Abstract
Objectives
The emergence of a SARS‐CoV‐2 variant with a point mutation in the spike (S) protein, D614G, has taken precedence over the original Wuhan isolate by May 2020. With an increased infection and transmission rate, it is imperative to determine whether antibodies induced against the D614 isolate may cross‐neutralise against the G614 variant.
Methods
Antibody profiling against the SARS‐CoV‐2 S protein of the D614 variant by flow cytometry and assessment of neutralising antibody titres using pseudotyped lentiviruses expressing the SARS‐CoV‐2 S protein of either the D614 or G614 variant tagged with a luciferase reporter were performed on plasma samples from COVID‐19 patients with known D614G status (n = 44 infected with D614, n = 6 infected with G614, n = 7 containing all other clades: O, S, L, V, G, GH or GR).
Results
Profiling of the anti‐SARS‐CoV‐2 humoral immunity reveals similar neutralisation profiles against both S protein variants, albeit waning neutralising antibody capacity at the later phase of infection. Of clinical importance, patients infected with either the D614 or G614 clade elicited a similar degree of neutralisation against both pseudoviruses, suggesting that the D614G mutation does not impact the neutralisation capacity of the elicited antibodies.
Conclusions
Cross‐reactivity occurs at the functional level of the humoral response on both the S protein variants, which suggests that existing serological assays will be able to detect both D614 and G614 clades of SARS‐CoV‐2. More importantly, there should be negligible impact towards the efficacy of antibody‐based therapies and vaccines that are currently being developed.
Keywords: clade, COVID‐19, cross‐reactivity, D614G variant, neutralising antibodies, SARS‐CoV‐2
A single point mutation from aspartic acid (D) to glycine (G) at position 614 of the SARS‐CoV‐2 spike (S) protein, termed D614G, has garnered global attention due to the observed increase in transmissibility and infection rate. Given that a majority of the developing antibody‐mediated therapies and serological assays are based on the S antigen of the original Wuhan reference sequence, it is crucial to determine whether humoral immunity acquired from the original SARS‐CoV‐2 isolate is able to induce cross‐detection and cross‐protection against the novel prevailing D614G variant. In this study, we demonstrated an overall equivalent neutralising capacity against both the D614 and G614 pseudoviruses, suggesting negligible impact towards the efficacy of antibody‐based therapies and vaccines that are currently being developed.

Introduction
Coronavirus disease 2019 (COVID‐19) is the consequence of an infection by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which emerged in Wuhan, China, in December 2019. 1 The rapid expansion of the COVID‐19 pandemic has affected 213 countries and territories, with a global count of more than 80 million laboratory‐confirmed human infection cases to date. 2 An inevitable impact of this pandemic is the accumulation of immunologically relevant mutations among the viral populations due to natural selection or random genetic drift, resulting in enhanced viral fitness and immunological resistance. 3 , 4 For instance, antigenic drift was previously reported in other common cold coronaviruses, OC43 and 229E, as well as in SARS‐CoV. 5 , 6 , 7
In early March 2020, a non‐synonymous mutation from aspartic acid (D) to glycine (G) at position 614 of SARS‐CoV‐2 spike (S) protein was identified. 8 This variant, G614, rapidly became the dominant SARS‐CoV‐2 clade in Europe by May 2020, suggesting a higher transmission rate over the original isolate, D614. 8 In vitro and animal studies have also indicated that the G614 variant may have an increased infectivity and may be associated with higher viral loads and more severe infections. 8 , 9 , 10 , 11 , 12 Notably, single point mutations have been shown to induce resistance to neutralising antibodies in other coronaviruses, including SARS‐CoV and Middle East respiratory syndrome (MERS‐CoV). 13 , 14 More importantly, mutations in the S protein of SARS‐CoV‐2 have been shown to induce conformational modifications that alter antigenicity. 15 , 16 Hence, determining any cross‐neutralising capability of antibodies developed against the earlier G614 variant is of paramount importance to validate the therapeutic efficacy of developing immune‐based interventions.
Results
Antibody profiling against the SARS‐CoV‐2 S protein was first assessed using plasma samples collected from COVID‐19 patients (n = 57) during the Singapore outbreak between January and April 2020, across the early recovery phase [median 31 days post‐illness onset (pio)] and a later post‐recovery time point (median 98 days pio) (Table 1, Figure 1a and b). All patients showed a decrease in IgM response (Figure 1a), and a prolonged IgG response over time (Figure 1b). Although one recent study has demonstrated similar neutralisation profiles against both D614 and G614 SARS‐CoV‐2 pseudoviruses, the virus clade by which the six individuals were infected with was not identified. 9 According to Singapore’s SARS‐CoV‐2 clade pattern from December 2019 till July 2020 based on n = 736 cases with genome availability, the D614G mutation, indicated as G clade following the GISAID clade nomenclature, only appeared in March 2020 (Figure 1c). Hence, with knowledge on the D614G status of a subset of COVID‐19 patients (n = 44 infected with D614, n = 6 infected with G614, n = 7 containing all other clades: O, S, L, V, G, GH or GR; Table 1, Figure 1c), the neutralising capacity of these anti‐SARS‐CoV‐2 antibodies was assessed using pseudotyped lentiviruses expressing the SARS‐CoV‐2 S protein tagged with a luciferase reporter as a surrogate of live virus. 17 The neutralisation EC50 values of each patient were interpolated from the respective dose–response neutralisation titration curves (Table 2, Figure 1d and e, Supplementary figure 1). Notably, these antibodies were able to neutralise both SARS‐CoV‐2 D614 and G614 pseudoviruses at similar levels, despite having a significantly lower neutralisation capacity at median 98 days pio in all COVID‐19 patients (Figure 1d and e, Supplementary figures 1 and 2). Corroborating other studies, severe patients have a higher and persisting level of neutralising antibodies as compared with both mild and moderate patients (Table 2, Supplementary figure 2). 18 , 19 Of clinical importance, all the patients infected with either the D614 or G614 clade elicited a similar degree of neutralisation against both D614 and G614 pseudoviruses (Figure 1f), suggesting that the D614G mutation does not impact the neutralisation capacity of the elicited antibodies. Our results support the notion that the locus where the point mutation occurred is not critical for antibody‐mediated immunity and may not have an impact on virus resistance towards antibody‐based interventions. 4 , 20
Table 1.
Demographic and clinical information of COVID‐19 patients
| Patients (n = 57) | |
|---|---|
| Demographics | |
| Age, years | 45 (13) |
| Sex | |
| Male | 38 (66.7%) |
| Female | 19 (33.3%) |
| Ethnicity | |
| Chinese | 42 (73.7%) |
| Others | 15 (26.3%) |
| Comorbidities | 29 (50.9%) |
| Hyperlipidaemia | 14 (24.6%) |
| Hypertension | 13 (22.8%) |
| Diabetes | 7 (12.3%) |
| Myocardial infection (history) | 5 (8.8%) |
| Others | 10 (17.5%) |
| D614G infection status | |
| D614 | 44 (77.2%) |
| G614 | 6 (10.5%) |
| Others a | 7 (12.3%) |
| Clinical outcome (clinical severity; group) | |
| No pneumonia (0; mild) | 25 (43.9%) |
| Pneumonia, without hypoxia (1; moderate) | 19 (33.3%) |
| Pneumonia, with hypoxia (2; severe) | 13 (22.8%) |
Data are presented as Mean (SD) or n (%). COVID‐19: Coronavirus Disease 2019.
Others: O, S, L, V, G, GH or GR clades.
Figure 1.
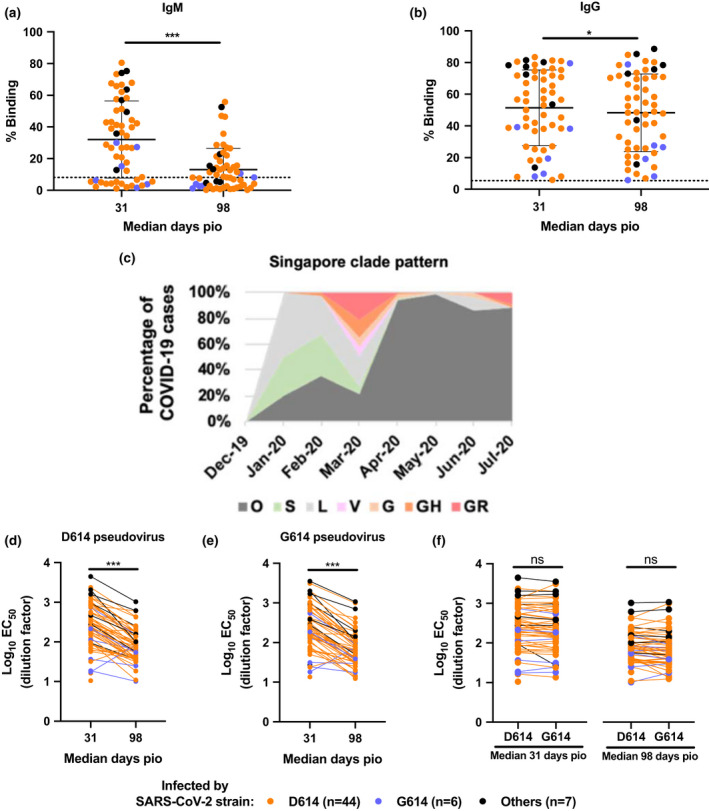
Timeline of events during the SARS‐CoV‐2 outbreak in Singapore, and the antibody profiles of COVID‐19 patients and their neutralising capacity against both D614 and G614 variants of SARS‐CoV‐2. Plasma samples of COVID‐19 patients (n = 57) at median 31 and median 98 days post‐illness onset (pio) were assessed for anti‐SARS‐CoV‐2 IgM and IgG antibody response. Plasma samples (1:100 dilution) were incubated with transduced HEK293T cells expressing SARS‐CoV‐2 spike protein, and (a) anti‐IgM and (b) anti‐IgG levels were quantified by flow cytometry. Percentage binding indicates the percentage of cells with antibody binding. Data are shown as mean ± SD of two independent experiments. Dotted line indicates mean + 3SD of healthy controls (n = 22). Statistical analysis was carried out with the Wilcoxon signed‐rank test (*P < 0.05, ***P < 0.001). (c) Percentage of COVID‐19 cases with genome available (n = 736) during the Singapore outbreak from December 2019 to July 2020, segregated by the clade with which the patients were infected following GISAID clade nomenclature. (d–f) Anti‐SARS‐CoV‐2 neutralising antibodies were assessed using luciferase expressing lentiviruses pseudotyped with SARS‐CoV‐2 spike (S) protein of either the original strain, D614, or the mutant variant, G614. Log10 neutralisation EC50 profiles against (d) D614 and (e) G614 pseudoviruses across both time points. Data represent the mean of two independent experiments, and statistical analysis was carried out using the paired t‐test (***P < 0.001). (f) Comparison of log10 neutralisation EC50 values between D614 and G614 pseudoviruses during both time points. Data represent the mean of two independent experiments, and statistical analysis was carried out using the paired t‐test. All data points are non‐significant (ns).
Table 2.
Neutralisation EC50 values of COVID‐19 patients
| Patient | Days post‐illness onset (pio) | Recovery phase | Infection by SARS‐CoV‐2 strain a | D614 (EC50) Dilution factor | D614 (Log 10 EC50) Dilution factor | G614 (EC50) Dilution factor | G614 (Log 10 EC50) Dilution factor |
|---|---|---|---|---|---|---|---|
| Mild (No pneumonia) | |||||||
| #1 | 39 | Early | Others | 93.821 | 1.972300058 | 27.088 | 1.432776941 |
| 95 | Late | 36.481 | 1.562066734 | ND | ND | ||
| #2 | 34 | Early | D614 | 59.67 | 1.775756038 | 59.527 | 1.774713996 |
| 152 | Late | 59.156 | 1.7719988 | 46.489 | 1.667350204 | ||
| #3 | 30 | Early | D614 | 84.26 | 1.925621455 | 100.33 | 2.001430812 |
| 111 | Late | 36.216 | 1.558900481 | 20.109 | 1.303390474 | ||
| #4 | 29 | Early | D614 | 264.7 | 2.422753941 | 371.63 | 2.570110765 |
| 92 | Late | 85.178 | 1.930327439 | 101.03 | 2.004450353 | ||
| #5 | 30 | Early | D614 | 401.03 | 2.603176862 | 229.98 | 2.36169007 |
| 100 | Late | 93.083 | 1.968870372 | 42.272 | 1.626052796 | ||
| #6 | 32 | Early | D614 | 56.708 | 1.753644331 | 49.807 | 1.697290384 |
| 96 | Late | 37.541 | 1.574505837 | 24.87 | 1.395675785 | ||
| #7 | 30 | Early | D614 | 182.16 | 2.260453018 | 179.26 | 2.253483392 |
| 107 | Late | 37.299 | 1.571697188 | 31.102 | 1.492788317 | ||
| #8 | 30 | Early | D614 | 70.715 | 1.849511546 | 64.52 | 1.809694359 |
| 88 | Late | 38.049 | 1.580343247 | 32.853 | 1.516575034 | ||
| #9 | 25 | Early | D614 | 61.803 | 1.791009557 | 67.785 | 1.8311336 |
| 101 | Late | 45.326 | 1.656347394 | 13.3 | 1.123851641 | ||
| #10 | 32 | Early | D614 | 123.21 | 2.090645958 | 72.937 | 1.862947896 |
| 110 | Late | 18.353 | 1.263707065 | ND | ND | ||
| #11 | 33 | Early | D614 | 312.72 | 2.495155657 | 135.08 | 2.130591052 |
| 91 | Late | 103.42 | 2.014604533 | 60.652 | 1.782845126 | ||
| #12 | 33 | Early | D614 | 365.85 | 2.563303059 | 233.92 | 2.369067355 |
| 96 | Late | 79.832 | 1.90217701 | 35.665 | 1.552242228 | ||
| #13 | 31 | Early | G614 | 110.63 | 2.043872912 | 127.51 | 2.105544246 |
| 94 | Late | 65.001 | 1.812920038 | 63.342 | 1.801691772 | ||
| #14 | 24 | Early | D614 | 151.32 | 2.179896333 | 143.27 | 2.156155261 |
| 100 | Late | 39.825 | 1.600155784 | 31.445 | 1.497551599 | ||
| #15 | 28 | Early | D614 | 242.06 | 2.383923029 | 241.44 | 2.382809222 |
| 98 | Late | 58.31 | 1.765743041 | 52.821 | 1.722806619 | ||
| #16 | 31 | Early | D614 | 169.39 | 2.228887768 | 134.4 | 2.128399269 |
| 92 | Late | 78.702 | 1.895985769 | 78.239 | 1.893423291 | ||
| #17 | 39 | Early | D614 | 89.4 | 1.951337519 | 77.364 | 1.888538916 |
| 97 | Late | 25.104 | 1.399742926 | 14.494 | 1.161188257 | ||
| #18 | 26 | Early | D614 | 16.219 | 1.210024074 | 13.513 | 1.130751777 |
| 99 | Late | ND | ND | ND | ND | ||
| #19 | 39 | Early | G614 | 18.721 | 1.272329043 | 24.532 | 1.389732956 |
| 99 | Late | 10.11 | 1.004751156 | 17.581 | 1.245043574 | ||
| #20 | 35 | Early | D614 | 941.37 | 2.973760354 | 856.37 | 2.932661445 |
| 99 | Late | 171 | 2.23299611 | 97.95 | 1.99100444 | ||
| #21 | 35 | Early | D614 | 312.28 | 2.494544171 | 150.83 | 2.178487731 |
| 99 | Late | 38.602 | 1.586609806 | 19.899 | 1.298831252 | ||
| #22 | 32 | Early | G614 | 17.385 | 1.240174695 | 18.098 | 1.257630584 |
| 98 | Late | 83.448 | 1.921415932 | 74.848 | 1.8741802 | ||
| #23 | 62 | Early | G614 | 36.553 | 1.562923026 | 31.281 | 1.495280628 |
| 104 | Late | 24.869 | 1.395658322 | 29.766 | 1.473720477 | ||
| #24 | 38 | Early | D614 | 10.477 | 1.020236944 | ND | ND |
| 99 | Late | ND | ND | ND | ND | ||
| #25 | 18 | Early | D614 | 849.23 | 2.929025328 | ND | ND |
| 105 | Late | 601.69 | 2.779372794 | ND | ND | ||
| Moderate (Pneumonia, without hypoxia) | |||||||
| #1 | 29 | Early | D614 | 325.6 | 2.512684396 | 311.41 | 2.493332555 |
| 99 | Late | 50.013 | 1.699082906 | 40.54 | 1.607883744 | ||
| #2 | 29 | Early | Others | 280.08 | 2.447282098 | 279.51 | 2.44639735 |
| 91 | Late | 55.82 | 1.746789832 | 49.937 | 1.698422448 | ||
| #3 | 37 | Early | D614 | 565.39 | 2.752348123 | 412.73 | 2.615666037 |
| 99 | Late | 176.37 | 2.246424715 | 192.41 | 2.28422764 | ||
| #4 | 29 | Early | D614 | 406.93 | 2.609519708 | 394.6 | 2.596157081 |
| 92 | Late | 58.04 | 1.763727404 | 70.882 | 1.850535963 | ||
| #5 | 29 | Early | D614 | 188.21 | 2.274642695 | 172.03 | 2.235604189 |
| 106 | Late | 197.85 | 2.296336055 | 157.28 | 2.1966735 | ||
| #6 | 25 | Early | D614 | 2349.4 | 3.370956964 | 2000.3 | 3.301095135 |
| 96 | Late | 432.12 | 2.635604367 | 319.05 | 2.503858749 | ||
| #7 | 34 | Early | D614 | 96.242 | 1.983364639 | 110.53 | 2.04348017 |
| 104 | Late | 10.932 | 1.038699623 | 12.366 | 1.092229242 | ||
| #8 | 28 | Early | D614 | 227 | 2.356025857 | 215.24 | 2.332922983 |
| 113 | Late | 41.09 | 1.613736141 | 28.984 | 1.462158321 | ||
| #9 | 31 | Early | D614 | 792.61 | 2.899059547 | 601.93 | 2.779545989 |
| 96 | Late | 182.48 | 2.261215272 | 132.86 | 2.123394248 | ||
| #10 | 32 | Early | D614 | 541.77 | 2.733814953 | 399.85 | 2.6018971 |
| 99 | Late | 136.61 | 2.135482491 | 121.88 | 2.085932446 | ||
| #11 | 29 | Early | D614 | 164.37 | 2.215822555 | 152.3 | 2.182699903 |
| 90 | Late | 34.63 | 1.539452492 | 41.678 | 1.61990687 | ||
| #12 | 32 | Early | D614 | 241.37 | 2.38268329 | 267.15 | 2.426755179 |
| 89 | Late | 35.053 | 1.544725193 | 39.4 | 1.595496222 | ||
| #13 | 58 | Early | D614 | 84.158 | 1.925095406 | 51.315 | 1.710244333 |
| 101 | Late | 34.56 | 1.538573734 | 25.507 | 1.406659382 | ||
| #14 | 25 | Early | D614 | 220.86 | 2.344117068 | 171.07 | 2.233173855 |
| 106 | Late | 31.918 | 1.50403567 | 33.142 | 1.520378713 | ||
| #15 | 36 | Early | D614 | 200.82 | 2.302806963 | 156.64 | 2.194902674 |
| 87 | Late | 70.748 | 1.849714167 | 65.35 | 1.815245592 | ||
| #16 | 27 | Early | D614 | 308.07 | 2.488649409 | 201.4 | 2.304059466 |
| 106 | Late | 90.322 | 1.955793546 | 56.963 | 1.755592854 | ||
| #17 | 34 | Early | D614 | 1079.6 | 3.033262876 | 1039.5 | 3.016824494 |
| 115 | Late | 100.36 | 2.001560653 | 119.98 | 2.079108858 | ||
| #18 | 42 | Early | D614 | 89.823 | 1.953387556 | 69.059 | 1.839220285 |
| 107 | Late | 31.172 | 1.493764668 | 31.425 | 1.497275286 | ||
| #19 | 30 | Early | G614 | 214.79 | 2.332014058 | 186.07 | 2.269676358 |
| 99 | Late | 54.362 | 1.735295426 | 38.613 | 1.586733545 | ||
| Severe (Pneumonia, with hypoxia) | |||||||
| #1 | 31 | Early | G614 | 740.24 | 2.869372549 | 548.74 | 2.739366619 |
| 92 | Late | 154.05 | 2.187661703 | 92.754 | 1.967332648 | ||
| #2 | 33 | Early | Others | 940.91 | 2.973548084 | 967.53 | 2.98566444 |
| 97 | Late | 250.17 | 2.398235229 | 199.92 | 2.300856243 | ||
| #3 | 29 | Early | D614 | 1597.5 | 3.203440867 | 1443.9 | 3.159537116 |
| 96 | Late | 173.92 | 2.240349527 | 236.97 | 2.374693369 | ||
| #4 | 29 | Early | D614 | 970.61 | 2.987044761 | 651.53 | 2.813934418 |
| 104 | Late | 106.39 | 2.026900809 | 86.982 | 1.939429389 | ||
| #5 | 34 | Early | D614 | 755.31 | 2.878125235 | 822.44 | 2.915104224 |
| 113 | Late | 71.959 | 1.857085119 | 74.804 | 1.873924822 | ||
| #6 | 33 | Early | Others | 2042.2 | 3.310098272 | 2007.9 | 3.30274208 |
| 110 | Late | 100.71 | 2.003072596 | 108.06 | 2.033664963 | ||
| #7 | 30 | Early | D614 | 1291.7 | 3.11116166 | 3109.8 | 3.492732459 |
| 87 | Late | 420.78 | 2.624055089 | 996.85 | 2.998629813 | ||
| #8 | 28 | Early | D614 | 1298.1 | 3.11330815 | 1391.8 | 3.143576832 |
| 109 | Late | 224.08 | 2.350403096 | 246.4 | 2.391640703 | ||
| #9 | 37 | Early | Others | 466.49 | 2.668842338 | 383.24 | 2.583470831 |
| 92 | Late | 156.93 | 2.195705975 | 140.67 | 2.148201487 | ||
| #10 | 39 | Early | Others | 4453.3 | 3.648681953 | 3528.8 | 3.547627045 |
| 116 | Late | 1024.2 | 3.010384771 | 1072.7 | 3.030478281 | ||
| #11 | 40 | Early | D614 | 529.25 | 2.723660867 | 730.88 | 2.863846078 |
| 60 | Late | 253.5 | 2.403977964 | 419.99 | 2.62323895 | ||
| #12 | 31 | Early | D614 | 891.98 | 2.950355117 | 1016.9 | 3.007278247 |
| 93 | Late | 136.02 | 2.133602771 | 108.15 | 2.034026524 | ||
| #13 | 40 | Early | Others | 1595.2 | 3.202815141 | 1691.3 | 3.228220649 |
| 60 | Late | 612.24 | 2.7869217 | 702.75 | 2.846800854 | ||
COVID‐19: Coronavirus Disease 2019; Early: median 31 days post‐illness onset (pio); Late: median 98 days pio; ND: not determined.
Others: O, S, L, V, G, GH or GR clades.
Discussion
The emergence of a new virus clade due to random mutations could heavily deter the therapeutic outcome of treatments and vaccines. Majority of the current immunoassays developed against SARS‐CoV‐2 are based on the S antigen of the original Wuhan reference sequence. 21 , 22 Moreover, pioneer batches of therapeutics and candidate vaccines were mostly designed based on earlier infections. As a result, mutations in the dominant variant sequence could potentially alter the viral phenotype and virulence, thereby rendering current immune‐based therapies less efficient and effective. 23 , 24 Fortunately, a recent pre‐print reported no observable difference in IgM, IgG and IgA profiles against either the D614 or G614 S variant in an antigen‐based serological assay, 25 providing preliminary findings on the effectiveness of current diagnostic approaches to detect SARS‐CoV‐2 G614 infections.
In addition, determining the level of cross‐reactivity is essential for immunosurveillance, as well as to identify broadly neutralising antibodies or epitopes. 26 Here, we confirm that cross‐reactivity occurs at the functional level of the humoral response on both the S protein variants. Of note, the stronger neutralising capacity observed during the early recovery phase may be due to the higher level of IgM response at median 31 days pio, as plasma IgM has been shown in a recent pre‐print to contribute towards SARS‐CoV‐2 neutralisation. 27 While IgA has also been reported to mediate neutralising activities during SARS‐CoV‐2 infection at a lower potency, 27 investigations on the IgA levels and neutralising capacity in patients infected by the G614 clade would be needed to confirm earlier findings. Interestingly, although there was no significant difference between the neutralising capacity against both D614 and G614 pseudoviruses, individuals infected by the G614 clade, albeit small patient numbers, appear to have a lower log10 EC50 value (Figure 1d–f). While it remains elusive, this observation may be associated to the lower IgM and IgG levels in these patients. Nonetheless, our results, together with the recent serological evaluation, 25 strongly suggest that existing serological assays will be able to detect both D614 and G614 clades of SARS‐CoV‐2 with a similar sensitivity. Recent studies have also demonstrated an overall equivalent sensitivity against both the D614 and G614 pseudotyped viruses, suggesting that the D614G mutation is not expected to hinder current vaccine development. 10 , 11 , 12 , 28 However, it is of clinical relevance to assess if cross‐reactivity between the variants may enhance viral infection when neutralising antibodies are present at suboptimal concentrations. 29 More importantly, further studies using monoclonal antibodies are necessary to validate the cross‐reactivity profiles between both SARS‐CoV‐2 S variants.
Overall, our study shows that the D614G mutation on the S protein does not impact SARS‐CoV‐2 neutralisation by the host antibody response, nor confer viral resistance against the humoral immunity. Hence, there should be negligible impact towards the efficacy of antibody‐based therapies and vaccines that are currently being developed.
Methods
Ethical approval
Written informed consent was obtained from participants in accordance with the tenets of the Declaration of Helsinki. The study design protocol was approved by National Healthcare Group (NHG) Domain Specific Review Board (DSRB) under study number 2012/00917. Specimens from healthy donors were collected under study numbers 2017/2806 and NUS IRB 04‐140.
COVID‐19 patients and sample collection
Fifty‐seven patients who tested PCR‐positive for SARS‐CoV‐2 in nasopharyngeal swabs in Singapore were recruited into the study from January to March 2020 30 , 31 (Table 1). Patients were categorised into three groups based on clinical severity during hospitalisation: mild (no pneumonia on chest radiographs (CXR), n = 25), moderate (pneumonia on CXR without hypoxia, n = 19) and severe (pneumonia on CXR with hypoxia (desaturation to ≤ 94%), n = 13). Whole blood of patients was collected in BD Vacutainer® CPT™ tubes (BD Biosciences, Franklin Lakes, NJ, USA) and centrifuged at 1700 g for 20 min to obtain plasma fractions. Plasma samples were either heat‐inactivated at 56°C for 30 min, 17 or treated with Triton™ X‐100 (Thermo Fisher Scientific, Waltham, MA, USA) to a final concentration of 1% for 2 h at room temperature (RT) for virus inactivation. 31 , 32
Determining D614G mutation status of COVID‐19 patients
Residual clinical RNA was subjected to tiled amplicon PCR using ARTIC nCoV‐2019 version 3 panel. 33 Sequencing libraries were prepared using the Nextera XT and sequenced on MiSeq (Illumina, San Diego, California, USA) to generate 300 bp paired‐end reads. The reads were subjected to a hard‐trim of 50 bp on each side to remove primer artefacts using BBMap 34 prior to consensus sequence generation by Burrows‐Wheeler Aligner‐MEM v0.7.17. Sequences with nucleotide mutation A23403G were assigned as D614G.
Cells
Human embryonic kidney (HEK) 293T (ATCC, Manassas, VA, USA) cells were maintained in DMEM (Cytiva Life Sciences, Marlborough, MA USA) with 10% heat‐inactivated foetal bovine serum (FBS; Cytiva Life Sciences). CHO cells expressing human ACE2 (CHO‐ACE2; kindly gifted by Professor Yee‐Joo Tan, Department of Microbiology, NUS & IMCB, A*STAR, Singapore) were cultured in DMEM with 10% FBS, 1% MEM non‐essential amino acid solution (Thermo Fisher Scientific), and 0.5 mg mL‐1 of Geneticin selective antibiotic (Thermo Fisher Scientific). Surface expression of ACE2 on CHO‐ACE2 cells was confirmed using anti‐human ACE2 Alexa Fluor 647 (Santa Cruz Biotechnology, Dallas, TX, USA). All cells were maintained at 37°C with 5% CO2.
S‐flow assay
Full‐length SARS‐CoV‐2 Spike (S) protein of the D614 variant‐expressing HEK293T cells was produced by transduction with lentiviral particles. 35 Cells were seeded at 1.5 × 105 per well in 96‐well plates and incubated with Triton™ X‐100 inactivated plasma samples (1:100 dilution) in 10% FBS in PBS (FACS blocking buffer), followed by a secondary incubation of Alexa Fluor 647‐conjugated anti‐human IgM or IgG (1:500 dilution; Thermo Fisher Scientific) and propidium iodide (1:2500 dilution; Sigma‐Aldrich, St. Louis, MO, USA). Cells were acquired on BDTM LSR II laser (BD Biosciences), and results were analysed with FlowJo (version 10, Tree Star Inc. Becton Dickinson, Ashland, OR). Results are presented as percentage of binding, which indicates the percentage of cells with antibody binding.
SARS‐CoV‐2 pseudovirus production
The pseudotyped lentiviruses were produced as previously described. 3 Briefly, using the third‐generation lentivirus system, pseudotyped viral particles expressing SARS‐CoV‐2 D614 strain or G614 variant S proteins were generated by reverse transfection of 3 × 107 of HEK293T cells with 12 μg pMDLg/PRRE (Addgene, Watertown, Massachusetts, USA), 6 μg pRSV‐Rev (Addgene), 12 μg pTT5LnX‐coV‐SP (SARS‐CoV‐2 wildtype S, a kind gift from Dr Brendon John Hanson, DSO National Laboratories, Singapore) or pTT5Lnx‐coV‐SP‐D614G (SARS‐CoV‐2 mutant D614G S), and 24 μg pHIV‐Luc‐ZsGreen (Addgen) using Lipofectamine 2000 transfection (Invitrogen, Carlsbad, California, USA). Cells were cultured for 3 days, before viral supernatant was harvested by centrifugation to remove cell debris and filtered through a 0.45 μm filter unit (Sartorius, Gottingen, Germany). Viral titres were quantified with Lenti‐X™ p24 Rapid Titre Kit (Takara Bio, Kusatsu, Shiga, Japan).
Pseudovirus neutralisation assay
The pseudotyped lentivirus neutralisation assay was performed as previously described, with slight modifications. 3 CHO‐ACE2 cells were seeded at 3.2 x 104 per well in a 96‐well black microplate (Corning, New York, NY) in culture medium without Geneticin. Serially diluted heat‐inactivated plasma samples (1:10 to 1:31 250 dilutions) were incubated with equal volume of pseudovirus expressing SARS‐CoV‐2 S proteins of either original wildtype or D614G mutant strain (0.4 ng μL−1 of p24) at 37°C for 1 h, before being added to pre‐seeded CHO‐ACE2 cells. Cells were refreshed with culture media after 1 h incubation. After 48 h, cells were washed with PBS and lysed with 1× Passive Lysis Buffer (Promega, Madison, Wisconsin, USA) with gentle shaking at 125 rpm for 30 min at 37°C. Luciferase activity was subsequently quantified with Luciferase Assay System (Promega) on a GloMax Luminometer (Promega).
Data and statistical analysis
Data were analysed using GraphPad Prism (version 8.4.3; GraphPad Software, San Diego, CA) and Microsoft Excel (version 16.39; Microsoft). The Wilcoxon signed‐rank test and the paired t‐test were carried out to compare the antibody and neutralisation profiles of COVID‐19 patients at median of 31 and 98 days’ post‐illness onset (pio). P‐values less than 0.05 are considered to be statistically significant.
Conflict of interest
All authors declare no conflicts.
Author contributions
Cheryl Lee: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing‐original draft; Writing‐review & editing. Siti Naqiah Amrun: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing‐review & editing. Rhonda Chee: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing‐review & editing. Yun Shan Goh: Data curation; Formal analysis; Investigation; Methodology; Writing‐review & editing. Tze‐Minn Mak: Data curation; Formal analysis; Investigation; Methodology; Writing‐review & editing. Sophie Octavia: Data curation; Formal analysis; Investigation; Methodology; Writing‐review & editing. Nicholas Yeo: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing‐review & editing. Ziwei Chang: Data curation; Investigation; Methodology; Writing‐review & editing. Matthew Tay: Data curation; Investigation; Methodology; Writing‐review & editing. Anthony Torres‐Ruesta: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing‐review & editing. Guillaume Carissimo: Formal analysis; Validation; Writing‐review & editing. Chek Meng Poh: Data curation; Investigation; Methodology; Writing‐review & editing. Siew‐Wai Fong: Formal analysis; Validation; Writing‐review & editing. Bei Wang: Resources; Supervision; Validation; Writing‐review & editing. Sandy Lee: Methodology; Validation; Writing‐review & editing. Barnaby Edward Young: Resources; Supervision; Validation; Writing‐review & editing. Seow‐Yen Tan: Resources; Supervision; Validation; Writing‐review & editing. Yee Sin Leo: Resources; Supervision; Validation; Writing‐review & editing. David Chien Lye: Resources; Supervision; Validation; Writing‐review & editing. Raymond Lin: Resources; Supervision; Validation; Writing‐review & editing. Sebastian Maurer‐Stroh: Data curation; Formal analysis; Investigation; Validation; Writing‐review & editing. Bernett Lee: Data curation; Formal analysis; Validation; Writing‐review & editing. Cheng‐I Wang: Resources; Supervision; Writing‐review & editing. Laurent Renia: Conceptualization; Methodology; Project administration; Supervision; Writing‐review & editing. Lisa FP Ng: Conceptualization; Funding acquisition; Methodology; Project administration; Supervision; Writing‐review & editing.
Supporting information
Acknowledgments
The authors thank the study participants who donated their blood samples to this project and the healthcare workers caring for the COVID‐19 patients. The authors also wish to thank Ding Ying and the Singapore Infectious Disease Clinical Research Network (SCRN) for their help in patient recruitment and the staffs at the National Centre for Infectious Diseases (NCID) who assisted with data analysis on viral sequences and determination of the D614G status. The authors also thank Professor Yee‐Joo Tan (Department of Microbiology, NUS; Institute of Molecular and Cell Biology (IMCB), A*STAR) for kindly providing the CHO‐ACE2 cells and Dr Brendon John Hanson (DSO National Laboratories, Singapore) for kindly providing the SARS‐CoV‐2 wildtype S protein. This study was supported by core and COVID‐19 (H20/04/g1/006) research grants from Biomedical Research Council (BMRC) and the A*ccelerate GAP‐funded project (ACCL/20‐GAP001‐C20H‐E) from Agency for Science, Technology and Research (A*STAR), and National Medical Research Council (NMRC) COVID‐19 Research fund (COVID19RF‐001, COVID19RF‐007 and COVID19RF‐060). ATR is supported by the Singapore International Graduate Award (SINGA), A*STAR. The funding sources had no role in the study design; collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Contributor Information
Laurent Renia, Email: renia_laurent@immunol.a-star.edu.sg.
Lisa FP Ng, Email: lisa_ng@immunol.a-star.edu.sg.
References
- 1. Cohen J, Normile D. New SARS‐like virus in China triggers alarm. Science 2020; 367: 234–235. [DOI] [PubMed] [Google Scholar]
- 2. Worldometer . Dover, Delaware, USA. Available from: https://www.worldometers.info/coronavirus/?
- 3. Sevajol M, Subissi L, Decroly E, Canard B, Imbert I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS‐coronavirus. Virus Res 2014; 194: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614G means for the COVID‐19 pandemic remains unclear. Cell 2020; 182: 794–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren L, Zhang Y, Li J et al Genetic drift of human coronavirus OC43 spike gene during adaptive evolution. Sci Rep 2015; 5: 11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chibo D, Birch C. Analysis of human coronavirus 229E spike and nucleoprotein genes demonstrates genetic drift between chronologically distinct strains. J Gen Virol 2006; 87: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 7. Song H‐D, Tu C‐C, Zhang G‐W et al Cross‐host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci USA 2005; 102: 2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Jackson CB, Mou H et al The D614G mutation in the SARS‐CoV‐2 spike protein reduces S1 shedding and increases infectivity. bioRxiv 2020: 2006.2012.148726.
- 9. Korber B, Fischer WM, Gnanakaran S et al Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell 2020; 182: 812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou YJ, Chiba S, Halfmann P et al SARS‐CoV‐2 D614G variant exhibits efficient replication ex vivo and transmission in vivo . Science 2020; 370: 1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plante JA, Liu Y, Liu J et al Spike mutation D614G alters SARS‐CoV‐2 fitness. Nature 2020. 10.1038/s41586-020-2895-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Jackson CB, Mou H et al SARS‐CoV‐2 spike‐protein D614G mutation increases virion spike density and infectivity. Nat Commun 2020; 11: 6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sui J, Aird DR, Tamin A et al Broadening of neutralization activity to directly block a dominant antibody‐driven SARS‐coronavirus evolution pathway. PLoS Pathog 2008; 4: e1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang X‐C, Agnihothram SS, Jiao Y et al Identification of human neutralizing antibodies against MERS‐CoV and their role in virus adaptive evolution. Proc Natl Acad Sci USA 2014; 111: E2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eaaswarkhanth M, Al Madhoun A, Al‐Mulla F. Could the D614G substitution in the SARS‐CoV‐2 spike (S) protein be associated with higher COVID‐19 mortality? Int J Infect Dis 2020; 96: 459–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phan T. Genetic diversity and evolution of SARS‐CoV‐2. Infection, Genetics and Evolution 2020; 81: 104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poh CM, Carissimo G, Wang B et al Two linear epitopes on the SARS‐CoV‐2 spike protein that elicit neutralising antibodies in COVID‐19 patients. Nat Commun 2020; 11: 2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Guo X, Xin Q et al Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis 2020; 71: 2688–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao J, Yuan Q, Wang H et al Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020; 71: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnes CO, West AP, Huey‐Tubman KE et al Structures of human antibodies bound to SARS‐CoV‐2 spike reveal common epitopes and recurrent features of antibodies. Cell 2020; 182: 828–842. e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CY‐P, Lin RTP, Renia L, Ng LFP. Serological approaches for COVID‐19: epidemiologic perspective on surveillance and control. Front Immunol 2020; 11: 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C, Gao Z, Shen K et al Safety and efficiency of endoscopic resection versus laparoscopic resection in gastric gastrointestinal stromal tumours: a systematic review and meta‐analysis. Eur J Surg Oncol 2020; 46: 667–674. [DOI] [PubMed] [Google Scholar]
- 23. Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. J Virol 2010; 84: 9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ojosnegros S, Beerenwinkel N. Models of RNA virus evolution and their roles in vaccine design. Immunome research 2010; 6(Suppl 2): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klumpp‐Thomas C, Kalish H, Hicks J et al D614G spike variant does not alter IgG, IgM, or IgA spike seroassay performance. medRxiv 2020. 2020.2007.2008.20147371.
- 26. Hicks J, Klumpp‐Thomas C, Kalish H et alSerologic cross‐reactivity of SARS‐CoV‐2 with endemic and seasonal Betacoronaviruses. medRxiv the preprint server for health sciences 2020. 2020.2006.2022.20137695.
- 27. Klingler J, Weiss S, Itri V et al Role of IgM and IgA antibodies in the neutralization of SARS‐CoV‐2. medRxiv 2020. 2020.08.18.20177303.
- 28. Weissman D, Alameh MG, de Silva T et al D614G spike mutation increases SARS CoV‐2 susceptibility to neutralization. Cell Host Microbe 2020. 10.1016/j.chom.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arvin AM, Fink K, Schmid MA et al A perspective on potential antibody‐dependent enhancement of SARS‐CoV‐2. Nature 2020; 584: 353–363. [DOI] [PubMed] [Google Scholar]
- 30. Pung R, Chiew CJ, Young BE et al Investigation of three clusters of COVID‐19 in Singapore: implications for surveillance and response measures. Lancet 2020; 395: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amrun SN, Lee CY‐P, Lee B et al Linear B‐cell epitopes in the spike and nucleocapsid proteins as markers of SARS‐CoV‐2 exposure and disease severity. EBioMedicine 2020; 58: e102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darnell ME, Taylor DR. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion 2006; 46: 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quick J.nCoV‐2019 sequencing protocol v1 protocols.io. [updated 26 August 2020]. Available from: https://www.protocols.io/view/ncov‐2019‐sequencing‐protocol‐v3‐locost‐bh42j8ye/metadata
- 34. Bushnell B. BBMap: A fast, accurate, splice‐aware aligner. Ernest Orlando Lawrence Berkeley National Laboratory, Berkeley, CA. Conference: 9th Annual Genomics of Energy & Environment Meeting, Walnut Creek, CA. 2014. Report No. LBNL‐7065E. Available from: https://www.osti.gov/servlets/purl/1241166.
- 35. Goh YS, Chavatte JM, Lim JLA et al Sensitive detection of total anti‐spike antibodies and isotype switching in asymptomatic and symptomatic COVID‐19 patients. Cell Rep Med 2021. 10.2139/ssrn.3713507 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


