Abstract
Primary peripheral blood monocytes are responsible for the hematogenous dissemination of human cytomegalovirus (HCMV) following a primary infection. In order to facilitate viral spread, HCMV extends the naturally short 48-hour lifespan of monocytes by stimulating a non-canonical activation of Akt during viral entry, which leads to the increased expression of a specific subset of antiapoptotic proteins. In this study, global analysis of the Akt signaling network showed HCMV induced a more robust activation of the entire network when compared to normal myeloid growth factors. Furthermore, we found a unique interplay between HCMV-activated Akt and the stress response transcription heat shock factor 1 (HSF1) that allowed for the synthesis of both cap- and internal ribosome entry site (IRES)-containing antiapoptotic mRNAs such as myeloid cell leukemia-1 (Mcl-1) and X-linked inhibitor of apoptosis (XIAP), respectively. As generally a switch from cap-dependent to IRES-mediated translation occurs during cellular stress, the ability of HCMV to concurrently drive both types of translation produces a distinct milieu of prosurvival proteins needed for the viability of infected monocytes. Indeed, we found inhibition of XIAP led to death of ~99% of HCMV-infected monocytes while having minimal effect on the viability of uninfected cells. Taken together, these data indicate that the aberrant activation of the Akt network by HCMV induces the upregulation of a unique subset of antiapoptotic proteins specifically required for the survival of infected monocytes. Consequently, our study highlights the possibility of exploiting these virus-induced changes to prevent viral spread in immunocompromised patients at high-risk for HCMV exposure.
Keywords: Cytomegalovirus, Monocytes, Small-molecule inhibitor
1. Introduction
Human cytomegalovirus (HCMV) is a ubiquitous betaherpervirus with a seroprevalence of 50–90% among adults in the United States (Staras et al., 2006). In immunocompetent individuals, HCMV infection is generally asymptomatic, although HCMV can manifest as mononucleosis syndrome and is linked to chronic inflammatory diseases, including atherosclerosis and inflammatory bowel disease (Lawlor and Moss, 2010; Nerheim et al., 2004). In contrast, HCMV infection is a significant health burden for immunocompromised and immunonaive patients such as transplant recipients and congenitally infected neonates, respectively (Ho, 1977; Masur et al., 1996; Stagno et al., 1982). Symptomatic HCMV disease in patients with ablated immunity can affect almost every organ in the body leading to end-organ damage and significant morbidity and mortality (Emery, 2001; Ljungman et al., 1992). The widespread inflammatory-based organ disease associated with an active HCMV infection is a direct consequence of the systemic spread that occurs during either asymptotic and symptomatic infections; a process crucial for the establishment of viral persistence within the host.
HCMV is predominantly cell-associated in the circulation with monocytes serving as the major cell type harboring quiescent (non-replicating) viral genomes during both asymptomatic and symptomatic infections, indicating blood monocytes function to deliver the virus into peripheral tissues (Manez et al., 1996; Sinclair and Sissons, 1996; Sinzger and Jahn, 1996; Soderberg-Naucler et al., 1997; Taylor-Wiedeman et al., 1991; Taylor-Wiedeman et al., 1994). Although the non-replicative state of HCMV would appear counterintuitive to monocytes being disseminators of the virus, we have found that the viral entry process directly stimulates differentiation of monocytes into macrophages, which are permissive for viral replication, and thus allow for the seeding of the virus in organs (Chan et al., 2012a; Chan et al., 2012b; Smith et al., 2004). In support, monocyte-derived macrophages appear to be the first cells to express viral antigens in infected organs of transplant recipients exhibiting an acute HCMV infection (Gnann et al., 1988; Sinzger et al., 1996). Additionally, a humanized mouse model of HCMV infection found the source of HCMV within peripheral organs to be from human macrophages derived from peripheral blood monocytes (Smith et al., 2010). Yet, a conundrum to monocytes being responsible for viral dissemination is their limited 48-hour (h) lifespan following release from the bone marrow, when these cells are programmed to undergo apoptosis in the absence of a survival stimuli (Gonzalez-Mejia and Doseff, 2009; Whitelaw, 1972). Despite HCMV encoding a vast array of viral antiapoptotic proteins, the lack of expression during the first 48 hours of infection precludes a viral gene product as the driver of infected monocyte survival through the 48-h “viability gate” (Chan et al., 2010). In order to overcome the intrinsic biological programming of monocytes to undergo apoptosis, HCMV stimulates a distinct prosurvival signalsome via the simultaneous engagement of epidermal growth factor receptor (EGFR) by viral glycoprotein gB and alpha v beta 3 (αvβ3) or β1 integrin receptor by gH during viral entry (Chan et al., 2012a; Chan et al., 2009b; Nogalski et al., 2011; Nogalski et al., 2013). Receptor co-signaling converges to mediate a unique activation of Akt whereby HCMV binding stimulates a chronic phosphorylation of Akt, while EGF (cognate EGFR ligand) induces a transient activation (Chan et al., 2009b). Moreover, HCMV stimulates a more robust activation of Akt when compared to normal myeloid growth factors and crosstalk between the gB/EGFR and gH/αvβ3 or β1 signaling axes is required for full HCMV-induced Akt signal strength (Chan et al., 2012a; Cojohari et al., 2016). These data suggest a coordinated signaling by gB and gH stimulates a non-traditional activation of Akt specifically required for the survival of HCMV-infected monocytes.
Akt is regulated by an intricate network of enzymes and phospholipid messengers, which are rapidly modified by HCMV infection to stimulate a non-canonical Akt activation pathway (Cojohari et al., 2016). Engagement of EGFR activates class 1A PI3Ks to recruit and phosphorylate Akt at the plasma membrane (Chan et al., 2009b; Wang et al., 2003). Although PI3K p110δ is the major isoform found in uninfected immature or growth factor treated monocytes to mediate survival (Cojohari et al., 2016; Voss et al., 2005), HCMV entry induces a switch from p110δ to p110β as the central PI3K isoform regulating the survival of infected monocytes (Cojohari et al., 2016). Concurrently, HCMV entry rapidly inhibits phosphatase and tensin homolog (PTEN), a negative PI3K regulator, to allow for maximum Akt activity (Cojohari et al., 2016). Most surprising, in contrast to myeloid growth factors, HCMV upregulates the expression of a negative regulator SH-2 containing inositol 5’polyphosphatase 1 (SHIP1) (Cojohari et al., 2016). However, rescue studies indicated SHIP1 functioned as a positive regulator of Akt during HCMV entry and was necessary for the HCMV-induced prosurvival state. Leukemia cells are also known to exhibit this unusual phenotype where overexpression of SHIP1 is required to promote an Akt-dependent cell survival (Franke et al., 1997; Li and Marshall, 2015; Ma et al., 2008); however, our study was the first to show the positive effects of SHIP1 on Akt activity in a non-cancer cell type (Cojohari et al., 2016). This non-canonical activation pathway required for the survival of infected monocytes indicates HCMV-activated Akt exerts unique biological functions when compared to Akt activated by myeloid growth factors.
The aberrant activation of Akt by HCMV leads to the upregulation of a select subset of Akt-dependent antiapoptotic proteins, including myeloid cell leukemia-1 (Mcl-1) and heat shock protein 27 (HSP27), that are not or weakly upregulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF) despite also stimulating Akt activity (Peppenelli et al., 2016). The phosphorylation profile of Akt is known to direct substrate specificity (Yung et al., 2011), suggesting that HCMV-activated Akt has distinct target substrates when compared to myeloid growth factors. Accordingly, HCMV infection leads to the phosphorylation of downstream Akt-dependent proteins not phosphorylated by either GM-CSF and M-CSF (Peppenelli et al., 2016). Regulators of protein translation appear to be the most differentially phosphorylated by HCMV- versus growth factor-activated Akt (Peppenelli et al., 2016). In particular, mammalian target of rapamycin (mTOR) is rapidly activated by HCMV infection (Kudchodkar et al., 2004; Moorman and Shenk, 2010; Peppenelli et al., 2016; Poglitsch et al., 2012), indicating that a selective activation of the PI3K/Akt/mTOR pathway is required for the synthesis of a subset of prosurvival proteins critical to the survival of infected monocytes. Indeed, while both HCMV infection or growth factor treatments increases the transcription of both Mcl-1 and HSP27, only HCMV infection stimulates the translation of these antiapoptotic proteins in a mTOR-dependent manner (Peppenelli et al., 2016). The full extent to which the Akt signaling network is aberrantly regulated is unclear but these data hint at the possibility of exploiting the unique changes as an antiviral strategy to eliminate HCMV-infected monocytes prior to dissemination and the initiation of viral replication.
In this study, we take a global approach using an antibody array consisting of >100 components of the Akt signaling network to define the distinct changes made to the Akt signalsome by HCMV infection. In comparative analyses with normal myeloid growth factors, we found that HCMV triggered a more robust activation of the entire network, although HCMV did not stimulate the phosphorylation of certain downstream substrates while growth factors were able to induce a similar or higher phosphorylation of other targets. Nonetheless, consistent with our previous findings, HCMV led to a significant increase in mTOR activation whereas GM-CSF and M-CSF had lesser effect (Peppenelli et al., 2016). The increased mTOR activity resulted in elevated S6 kinase (S) activity, a downstream regulator of translation. However, loss S6K showed no effect on the HCMV-induced synthesis of Mcl-1 and HSP27. Instead, the expression of Mcl-1 was dependent on another downstream mTOR translation effector protein, eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), while HSP27 expression was regulated by a stress response transcription factor, HSF1, which was downstream of Akt. In addition to stimulating monocyte survival through the increased translation of select antiapoptotic proteins, our microarray analysis also identified a new direct Akt antiapoptotic target, X-linked inhibitor of apoptosis (XIAP), that was activated by HCMV infection significantly beyond GM-CSF or M-CSF treatment. Importantly, we found inhibition of XIAP led to death of ~99% of HCMV-infected monocytes while having minimal effect on the viability of uninfected cells. Taken together, our data indicates that the non-canonical activation of the Akt network during HCMV infection induces the upregulation and/or activity of a select subset of antiapoptotic proteins specifically required for the survival of infected monocytes, thus raising the possibility of using these virus-induced changes as the basis of novel antiviral therapies to prevent viral spread.
2. Materials and Methods
2.1. Human peripheral blood monocyte isolation
Isolation of human peripheral blood monocytes was performed as previously described (Chan et al., 2010; Smith et al., 2004; Yurochko and Huang, 1999). Briefly, blood was drawn from random donors by venipuncture, diluted in Roswell Park Memorial Institute medium (RPMI) 1640, and centrifuged through Histopaque 1077 (Sigma Aldrich, St. Louis, MO) to remove red blood cells and neutrophils. Mononuclear cells were collected and washed with saline to remove the platelets and then separated by centrifugation through a Percoll (GE Healthcare, Wilkes-Barre, PA) gradient (40.48% and 47.7%). More than 90% of isolated peripheral blood mononuclear cells were monocytes as determined by CD14-positive staining (Chan et al., 2012b). Cells were washed with saline, resuspended in RPMI 1640 (Lonza, Walkersville, MD) supplemented with 1% human AB serum (Lonza), and counted. All experiments were performed in 1–2% human serum at 37°C in a 5% CO2 incubator, unless otherwise stated. University Institutional Review Board and Health Insurance Portability and Accountability Act guidelines for the use of human subjects were followed for all experimental protocols in our study.
For the inhibitor studies, the following reagents were used: MK2206 2HCL (MK; an Akt inhibitor), LY2584702 (a S6K inhibitor), and embelin (a XIAP inhibitor) from Selleckchem (Houston, TX); KRIBB11 (a HSF1 inhibitor) and LY294002 (a pan-PI3K inhibitor) from Calbiochem (Billerica, MA); 4EGI-1 (an eIF4E inhibitor) from Tocris (Bristol, UK).
2.2. Virus preparation and infection
Human embryonic lung (HEL) 299 fibroblasts (CCL-137, American Type Culture Collection, Manassas, VA) of low passage (P7–15) were subcultured in Dulbecco’s Modified Eagle medium (DMEM) (Lonza) with 2.5 μg/ml plasmocin (Invivogen, San Diego, CA) and 10% fetal bovine serum (FBS) (Sigma). When culture reached confluency, cells were infected with HCMV (strain TB40E) in DMEM + 4% FBS. Virus was purified from supernatant on a 20% sorbitol cushion to remove cellular contaminants and resuspended in RPMI 1640 medium. A multiplicity of infection (MOI) of 5 was used for each experiment as >99% of monocytes were infected with TB40E (Chan et al., 2009b). Mock infection was performed by adding an equivalent volume of RPMI 1640 medium to monocytes, while GM-CSF or M-CSF treatment was performed by adding an equivalent volume of RPMI 1640 medium with recombinant human GM-CSF or M-CSF at 100 ng/ml (R&D Systems, Minneapolis, MI).
2.3. Protein Microarray Analysis
Peripheral blood monocytes (7 × 106 / treatment) were mock or HCMV infected, or GM-CSF or M-CSF treated for 24 h. Additionally, monocytes were pretreated with MK2206 inhibitor for 1 h prior to mock or HCMV infection. Akt/PKB phospho-protein array was performed as described by the manufacturer (Full Moon Biosystems, Sunnyvale, CA). Briefly, cells were lysed and protein biotin labelled in 10 μg/μl DMF (N,N-Dimethyformamide). To prepare slides for microarray analyses, slides were first blocked for 1 h in blocking solution at room temperature (RT), rinsed with Milli-Q grade water for 5 m (minutes), and incubated with biotin-labeled cell lysates at 4°C overnight. Following washing, conjugated labeled proteins were detected using cyanine 3 (Cy3)-streptavidin (Invitrogen, Grand Island, NY). Processed arrays were then sent to Full Moon Biosystems for reading. Protein expression levels for each donor were normalized to S473-Akt in the HCMV-infected treatment group, which was set to 100%. From a n=3, the mean, standard deviation, and subsequent 95% confidence intervals were calculated.
2.4. Western blot analysis
Monocytes were harvested in radioimmunoprecipitation assay buffer (RIPA) buffer (1 M Tris-HCl [pH 7.5], 0.5 M EDTA, 5 M NaCl, 1% Triton X-100, 10% NaDodSO4 [SDS], and 10% glycerol) containing one times protease inhibitor mixture (Sigma-Aldrich), one times phosphatase inhibitor mixture II (Sigma-Aldrich), and one times phosphatase inhibitor mixture III (Sigma-Aldrich) for 15 m on ice. The lysates were cleared by centrifugation at 4°C (5 m, 16,000 × g) and stored at −20°C until analyzed. Sample protein was solubilized in six times sample buffer (Sigma-Aldrich) by boiling for 10 m. Equal amounts of total cellular protein from each sample were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting. Blots were blocked in 5% bovine serum albumin (BSA) (Fisher Scientific, Waltham, MA) for 1 h at RT and then incubated with primary antibodies overnight at 4°C. Antibodies were purchased from the following companies: anti-Mcl-1, anti-HSP27, anti-XIAP, and anti-caspase-3 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); anti-phospho (p)-P70S6K (T389), anti-4E-BP1, anti-p-4E-BP1 (T37/46), anti-eIF4E, anti-p-eIF4E (S209), anti-HSF1, anti-mTOR, anti-p-mTOR (S2448) antibodies from Cell Signaling (Danvers, MA); anti-p-HSF1 (S326), anti-S6K1, anti-p-S6K (T421/S424), anti-p-XIAP (S87), and anti-β-actin antibodies from Abcam (Cambridge, MA). Blots were then incubated with diluted horseradish peroxidase (HRP)-conjugated secondary antibodies for 30 m at RT and developed using ECL Plus (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s protocol.
2.5. Flow Cytometry
Monocytes were washed in phosphate-buffered saline (PBS) and incubated in blocking solution consisting of fluorescence-activated cells sorting buffer, 5% BSA, and human FcR binding inhibitor (eBioscience, San Diego, CA), followed by staining with an allophycocyanin (APC)–anti-CD14 or APC–anti-mouse IgG1 isotype control antibody (BioLegend, San Diego, CA) on ice. The cells were then washed and stained with propidium iodide (PI) and phycoerythrin-annexin V (BD Pharmingen, Franklin Lakes, NJ) to detect dead and dying cells. After staining, the cells were analyzed by flow cytometry using an LSRFortessa cell analyzer and BD FACSDiva software (BD Biosciences). Our gating strategy on forward scatter (FSC)/side scatter (SSC) was set to include both cells in the early stages of apoptosis (decreased FSC and increased SSC compared to those for viable cells) and cells in the late stages of apoptosis (decreased FSC and decreased SSC compared to those of viable cells).
2.6. Transient Transfection and RNA Silencing
Monocytes (3 × 106 / transfection) were resuspended in 100 μl of RT nucleofection solution (Amaxa P3 primary cell solution; Amaxa Biosystems, Cologne, Germany) containing validated 1 μM S6K-targeting siRNA (Ambion-Thermo Fisher Scientific, Carlsbad, CA) or 1 μM control siRNA (Ambion-Thermo Fisher Scientific), and transfected with a 4D-Nucleofector system using program EI-100. Following transfection, cells were incubated in RPMI 1640 (Cellgro) supplemented with 5% human AB serum in RPMI at 37°C (Lonza) for 24 h. Monocytes were then mock or HCMV infected for 24 h and subjected to western blot analysis.
3. Results
3.1. Convergence of receptor signaling onto the Akt hub triggers a robust activation of the downstream network.
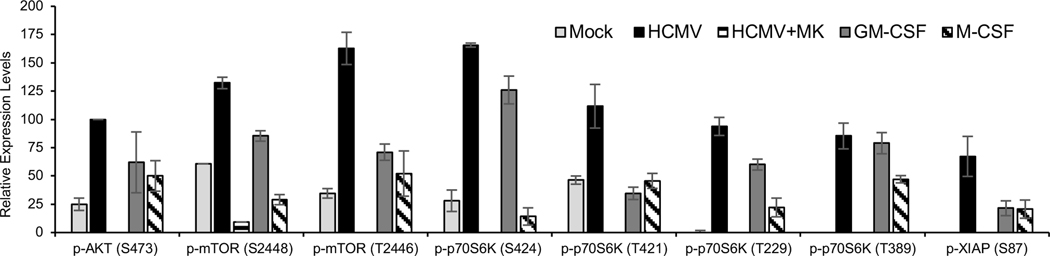
In the absence of viral antiapoptotic proteins, HCMV’s triggering of EGFR and αvβ3 signaling leads to an enhanced activation of Akt in order to support a virus-specific antiapoptotic microenvironment within infected monocytes (Chan et al., 2010; Chan et al., 2012a; Chan et al., 2012b; Nogalski et al., 2011; Nogalski et al., 2013; Peppenelli et al., 2016). Unlike Akt activated by GM-CSF- and M-CSF treatment, HCMV-activated Akt stimulates the translation of Mcl-1 and HSP27 in a mTOR-dependent manner (Peppenelli et al., 2016), suggesting a distinct regulation of the Akt signaling system is needed to promote the survival of infected monocytes. Biologically, this difference highlights the possibility of selectively targeting HCMV-infected monocytes, while allowing uninfected monocytes to maintain normal immunosurveillance function and respond to myeloid growth factors. To examine the extent to which HCMV infection aberrantly modifies the Akt network, a protein microarray was used to monitor the phosphorylation status of known components within the Akt signaling circuitry following 24 hpi, when Mcl-1 and HSP27 are maximally expressed (Chan et al., 2010; Chan et al., 2012b; Peppenelli et al., 2016). In general, we found that HCMV induced a more robust activation of the Akt signaling network when compared normal myeloid growth factors, albeit certain elements were phosphorylated equally by all treatments, such as p53 T81, or upregulated exclusively by growth factors, such as 14-3-3- theta/tau (supplemental Fig. 1). The increased phosphorylation of Akt substrates following infection was confirmed to be dependent on Akt as phosphorylation events were abrogated by MK2206 (an Akt inhibitor). Consistent with our previous findings, HCMV increased the phosphorylation of Akt S473 and mTOR S2448 at 24 hpi (Fig. 1) (Peppenelli et al., 2016). In addition, we found mTOR S2481 and mTOR T2446 phosphorylation to be more prominent in HCMV-infected monocytes compared to GM-CSF- or M-CSF-treated cells, which displayed negligible to nominal increases relative to mock-infected cells (Fig. 1). The hyperphosphorylation of mTOR at S2448, S2481, and T2446 indicate an increase in mTOR-dependent protein translation within infected monocytes; however, HCMV-activated Akt also appears to regulate mTOR-independent translation events. Inactivation of glycogen synthase kinase (GSK3) causes a dephosphorylation and subsequent activation of eukaryotic translation initiation factor 2B (eIF2B), which globally enhances translation initiation (Proud, 2007). HCMV stimulated an enhanced inhibitory phosphorylation of GSK-3β S9 and GSK-3βα S21 (Supplemental Fig. 1), suggesting an overall requirement of infected monocytes to acquire a cellular environment geared towards accelerated protein translation. Additionally, our Akt network analysis also suggests HCMV-activated Akt processes a distinct activity from Akt activated by normal myeloid growth factors aimed at driving the translation of specific cellular antiapoptotic proteins critical to the survival of infected monocytes; thus, potentially allowing for the selective targeting of infected monocytes.
Fig. 1. HCMV infection stimulates a robust activation of the Akt signaling network.
Primary monocytes were mock infected, HCMV infected, GM-CSF treated, or M-CSF treated for 24 hours. Monocytes were also pretreated with MK (an AKT inhibitor) for one hour prior to a 24 h infection. Cells were then lysed and subjected to protein microarray analysis from Fullmoon Biosystems. Data are the mean from 3 independent blood donors.
3.2. HCMV stimulates the translation of select antiapoptotic mRNAs via the mTOR-dependent activation of eIF4E.
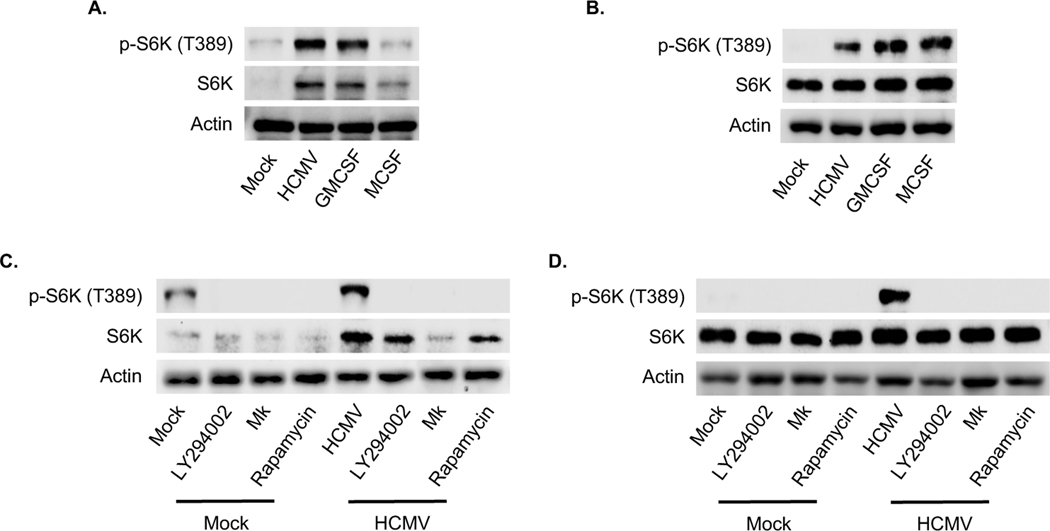
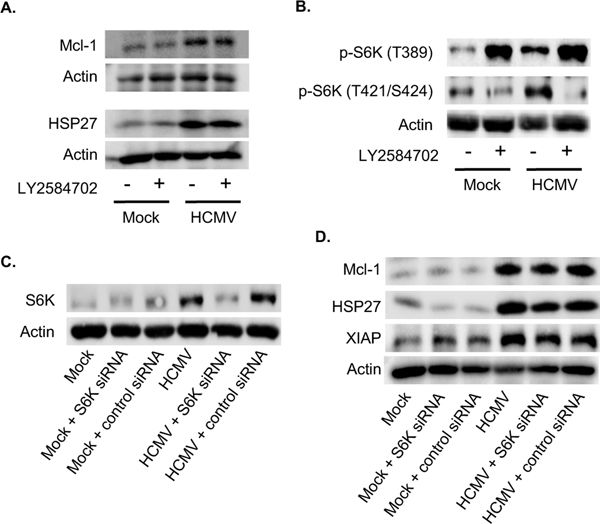
Based on the network analysis, HCMV-induced Akt appears to regulate protein translation through multiple mechanisms. However, the upregulation of the select Akt-dependent antiapoptotic proteins during HCMV infection requires mTOR (Peppenelli et al., 2016). S6K (also known as p70S6 kinase), is a direct target of mTOR that regulates protein synthesis by phosphorylation of S6 ribosomal protein. Phosphorylation of T229 in the catalytic domain and T389 by mTOR are important for kinase function, although T389 appears to be the predominant site responsible of kinase activity (Alessi et al., 1998; Avruch et al., 2001; Pullen et al., 1998). Phosphorylation of S371 within the turn motif allows for efficient T389 phosphorylation. In addition, phosphorylation of S411, S418, S424, and T421 residing in the autoinhibitory domain relieves suppression of S6K (Dufner and Thomas, 1999). HCMV induced a more robust phosphorylation of all regulatory sites on S6K when compared to M-CSF (Fig. 1). GM-CSF induced a S6K phosphorylation pattern similar to HCMV with the exception of S371 and T421, which were not phosphorylated by GM-CSF treatment. These data suggest HCMV to be the most potent activator of S6K despite similar T389 phosphorylation induced by HCMV and GM-CSF. Indeed, S6 ribosomal protein phosphorylation was highest in HCMV-infected monocytes when compared to growth factors (Fig. 1). Western blot confirmed S6K was efficiently phosphorylated at T389 in both HCMV-infected and GM-CSF-treated monocytes at 24 hours (Fig. 2A), although M-CSF rapidly induced phosphorylation comparable to HCMV and GM-CSF at 15 min post treatment (Fig. 2B). The maintaining of elevated levels of activated S6K in HCMV-infected and GM-CSF-treated monocytes may in part be due to the increased total protein levels (Fig. 2A). As expected, inhibition of the PI3K/Akt/mTOR signaling cascade with small-molecule antagonist abrogated the activation S6K following HCMV infection (Fig. 2C and D). Next, monocytes were treated with LY2584702 (a S6K inhibitor) prior to infection to address the possible involvement of S6K on the upregulation of Mcl-1 and HSP27. Despite the dependency of HCMV-induced Mcl-1 and HSP27 on mTOR-mediated translation, the loss of S6K activity had no effect on protein expression (Fig. 3A). However, LY2584702 pretreatment selectively blocked phosphorylation of S6K at T421/424 without effecting the major kinase regulatory site T389 (Fig. 3B). To circumvent the incomplete inhibition of S6K by LY2584702, siRNAs were used to lower total protein levels (Fig. 3C). Consistent with LY2584702 treatment, a reduction in S6K expression did not block the upregulation of Mcl-1 and HSP27 following infection (Fig. 3D), indicating S6K is not involved in the regulation of critical Akt-dependent antiapoptotic proteins needed for the survival of HCMV-infected monocytes.
Fig. 2. HCMV upregulates S6K activity via the PI3K/Akt/mTOR signaling pathway.
(A-B) Peripheral blood monocytes were mock or HCMV infected, or treated with GM-CSF or M-CSF for (A) 24 h or (B) 30 m. (C-D) Monocytes were pretreated with LY294002 (a PI3K inhibitor), MK, or rapamycin (a mTOR inhibitor) for 1 h. Following treatment with inhibitors, cells were mock or HCMV infected for (C) 24 h or (D) 30 min. (A-D) Levels of p-S6K (T389), and S6K were detected by immunoblotting from whole cell lysates. Membranes were then reprobed for β-actin as a loading control. Data are representative of 3–6 independent blood donors.
Fig. 3. Activation of S6K is not responsible for the increased expression of Mcl-1 and HSP27 following HCMV infection.
(A-B) Monocytes were pretreated with LY2584702 (a S6K inhibitor) for 1 h, then mock or HCMV infected for 24 h. (A) Mcl-1 and HSP27 or (B) pS6K (T389) and pS6K (T421/S424) were detected by immunoblotting. (C-D) Monocytes were transfected with a scrambled control siRNA or S6K siRNA and incubated for 24 h. Following incubation, cells were mock infected or HCMV infected for an additional 24 h. (C) S6K or (D) Mcl-1, HSP27 and XIAP expression were determined by immunoblotting. (A-D) Membranes were reprobed for β-actin as a loading control. Data are representative of 3–6 independent blood donors.
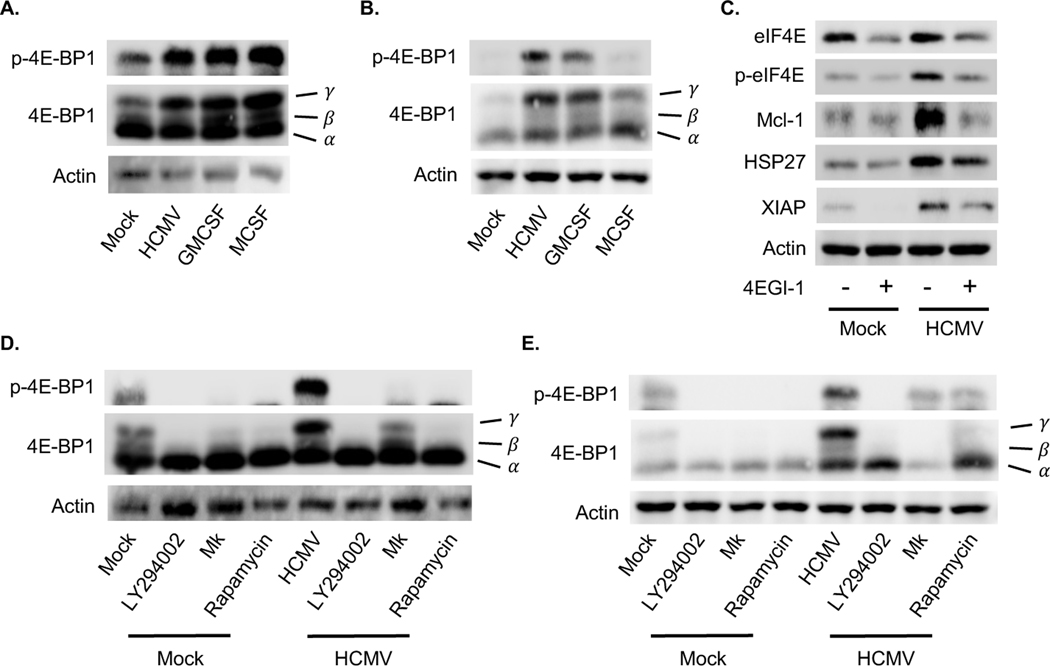
4E-BP1 is a second well-characterized mTOR target that acts as an inhibitor of translation initiation by binding and inactivating eukaryotic translation initiation factor 4E (eIF4E) (Nandagopal and Roux, 2015; Shahbazian et al., 2006). mTOR phosphorylation of 4E-BP1 promotes the dissociation of the eIF4E/4E-BP1 complex, consequently mitigating the inhibitory effects of 4E-BP1 on eIF4E-dependent translation (Pause et al., 1994). HCMV and growth factors rapidly stimulated 4E-BP1 phosphorylation with 15 minutes post treatment (Fig. 4A). However, HCMV infection sustained higher levels of p-4E-BP1 relative to GM-CSF and M-CSF treatment at 24 hpi, which led to the activation of eIF4E (Fig. 4C). PI3K/Akt/mTOR signaling was required for the inhibition of 4E-BP1, as blockade of the pathway prevented 4E-BP1 phosphorylation (Fig. D and E). To assess the role of eIF4E on the acquisition of the antiapoptotic state, monocytes were treated with 4EGI-1 (an eIF4E inhibitor) prior to infection. We found the loss of eIF4E abrogated Mcl-1 expression while having minimal effect on HSP27 expression in HCMV-infected monocytes (Fig. 4C), indicating eIF4E is required for the production of specific HCMV-induced Akt-dependent antiapoptotic proteins. Moreover, these results suggest a branching of Akt signaling is responsible for the upregulation of different survival proteins within HCMV-infected monocytes.
Fig. 4. HCMV stimulates a sustained inactivation of 4E-BP1 in a PI3K/Akt/mTOR dependent manner.
(A-B) Peripheral blood monocytes were mock or HCMV infected, or treated with GM-CSF or M-CSF for (A) 30 m or (B) 24 h. (C) Monocytes were pretreated with 4EGI-1 (an eIF4E inhibitor) for 1 h, then mock or HCMV infected for 24 h. Levels of Mcl-1, HSP27, and XIAP expression were determined by immunoblotting. (D-E) Monocytes were pretreated with LY294002, MK, or rapamycin for 1 h. Following pretreatment, cells were mock or HCMV infected for (D) 30 m or (E) 24 h. 4E-BP1 and p-4E-BP1 expression were determined by immunoblotting. Electrophoresis of 4E-BP1 separates into three different forms (Constantinou and Clemens, 2005). The γ band corresponds to the most highly phosphorylated form of 4E-BP1, whereas the β and α bands represent the intermediate and least phosphorylated form, respectively. (A-E) Membranes were reprobed for β-actin as a loading control. Data are representative of 4–6 independent blood donors.
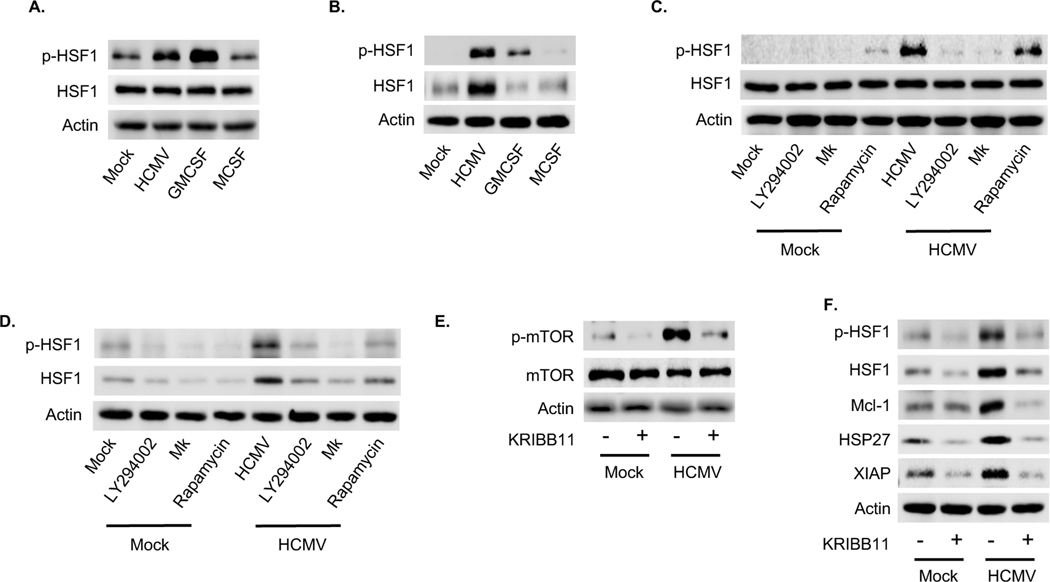
3.3. HCMV activates the stress-responsive transcription factor HSF1 in a Akt-dependent manner to increase the expression of prosurvival proteins.
HCMV induces host stress responses during infection due to nutrient and energy depletion (Alwine, 2008; McArdle et al., 2012; Shenk and Alwine, 2014). Heat shock factor 1 (HSF1), a heat (stress)-activated transcription factor, is a critical player in the stress response required for the expression of heat shock proteins (HSPs) (Calderwood et al., 2010; Wu, 1995). We found HCMV and GM-CSF phosphorylated HSF1 within 15 minutes post treatment while M-CSF had no effect on activity (Fig. 5A). Total HSF1 levels were exclusively increased within HCMV-infected monocytes leading to the sustained elevated levels of activated protein through 24 hpi (Fig. 5B), suggesting that maintaining activated HSF1 is crucial to the long-term survival of infected monocytes. Consistent with other studies, inhibition of the PI3K/Akt/mTOR cascade prevented both the rapid phosphorylation and upregulation of protein levels following HCMV infection (Fig. 5C and D). However, inhibition of mTOR had a significantly reduced effect on the rapid activation of HSF1 relative to PI3K and Akt inhibition (Fig. 5C). Since mTOR, PI3K and Akt are all essential to S6K and 4E-BP1 phosphorylation, these data indicate a diverging pathway from Akt is responsible for regulating HSF1. Moreover, the loss of HSF1 activity also blocked HCMV-induced mTOR activation, suggesting the presence of a positive feedback loop between HSF1 and mTOR signaling (Fig. 5E). Although we cannot exclude the possibility that decreased mTOR activation is due to the off-target effects of KRIBB1, our data is consistent with other studies showing increased HSF1 can sustain mTOR activity (Cigliano et al., 2017). Regardless, the initial triggering of the Akt-to-HSF1 pathway was responsible for the modulation of HSP27 following HCMV infection as HSF1 activation was essential for the increase of HSP27 protein (Fig. 5F). In addition, we found inhibition of HSF1 abrogated the ability of HCMV to upregulate Mcl-1 (Fig. 5F), demonstrating that, although Mcl-1 transcription is not directly modulated by HSF1, the positive feedback loop existing between HSF1 and the PI3K/Akt/mTOR pathway allows for the HSF1 regulation of Mcl-1 expression. Overall, these data provide evidence that HCMV infection drives a chronic stress response mediated by the interplay between the mTOR and HSF1, which is necessary for long-term survival of infected monocytes.
Fig. 5. A HSF1 positive feedback loop amplifies PI3K/Akt/mTOR signaling within HCMV-infected monocytes.
(A-B) Monocytes were mock or HCMV infected, or treated with GM-CSF or M-CSF for (A) 30 m or (B) 24 h. (C-D) Monocytes were pretreated with LY294002, MK, or rapamycin for 1 h, after which cells were either mock or HCMV infected for (C) 30 m or (D) 24 h. (A-D) HSF1 and p-HSF1 expression were determined by immunoblotting. (E-F) Prior to 24 h mock or HCMV infection, monocytes were pretreated with KRIBB11 (a HSF1 inhibitor) for 1 h. (E) mTOR and p-mTOR or (F) HSF1, p-HSF1, Mcl-1, HSP-27, and XIAP expression were assessed by immunoblot. (A-F) Membranes were then reprobed for β-actin as a loading control. Data are representative of 3–5 independent blood donors.
3.4. XIAP is critical to the survival of HCMV-infected monocytes.
Our previous studies demonstrated the aberrant activation of Akt led to the increased synthesis of select antiapoptotic proteins such as Mcl-1 and HSP27 in order to stimulate a prosurvival state within infected monocytes (Chan et al., 2010; Chan et al., 2012b; Peppenelli et al., 2016). Global analysis of the Akt signaling pathway now indicates an additional mechanism whereby HCMV-activated Akt can directly activate specific prosurvival effectors. X-linked inhibitor of apoptosis (XIAP) was identified as a direct downstream Akt target differentially regulated during HCMV infection and growth factor treatment (Fig. 1). XIAP inhibits caspase 3 through direct binding and blocking the proteolytic cleavage required for activation (Riedl and Shi, 2004). As inhibition of caspase 3 is critical for HCMV-infected monocytes to successfully navigate the 48-h viability checkpoint (Chan et al., 2010; Chan et al., 2012b), we sought to determine the role of XIAP in promoting the antiapoptotic state of infected monocytes. Despite total protein being upregulated by HCMV and normal myeloid growth factors, XIAP was exclusively activated by HCMV (Fig. 6A). Multiple HCMV strains induced a similar increase in expression and activation of XIAP demonstrating a critical function of this caspase inhibitor to the survival of infected monocytes across multiple strains (Fig. 6B). The induction of XIAP was dependent on PI3K/Akt/mTOR signaling, since pathway inhibition completely abrogated the ability of HCMV to upregulate XIAP (Fig. 6C and D). However, loss of XIAP activity with the use of a small-molecule inhibitor (embelin) led to the proteolytic cleavage of procaspase 3 and the accumulation of active caspase within infected monocytes (Fig. 7A and B). To determine the contribution of XIAP to the survival of HCMV-infected monocytes, cells were treated with embelin at 24 hpi, a time when maximum resistance to apoptosis is reached, and viability examined. HCMV infection induced the survival of monocytes from 56.7% to 80.5% and the inhibition of XIAP led to death of ~99% of viable infected monocytes (Fig. 7C). In contrast, XIAP inhibition had a significantly less effect on the viability of “live” uninfected monocytes decreasing viability from 56.4% to 35.6%, suggesting a specific requirement of HCMV-infected monocytes for the increased activity of XIAP. Taken together, these data indicate the aberrant activation of the Akt network by HCMV selectively stimulates the increased activity of select antiapoptotic proteins required for the survival and differentiation of newly infected monocytes, a crucial step in the viral dissemination strategy.
Fig. 6. HCMV induces XIAP activity in a PI3K/Akt/mTOR dependent manner.
(A) Monocytes were mock or HCMV infected, or treated with GM-CSF or M-CSF for 24 h. (B) Monocytes were infected with HCMV strain TB40E or Towne/E for 24 h. (C-D) Monocytes were pretreated with LY, MK, or rapamycin for 1 h. Cells were then mock or HCMV infected for 24 h. (A-D) Levels of p-XIAP and XIAP were determined by immunoblotting. Membranes were then reprobed for β-actin as a loading control. Data are representative of 3–6 independent blood donors.
Fig. 7. HCMV utilizes XIAP to prevent apoptosis in newly infected monocytes by blocking the activation of caspase 3.
(A-B) Monocytes were mock or HCMV infected for 24 h, after which infected cells were treated with embelin (a XIAP inhibitor) for an additional 24 h. (A) p-XIAP and (B) caspase 3 cleavage was assessed by immunoblotting. Membranes were then reprobed for β-actin as a loading control. (C) Viability was measured by flow cytometry using propidium iodide (PI) and Annexin V staining. Gates represent live cells (PI and Annexin V negative), apoptotic cells (PI low and Annexin V high), and late apoptotic or dead cells (PI and Annexin V positive). (A to D) Results are representative of 3–8 independent experiments using monocytes from different donors.
4. Discussion
The prevention and treatment of HCMV-associated diseases has been hampered by the unusual viral dissemination strategy employed by HCMV. In circumventing the short lifespan of monocytes without the use of de novo synthesized viral gene products (Chan et al., 2008; Chan et al., 2009a; Chan et al., 2010; Chan et al., 2012a; Chan et al., 2012b; Smith et al., 2004), HCMV is able to utilize monocytes as “Trojan horses” to penetrate into peripheral tissue while being sheltered from the effects of replication inhibitors. Consequently, prophylactic treatment has simply delayed the kinetics of HCMV-disease in high risk transplant patients as viral replication is initiated upon termination of the antiviral therapy (Fishman et al., 2007; Sagedal et al., 2004). Thus, identifying new targets aimed at eliminating persistently infected myeloid cells is critical for the long-term resolution of disease. We have previously shown that HCMV binding stimulates a distinct signalsome through the simultaneous activation of cellular receptors EGFR and αvβ3 or β1 (Chan et al., 2012a; Chan et al., 2009b; Nogalski et al., 2011; Nogalski et al., 2013). The convergence of receptor signaling onto Akt leads to a different phosphorylation signature when compared to Akt activated by myeloid growth factors and subsequently the upregulation of a subset of Akt-dependent antiapoptotic proteins (Cojohari et al., 2016; Peppenelli et al., 2016). We now demonstrate in this study that HCMV infection induces a unique interplay between the Akt signaling network and the stress response, which mediates the activation and upregulation of select survival proteins within HCMV-infected monocytes.
The canonical activation of Akt by normal growth factor receptor signaling requires a PI3K mediated conformational change to unmask T308 and S473, which are phosphorylated by phosphoinositide kinase-1 (PDK1) and mTOR complex 2 (mTORC2), respectively. However, simultaneous to PI3K activation, HCMV entry into monocytes modifies the activities of two major Akt negative regulators, PTEN and SHIP1 (Cojohari et al., 2016). PTEN, which directly reverses PI3K activity, is rapidly shutdown by HCMV (Cojohari et al., 2016). SHIP1, which antagonizes Akt activation under homeostatic conditions (Kerr, 2011), is rapidly upregulated and converted into a positive regulator of Akt further contributing to the HCMV-induced prosurvival state (Cojohari et al., 2016). The activation of Akt via SHIP1 results in a more robust phosphorylation of Akt at S473 (Franke et al., 1997; Li and Marshall, 2015; Ma et al., 2008), suggesting the initiation of the non-canonical Akt activation pathway may contribute to the hyperphosphorylation of Akt following HCMV infection that allows for distinct functional outcomes when compared to Akt activated by normal myeloid growth factors. Indeed, our global analysis indicated the totality of converging signaling pathways onto Akt during viral entry stimulates a more robust activation of the entire signaling network compared to growth factors. The enhanced activity of the Akt network appears to be aimed at driving protein translation through the increased activity of mTOR and decreased activity of GSK3s, which is consistent with our previous studies demonstrating HCMV-activated Akt drives the synthesis of select antiapoptotic proteins (Chan et al., 2010; Chan et al., 2012b; Peppenelli et al., 2016).
HCMV stimulates the synthesis of Mcl-1 and HSP27 in an mTOR-dependent manner (Peppenelli et al., 2016). mTOR controls translation via the regulation of translation factors S6K and eIF4E (Nandagopal and Roux, 2015). S6K targets a number of proteins that control protein translation including eIF4B (Raught et al., 2004), programmed cell death protein-4 (PDCD4) (Yang et al., 2003), eukaryotic translation initiation factor 2K (eIF2K) (Wang et al., 2001), and S6 ribosomal protein (Ferrari et al., 1991). The activation of S6K has been shown to induce the translation of survival factors such as XIAP and antiapoptotic B-cell lymphoma (Bcl-2) family members (Basu and Sridharan, 2017; Liwak et al., 2012). However, S6K was not involved in the upregulation of select antiapoptotic proteins required for the survival of infected monocytes despite being rapidly activated following HCMV infection. Although these studies demonstrate S6K as being dispensable for virus-induced upregulation of Mcl-1, HSP27, and XIAP, further work is needed to determine if S6K regulates the expression of other survival proteins required of the survival of infected monocytes. Alternatively, S6K1 also directly phosphorylates apoptosis regulators such as Mdm2 and BAD, which may allow for direct regulation on the activities of apoptotic regulators rather than through the increased expression.
In addition to S6K, mTOR signaling activates eIF4E to promote translation by phosphorylating and inactivating 4E-BP1, an inhibitor of translation initiation (Pause et al., 1994). EIF4E activation typically does not have global effects on translation rates, but has been found to selectively favor cap-dependent translation over internal ribosome entry site (IRES)-mediated translation (Mamane et al., 2006; Mamane et al., 2004). HCMV and growth factors rapidly phosphorylated 4E-BP1, although HCMV maintained the highest level of inactivation through 24 hours followed sequentially by GM-CSF and M-CSF. The amount of inactivated 4E-BP1 correlated with Mcl-1 expression levels induced by HCMV and myeloid growth factors (Peppenelli et al., 2016), suggesting a central role for eIF4E in regulating Mcl-1. Indeed, Mcl-1 expression in HCMV-infected monocytes was dependent on eIF4E, which was consistent with other studies demonstrating eIF4E cap-dependent regulation of Mcl-1 expression via the PI3K/Akt/eIF4E pathway (Wendel et al., 2007). Interestingly, during times of cellular stress 4E-BP1 typically undergoes dephosphorylation leading from a switch from cap-dependent translation to cap-independent mechanisms such as IRES-mediated translation (Nandagopal and Roux, 2015). Yet, cap-dependent translation is maintained in monocytes during the HCMV-induced stress response, which is consistent with infected fibroblasts (Alwine, 2008). We also now demonstrate that HCMV concurrently initiates translation of IRES-containing XIAP and HSP27 mRNAs, indicating HCMV modulates the stress response in a highly specific manner to allow for the upregulation of an unusual mixture of antiapoptotic factors critical for survival of infected monocytes. The ability of HCMV to concurrently drive cap- and IRES-dependent translation highlights a fundamental biological difference between HCMV- and growth factor-mediated monocyte survival, whereby targeting cellular factors only induced during HCMV infection may allow for the selective in vivo elimination of infected monocytes.
Normal cellular stress responses alter the intracellular environment to promote cell survival through stresses such as nutrient depletion, energy depletion, and hypoxia. The shutdown of PI3K/Akt/mTOR signaling is a common consequence of stress responses that reduces translation, and thus energy consumption, which allows the cell to recover from a stress insult (Holcik and Sonenberg, 2005; Kaufman et al., 2002; Wek et al., 2006; Wouters et al., 2005). However, the continuation of translation is essential for the monocyte-to-macrophage differentiation process to occur and ultimately HCMV replication. To date, the mechanism by which HCMV sustains mTOR driven cap-dependent translation is unclear. Akt activation is necessary but not sufficient alone for cap-dependent translation to occur as stress responses often interrupt the Akt-to-mTOR pathway at several points downstream of Akt. Our data here suggests that HSF1 may play an integral role in ensuring continued mTOR signaling during times of stress. Although mTOR can directly phosphorylate HSF1 (Chou et al., 2012), HSF1 has also been shown to be upregulated in hepatocarcinoma cells where it sustains the activity of mTOR (Cigliano et al., 2017). Loss of mTOR completely ablated HCMV-induced 4E-BP1 and S6K phosphorylation while having marginal effects on HSF1 activity, indicating minimal involvement of mTOR in regulating the activity of HSF1. In contrast, inhibition of HSF1 completely abrogated HCMV-induced mTOR phosphorylation, suggesting the presence of a feedback loop between HSF1 and the PI3K/Akt/mTOR cascade. Signal analysis following HCMV infection revealed bifurcation of signal at Akt towards either mTOR or HSF1. The distinct signaling branch from HCMV-activated Akt towards stress response factors that are able to positively feedback onto the PI3K/Akt/mTOR pathway provides a mechanism by which HCMV, but not normal myeloid growth factors, is able to activate mTOR. The positive feedback loop also allows for HCMV to regulate cap-dependent survival factors that are not normally associated with the stress response. Whether the relationship between the PI3K/Akt/mTOR pathway and HSF1 occurs during infection of other cell types remains to be determined. Moreover, the molecular mechanism whereby HSF1, a transcription factor, can rapidly modulate the phosphorylation levels of signaling components within the PI3K/Akt/mTOR pathway is unclear. Regardless, although generally a switch from cap-dependent to IRES-mediated translation occurs during cellular stress, HCMV appears to simultaneously drive the translation of both cap- and IRES-dependent survival proteins within infected monocytes via the unique interaction between Akt and stress response elements.
The requirement for different survival factors likely reflects a complex interplay between pro- and anti-death components, where the cell seeks to maintain antiapoptotic signaling until a proapoptotic threshold is exceeded and cells become committed to executing the apoptotic program. Both HCMV and growth factors must subvert the proapoptotic programming of monocytes (Gonzalez-Mejia and Doseff, 2009; Whitelaw, 1972); however, HCMV must concurrently abrogate the surge in the intracellular proapoptotic protein expression that occurs during the antiviral response (Chan et al., 2008; Chan et al., 2009a). Here, we demonstrate that HCMV increases the proapoptotic threshold of infected monocytes by stimulating the synthesis of a unique milieu of cap- and IRES-dependent intracellular antiapoptotic proteins, which is in contrast to the predominantly cap-dependent translation that occurs in monocytes induced to survive via growth factor stimulation. Current therapies against HCMV block specific steps along the virus replication cycle, rendering these antiviral drugs ineffective at preventing viral spread in transplant patients at high risk for HCMV exposure and often simply shift the disease to late onset due to the absence of initial HCMV replication in monocytes and the long-lifespan of infected macrophages (Fishman et al., 2007; Sagedal et al., 2004). Thus, deciphering the distinct mechanisms by which HCMV induces monocyte-to-macrophage survival when compared to normal myeloid growth factors is critical to identifying novel cellular antiviral targets that directly eliminate HCMV-infected monocytes with minimal effect on uninfected cells. Indeed, the selective cytotoxicity of the XIAP inhibitor embelin against HCMV-infected monocytes may allow for the targeting of HCMV-infected while permitting uninfected monocytes to maintain their immune surveillance function.
Supplementary Material
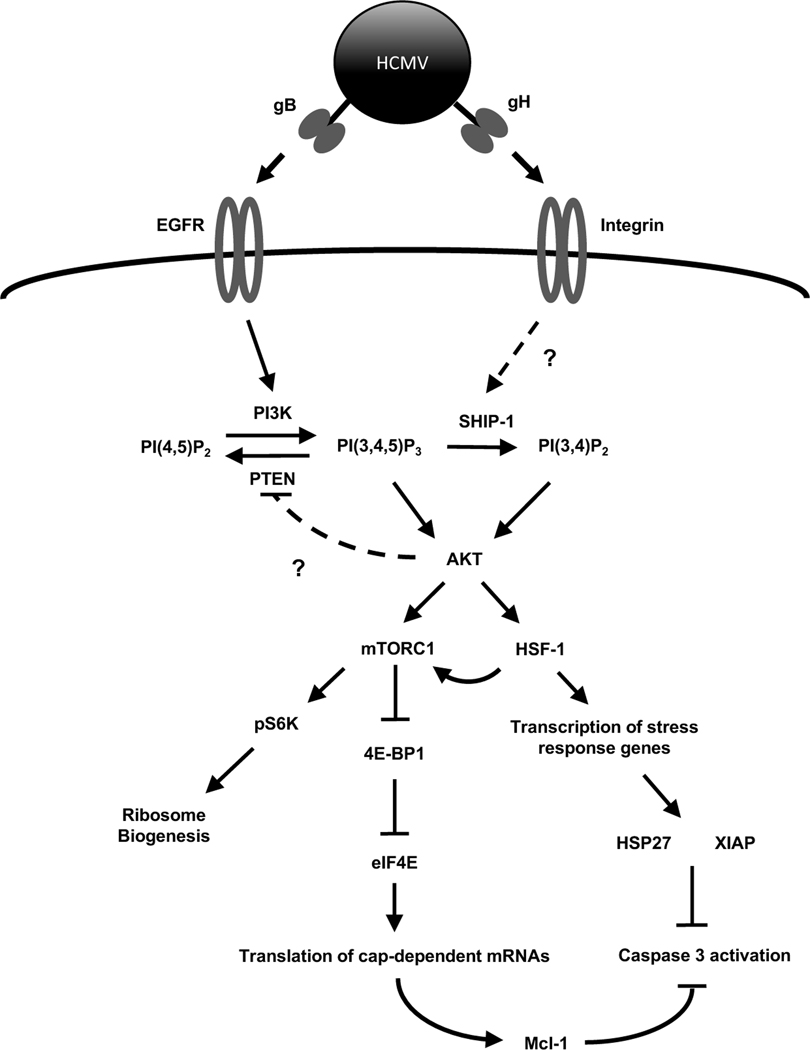
Fig. 8. Proposed model for HCMV regulation of Akt-dependent survival proteins during infection of monocytes.
Canonical Akt signaling initiated by normal myeloid growth factors induces PI3K activation through a receptor tyrosine kinase receptor. Similarly, HCMV initiation of Akt is triggered by gB binding to EGFR and the subsequent activation of PI3K. To ensure maximum Akt activity, HCMV and growth factors induce an early phosphorylation-dependent inactivation of the negative regulator PTEN by an unknown mechanism. However, unlike myeloid growth factors, HCMV concurrently stimulates a rapid upregulation of SHIP1 activity leading to the non-canonical activation of Akt. This non-traditional activation of Akt by HCMV allows for the increased translation of cap-dependent prosurvival mRNAs while also activating the stress response transcription factor HSF1. In addition to mediating an increase in stress response survival factors, HSF1 positively regulates mTOR activity resulting in the continued expression of cap-dependent antiapoptotic proteins along with stressed-induced IRES-dependent survival factors. The interplay between Akt and the stress response during HCMV infection leads to the upregulation of a unique subset of Akt-dependent antiapoptotic proteins required for the survival of infected monocytes past the critical 48-h cell-fate checkpoint.
Acknowledgments
This work was supported by grants from the Sinsheimer Scholar Award to G.C. Chan, American Heart Association to G.C. Chan, National Institute of Allergy and Infectious Disease (R56AI110803) to G.C. Chan, National Institute of Allergy and Infectious Disease (R56AI125693) to G.C. Chan, and National Heart, Lung, and Blood Institute (R01HL139824).
Abbreviations
- HCMV
human cytomegalovirus
- h
hour
- EGF
epidermal growth factor
- EGFR
EGF receptor
- αvβ3
alpha v beta 3 integrin
- gB
glycoprotein B
- gH
glycoprotein H
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- SHIP1
SH-2 containing inositol 5’polyphosphatase 1
- Mcl-1
myeloid cell leukemia-1
- HSP27
heat shock protein 27
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- M-CSF
macrophage colony-stimulating factor
- mTOR
mammalian target of rapamycin
- S6K
S6 kinase
- 4E-BP1
eukaryotic initiation factor 4E-binding protein 1
- XIAP
X-linked inhibitor of apoptosis
- RPMI
Roswell Park Memorial Institute medium
- HEL
human embryonic lung cells
- DMEM
Dulbecco’s Modified Eagle medium
- FBS
fetal bovine serum
- DMF
N,N-dimethyformamide
- RT
room temperature
- Cy3
cyanine 3
- RIPA
radioimmunoprecipitation assay buffer
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- BSA
bovine serum albumin
- HRP
horse radish peroxidase
- APC
allophycocyanin
- PI
propidium iodide
- FSC
forward scatter
- SSC
side scatter
- GSK3
glycogen synthase kinase 3
- eIF2B
eukaryotic translation initiation factor 2B
- eIF4B
eukaryotic translation initiation factor 4B
- HSF1
heat shock factor 1
- PDK1
phosphoinositide kinase 1
- mTORC2
mTOR complex 2
- PDCD4
programmed cell death protein 4
- eIF2K
eukaryotic translation initiation factor 2K
- Bcl-2
B-cell lymphoma 2
- IRES
internal ribosome entry site
Footnotes
Conflicts of interest
All authors declare that they have no conflicts of interest with the contents of this article.
References
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J, 1998. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 8, 69–81. [DOI] [PubMed] [Google Scholar]
- Alwine JC, 2008. Modulation of host cell stress responses by human cytomegalovirus. Curr Top Microbiol Immunol 325, 263–279. [DOI] [PubMed] [Google Scholar]
- Avruch J, Belham C, Weng Q, Hara K, Yonezawa K, 2001. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog Mol Subcell Biol 26, 115–154. [DOI] [PubMed] [Google Scholar]
- Basu A, Sridharan S, 2017. Regulation of anti-apoptotic Bcl-2 family protein Mcl-1 by S6 kinase 2. PLoS One 12, e0173854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Xie Y, Wang X, Khaleque MA, Chou SD, Murshid A, Prince T, Zhang Y, 2010. Signal Transduction Pathways Leading to Heat Shock Transcription. Sign Transduct Insights 2, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD, 2008. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol 181, 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Yurochko AD, 2009a. NF-kB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res 144, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD, 2010. PI(3)K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol 184, 3213–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Stevenson EV, Yurochko AD, 2012a. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination. J Leukoc Biol 92, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD, 2009b. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 106, 22369–22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD, 2012b. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J Virol 86, 10714–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SD, Prince T, Gong J, Calderwood SK, 2012. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One 7, e39679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigliano A, Wang C, Pilo MG, Szydlowska M, Brozzetti S, Latte G, Pes GM, Pascale RM, Seddaiu MA, Vidili G, Ribback S, Dombrowski F, Evert M, Chen X, Calvisi DF, 2017. Inhibition of HSF1 suppresses the growth of hepatocarcinoma cell lines in vitro and AKT-driven hepatocarcinogenesis in mice. Oncotarget 8, 54149–54159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojohari O, Peppenelli MA, Chan GC, 2016. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. J Virol 90, 6443–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C, Clemens MJ, 2005. Regulation of the phosphorylation and integrity of protein synthesis initiation factor eIF4GI and the translational repressor 4E-BP1 by p53. Oncogene 24, 4839–4850. [DOI] [PubMed] [Google Scholar]
- Dufner A, Thomas G, 1999. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253, 100–109. [DOI] [PubMed] [Google Scholar]
- Emery VC, 2001. Investigation of CMV disease in immunocompromised patients. J Clin Pathol 54, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Bandi HR, Hofsteenge J, Bussian BM, Thomas G, 1991. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem 266, 22770–22775. [PubMed] [Google Scholar]
- Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, Sgarabotto D, Torre-Cisneros J, Uknis ME, 2007. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant 21, 149–158. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A, 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665–668. [DOI] [PubMed] [Google Scholar]
- Gnann JW Jr., Ahlmen J, Svalander C, Olding L, Oldstone MB, Nelson JA, 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am J Pathol 132, 239–248. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mejia ME, Doseff AI, 2009. Regulation of monocytes and macrophages cell fate. Front Biosci (Landmark Ed) 14, 2413–2431. [DOI] [PubMed] [Google Scholar]
- Ho M, 1977. Virus infections after transplantation in man. Brief review. Arch Virol 55, 1–24. [DOI] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N, 2005. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6, 318–327. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM, 2002. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol 3, 411–421. [DOI] [PubMed] [Google Scholar]
- Kerr WG, 2011. Inhibitor and activator: dual functions for SHIP in immunity and cancer. Annals of the New York Academy of Sciences 1217, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar SB, Yu Y, Maguire TG, Alwine JC, 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol 78, 11030–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor G, Moss AC, 2010. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis 16, 1620–1627. [DOI] [PubMed] [Google Scholar]
- Li H, Marshall AJ, 2015. Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: A distinct branch of PI3K signaling. Cell Signal 27, 1789–1798. [DOI] [PubMed] [Google Scholar]
- Liwak U, Thakor N, Jordan LE, Roy R, Lewis SM, Pardo OE, Seckl M, Holcik M, 2012. Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell Biol 32, 1818–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P, Engelhard D, Link H, Biron P, Brandt L, Brunet S, Cordonnier C, Debusscher L, de Laurenzi A, Kolb HJ, et al. , 1992. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: experience of European Bone Marrow Transplant Group. Clin Infect Dis 14, 831–835. [DOI] [PubMed] [Google Scholar]
- Ma K, Cheung SM, Marshall AJ, Duronio V, 2008. PI(3,4,5)P3 and PI(3,4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell Signal 20, 684–694. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N, 2006. mTOR, translation initiation and cancer. Oncogene 25, 6416–6422. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N, 2004. eIF4E--from translation to transformation. Oncogene 23, 3172–3179. [DOI] [PubMed] [Google Scholar]
- Manez R, Kusne S, Rinaldo C, Aguado JM, St George K, Grossi P, Frye B, Fung JJ, Ehrlich GD, 1996. Time to detection of cytomegalovirus (CMV) DNA in blood leukocytes is a predictor for the development of CMV disease in CMV-seronegative recipients of allografts from CMV-seropositive donors following liver transplantation. J Infect Dis 173, 1072–1076. [DOI] [PubMed] [Google Scholar]
- Masur H, Whitcup SM, Cartwright C, Polis M, Nussenblatt R, 1996. Advances in the management of AIDS-related cytomegalovirus retinitis. Ann Intern Med 125, 126–136. [DOI] [PubMed] [Google Scholar]
- McArdle J, Moorman NJ, Munger J, 2012. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog 8, e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman NJ, Shenk T, 2010. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol 84, 5260–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandagopal N, Roux PP, 2015. Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation (Austin) 3, e983402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerheim PL, Meier JL, Vasef MA, Li WG, Hu L, Rice JB, Gavrila D, Richenbacher WE, Weintraub NL, 2004. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am J Pathol 164, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalski MT, Chan G, Stevenson EV, Gray S, Yurochko AD, 2011. Human cytomegalovirus-regulated paxillin in monocytes links cellular pathogenic motility to the process of viral entry. J Virol 85, 1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalski MT, Chan GC, Stevenson EV, Collins-McMillen DK, Yurochko AD, 2013. The HCMV gH/gL/UL128–131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes. PLoS Pathog 9, e1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC Jr., Sonenberg N, 1994. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature 371, 762–767. [DOI] [PubMed] [Google Scholar]
- Peppenelli MA, Arend KC, Cojohari O, Moorman NJ, Chan GC, 2016. Human cytomegalovirus stimulates the synthesis of select Akt-dependent antiapoptotic proteins during viral entry to promote survival of infected monocytes. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, Krmpotic A, Jonjic S, Horl WH, Zlabinger GJ, Puchhammer E, Saemann MD, 2012. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant 12, 1458–1468. [DOI] [PubMed] [Google Scholar]
- Proud CG, 2007. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J 403, 217–234. [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G, 1998. Phosphorylation and activation of p70s6k by PDK1. Science 279, 707–710. [DOI] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW, 2004. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J 23, 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y, 2004. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 5, 897–907. [DOI] [PubMed] [Google Scholar]
- Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degre M, Fauchald P, Rollag H, 2004. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66, 329–337. [DOI] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N, 2006. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J 25, 2781–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T, Alwine JC, 2014. Human Cytomegalovirus: Coordinating Cellular Stress, Signaling, and Metabolic Pathways. Annu Rev Virol 1, 355–374. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Sissons P, 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39, 293–301. [DOI] [PubMed] [Google Scholar]
- Sinzger C, Jahn G, 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39, 302–319. [DOI] [PubMed] [Google Scholar]
- Sinzger C, Plachter B, Grefte A, The TH, Jahn G, 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis 173, 240–245. [DOI] [PubMed] [Google Scholar]
- Smith MS, Bentz GL, Alexander JS, Yurochko AD, 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol 78, 4444–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, Nelson JA, 2010. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell host & microbe 8, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA, 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91, 119–126. [DOI] [PubMed] [Google Scholar]
- Stagno S, Pass RF, Dworsky ME, Henderson RE, Moore EG, Walton PD, Alford CA, 1982. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N Engl J Med 306, 945–949. [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ, 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 43, 1143–1151. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH, 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol 72 ( Pt 9), 2059–2064. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons P, Sinclair J, 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol 68, 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss OH, Kim S, Wewers MD, Doseff AI, 2005. Regulation of monocyte apoptosis by the protein kinase Cdelta-dependent phosphorylation of caspase-3. J Biol Chem 280, 17371–17379. [DOI] [PubMed] [Google Scholar]
- Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES, 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG, 2001. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J 20, 4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG, 2006. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34, 7–11. [DOI] [PubMed] [Google Scholar]
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW, 2007. Dissecting eIF4E action in tumorigenesis. Genes Dev 21, 3232–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw DM, 1972. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 5, 311–317. [DOI] [PubMed] [Google Scholar]
- Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C, 2005. Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol 16, 487–501. [DOI] [PubMed] [Google Scholar]
- Wu C, 1995. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11, 441–469. [DOI] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH, 2003. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 23, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung HW, Charnock-Jones DS, Burton GJ, 2011. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6, e17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Huang ES, 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol 162, 4806–4816. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.