&D (CSRD). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the US Department of Veterans Affairs or the United States government. This study was also supported by the Canadian Institutes of Health Research awarded to EB. EB is recipient of senior award from the Fonds de Recherche du Québec en Santé (FRQS).
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. 2020.
- 2.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC State‐of‐the‐art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)thrombosis in COVID‐19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al‐Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manne B.K., Denorme F., Middleton E.A., et al. Platelet gene expression and function in patients with COVID‐19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hottz E.D., Azevedo‐Quintanilha I.G., Palhinha L., et al. Platelet activation and platelet‐monocyte aggregate formation trigger tissue factor expression in patients with severe COVID‐19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett T.J., Lee A.H., Xia Y., et al. Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circ Res. 2020;127:945–947. doi: 10.1161/CIRCRESAHA.120.317803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S., Liu Y., Wang X., et al. SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaid Y., Puhm F., Allaeys I., et al. Platelets can contain SARS‐CoV‐2 RNA and are hyperactivated in COVID‐19. Circ Res. 2020 doi: 10.1101/2020.06.23.20137596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen B., Yi X., Sun Y., et al. Proteomic and metabolomic characterization of COVID‐19 patient sera. Cell. 2020;182:59–72. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell R.A., Schwertz H., Hottz E.D., et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood. 2019;133:2013–2026. doi: 10.1182/blood-2018-09-873984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rondina M.T., Brewster B., Grissom C.K., et al. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1) Chest. 2012;141:1490–1495. doi: 10.1378/chest.11-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt M.B., Lahon A., Arya R.P., Spencer Clinton J.L., Rico‐Hesse R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koupenova M., Corkrey H.A., Vitseva O., et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10:1780. doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koupenova M., Vitseva O., MacKay C.R., et al. Platelet‐TLR7 mediates host survival and platelet count during viral infection in the absence of platelet‐dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youssefian T., Drouin A., Masse J.M., Guichard J., Cramer E.M. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 22.Marcoux G., Laroche A., Espinoza Romero J., Boilard E. Role of platelets and megakaryocytes in adaptive immunity. Platelets. 2020:1–12. doi: 10.1080/09537104.2020.1786043. [DOI] [PubMed] [Google Scholar]
- 23.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine‐Weber H., Schroeder S., et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatlekar S., Basak I., Edelstein L.C., et al. Anti‐apoptotic BCL2L2 increases megakaryocyte proplatelet formation in cultures of human cord blood. Haematologica. 2019;104:2075–2083. doi: 10.3324/haematol.2018.204685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell R.A., Franks Z., Bhatnagar A., et al. Granzyme A in human platelets regulates the synthesis of proinflammatory cytokines by monocytes in aging. J Immunol. 2018;200:295–304. doi: 10.4049/jimmunol.1700885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davizon‐Castillo P., McMahon B., Aguila S., et al. TNF‐alpha‐driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134:727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middleton E.A., Rowley J.W., Campbell R.A., et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood. 2019;134:911–923. doi: 10.1182/blood.2019000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon L.M., Edelstein L.C., Nagalla S., et al. Human platelet microRNA‐mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123:e37–e45. doi: 10.1182/blood-2013-12-544692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowley J.W., Oler A.J., Tolley N.D., et al. Genome‐wide RNA‐seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkhart J.M., Vaudel M., Gambaryan S., et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120:e73–e82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 33.Machlus K.R., Wu S.K., Stumpo D.J., et al. Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation. Blood. 2016;127:1468–1480. doi: 10.1182/blood-2015-08-663146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCray P.B., Jr, Pewe L., Wohlford‐Lenane C., et al. Lethal infection of K18‐hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radzikowska U., Ding M., Tan G., et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75:2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt R., Bultmann A., Fischel S., et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB‐dependent inflammation in monocytes. Circ Res. 2008;102:302–309. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Mi L., Xu J., et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K., Chen W., Zhou Y.‐.S., et al. SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 39.Watanabe A., Yoneda M., Ikeda F., Terao‐Muto Y., Sato H., Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J Virol. 2010;84:4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushkarsky T., Zybarth G., Dubrovsky L., et al. CD147 facilitates HIV‐1 infection by interacting with virus‐associated cyclophilin A. Proc Natl Acad Sci USA. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shilts J., Wright G.J. No evidence for basigin/CD147 as a direct SARS‐CoV‐2 spike binding receptor. bioRxiv. 2020 doi: 10.1101/2020.07.25.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seizer P., Borst O., Langer H.F., et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI‐EMMPRIN interaction. Thromb Haemost. 2009;101:682–686. doi: 10.1160/th08-06-0368. [DOI] [PubMed] [Google Scholar]
- 43.Crosnier C., Bustamante L.Y., Bartholdson S.J., et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vankadari N., Wilce J.A. Emerging WuHan (COVID‐19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koupenova M. Potential role of platelets in COVID‐19: Implications for thrombosis. Res Pract Thromb Haemost. 2020;4:737–740. doi: 10.1002/rth2.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto Y., Moki T., Takizawa T., Shiratsuchi A., Nakanishi Y. Evidence for phagocytosis of influenza virus‐infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 47.French S.L., Butov K.R., Allaeys I., et al. Platelet‐derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4:3011–3023. doi: 10.1182/bloodadvances.2020001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edinger T.O., Pohl M.O., Stertz S. Entry of influenza A virus: host factors and antiviral targets. J Gen Virol. 2014;95:263–277. doi: 10.1099/vir.0.059477-0. [DOI] [PubMed] [Google Scholar]