Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disorder associated with exposure to head trauma. In 2015, a panel of neuropathologists funded by the NINDS/NIBIB defined preliminary consensus neuropathological criteria for CTE, including the pathognomonic lesion of CTE as “an accumulation of abnormal hyperphosphorylated tau (p-tau) in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern,” based on review of 25 tauopathy cases. In 2016, the consensus panel met again to review and refine the preliminary criteria, with consideration around the minimum threshold for diagnosis and the reproducibility of a proposed pathological staging scheme. Eight neuropathologists evaluated 27 cases of tauopathies (17 CTE cases), blinded to clinical and demographic information. Generalized estimating equation analyses showed a statistically significant association between the raters and CTE diagnosis for both the blinded (OR = 72.11, 95% CI = 19.5–267.0) and unblinded rounds (OR = 256.91, 95% CI = 63.6–1558.6). Based on the challenges in assigning CTE stage, the panel proposed a working protocol including a minimum threshold for CTE diagnosis and an algorithm for the assessment of CTE severity as “Low CTE” or “High CTE” for use in future clinical, pathological, and molecular studies.
Keywords: Brain trauma, Chronic traumatic encephalopathy, Neurodegenerative disorders, Tauopathy, Traumatic brain injury
INTRODUCTION
Ninety-three years ago, New Jersey medical examiner Harrison Stanford Martland postulated that repetitive traumatic brain injuries (TBI) sustained during amateur and professional boxing careers could lead to lasting neuropsychiatric, cognitive and motor sequelae (1, 2). Increasing recognition of this distinct clinical entity, variably referred to as “punch drunk,” “dementia pugilistica,” and “chronic traumatic encephalopathy” (3, 4) led to the publication of several neuropathological case reports in the 1950s and 1960s, and culminated in the 1973 cohort study of 15 male pugilists by Corsellis, Bruton, and Freeman-Browne (5–11). These studies collectively detailed cortical atrophy, ventricular enlargement, cavum and fenestrated septum pellucidum, thinning of the corpus callosum, substantia nigra depigmentation, cerebellar scarring, neuronal loss, gliosis, and argyrophilic neurofibrillary tangles (NFTs) in neurons of the medial temporal lobe, cortex, and brainstem. In the 1990s, 2 independent research teams described the neuronal pathology of “dementia pugilistica” consisting of thioflavin-positive and hyperphosphorylated tau (p-tau)-immunoreactive NFTs and neurites with a predilection to surround small cortical blood vessels at the depths of sulci (12–15). In 2005 and 2006, the identification of p-tau pathology in middle-aged American football players by Omalu et al brought worldwide awareness to chronic traumatic encephalopathy (CTE) and to the concept that repetitive head trauma sustained in modern day contact sports could trigger the same neurodegenerative disorder as boxing (16). Today, CTE pathology has been reported in other contact sports including soccer (17–19), ice hockey (20), wrestling (21), rugby (19, 22, 23), baseball (17), mixed martial arts, bull-riding; in military-related activities, including exposure to explosive blasts (24, 25); in repetitive head trauma resulting from physical abuse (26), “head banging” (15), poorly controlled epilepsy (15), and “dwarf-throwing” (27), and even demonstrated following exposure to a single moderate or severe TBI (28–30).
In 2013, a clinicopathological case series of 68 men with a history of exposure to repetitive head impacts and neuropathological evidence of CTE reported “epicenters” of p-tau NFTs, astrocytic tangles, and neurites clustered at the depths of the cortical sulci, ranging in severity from isolated, discrete foci in the neocortex to widespread p-tau pathology involving the medial temporal lobe, diencephalon, and brainstem. McKee et al proposed neuropathological criteria for the diagnosis of CTE and a staging scheme for characterizing the severity of p-tau pathology (20). The method of staging CTE p-tau pathology was based on large hemispheric 50-µm-thick slides immunostained as free-floating sections for p-tau, largely adopted from Braak’s method of staging p-tau pathology of Alzheimer disease (AD) (31). McKee et al proposed 4 pathological stages of CTE: stages I (mild) thru IV (severe). In stage I CTE, there were 1 or 2 isolated epicenters of NFTs, astrocytic tangles and dot-like neurites arranged around small blood vessels at the depths of the sulci, most frequently in the frontal cortex. In stage II CTE, 3 or more CTE lesions were found in multiple cortical regions. The lesions were larger and superficial NFTs were found in adjacent cortices. There was also neurofibrillary pathology in the locus coeruleus and nucleus basalis of Meynert. In stage III CTE, larger, confluent perivascular patches of p-tau-immunoreactive NFTs, dot-like and thread-like neurites and astrocytic tangles were found at the sulcal depths, as well as NFTs in the superficial cortical laminae. Diffusely distributed NFTs were also found in medial temporal lobe structures, including the hippocampus, entorhinal and perirhinal cortices, and amygdala. There was also more widespread brainstem p-tau pathology. By stage III, macroscopic features, such as cerebral atrophy, ventricular enlargement, and abnormalities of the septum pellucidum, were often found. Neurofibrillary degeneration in stage III CTE involved CA4 and CA2, as well as CA1 of the hippocampus. In CTE stage IV, cerebral, medial temporal lobe and diencephalic atrophy were found grossly. There was depigmentation of the substantia nigra and locus coeruleus. Perivascular p-tau lesions and NFTs were distributed throughout the cerebral cortex, and there was pronounced neurofibrillary degeneration of the medial temporal lobe, diencephalon and brainstem. In addition, NFTs were found in the cerebellar dentate nucleus, basis pontis and spinal cord. Neuronal loss and gliosis were prominent in the frontal and temporal cortices. Other pathologic lesions, including myelin and axonal loss, as well as transactive response DNA-binding protein 43 (TDP-43) pathology were also found in the most severe CTE cases.
In March 2013, the National Institutes of Health (NIH), supported by the Foundation for NIH’s Sports Health Research Program with funding from the National Football League (NFL), launched a major effort to define the neuropathologic characteristics of CTE. The first NINDS/NIBIB consensus panel met in February 2015 to blindly evaluate 25 cases of various tauopathies, including CTE, AD, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Guamanian parkinsonism dementia complex (GPDC), argyrophilic grain disease (AGD), and primary age-related tauopathy (PART) using the preliminary criteria proposed by McKee et al (32). Eight neuropathologists, blinded to all clinical and demographic information, successfully identified CTE pathology amongst 25 cases of various tauopathies and determined that CTE was a distinct disease. The panel also defined a pathognomonic lesion of CTE as “an accumulation of abnormal hyperphosphorylated tau (p-tau) in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern.” In addition, the group defined supportive but nonspecific p-tau-immunoreactive features of CTE (Table 1). Since their publication, the NINDS/NIBIB criteria for the neuropathological diagnosis of CTE have been widely adopted and used in neuropathological evaluation of CTE in diverse research brain banks and autopsy cohorts (18, 23, 32–39).
TABLE 1.
Preliminary NINDS Criteria for the Pathological Diagnosis of Chronic Traumatic Encephalopathy (CTE) (31)
| Required for the diagnosis of CTE (pathognomonic CTE lesion):* | |
| 1) | Phosphorylated tau aggregates in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci. |
| Supportive tau-related neuropathological features of CTE: | |
| 1) | Abnormal tau-immunoreactive pretangles and neurofibrillary tangle (NFTs) preferentially affecting superficial layers (layers II–III), in contrast to layers III and V as in AD. |
| 2) | In the hippocampus, pretangles, NFTs or extracellular tangles preferentially affecting CA2 and pretangles and prominent proximal dendritic swellings in CA4. These regional p-tau pathologies differ from the preferential involvement of CA1 and subiculum found in AD. |
| 3) | Abnormal p-tau immunoreactive neuronal and astrocytic aggregates in subcortical nuclei, including the mammillary bodies and other hypothalamic nuclei, amygdala, nucleus accumbens, thalamus, midbrain tegmentum, and isodendritic core (nucleus basalis of Meynert, raphe nuclei, substantia nigra and locus coeruleus). |
| 4) | Tau-immunoreactive thorny astrocytes at the glial limitans most commonly found in the subpial and periventricular regions. |
| 5) | Tau-immunoreactive large grain-like and dot-like structures (in addition to some threadlike neurites). |
| Supportive nontau-related neuropathological features of CTE: | |
| 1) | Macroscopic features: disproportionate dilatation of the third ventricle, septal abnormalities, mammillary body atrophy, and contusions or other signs of previous traumatic injury. |
| 2) | TDP-43 immunoreactive neuronal cytoplasmic inclusions and dot-like structures in the hippocampus, anteromedial temporal cortex and amygdala. |
| Aging-related tau astrogliopathy (ARTAG) may be present but is neither diagnostic nor supportive (40) | |
The second consensus panel made refinements in the description of a pathognomonic lesion. They determined that the perivascular p-tau aggregates should include neurofibrillary tangles, with or without astrocytes, and that the focus had to be in deeper cortical layers not restricted to subpial and superficial regions.
The NINDS/NIBIB panel convened for a second meeting in Boston, MA, on November 15 and 16, 2016, to further refine the diagnostic criteria for CTE, including the minimum threshold for diagnosis. The panel sought to determine whether CTE pathology could be reliably distinguished from other neurodegenerative processes common to aging and from other primary tauopathies, such as aging-related tau astrogliopathy (ARTAG) and PART, based on the preliminary 2015 consensus definition (32). The panel also addressed whether p-tau pathology in CTE could be progressively staged in a consistent neuroanatomical pattern of distribution and burden as proposed by McKee et al (20).
MATERIALS AND METHODS
Twenty-nine cases of various tauopathies were selected for evaluation, including CTE (n = 19), ARTAG (n = 4), AGD (n = 2), PART (n = 2), and mild AD (n = 2). Cases were selected from the Veterans Affairs—Boston University—Concussion Legacy Foundation (VA-BU-CLF)/Understanding Neurological Injury and Traumatic Encephalopathy (UNITE) brain bank in Bedford MA and the Mayo Clinic Brain Bank in Jacksonville, FL. All cases were selected by individuals not directly involved in the consensus case evaluation (KFB, VEA, ACM). To assess diagnostic reproducibility, 3 CTE cases previously evaluated in the first consensus conference meeting were included. The remaining 26 cases (16 CTE, 10 other) had not been previously evaluated by the participating neuropathologists. CTE cases were selected for a wide range of age at death (23–82, mean 52.7 years). The selected CTE cases had been previously evaluated by Boston University UNITE neuropathologists (ACM, VEA) using a full set of paraffin-embedded 10-µm-thick slides and large hemispheric 50-µm-thick slides and assigned a CTE stage as previously described (20). The cases included CTE stage I (n = 1), CTE stage II (n = 3), CTE stage III (n = 11), and CTE stage IV (n = 4).
Formalin-fixed, paraffin-embedded tissue blocks for each case were recut and processed into slides from the following brain regions: (1) superior frontal gyrus, (2) dorsolateral superior frontal gyrus, (3) striatum (caudate, nucleus accumbens, and putamen), (4) temporal pole, (5) superior temporal gyrus, (6) amygdala (with entorhinal cortex), (7) hippocampus (at the level of the lateral geniculate), (8) thalamus (with mammillary body), and (9) cerebellum (with dentate nucleus), at the Bedford VA Neuropathology Lab. Hematoxylin and eosin staining with Luxol fast blue counterstain and p-tau immunohistochemistry were performed on all sampled regions. Phosphorylated-TDP-43 immunohistochemistry was performed on the hippocampus and superior temporal gyrus. Beta-amyloid 42 immunohistochemistry was performed on the dorsolateral superior frontal gyrus and hippocampus. Bielschowsky silver impregnation was performed on the hippocampus. Immunohistochemistry was performed on a Leica BOND RX autostainer. Antibodies used were p-tau (AT8; Pierce Endogen, Rockford, IL; 1:2000), Aβ (4G8; BioLegend, San Diego, CA; 1:100 000; 2-minute formic acid pretreatment), and pTDP-43 (pS409/410 mouse monoclonal; Cosmo Bio Co, Tokyo, Japan; 1:2000). Sections were incubated with primary antibodies overnight at 4°C. Subsequently, sections were treated with biotinylated secondary antibody after 3 PBS washes and labeled with a horseradish peroxidase substrate kit (Vector Laboratories, Burlingame, CA), counterstained with Gill’s hematoxylin (Vector Laboratories H-3401), and coverslipped with Permount mounting medium (Thermo Scientific, Rockford, IL).
The 29 cases were assigned a unique numeric identifier. A total of 598 newly processed histologic slides (26 cases with 23 slides per case) were scanned at the Mayo Clinic in Jacksonville, FL by a researcher (KFB), blinded to slide origin and identity, using an Aperio scanner (Leica Biosystems, Buffalo Grove, IL). Sixty-seven digital images from 3 CTE cases (23 slides per case; 2 slides unavailable for Case #11) from the first CTE consensus meeting were also included. The resulting high resolution (20× magnification) digital images were uploaded to the Aperio Slide-hosting portal. Slide images were linked with their corresponding unique numeric identifier, neuroanatomic region, and histologic stain. Information pertaining to the individual’s name, age, sex, race, clinical diagnosis, clinical symptoms, athletic exposure, or gross neuropathologic features was not accessible to slide evaluators through the online portal. Assessment of the study slides followed the same parameters as the first consensus. Neuropathologists used digital images of scanned slides prepared by a single laboratory and were blinded to clinical histories and gross pathological findings.
Eight neuropathologists (NJC, JFC, DWD, RDF, CDK, DDP, TDS, JPV) experienced in the pathology of neurodegenerative disorders participated in the evaluation of the digital images. Seven of the 8 neuropathologists had previously performed blinded analysis of the study set in the first CTE consensus conference. The neuropathologists were provided a tauopathy criteria guide regarding the neuropathologic diagnoses of AD, PART, GPDC, AGD, CBD, PSP, and ARTAG as well as CTE criteria outlined from the first CTE consensus conference (32) and the staging scheme proposed by McKee et al (20). (See Supplementary Material for guides.) Evaluating neuropathologists were not aware of the number of cases representing each tauopathy (including CTE) or that some of the cases had been evaluated in the first consensus conference. Through the Aperio Slide-hosting portal, the neuropathologists evaluated the cases independently at their respective institutions, in any order and at any pace. For each of the 29 cases, the neuropathologists completed an evaluation form in which they specified their name, case identifier, whether the case met CTE neuropathologic criteria (yes or no). If the neuropathologist felt the case met CTE criteria, they specified the CTE stage (stage I, stage II, stage III, or stage IV) and whether the case met additional neuropathological diagnoses. If the neuropathologist felt the case did not meet CTE criteria, they specified the alternative neuropathological diagnosis. Following complete evaluation of all 29 cases and submission of evaluation forms to the data core of Boston University School of Medicine (BUSM), the evaluators were provided additional information regarding the gross neuropathological features for each case and clinical summaries, including age and athletic exposure, and asked to repeat their evaluation. Results from the second evaluation round were again submitted to BUSM data core, and the data were anonymously compiled prior to the in-person meeting. Only neuropathological assessments submitted to the data core prior to the meeting were included in the analysis.
A larger panel of neuropathologists, clinicians, and biomedical researchers (including KFB, KDO, WS, IL, WG, ACM, and the NINDS TBI/CTE Group) convened on November 15–16, 2016 in Boston, MA. Meeting participants reviewed the clinical vignettes, digitized images of the cases and results from the neuropathological evaluations to facilitate group discussion. Based on these discussions, the consensus panel proposed the minimum threshold for the diagnosis of CTE, strict features necessary for a “pathognomonic” lesion, and a methodological workflow for the assessment of CTE. The panel also developed an algorithm for judging whether the severity of CTE was “High” or “Low.” They also discussed challenges in CTE characterization in the context of coexistent neurodegenerative and aging pathologies, as well as strategies for teaching and disseminating neuropathology practice measures.
Generalized estimating equation was used to assess the association between the rater’s diagnosis of CTE (absent/present) and the BU UNITE diagnosis of CTE (absent/present). The latter had been made prior to the study using a full set of paraffin-embedded 10-µm-thick slides and large hemispheric 50-µm-thick slides. Generalized estimating equation was performed in order to account for correlations between the raters for the same case. This association was assessed before (blinded) and after (unblinded) raters were provided with the gross neuropathology and clinical data of the cases. Kappa statistic was also calculated to evaluate agreement among the raters on CTE status before (blinded) and after (unblinded) raters were provided with the clinical data of the cases. Lastly, kappa statistic was again calculated to evaluate agreement among the raters on the stage of CTE before and after raters were provided with clinical data. Individuals without CTE were not included in these determinations given that the objective was to evaluate agreement of CTE severity.
RESULTS
The neuropathological and demographic features of the 29 study cases are shown in Table 2. The recut and stained slides for 2 of the 19 CTE cases (Case #16, CTE stage I; Case #5, CTE stage II) failed to include diagnostic p-tau pathology, consequently those 2 cases were removed from analysis. For the remainder of the cases submitted as CTE (n = 17), the evaluating neuropathologists were highly successful in detecting CTE neuropathological changes. In the first round, blinded to clinical data and gross neuropathological features, 88% of the responses indicated the diagnosis of CTE, and after the clinical data and gross neuropathological features were supplied, 97% of the responses indicated CTE (Fig. 1). Generalized estimating equation analyses showed a statistically significant association, with large effect sizes, between the raters and the cases submitted as CTE for both the blinded (OR = 72, 95% CI = 19–267) and unblinded rounds (OR = 257, 95% CI = 64–1559). Amongst the 8 raters there was also substantial agreement in the diagnosis of CTE for the blinded first round (Kappa = 0.63) and there was a notable improvement in agreement for the unblinded second round (Kappa = 0.79, substantial agreement).
TABLE 2.
Demographic Features and Evaluation Results of CTE Study Cases
| Case | Age/Sex | Sport (Duration, Year) | Submission Dx (Stage) | Evaluation (CTE Dx) |
Outcome (✓) | |
|---|---|---|---|---|---|---|
| Round 1 | Round 2 | |||||
| 16 | 23/M | FB (9) | CTE (I) | No diagnostic pathology | Eliminated | |
| 23 | 25/M | FB (15) | CTE (II) | 8/8 | 8/8 | Low CTE (3) |
| 15 | 27/M | FB (14) | CTE (II) | 8/8 | 8/8 | Low CTE (4) |
| 5 | 38/M | FB (14) | CTE (II) | No diagnostic pathology | Eliminated | |
| 14 | 40/M | FB (13) | CTE (III) | 6/8 | 7/8 | High CTE (10) |
| 27 | 41/M | FB (17) | CTE (III) | 7/8 | 8/8 | High CTE (7) |
| 21 | 46/M | FB (20) | CTE (III) | 8/8 | 8/8 | High CTE (9) |
| 17 | 48/M | FB (17) | CTE (III) | 8/8 | 8/8 | High CTE (7) |
| 25 | 49/M | FB (23) | CTE (III) | 8/8 | 8/8 | High CTE (9) |
| 29 | 53/M | FB (15) | CTE (III) | 4/8 | 7/8 | High CTE (8) |
| 1 | 53/M | FB (14) | CTE (III) | 8/8 | 8/8 | High CTE (7) |
| 8 | 61/M | BX (26) | CTE (III) | 8/8 | 8/8 | High CTE (10) |
| 6 | 66/M | FB (17) | CTE (III) | 8/8 | 8/8 | High CTE (9) |
| 7 | 66/M | FB (16) | CTE (III) | 8/8 | 8/8 | High CTE (6) |
| 9 | 69/M | FB (28) | CTE (IV) | 8/8 | 8/8 | High CTE (10) |
| 20 | 68/M | FB (19) | CTE (IV) | 7/8 | 8/8 | High CTE (10) |
| 18 | 70/M | FB (≥8) | CTE (III) | 6/8 | 8/8 | High CTE (5) |
| 11 | 75/M | FB (18) | CTE (IV) | 4/8 | 6/8 | High CTE (8) |
| 24 | 82/M | FB (12) | CTE (IV) | 6/8 | 8/8 | High CTE (10) |
CTE, chronic traumatic encephalopathy; Dx, diagnosis; FB, football; M, male; Outcome (✓), the results of the workflow protocol for the assessment of high and low CTE, number in brackets represents the number of regions with NFTs (derived from Workflow Diagram).
FIGURE 1.
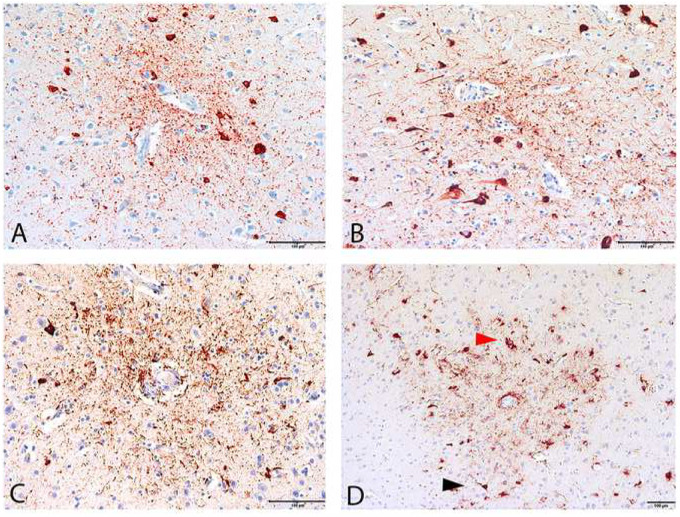
Diagnostic lesions of chronic traumatic encephalopathy (CTE) in 4 cases. (A–D) Pathognomonic CTE lesions in 4 cases immunolabeled by the anti-phosphorylated tau antibody AT8. (A) 25-Year-old former collegiate football player (CTE stage II, case #23). The CTE focus appears neuronal and composed of perivascular NFTs and dotlike neurites surrounding a small cerebral blood vessel. Glial tau pathology is not immediately evident. (B) A 27-year-old former professional football player (CTE stage II, case #15). The CTE focus is neuronal and composed of perivascular NFTs and dotlike neurites surrounding a small cerebral blood vessel. (C) A 40-year-old former professional football player (CTE stage III, case #14). The CTE focus is neuronal and astrocytic, and composed of p-tau-immunoreactive NFTs, dotlike and threadlike neurites and cellular processes, surrounding a small cerebral blood vessel. (D) A 69-year-old former professional football player (CTE stage IV, Case #9). The CTE focus is composed of tau-immunoreactive NFT (black arrowhead), astrocytes (red arrowhead), dot and threadlike neurites and cell processes surrounding a small cerebral blood vessel. Scale bars: 100 µm.
For 10 of the 17 suspected CTE cases (#1, 6, 7, 8, 9, 15, 17, 21, 23, 25, including 2 cases from the first consensus), there was perfect agreement among the neuropathologists regarding a CTE diagnosis in both the first and second rounds. In 2 cases (#20, 27) from the first round, one reviewer gave an alternate diagnosis of CBD (88% agreement), which was changed to CTE after the clinical data and gross neuropathology were revealed (100% agreement). In 3 cases (#14,18, 24), 6 of the 8 (75%) reviewers considered the diagnosis to be CTE in the first round; in the second round, all but one response identified the case as CTE (95%). The alternate diagnoses included GPDC (2), ARTAG (2), PSP (1), and AGD (1). The panel re-evaluated and discussed these 5 cases (#14, 18, 20, 24, 27) at the face-to-face meeting and agreed that pathognomonic lesions were present in each case, warranting the diagnosis of CTE. In case #29, 4 of 8 (50%) reviewers considered the diagnosis to be CTE in the first round; the alternative diagnoses were ARTAG and PART (3) and AGD (1). After review of the clinical and gross neuropathological information, most reviewers agreed the diagnosis was CTE, although one reviewer maintained the diagnosis of PART and ARTAG. Review of this case by the panel found that despite a low burden of p-tau pathology in the cortex, there was a pathognomonic lesion of CTE in the inferior temporal cortex supporting the diagnosis of CTE. The panel also suggested the case warranted additional cortical sampling for greater clarity regarding the frequency of pathognomonic CTE lesions and cortical NFT burden. Subpial thorn-shaped astrocytes (TSA) at the depths of the sulci were also a prominent feature of the case. This was interpreted by the in-person panel as a feature of ARTAG and not diagnostic for CTE. NFT in the medial temporal lobe were also found in case #29 and interpreted by one reviewer as consistent with PART. After panel discussion, the group determined that if a case is diagnosed as CTE based on cortical pathology, the presence of NFT in the medial temporal lobe is considered a feature of CTE and an additional diagnosis of PART is not warranted. The group also noted that CA4 p-tau pathology was uncommon in PART, whereas in CTE, if present, CA4 p-tau pathology consists of pretangles, NFTs and dendritic dystrophy which can be useful for distinguishing CTE from PART. Case #11, a case with widespread neuritic plaques that was also evaluated in the first consensus, also posed difficulties for the reviewers. Prior to learning the clinical and gross neuropathological information, 3 reviewers made a diagnosis of AD and one of ARTAG. After learning the clinical information, 2 reviewers still considered the diagnosis to be AD. Re-assessment of the case by the in-person panel found that the case satisfied criteria for CTE in that multiple cortical pathognomonic CTE lesions were present. The cortical NFT burden was predominantly superficial in the temporal lobe; however, the group agreed that the case warranted a second diagnosis of AD neuropathological change.
In addition, the group discussed the minimum threshold for the diagnosis of CTE and the pathological features critical to a strict definition of “pathognomonic CTE lesion.” The group endorsed a single pathognomonic lesion in the cortex as the minimum threshold for CTE. The group also endorsed the following features of the pathognomonic lesion as necessary: p-tau aggregates in neurons, with or without concomitant p-tau-immunoreactive TSA, at the depth of the sulcus around small blood vessels, in deeper cortical layers not restricted to subpial and superficial region (Fig. 1). The panel unanimously confirmed that, based on case material available, purely astrocytic perivascular p-tau lesions (including subpial ARTAG) did not meet criteria for CTE (Fig. 2). Furthermore, clusters of p-tau-immunoreactive astrocytes in the white matter of the frontal and temporal cortex, basal ganglia, lateral or medial brainstem were considered consistent with ARTAG (40–43), and not specific features of CTE.
FIGURE 2.
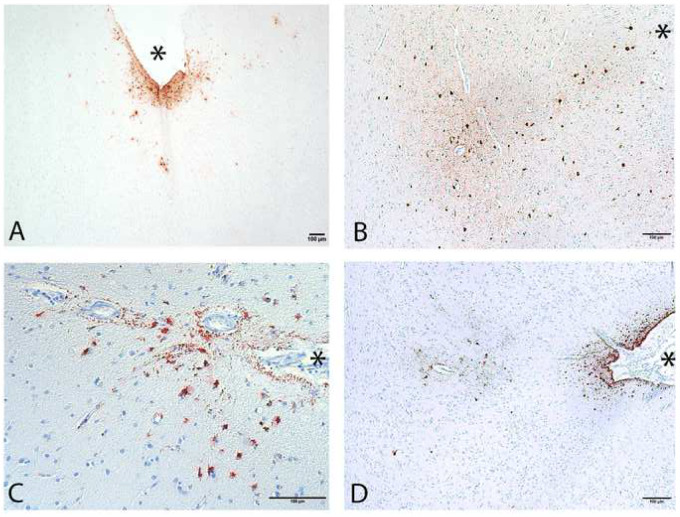
ARTAG and CTE p-tau pathology immunolabeled by the anti-phosphorylated tau antibody AT8. (A) Subpial ARTAG with superficial astrocytic p-tau pathology, not diagnostic of CTE. (B) CTE focus at depth of the sulcus. (C) Subpial ARTAG. (D) CTE focus at sulcal depth in addition to subpial ARTAG. (Sulcal depths indicated by asterisks; scale bars: 100 µm).
For the 10 non-CTE cases submitted, 95% of responses in the first blinded round of evaluation correctly indicated that CTE was not present. In the unblinded second round, 99% of the responses correctly indicated that CTE was not present. The submitted diagnoses on the cases misidentified as CTE were: ARTAG (2), PART (1), and mild AD (1). The only incorrect response in the second round was a case that had been submitted as ARTAG.
Evaluators were less consistent assigning a CTE stage. Unanimous consensus on CTE stage was not reached on any case in either round of evaluation. Of the 17 CTE cases evaluated, 5 (29%) cases were given a CTE stage by the majority of neuropathologists that matched the CTE stage originally assigned by UNITE neuropathologists. This was true for both first and second rounds of evaluation. For 12/17 CTE cases (71%), the majority of the neuropathologists assigned a CTE stage that was one level lower than the UNITE neuropathologists assigned CTE stage. For 2/17 CTE cases, consensus was split between two CTE stages (one corresponding to the assigned level). For both rounds, 2 different CTE stages were assigned on 5 CTE cases, 3 different CTE stages were assigned on 11 CTE cases, and for one case (Case #9), neuropathologists were split between all 4 CTE stages. Amongst the 8 raters, Kappa values were 0.22 and 0.19 for the blinded first round and unblinded second round, respectively.
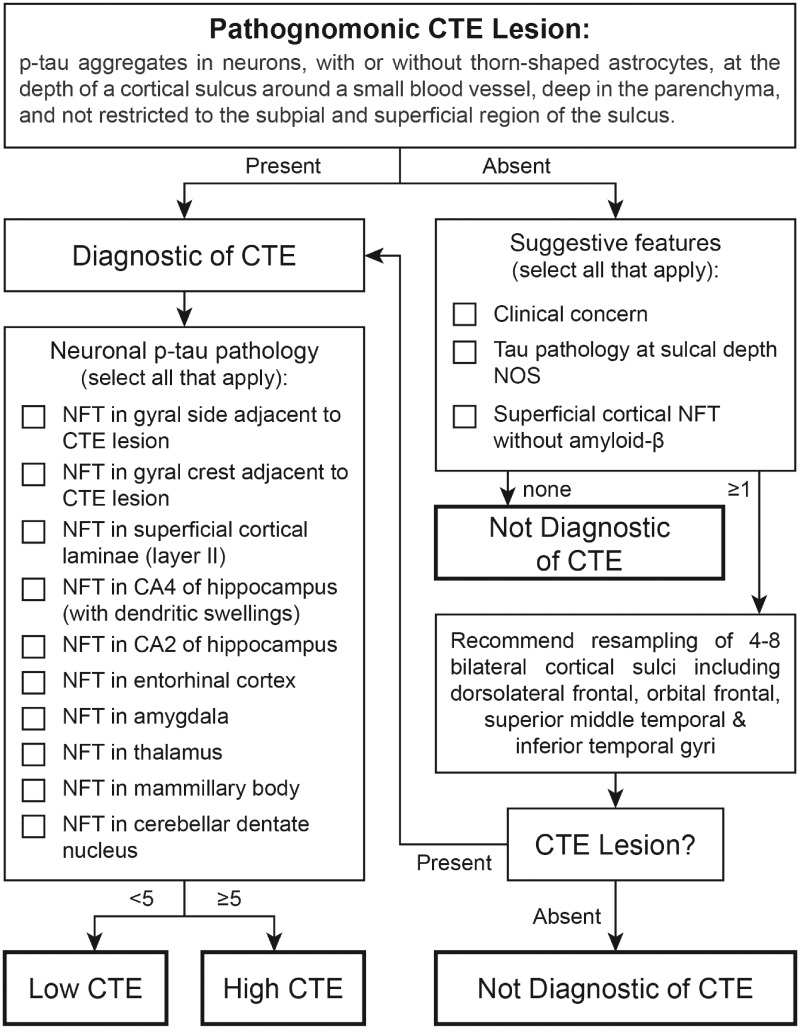
After discussion, the neuropathologists agreed that a simple and practical tool for the assessment of CTE pathological severity would be helpful for general use. The panel, therefore, proposed an operational workflow for the diagnosis and binary classification of CTE as mild “Low CTE” or severe “High CTE” requiring evaluation of a limited number of paraffin-embedded, 10-µm-thick tissue sections (Fig. 3).
FIGURE 3.
Working protocol for the diagnosis of chronic traumatic encephalopathy (CTE).
Proposed Operational Workflow:
The diagnosis of CTE is made using 10-µm-thick paraffin-embedded tissue sections from the following brain regions: (1) Middle frontal gyrus, (2) Superior and middle temporal gyri, (3) Inferior parietal lobule, (4) hippocampus, (5) amygdala, (6) basal ganglia, (7) thalamus, (8) midbrain with substantia nigra, (9) pons with locus coeruleus, (10) medulla oblongata, and (11) cerebellar cortex, as recommended by the first consensus conference (32).
The minimum threshold for diagnosis of CTE is the presence of a single pathognomonic lesion in the cortex (Fig. 1). A pathognomonic lesion consists of p-tau aggregates in neurons, with or without glial tau in TSA, at the depth of a cortical sulcus around a small blood vessel, in deeper cortical layers not restricted to subpial and superficial region of the sulcus.
To stage CTE severity, in the cortical region containing the CTE focus, the presence of NFT in the bank or crest of the gyrus each count as 1 point, NFT in the superficial cortical laminae (often most prominent in the temporal lobe) also count as 1 point. Should multiple CTE foci be present, scoring is to be performed on the single, most definitive CTE focus (i.e. maximum of 3 points from the cortex per case). The presence of NFTs in CA1 and CA4 of the hippocampus, entorhinal cortex, amygdala, thalamus, mammillary body, and cerebellar dentate nucleus also count as 1 point each for a total of 10 possible points per case.
Cases with <5 points are considered mild cases of CTE (“Low CTE”; Fig. 1A, B). Cases with 5 or more points are considered severe cases of CTE (“High CTE”; Fig. 1C, D). This approach accounts for variability in neuroanatomical patterns of p-tau pathology and distinguishes cases with focal involvement of the cortex (Low CTE) from those with more widely distributed neurofibrillary pathology (High CTE).
Given the patchy nature of CTE, in instances when CTE is suspected but not evident in the initial slides, additional cortical sampling is recommended. It is recommended that the resampled tissue capture 4–8 cortical sulci (preferably bilateral) from the superior frontal, dorsolateral superior frontal, superior middle temporal, and/or inferior temporal gyri.
In cases with only subpial p-tau TSA, superficial laminar NFT or nonspecific sulcal p-tau pathology, the case is not diagnostic for CTE and resampling is recommended.
If a CTE focus is detected with resampling, the case is diagnosed as CTE and assessed for severity as Low or High CTE. If no CTE foci are identified with resampling, the case is considered not diagnostic for CTE.
For the 17 CTE cases evaluated by the consensus panel, these guidelines accurately categorized the level of severity of CTE pathology as Low (n = 2) or High (n = 15) (Table 2).
DISCUSSION
Following independent analysis and in-person review of 27 study cases by the panel, including 17 cases of suspected CTE from subjects (ranging from 25 to 82 years, mean 52.7) and 10 cases representing ARTAG, PART, AGD, and mild AD (ranging from 81 to 94 years, mean 88.0), there was strong agreement in the diagnosis of CTE, both blinded to clinical and gross neuropathological information and unblinded. These results confirm the robustness of the consensus recommendations defined by the first NINDS/NIBIB consensus conference (32). The panel upheld the definition of the pathognomonic lesion of CTE as “phosphorylated tau aggregates in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci”; however, the panel made several refinements to the criteria. In particular, based on the case material reviewed, the panel concluded that the perivascular p-tau aggregates had to include neuronal profiles, with or without p-tau in astrocytes, and that the focus had to be in deeper cortical layers not restricted to subpial and superficial regions. In addition, the panel cautioned that subpial TSA or perivascular astrocytic pathology in isolation were consistent with ARTAG and did not meet the minimum criteria for CTE. However, members of the panel did acknowledge that the exact role of glial tau accumulation in CTE is an issue requiring further study. The consensus panel agreed that recognition of CTE pathology was more challenging in older individuals due to the increased prevalence of aging-related tau pathologies, notably ARTAG and PART. Community-based studies from Forrest et al and Bieniek et al together suggest that while cortical ARTAG frequency is notable in the elderly (38%), the presence/absence of CTE pathology varies by sex and head trauma history in the autopsy cohort evaluated (36, 44). Furthermore, NFTs in medial temporal structures in the absence of amyloid plaques are characteristic of both PART and CTE although there is some suggestion that CA4 p-tau pathology is more consistent with CTE. While further work needs to be done to differentiate CTE medial temporal lobe p-tau pathology from PART, the panel decided that, for the time-being, cases with cortical CTE lesions and NFT in the medial temporal lobe do not warrant an additional diagnosis of PART.
The results of applying the McKee staging system to the cases in this consensus evaluation proved to be inconsistent. P-tau pathology of CTE is, by definition, patchy, and diagnostic lesions in mild cases may be widely dispersed in cortical regions. The McKee CTE staging scheme was originally proposed following review of extended case material, as well as large hemispheric sections. As such, it is conceivable that the more limited sampling provided for this consensus review underrepresented the pathology in selected cases. Consistent with this, the consensus panel failed to identify CTE pathology in 2 of the cases included for evaluation in which CTE had previously been diagnosed. While large hemispheric sections offer an improved way to assess global p-tau pathology, the objective of this study was to develop a practical method of assessing CTE that would be available to a general pathologist. Given that, this was not entirely unexpected. As a result, the consensus panel proposed a simplified working protocol for the assessment of CTE and an algorithm for evaluation of CTE severity. The algorithm classifies cases with pathognomonic CTE foci as either “Low CTE” or “High CTE,” which is determined by the presence of NFT in widespread brain regions, including the thalamus, mammillary bodies, hippocampus, amygdala and entorhinal cortex. The designation “Low CTE” roughly equates to CTE stages I and II, and “High CTE” to CTE stages III and IV. The proposed assessment scheme for CTE severity is provisional, it does not reflect NFT burden, and the usefulness of the working protocol will need to be continuously evaluated in a larger number of CTE cases to confirm its reliability. Nevertheless, in the small number of CTE cases evaluated by the panel, the proposed algorithm accurately distinguished between mild (Low) and severe (High) cases of CTE (Fig. 3).
Future measurement of p-tau burden in CTE, as well as regional involvement, will allow for the comparison of antemortem variables to varying degrees of pathology and facilitate analysis of disease risk factors (e.g. type of exposure, exposure duration, etc.) and disease modifiers (e.g. genetic variants, clinical or behavioral therapies, etc.). Neuroanatomical regions assessed in the second consensus meeting were comparable to the first consensus meeting (for the sake of uniformity and intrarater reliability); however, it should be noted that proposed workflow from both meetings includes additional structures, notably brainstem, as abnormal subcortical tau pathology is a supportive feature of CTE (Table 1). Uniform assessment of CTE severity will also permit comparison between cohorts and centers.
One of the strengths of this study is that all slides were processed by a single research center, the VA-BU-CLF/UNITE brain bank, producing consistency in histologic preparation. Similarly, the evaluating neuropathologists were provided the same set of digital images for all cases. In addition, the neuropathologists evaluated the cases independently, at their own pace, and in their own sequence. Limitations of the study are that nearly all CTE cases were former professional American football players; one was a former college football player and one was a former professional boxer. Furthermore, the study cases were relatively “pure” CTE (with the exception of some AD pathology) despite reports indicating that comorbid neurodegeneration (AD, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, Lewy body disease, multiple system atrophy, etc.) is common in older individuals with CTE (19, 20, 45–47). Distinguishing CTE from concomitant neurodegenerative and aging-related pathologies represents a topic of interest for future studies, and the consensus committee makes no assertions regarding CTE in the presence of AD or other neurodegenerative disorders at this time. Additional educational training for general neuropathologists, neuropathology fellows, and biomedical neuroscience researchers regarding the CTE criteria is also another area that needs to be developed more fully in the future.
In summary, blinded assessment of 27 cases of various tauopathies resulted in high diagnostic agreement in cases of CTE and non-CTE using the NINDS/NIBIB criteria for the diagnosis of CTE (32). However, attempts at CTE staging using the McKee CTE staging scheme were unsuccessful. In view of this difficulty assigning CTE stage, the panel proposed an alternate operational workflow for the diagnostic evaluation of potential CTE cases and a recommendation that CTE p-tau pathological severity be designated as “High CTE” or “Low CTE.” Low CTE cases are characterized by cortical pathology, whereas high CTE cases have pathology widely distributed in hippocampus, amygdala, entorhinal cortex, thalamus, mammillary bodies and cerebellar dentate nucleus. Validation of the operational workflow for potential CTE cases using additional cohorts and improved identification of CTE in the presence of comorbid neurodegenerative diseases and aging-related pathologies remain future objectives.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the use of the resources and facilities at the Bedford VA Medical Center (Bedford, MA), Boston University School of Medicine, (Boston, MA), and Mayo Clinic Jacksonville (Jacksonville, FL). We also gratefully acknowledge the help the individuals and families whose participation and contributions made this work possible.
Contributor Information
the TBI/CTE Research Group:
Debra Babcock, Patrick Bellgowan, Paul Crane, Brian Edlow, Bertrand Russ Huber, Patrick Kiernan, and Walter Koroshetz
This study was supported by the National Institute of Neurological Disorders and Stroke (1U01NS086659-01, 1U01NS086625-01, R01NS078337, R56NS078337, R01NS095252), Department of Defense (W81XWH-13-2-0064, W81XWH-14-1-0399), Department of Veterans Affairs, the Veterans Affairs Biorepository (CSP 501), the National Institute of Aging (1R01AG061028-01), the National Institute of Aging Boston University Alzheimer’s Disease Center (P30AG13846; supplement 0572063345-5), Department of Defense Peer Reviewed Alzheimer’s Research Program (DoD-PRARP #13267017), the National Institute of Aging Boston University Framingham Heart Study (R01AG1649), the National Operating Committee on Standards for Athletic Equipment and the Concussion Legacy Foundation. This study was also supported by unrestricted gifts from the Andlinger Foundation, the World Wrestling Entertainment and the National Football League. This study was also supported by grants P50 AG05681 and P01 AG03991 from the National Institute on Aging (NJC).
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
TBI/CTE Research Group: Debra Babcock, MD, PhD, Patrick Bellgowan, PhD, Paul Crane, MD, Brian Edlow, MD, Bertrand Russ Huber, MD, PhD, Patrick Kiernan, and Walter Koroshetz, MD
REFERENCES
- 1. Martland HS, Beling CC. Traumatic cerebral hemorrhage. Arch Neurpsych 1929;22:1001–23 [Google Scholar]
- 2. Martland HS. Punch drunk. JAMA 1928;91:1103–7 [Google Scholar]
- 3. Bowman KM, Blau A. Psychotic states following head and brain injury in adults and children. In: Injuries of the Skull, Brain and Spinal Cord: Neuro-Psychiatric, Surgical, and Medico-Legal Aspects. Baltimore, MD: Williams & Wilkins Co. 1940:309–60. [Google Scholar]
- 4. Montenigro PH, Corp DT, Stein TD, et al. Chronic traumatic encephalopathy: Historical origins and current perspective. Annu Rev Clin Psychol 2015;11:309–30 [DOI] [PubMed] [Google Scholar]
- 5. Brandenburg W, Hallervorden J. Dementia pugilistica mit anatomischem Befund. Virchows Arch Path Anat 1954;325:680–709 [DOI] [PubMed] [Google Scholar]
- 6. Grahmann H, Ule G. Beitrag zur Kenntnis der chronischen cerebralen Krankheitsbilder bei Boxern. Eur Neurol 1957;134:261–83 [PubMed] [Google Scholar]
- 7. Neubuerger KT, Sinton DW, Denst J. Cerebral atrophy associated with boxing. Arch NeurPsych 1959;81:403–8 [DOI] [PubMed] [Google Scholar]
- 8. Spillane JD. Five boxers. Br Med J 1962;2:1205–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet Lond Engl 1963;282:795–801 [DOI] [PubMed] [Google Scholar]
- 10. Payne EE. Brains of boxers. Minim Invasive Neurosurg 1968;11:173–88 [DOI] [PubMed] [Google Scholar]
- 11. Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973;3:270–303 [DOI] [PubMed] [Google Scholar]
- 12. Hof PR, Knabe R, Bovier P, et al. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol 1991;82:321–6 [DOI] [PubMed] [Google Scholar]
- 13. Hof PR, Bouras C, Buée L, et al. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol 1992;85:23–30 [DOI] [PubMed] [Google Scholar]
- 14. Geddes JF, Vowles GH, Robinson SF, et al. Neurofibrillary tangles, but not Alzheimer-type pathology, in a young boxer. Neuropathol Appl Neurobiol 1996;22:12–6 [PubMed] [Google Scholar]
- 15. Geddes JF, Vowles GH, Nicoll JA, et al. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol 1999;98:171–8 [DOI] [PubMed] [Google Scholar]
- 16. Omalu BIMD, DeKosky STMD, Minster R, et al. Chronic traumatic encephalopathy in a national football league player. Neurosurgery 2005;57:128–34 [DOI] [PubMed] [Google Scholar]
- 17. McKee AC, Daneshvar DH, Alvarez VE, et al. The neuropathology of sport. Acta Neuropathol 2014;127:29–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling H, Morris HR, Neal JW, et al. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 2017;133:337–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee EB, Kinch K, Johnson VE, et al. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 2019;138:389–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain J Neurol 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omalu BI, Fitzsimmons RP, Hammers J, et al. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs 2010;6:130–6 [Database]. [DOI] [PubMed] [Google Scholar]
- 22. Stewart W, McNamara PH, Lawlor B, et al. Chronic traumatic encephalopathy: A potential late and under recognized consequence of rugby union. QJM 2016;109:11–5 [DOI] [PubMed] [Google Scholar]
- 23. Buckland ME, Sy J, Szentmariay I, et al. Correction to: Chronic traumatic encephalopathy in two former Australian National Rugby League players. Acta Neuropathol Commun 2019;7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omalu B, Hammers JL, Bailes J, et al. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus 2011;31:E3. [DOI] [PubMed] [Google Scholar]
- 25. Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012;4:134ra60 [Database]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts GW, Whitwell HL, Acland PR, et al. Dementia in a punch-drunk wife. Lancet Lond Engl 1990;335:918–9 [DOI] [PubMed] [Google Scholar]
- 27. Williams DJ, Tannenberg AE. Dementia pugilistica in an alcoholic achondroplastic dwarf. Pathology (Phila) 1996;28:102–4. [DOI] [PubMed] [Google Scholar]
- 28. Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 2012;22:142–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zanier ER, Bertani I, Sammali E, et al. Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018;141:2685–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iacono D, Lee P, Edlow BL, et al. Early-onset dementia in war veterans: Brain polypathology and clinicopathologic complexity. J Neuropathol Exp Neurol 2020;79:144–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 32. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keene CD, Latimer CS, Steele LM, et al. First confirmed case of chronic traumatic encephalopathy in a professional bull rider. Acta Neuropathol 2018;135:303–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grinberg LT, Anghinah R, Nascimento CF, et al. Chronic traumatic encephalopathy presenting as Alzheimer’s disease in a retired soccer player. J Alzheimers Dis 2016;54:169–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bieniek KF, Blessing MM, Heckman MG, et al. Association between contact sports participation and chronic traumatic encephalopathy: A retrospective cohort study. Brain Pathol 2020;30:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ling H, Holton JL, Shaw K, et al. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015;130:891–3 [DOI] [PubMed] [Google Scholar]
- 39. Noy S, Krawitz S, Del Bigio MR. Chronic traumatic encephalopathy-like abnormalities in a routine neuropathology service. J Neuropathol Exp Neurol 2016;75:1145–54 [DOI] [PubMed] [Google Scholar]
- 40. Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol 2016;131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kovacs GG, Molnár K, László L, et al. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 2011;122:205–22 [DOI] [PubMed] [Google Scholar]
- 42. Kovacs GG, Milenkovic I, Wöhrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathol 2013;126:365–84 [DOI] [PubMed] [Google Scholar]
- 43. Kovacs GG, Xie SX, Lee EB, et al. Multisite assessment of Aging-Related Tau Astrogliopathy (ARTAG). J Neuropathol Exp Neurol 2017;76:605–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forrest SL, Kril JJ, Wagner S, et al. Chronic traumatic encephalopathy (CTE) is absent from a European community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019;78:398–405 [DOI] [PubMed] [Google Scholar]
- 45. McKee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010;69:918–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015;130:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koga S, Dickson DW, Bieniek KF. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 2016;75:963–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol 2009;3:1–23 [PMC free article] [PubMed] [Google Scholar]
- 51. Oyanagi K, Hashimoto T, Yamazaki M. Parkinsonism-dementia complex of Guam. In: Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. John Wiley & Sons, Ltd; 2011:171–178. doi: 10.1002/9781444341256.ch18 [DOI] [Google Scholar]
- 52. Tolnay M, Clavaguera F. Argyrophilic grain disease: A late-onset dementia with distinctive features among tauopathies. Neuropathol Off J Jpn Soc Neuropathol 2004;24:269–83 [DOI] [PubMed] [Google Scholar]
- 53. Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007;114:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kouri N, Whitwell JL, Josephs KA, et al. Corticobasal degeneration: A pathologically distinct 4R tauopathy. Nat Rev Neurol 2011;7:263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996;55:97–105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.